Abstract
To elucidate the potential function of lncRNA CACS15 in the progression of ovarian cancer (OC) and its underlying mechanism. CACS15 level in OC tissues and cell lines was determined by qRT-PCR. Correlation between CACS15 level and survival of OC patients was analyzed through Kaplan-Meier method. Regulatory effects of CACS15 on cellular behaviors of OC cells were evaluated through CCK-8 and Transwell assay. Subsequently, RIP and RNA pull-down were performed to uncover the interaction between CACS15 and EZH2. Through ChIP assay, the interaction between EZH2 and APC was illustrated. A series of rescue experiments were finally conducted to elucidate the role of CACS15/APC axis in the malignant progression of OC. CACS15 was upregulated in OC tissues and cell lines relative to matched ones. High-level of CACS15 predicted worse survival in OC patients. Knockdown of CACS15 attenuated proliferative, migratory and invasive abilities of OC cells. CACS15 was mainly distributed in cytoplasm of OC cells, which was interacted with EZH2 at post-transcriptional level. Knockdown of CACS15 reduced the occupancies of EZH2 and H3K27me3 in APC promoter regions. Notably, knockdown of APC could reverse the regulatory effect of CACS15 on cellular behaviors of OC cells. LncRNA CACS15 inhibits the expression of APC by recruiting EZH2, thus accelerating the progression of ovarian cancer as an oncogene.
Keywords: Ovarian cancer, LncRNA CACS15, EZH2, APC, metastasis
Introduction
Ovarian cancer (OC) has a high incidence in female reproductive organs secondary to cervical cancer and endometrial cancer [1]. Epithelial cancer is the most common subtype of ovarian malignancies. Its mortality remains high, posing a serious threat to female health [2]. Owing to the deep location of ovaries in the pelvic cavity, small size and lack of typical symptoms, the diagnosis of early-stage OC is a big challenge nowadays [3]. Most of OC patients have already developed metastases in the pelvic and abdominal cavity at the time of diagnosis.
EZH2 is highly expressed in many types of malignant tumors [4-7]. It participates in the occurrence and progression of tumors via mediating tumor cell behaviors [8]. EZH2 could indirectly mediate cellular activities through targeting its downstream genes as well [9].
LncRNA is a functional non-coding RNA with over 200 nucleotides [10]. A growing number of evidences have proved the involvement of lncRNAs in pathological processes, especially in tumorigenesis [11]. Dysregulated lncRNAs are usually observed in tumor tissues. They are capable of mediating malignant phenotypes of tumor cells, and thus influence the progression and metastasis of tumors [12]. The vital function of lncRNAs in ovarian cancer has been extensively concerned [13,14]. LncRNA ABHD11-AS1 influences the occurrence and progression of epithelial OC through targeting RhoC [15]. By inducing the formation of inflammasomes, lncRNA GAS5 suppresses tumor progression of OC [13]. LncRNA SNHG15 serves as an oncogene that predicts the poor prognosis of epithelial OC [16]. LncRNA JPX predicts the poor prognosis of OC patients, which accelerates tumor cells to proliferate, migrate and invade through PI3K/Akt/mTOR pathway [17].
LncRNA CACS15 is upregulated in many tumors, including breast cancer, colorectal cancer, osteosarcoma and bladder cancer [18,19]. Its role in OC, however, remains unclear. This study aims to uncover the function of lncRNA CACS15 in regulating the malignant progression of OC and the possible mechanism.
Methods
Sample collection
OC tissues (n = 58) were collected from OC patients undergoing the surgery for the first time in the Second Hospital of Shandong University from April 2016 to October 2018. Normal ovarian tissues (n = 30) were harvested from healthy controls. None of enrolled patients were treated with anti-tumor therapy before surgery. The experiment was approved by the Medical Ethics Committee of the Second Hospital of Shandong University and patients were informed consent.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA), quantified and purified by UV spectrophotometer. RNA was reversely transcribed into cDNA. QRT-PCR was performed under the conditions at 94°C for 5 min, followed by 40 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 90 s.
