Abstract
Lycium barbarum polysaccharides (LBP) is commonly known as a traditional Chinese medicine, which has protective effects against diabetic complications in clinic, such as diabetic retinopathy (DR). Previous studies have revealed that Rho/ROCK pathway play an important role in DR development. However, the mechanism between LBP and DR remains unknown. This study aims to explore the clear mechanism of the protective effect of LBP in diabetic retinopathy. In this study, streptozocin (STZ, 45 mg/kg) was administered for diabetic rats modeling. Weight, blood glucose levels and blood lipid were measured to assess the metabolic changes by LBP on diabetic rats. Evans blue (EB) extravasation was determined to assess blood-retinal barrier (BRB) disruption. Hematoxylin and Eosin (HE) staining and immunohistochemistry assay were applied for retina morphology exploration. The membranous disks of retina were examined by transmission electron microscope. Further, high glucose condition was induced in choroidal-retinal endothelial cells (RF/6A). Western blotting was performed for P-Occludin, ROCK1 and P-MLC protein expression. The results indicated that the blood glucose levels, blood lipid and EB infiltration capacity were decreased while the weight was increased in LBP-treated diabetic rats compared with model rats. Moreover, LBP could thicken the overall retina, prevent the disturbance of photoreceptor cell membranous disks and inhibit pathological angiogenesis in diabetes. In addition, the decreased expression of P-Occludin and increased expression of RhoA-associated protein kinase (ROCK) or phosphorylated myosin light chain (P-MLC) were observed in retinal tissue of diabetic rats and high glucose induced by RF/6A cells, which could be rescued by LBP and/or Fasudil. LBP has the protective effects on blood-retinal barrier by regulating the Rho/ROCK signaling pathway in diabetic rats. LBP may be served as a Rho/ROCK inhibitor for the treatment of DR.
Keywords: Lycium barbarum, blood-retinal barrier, ROCK, diabetic mellitus, protective effect
Introduction
Diabetic mellitus (DM) is the most prevalent metabolic disorder, which also causes the long-term health problems of individuals, societies and nations around the world [1,2]. Diabetic retinopathy (DR), one of the most common complications of DM, is one of the major causes of vision loss all over the world. It has long been believed that DR is a microvascular complication associated with endothelial dysfunction, which is characterized by leakage of blood-retina barrier (BRB), thickening of the retinal capillary basement membrane, loss of pericyte and endothelial cell, disturbance of photoreceptor cell membranous disks and neovascularization [3-5]. However, it is difficult to timely control the level of blood glucose, blood pressure along with the lipids in clinic [6], and the present clinical treatments of diabetic retinopathy are limited to laser photocoagulation and vitrectomy [7]. Therefore, it is an urgent clinical need to investigating innovative therapies target for DR.
Lycium barbarum (Gouqizi, Wolfberry) is commonly known as a traditional Chinese medicine used for anti-tumor, anti-oxidant, anti-inflammatory, immunoregulatory, anti-ageing, hepatoprotective, anti-glaucoma and neuroprotective [8,9]. Lycium barbarum polysaccharide (LBP) is the major liquid fraction of Lycium barbarum. Recent studies have reported LBP mediated clinical antidiabetic potential and protective actions against diabetic complications such as nephritic and retinopathy effects in animal models. In vitro, LBP could upregulate the expression of anti-apoptotic protein Bcl-2 levels in lens epithelial cells and retinal pigment epithelial cells from oxidative stress-induced apoptosis [10,11]. However, the studies focusing on the mechanisms of LBP action in DR is still poor.