Cell culture and transfection
OC cell lines (SKOV3, HO8910, A2780) and ovarian cell line (IOSE) were provided by ATCC, USA. Cells were cultured in Roswell Park Memorial Institute 1640 (RPMI 1640) containing 10% FBS (fetal bovine serum) (Gibco, Rockville, MD, USA) and 1% penicillin/streptomycin, and preserved in a 37°C, 5% CO2 incubator. One day prior to transfection, cells were seeded into a 6-well plate with 1 × 104 cells per well. Until 75-85% of confluence, cell transfection was performed using LipofectamineTM 2000. Complete medium was replaced at 4-6 h. Transfected cells for 24-48 h were harvested for the following experiments. The small inference RNA were siRNA-NC 5’-ACUACCGUUGUUAUAGGUGTT-3’, si-CASC15 1# 5’-GTGACACAGTTAACTTAAATT-3’, si-CASC15 2# 5’-GAATTGAACACACAGTTTTAT-3’.
Cell counting kit (CCK-8)
Transfected cells were seeded in a 96-well plate with 2 × 103 cells per well. At the appointed time points, 10 μL of CCK-8 (Beyotime Biotechnology, Shanghai, China) solution was applied per well. After incubation for 2 hours, the recorded absorbance at 450 nm using a microplate reader was used for plotting the growth curve.
Transwell assay
After 48 hours of transfection, cells were digested and resuspended in serum-free medium. Cell density was adjusted to 1.0 × 106/ml. Transwell chambers with pre-coated Matrigel were placed in 24-well plates. 200 μl of cell suspension and 500 μl of medium containing 10% FBS were added in the upper and lower chamber, respectively. After cell culture for 48 h, cells were fixed with 4% paraformaldehyde for 15 min and stained with crystal violet for 15 min. Inner cells were carefully cleaned. Penetrating cells were captured in 5 randomly selected fields of each sample. Cell invasion procedures were as the same as the above except for Matrigel pre-coating.
Western blot
Proteins were extracted from cells and loaded for electrophoresis and transferred on a polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) membrane. After blocking in 5% skim milk for 2 hours, membranes were subjected to incubation with primary antibodies at 4°C overnight and secondary antibodies for 2 hours. Bands were exposed by electrochemiluminescence (ECL) (Pierce, Rockford, IL, USA) and analyzed by Image Software.
RNA pull-down assay
T7 RNA polymerase (Promega, Madison, WI, USA) and biotin RNA tagged mixtures (Roche, Indianapolis, IN, USA) were used to label the transcriptional CACS15 in vitro. Biotinylation CACS15 was denaturalized at 90°C for 2 min, placed on ice for another 2 min and incubated with RNA binding buffer for 25 min. The biotin-coupled RNA complex was pulled down by incubating the cell lysates with streptavidin-coated beads. The expression of CACS15 in the bound fraction was detected by Western blot.
Chromatin immunoprecipitation (ChIP)
Cells were cross-linked with 1% formaldehyde for 10 min at room temperature. Subsequently, the cross-linked cells were lysed using lysis buffer and sonicated for 30 min. Finally, the sonicated lysate was immuno-precipitated with antibodies and IgG.
RNA immunoprecipitation (RIP)
Cells were collected and treated according to the procedures of Millipore Magna RIPTM RNA-Binding Protein Immunoprecipitation Kit. Cell lysate was incubated with anti-EZH2 or IgG antibody at 4°C for 6 h. A protein-RNA complex was captured and digested with 0.5 mg/ml proteinase K containing 0.1% sodium dodecyl sulphate (SDS) to extract RNA. The magnetic beads were repeatedly washed with RIP washing buffer to remove non-specific adsorption as much as possible. Finally, the extracted RNA was subjected to mRNA level determination using qRT-PCR.
Statistical analysis
SPSS 19.0 (SPSS IBM, Armonk, NY USA) was used for all statistical analysis. Data were represented as mean ± SD. The t-test was used for analyzing intergroup differences. Kaplan-Meier method was introduced for survival analysis. P < 0.05 indicated the significant difference.
Results
CACS15 was upregulated in OC and negatively correlated to the disease prognosis
Compared with the 30 cases of normal ovarian tissues, CACS15 was upregulated in 58 cases of OC tissues (Figure 1A). Identically, CACS15 was highly expressed in OC cell lines (Figure 1B). Kaplan-Meier method was introduced for analyzing the prognosis of OC. Both overall survival and disease-free survival were worse in OC patients with high level of CACS15 relative to those with low level of CACS15 (Figure 1C, 1D). It is suggested that CACS15 may be involved in the progression of OC.
Figure 1.
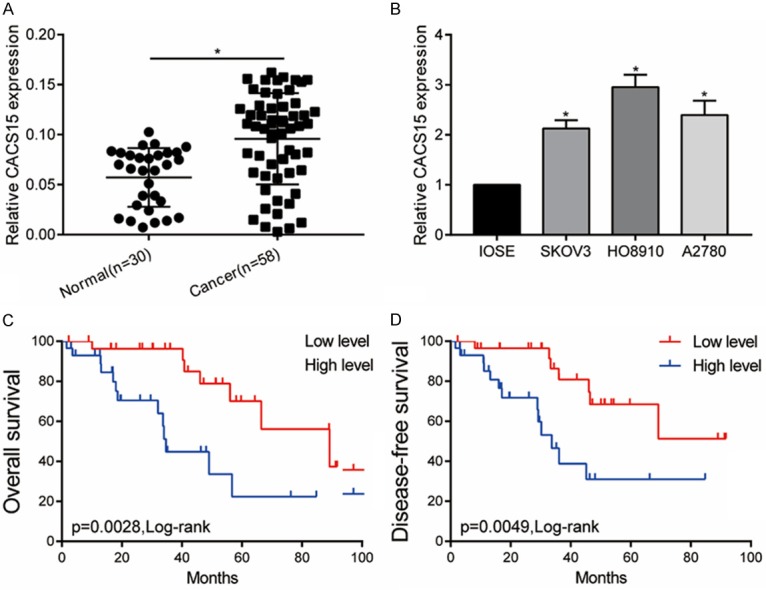
CACS15 was upregulated in OC and negatively correlated to the disease prognosis. A. Relative level of CACS15 in 30 cases of normal ovarian tissues and 58 cases of OC tissues. B. Relative level of CACS15 in normal ovarian cancer cell line (IOSE) and OC cell lines (SKOV3, HO8910, A2780). C. Kaplan-Meier introduced for analyzing the overall survival in OC patients with high level and low level of CACS15. D. Kaplan-Meier introduced for analyzing the disease-free survival in OC patients with high level and low level of CACS15.
Knockdown of CACS15 suppressed OC cells to proliferate, migrate and invade
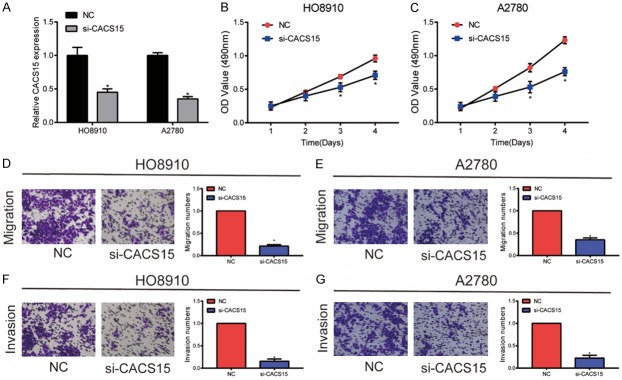
Transfection of si-CACS15 markedly downregulated CACS15 level in HO8910 and A2780 cells (Figure 2A). CCK-8 assay revealed the inhibited viability in OC cells transfected with si-CACS15 (Figure 2B, 2C). Transwell assay demonstrated that knockdown of CACS15 attenuated migratory and invasive abilities in HO8910 cells (Figure 2D, 2F). Similarly, inhibited migratory and invasive abilities were identified in A2780 cells with CACS15 knockdown (Figure 2E, 2G).
Figure 2.
Knockdown of CACS15 suppressed OC cells to proliferate, migrate and invade. A. Transfection efficacy of si-CACS15 in HO8910 and A2780 cells. B. CCK-8 assay revealed the viability in HO8910 cells transfected with NC or si-CACS15. C. CCK-8 assay revealed the viability in A2780 cells transfected with NC or si-CACS15. D. Transwell assay revealed the migration in HO8910 cells transfected with NC or si-CACS15. E. Transwell assay revealed the migration in A2780 cells transfected with NC or si-CACS15. F. Transwell assay revealed the invasion in HO8910 cells transfected with NC or si-CACS15. G. Transwell assay revealed the invasion in A2780 cells transfected with NC or si-CACS15.