RhoA is a small, guanosine-5’-triphosphate binding protein, bound to the plasma membrane, which activates RhoA-associated protein kinase (ROCK). ROCK pathway has been reported to regulate the expression and function of intercellular adhesion molecule-1 (ICAM-1) in endothelial cells and could be activated in vascular cells by serum from diabetic retinopathy patients [12]. In addition, VEGF plays a critical role in the pathogenesis of DR-related hyperpermeability and angiogenesis [13]. ROCK inhibition could block VEGF induced endothelial hyperpermeability [14]. These observation suggested that endothelial cells in diabetic retinopathy patients could be in a “ROCK-activated status” at the systemic level [15]. ROCK contains two isoforms, ROCK1 and ROCK2, which occupy an overall homology of 65%. Recent studies have revealed that RhoA and its downstream target ROCK1 regulate cellular adherence, migration, proliferation, and apoptosis through the control of the actin cytoskeletal assembly and cell contraction [16,17]. Inhibition of RhoA/ROCK1 signaling significantly reduced endothelial damage and vascular leakage which is stimulated by high glucose/advanced glycation end products (AGEs) in cultured endothelial cells and in the presence of diabetes mellitus [18]. A recent paper by Zandi et al. showed that macrophage polarization was triggered by ROCK2 signaling [19]. ROCK-2 is expressed in RPE and endothelial cells but its cellular distribution is not modified by diabetes. Thus, Rho/ROCK pathway play an important role in DR development, ROCK inhibition could be a new strategy in the total management of DR from its early to late stages.
Therefore, in the present study, we investigated the role of LBP in DR protection. Further, we clarified potential mechanisms by which LBP regulated DR in diabetic rats and choroidal-retinal endothelial cells.
Material and methods
Animals
A total of 152 rats purchased from Department of Experimental Animal Science, Liaoning Medical College (8-12 weeks, 180-220 kg) were housed in 18-23°C with 60% humidity. All the animal experiments performed in this present study complied with the Ethical Rules applicable for Molecular and Cellular Biochemistry.
Induction of diabetes mellitus model
After starvation for 12 h, streptozotocin (STZ, 45 mg/kg, Sigma, USA) was freshly dissolved in a 0.1 mol/L citrate buffer (pH 4.5). Tail vein injection of STZ once per day was utilized to induce diabetic rat model, while the control rats were only injected with the citrate buffer. Moreover, the fasting blood-glucose more than 16.5 mmol/L with polyuria, polydipsia and intense hunger symptoms was defined as the successful diabetes mellitus rats (DM). Healthy rats received an injection of a corresponding volume of saline as control group (CON).
Group assignment and drug treatment
The experiments were divided into 3 parts. In part 1, rats were assigned into control group (CON, n=36), diabetes mellitus group (DM, n=36) and Lycium barbarum polysaccharide group (LBP, n=36). LBP (250 mg/kg/day) was administered by oral gavage daily for 12 weeks and was dissolved in normal saline (2 ml/day). The control and DM group rats received normal saline daily under similar conditions.
In part 2, rats were assigned into control group (CON, n=12), diabetes mellitus group (DM, n=12), Lycium barbarum polysaccharide group (LBP, n=12), Fasudil group (F, n=4) and Lycium barbarum polysaccharide + Fasudil group (LBP + F, n=4). LBP (250 mg/kg/day) and Fasudil (15 mg/kg/day) were respectively administered by oral gavage and intraperitoneal injection daily for 12 weeks and was dissolved in normal saline (2 ml/day). The control and DM group rats received normal saline daily under similar conditions.
In part 3, choroidal-retinal endothelial cells (RF/6A) were seeded into glucose added medium for 5 groups: Group A (glucose, 5.5 mmol/L), Group B (glucose, 30 mmol/L), Group C (glucose, 40 mmol/L), Group D (glucose, 30 mmol/L; LBP, 1 g/L) and Group E (glucose, 40 mmol/L; LBP, 1 g/L).
In overall study, LBP was purchased from the Ningxia Agricultural and Forestry College and the fasudil was purchased from Apexbio. The experimental design is presented in Figure 1.
Figure 1.