CACS15 inhibited APC transcription via recruiting EZH2
To uncover the regulatory mechanism of CACS15 in OC, cellular distribution of CACS15 was first determined by qRT-PCR. As the data showed, CACS15 was mainly distributed in the cytoplasm of HO8910 cells, suggesting the regulatory role of CACS15 at post-transcriptional level (Figure 3A). Recent studies have indicated a novel regulatory network of lncRNA-protein to mediate gene expression [17]. Both RIP and RNA pull-down assay confirmed the interaction between EZH2 and CACS15 (Figure 3B, 3C). A previous study has suggested that EZH2 could suppress APC transcription in liver cancer [18]. Here, we speculated that CACS15 may inhibit APC transcription in OC via interacting with EZH2. Both mRNA and protein levels of APC were upregulated in OC cells transfected with si-CACS15 (Figure 3D, 3E). In addition, ChIP assay verified that EZH2 could bind to promoter regions of APC, thus inducing H3K27me3 modification in OC cells (Figure 3F, 3G). Notably, knockdown of CACS15 attenuated the enrichment of EZH2 and H3K27me3 in the promoter regions of APC (Figure 3H, 3I). Collectively, CACS15 interacted with EZH2 to suppress APC transcription in OC.
Figure 3.
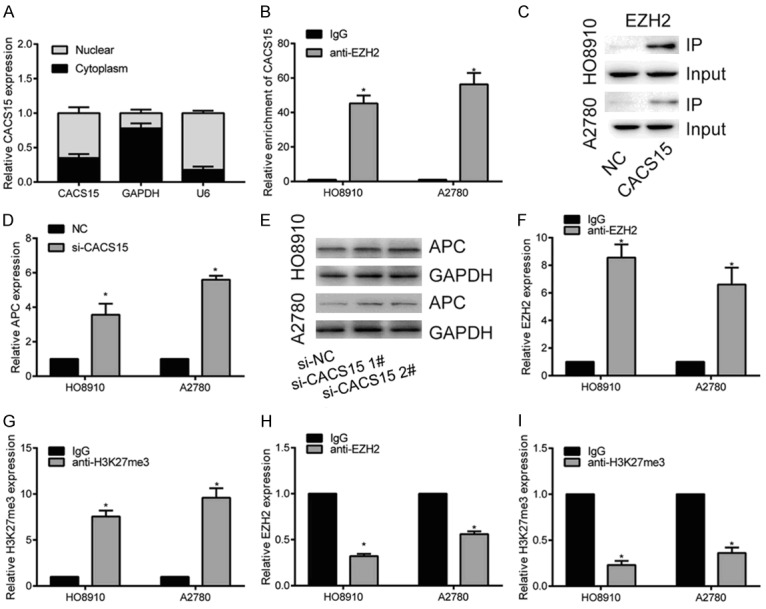
CACS15 inhibited APC transcription via recruiting EZH2. A. Cellular distribution of CACS15 in HO8910 cells. U6 was the internal reference for nucleus and GAPDH was for cytoplasm. B. RIP assay revealed the relative enrichment of CACS15 in immunoprecipitates with IgG or anti-EZH2. C. RNA pull-down assay revealed the protein level of CACS15 in IP and input of HO8910 and A2780 cells. D. Relative level of APC in HO8910 and A2780 cells transfected with NC or si-CACS15. E. Protein level of APC in HO8910 and A2780 cells transfected with NC or si-CACS15 (1# and 2#). F. ChIP assay revealed the relative level of EZH2 in APC promoter regions. G. ChIP assay revealed the relative level of H3K27me3 in APC promoter regions. H. EZH2 occupancy in APC promoter regions after transfection of si-CACS15 of HO8910 and A2780 cells. I. H3K27me3 occupancy in APC promoter regions after transfection of si-CACS15 of HO8910 and A2780 cells.
Knockdown of APC and CACS15 suppressed OC cells to proliferate, migrate and invade
A series of rescue experiments were conducted to explore the involvement of APC in CACS15-mediated malignant progression of OC. The upregulated APC in OC cells transfected with si-CACS15 was greatly downregulated after co-transfection of si-APC (Figure 4A). Transfection of si-CACS15 markedly inhibited the viability in OC cells, which was reversed by APC knockdown (Figure 4B, 4C). Identically, the inhibited migratory and invasive abilities in OC cells with CACS15 knockdown were reversed after APC knockdown (Figure 4D-G). Therefore, it is concluded that CACS15 exerted its carcinogenic role in OC via regulating APC.
Figure 4.
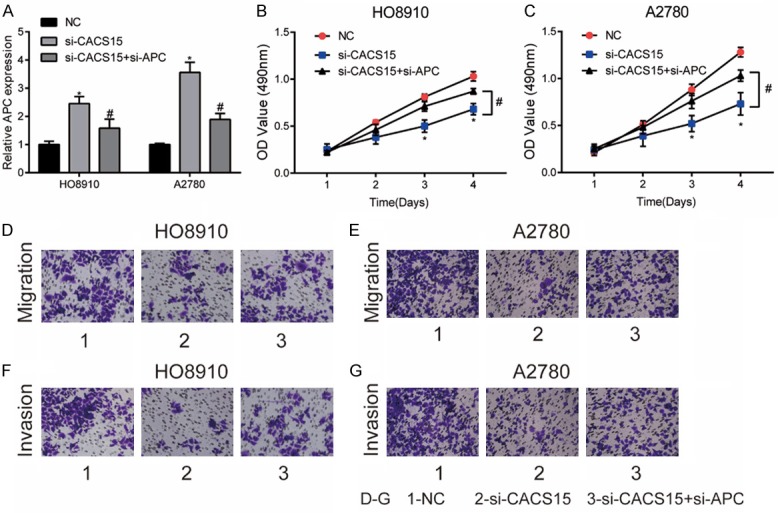
Knockdown of APC and CACS15 suppressed OC cells to proliferate, migrate and invade. HO8910 and A2780 cells were transfected with NC, si-CACS15 or si-CACS15+si-APC. A. Relative level of APC in each group. B, C. CCK-8 assay revealed the viability in each group. D, E. Transwell assay revealed the migration in each group. F, G. Transwell assay revealed the invasion in each group.
Discussion
Molecular mechanisms underlying the occurrence and progression of OC are very complex [20]. This study mainly explored the biological function of lncRNA CACS15 in the progression of OC.
LncRNAs are important regulators in tumors, including OC [21]. Increasing evidences have confirmed the crucial impact of lncRNAs on the malignant progression of OC [22]. For example, overexpression of lncRNA FER1L4 suppresses paclitaxel-resistance in OC cells via MAPK pathway [23]. It is reported that lncRNA FEZF1-AS1 stimulates the proliferative ability and inhibits apoptosis of OC cells through activating JAK-STAT3 pathway [24]. LncRNA myocardial infarction-associated transcript accelerates proliferation and suppresses apoptosis of epithelial OC by sponging miR-330-5p [25].
Previous studies have illustrated the great abundance of lncRNA CACS15 in tumors as an oncogene, which predicts a poor prognosis. In this study, CACS15 was upregulated in OC tissues and cell lines. Knockdown of CACS15 markedly attenuated proliferative, migratory and invasive abilities of OC cells. It is confirmed that CACS15 exerted a carcinogenic role in OC.
LncRNAs are able to influence tumor behaviors via different mechanisms. EZH2 was verified here to bind to CACS15, which further promoted the promoter methylation to inhibit gene expression of APC. APC is a key component in the Wnt transduction signaling. APC inactivation leads to degradation dysfunction of β-catenin. Free β-catenin accumulates in the cytoplasm and thereafter translocates into the nucleus, where Tcf/Lef is activated. Subsequently, activation of Tcf/Lef results in transcription abnormalities in c-myc, c-jun and Cyclin D1, eventually leading to cell malignancy [26]. Sohlh2 functions as a tumor suppressor in breast cancer, which alleviates tumor progression by APC upregulation to repress Wnt/β-catenin signaling pathway [27]. Mutations of APC in the distal oviduct result in precursor lesions that further develops into ovarian tumors [28]. A previous study demonstrated that EZH2 in liver cancer led to transcription inhibition of APC, a classical negative regulator in Wnt/β-catenin pathway [29]. We speculated that CACS15 may regulate EZH2 to further inhibit APC expression in OC. Our studies indicated that lncRNA CACS15 knockdown markedly upregulated APC expression in OC cells. Furthermore, lncRNA CACS15 knockdown reduced the occupancies of EZH2 and H3K27me3 in APC promoter. Notably, transfection of si-APC reversed the carcinogenic role of CACS15. It is concluded that upregulated CACS15 in OC accelerated the malignant progression through mediating EZH2-induced epigenetic inhibition of APC.