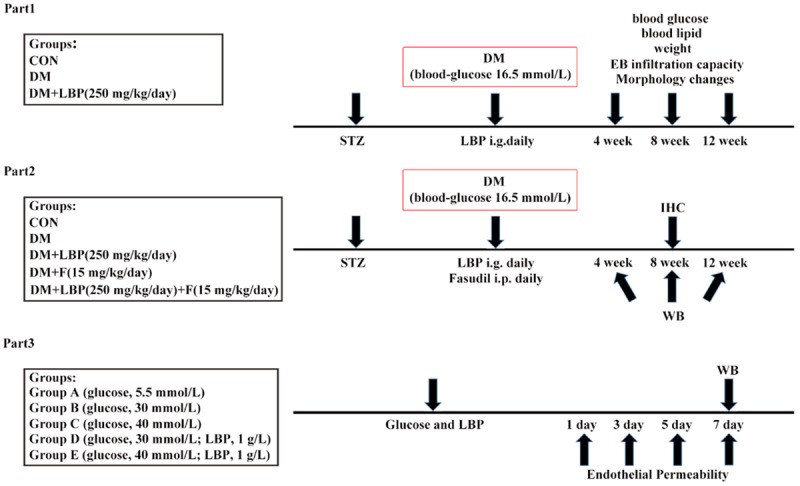
Study design and experimental groups. In part 1, STZ was injected once per day to induce DM. LBP was administered by oral gavage daily for 12 weeks. In part 2, STZ was injected once per day to induce DM. LBP and/or Fasudil were respectively administered by oral gavage and intraperitoneal injection daily for 12 weeks. In part 3, different level of glucose or 1 g/L LBP + glucose were added in medium of RF/6A cell. All animals and tissues were measured at 4, 8 and 12 weeks after LBP and/or Fasudil administration. Cells were measured at 3, 5 and 7 days after seeding in different level of glucose or 1 g/L LBP + glucose. STZ, streptozotocin; I.P, intraperitoneal; I.G, intragastric administration; WB, Western blot; IHC, Immunohistochemistry.
Blood-retinal barrier permeability
The tail vein injection of Evans Blue (EB: 45 mg/kg) was performed at the time point of 4, 8 and 12 weeks (w) after administration of LBP. 4 rats of each group (LBP, DM and CON groups) were anesthetized by intraperitoneal injection of 10% chloral hydrate (50 mg/kg). Then, the EB was administrated to rats by tail vein injection for 1 min. After 2 h circulation, eyeballs were taken out and the retina was detached from the eyeballs. Subsequently, the detached retinas were dried for 4 h within vacuum dryer and incubated with formamide (Sigma) for 18 h at 70°C. After 45 min centrifugation at 4°C with 12000 r/min, the 100 μl supernatant was collected for EB concentration determination using spectrophotometer at 620 nm and 740 nm wavelength. Finally, the EB infiltration capacity was defined as the content of EB (ng) in per retinal dry weight (mg).
Levels of blood glucose, weight and blood lipids in diabetic rats
Tail vein blood was taken for blood glucose detection by OneTouchll (Johnson & Johnson, USA). In addition, weight of rats was measured using a MPZOOB electronic balance. Moreover, triglyceride and total cholesterol concentration was determined employing Micro glycerin trilaurate (TG) content assay kit (BC0625) and Total cholesterol (TC) content assay kit (BC1980) purchased from Beijing solarbio life sciences Co., Ltd.
Hematoxylin and eosin staining
After fixation for 72 h, the 5 μm retina tissue paraffin slice was made employing paraffin slicing machine. Retina tissue paraffin slice was stained with hematoxylin for 5 min and eosin for 2 min at room temperature (Hematoxylin-Eosin Staining Kit, G1120, Beijing solarbi life sciences Co. Ltd., China) after dewaxing. The slice was sealed with neutral resin after washing. The morphology of retinal vessel was detected using light microscope.
Immunohistochemistry assay
The detached retina was fixed and digested as previously mentioned. The primary antibodies of VEGF (#P931; dilution 1:500) was purchased from Invitrogen. The primary antibodies of P-Occludin (#AF7069, 1:1000, Affinity Biosciences) and ROCK1 (ab45171, dilution 1:100, Abcam) were purchased from Abcam. The retinal vascular network was blocked with primary antibodies for 1 h and secondary antibodies (#21543; dilutiuon: 1:200, Wuhan Khayal Bio-Technology Co., Ltd, Wuhan, China) for 45 min at room temperature. Subsequently, sections were visualized with diaminobenzidine (DAB; Shanghai Jierdun Biotech) and counterstained with hematoxylin and eosin (H&E). Images were captured with a digital camera (Nikon).
Transmission electron microscopy
Retina was fixed in 1% osmic acid for 3 h and washed with ethyl alcohol and acetone under 4°C. Then, the retina was soaked in EPnosZl Epoxy resin impregnated mixed with 100% acetone (1:1) for 30 min and permeated with EPnosZl Epoxy resin embedding agent for 12 h. Subsequently, the retina was sliced and collected using copper mesh. The copper mesh with retina was stained with acetic acid and lead nitrate for 30 min respectively. The transmission electron microscope was used for the photoreceptor cell membranous disks observation.
Cell culture
Choroidal-retinal endothelial cells (RF/6A) were purchased from the Shanghai Institute for Biological Science, Chinese Academy of Sciences. Cells were subultured with MEM complete medium (containing 1% sodium pyruvate, 1% essential amino acid, 15% fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin) at 37°C with 5% CO2. Moreover, D-glucose and LBP were added in medium, which concentration and groups were mentioned above. After 1, 3, 5 and 7 days incubation, the morphology and density were observed using microscope.
Western blot assay
The detached retina was smashed with ultrasonic grinder and the protein was collected from the supernatant after the centrifugation at 4°C at with 12000 r/min for 30 min. Then, proteins were separated using 15% resolving gel. PVDF membrane was blocked with 5% skim milk prior to protein transfer. GAPDH was regarded as the internal reference. Furthermore, the membrane was incubated with primary antibodies of Occludin (#ab216327, dilution 1:1000, Abcam), ROCK1 (#ab45171, dilution 1:2000, Abcam), P-Occludin (#AF7069, 1:1000, Affinity Biosciences) and P-MLC (#3671, 1:1000, Cell Signaling Technology) for 1 h and secondary antibodies for 45 min at room temperature. ECL kit (#32209, Suzhou Biotsith Bioscience Co., Ltd, Suzhou, China) was applied for band visualization. The image was detected with ImageJ.
Measurement of endothelial permeability
According to the methods previously (6), RF/6A cells (3×105/well) were seeded into the transwell chamber (3 μm pore size, 12 mm diameter) with polycarbonate filter and cultured at 37°C with 5% CO2. The permeability detection was performed at 1, 3, 5 and 7 d incubation. 100 μl (0.34 mg/ml) HRP solution was added to each well and incubated for 5 min. Then, 50 μL cultures from the lower chamber was mixed with 850 μl HRP for 15 min at room temperature and the reaction was terminated by 100 μl H2SO4 (2 mol/l). Subsequently, the HRP absorbency was determined by microplate reader under 470 nm wavelength.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 (IBM, Armonk, NY, USA). The results are means ± S.D. Statistical comparisons of the results were performed using one-way ANOVA. Differences among treatments with a value of P<0.05 were considered to be statistically significant.