To sum up, our results demonstrated the upregulated lncRNA CACS15 was closely related to clinical characteristics of OC, which accelerated the progression of ovarian cancer through inhibiting APC. However, the deeper mechanism should be uncovered in the future. Our research suggested that lncRNA CACS15 may be a promising therapeutic target of OC.
Conclusions
LncRNA CACS15 inhibits the expression of APC by recruiting EZH2, thus accelerating the progression of ovarian cancer as an oncogene.
Disclosure of conflict of interest
None.
References
- 1.Liu J, Zhang X, Huang Y, Zhang Q, Zhou J, Zhang X, Wang X. miR-200b and miR-200c co-contribute to the cisplatin sensitivity of ovarian cancer cells by targeting DNA methyltransferases. Oncol Lett. 2019;17:1453–1460. doi: 10.3892/ol.2018.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JY, Cho CH, Song HS. Targeted therapy of ovarian cancer including immune check point inhibitor. Korean J Intern Med. 2017;32:798–804. doi: 10.3904/kjim.2017.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin B, Xu W, Li Y. Are omentectomy and lymphadenectomy necessary in patients with apparently early-stage malignant ovarian germ cell tumors? Int J Gynecol Cancer. 2019 doi: 10.1136/ijgc-2018-000078. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Lu B, Shi H. An in-depth look at small cell carcinoma of the ovary, hypercalcemic type (SCCOHT): clinical implications from recent molecular findings. J Cancer. 2019;10:223–237. doi: 10.7150/jca.26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y, Xu L, Chang Y, Zeng T, Chen X, Wang A, Groth J, Foo WC, Liang C, Hu H, Huang J. N-Myc promotes therapeutic resistance development of neuroendocrine prostate cancer by differentially regulating miR-421/ATM pathway. Mol Cancer. 2019;18:11. doi: 10.1186/s12943-019-0941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng H, Guan X, Gong L, Zeng J, Zhang H, Chen MY, Li G. CBX6 is negatively regulated by EZH2 and plays a potential tumor suppressor role in breast cancer. Sci Rep. 2019;9:197. doi: 10.1038/s41598-018-36560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, Chen X, Lin K, Zeng K, Liu X, Xu X, Pan B, Xu T, Sun L, He B, Pan Y, Sun H, Wang S. lncRNA SNHG6 regulates EZH2 expression by sponging miR-26a/b and miR-214 in colorectal cancer. J Hematol Oncol. 2019;12:3. doi: 10.1186/s13045-018-0690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, Riquelme E, Galindo H, Moran CA, Kalhor N, Swisher SG, Simon GR, Stewart DJ, Lee JJ, Wistuba II. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–6565. doi: 10.1158/1078-0432.CCR-12-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Gu M, Liu C, Wan X, Shi Q, Chen Q, Wang Z. Long noncoding RNA FOXC2-AS1 facilitates the proliferation and progression of prostate cancer via targeting miR-1253/EZH2. Gene. 2019;686:37–42. doi: 10.1016/j.gene.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 10.Su W, Tang J, Wang Y, Sun S, Shen Y, Yang H. Long non-coding RNA highly up-regulated in liver cancer promotes epithelial-to-mesenchymal transition process in oral squamous cell carcinoma. J Cell Mol Med. 2019;23:2645–2655. doi: 10.1111/jcmm.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:3850–3856. [PubMed] [Google Scholar]
- 12.Zhang H, Feng X, Zhang M, Liu A, Tian L, Bo W, Wang H, Hu Y. Long non-coding RNA CASC2 upregulates PTEN to suppress pancreatic carcinoma cell metastasis by downregulating miR-21. Cancer Cell Int. 2019;19:18. doi: 10.1186/s12935-019-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Yang C, Li Y, Chen A, Li L, You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci Rep. 2017 doi: 10.1042/BSR20171150. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu JJ, Lin XJ, Zheng TT, Tang XY, Hua KQ. Natural antisense transcript of hypoxia-inducible factor 1 regulates hypoxic cell apoptosis in epithelial ovarian cancer. Onco Targets Ther. 2018;11:9101–9110. doi: 10.2147/OTT.S173816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu DD, Chen X, Sun KX, Wang LL, Chen S, Zhao Y. Role of the lncRNA ABHD11-AS1 in the tumorigenesis and progression of epithelial ovarian cancer through targeted regulation of RhoC. Mol Cancer. 2017;16:138. doi: 10.1186/s12943-017-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu C, Dai C, Guo Y, Qin R, Liu J. Long noncoding RNA SNHG15 serves as an oncogene and predicts poor prognosis in epithelial ovarian cancer. Onco Targets Ther. 2019;12:101–111. doi: 10.2147/OTT.S182657. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li J, Feng L, Tian C, Tang YL, Tang Y, Hu FQ. Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:8135–8144. doi: 10.26355/eurrev_201812_16505. [DOI] [PubMed] [Google Scholar]
- 18.Gao R, Fang C, Xu J, Tan H, Li P, Ma L. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch Biochem Biophys. 2019;663:183–191. doi: 10.1016/j.abb.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Kong D, Li C, Yang Q, Wei B, Wang L, Peng C. Long noncoding RNA LSINCT5 acts as an oncogene via increasing EZH2-induced inhibition of APC expression in osteosarcoma. Biochem Biophys Res Commun. 2018;507:193–197. doi: 10.1016/j.bbrc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28:i61–i65. doi: 10.1093/annonc/mdx443. [DOI] [PubMed] [Google Scholar]
- 21.Mitra R, Chen X, Greenawalt EJ, Maulik U, Jiang W, Zhao Z, Eischen CM. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun. 2017;8:1604. doi: 10.1038/s41467-017-01781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao N, Yu L, Zhu B, Gan HY, Guo BQ. LncRNA GIHCG promotes development of ovarian cancer by regulating microRNA-429. Eur Rev Med Pharmacol Sci. 2018;22:8127–8134. doi: 10.26355/eurrev_201812_16504. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zou B, Tian T, Luo X, Mao B, Zhang X, Lei H. Overexpression of the lncRNA FER1L4 inhibits paclitaxel tolerance of ovarian cancer cells via the regulation of the MAPK signaling pathway. J Cell Biochem. 2018 doi: 10.1002/jcb.28032. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Cheng Z, Wang J. Long noncoding RNA FEZF1-AS1 promotes proliferation and inhibits apoptosis in ovarian cancer by activation of JAK-STAT3 pathway. Med Sci Monit. 2018;24:8088–8095. doi: 10.12659/MSM.911194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao S, Tian J, Zhang H, Wang S. LncRNA myocardial infarction-associated transcript promotes cell proliferation and inhibits cell apoptosis by targeting miR-330-5p in epithelial ovarian cancer cells. Arch Med Sci. 2018;14:1263–1270. doi: 10.5114/aoms.2018.75535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Liu R, Zhao N, Ji S, Hao C, Cui W, Zhang R, Hao J. Sohlh2 inhibits breast cancer cell proliferation by suppressing Wnt/beta-catenin signaling pathway. Mol Carcinog. 2019;58:1008–1018. doi: 10.1002/mc.22989. [DOI] [PubMed] [Google Scholar]
- 28.van der Horst PH, van der Zee M, Heijmans-Antonissen C, Jia Y, DeMayo FJ, Lydon JP, van Deurzen CH, Ewing PC, Burger CW, Blok LJ. A mouse model for endometrioid ovarian cancer arising from the distal oviduct. Int J Cancer. 2014;135:1028–1037. doi: 10.1002/ijc.28746. [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Lin J, Qin F, Yang Z, Ding Y, Zhang Y, Han L, Zhu X, Zhang Q. LncAPC drives Wnt/beta-catenin activation and liver TIC self-renewal through EZH2 mediated APC transcriptional inhibition. Mol Carcinog. 2018;57:408–418. doi: 10.1002/mc.22764. [DOI] [PubMed] [Google Scholar]