Results
LBP decreased blood glucose levels, blood lipid levels and EB infiltration capacity while increased the weight of diabetic rats
In this study, 54 rats were divided and treated as part 1. The blood glucose levels, blood lipid levels, weight and EB infiltration capacity were measured at 4, 8 and 12 weeks after LBP administration by gavage in STZ-induced rats. In Figure 2A, the blood glucose levels were significantly increased in DM and LBP groups compared with control group. And the blood glucose was reduced by 57% at 4 weeks, 40% at 8 weeks and 36% at 12 weeks in LBP-treated group compared with DM group. Furthermore, the weight of rats in DM group was dramatically lower than control group, which could be significantly rescued in LBP group (Figure 2B). For blood lipid level, the triglyceride was much higher in DM group compared with control group after 4 weeks, which could be significantly rescued in LBP group after 4 weeks (Figure 2C). And total cholesterol showed the similar tendency after 8 weeks (Figure 2D). In addition, as shown in Figure 2E, the EB infiltration capacity in DM group was increased compared with control group at 4, 8 and 12 weeks, which could be rescued in LBP group likewise. Therefore, these results implied that LBP was able to reduce the blood glucose levels, blood lipids levels, EB infiltration capacity and increase the weight of diabetic rats.
Figure 2.
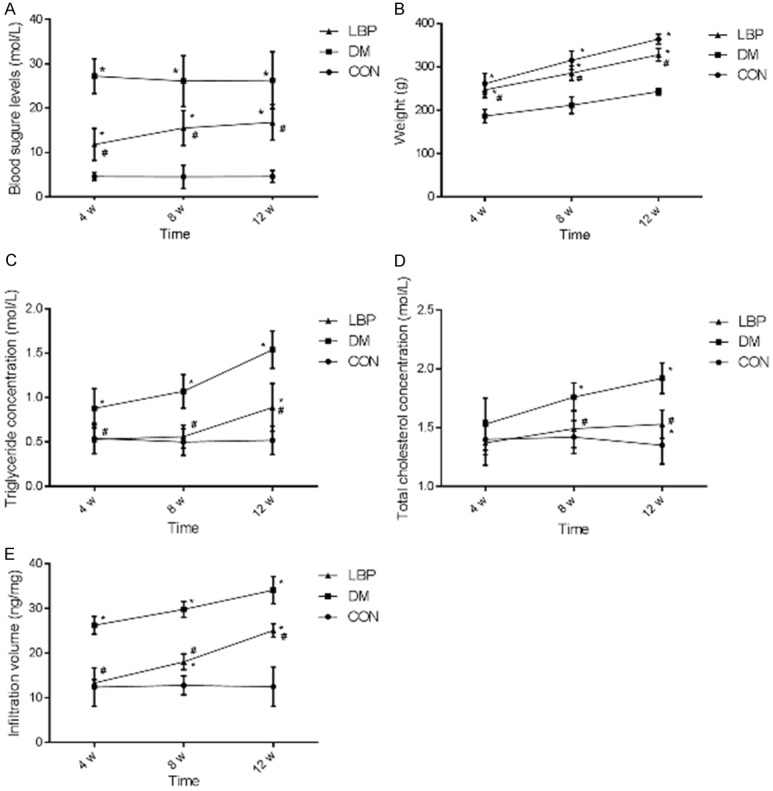
Effects of LBP on blood glucose levels, blood lipid levels, weight and EB infiltration capacity of diabetic rats. After the rats modeling and LBP treatment, the blood glucose level, weight, TG, TC and EB infiltration capacity was measured at the 4, 8 and 12 weeks in CON, DM and LBP groups as shown in (A-E) respectively. CON: control group treated with normal saline; DM: model group treated with normal saline; LBP: model group treated with LBP. Overall retina was shown in frame. *P<0.05 vs. CON group, #P<0.05 vs. DM group.
Effects of LBP on morphological changes of blood-retinal barrier in diabetic rats
In HE staining results, 54 rats were also divided and treated as part 1. As shown in Figure 3, compared with control group, the results showed that overall thickness of the retina in the DM group was reduced compared with control group at 12 weeks. However, the thickness of the overall retina in LBP treated group were almost the same as the control group at 4 and 8 weeks. Therefore, LBP may play protective roles in BRB in DM rats.
Figure 3.
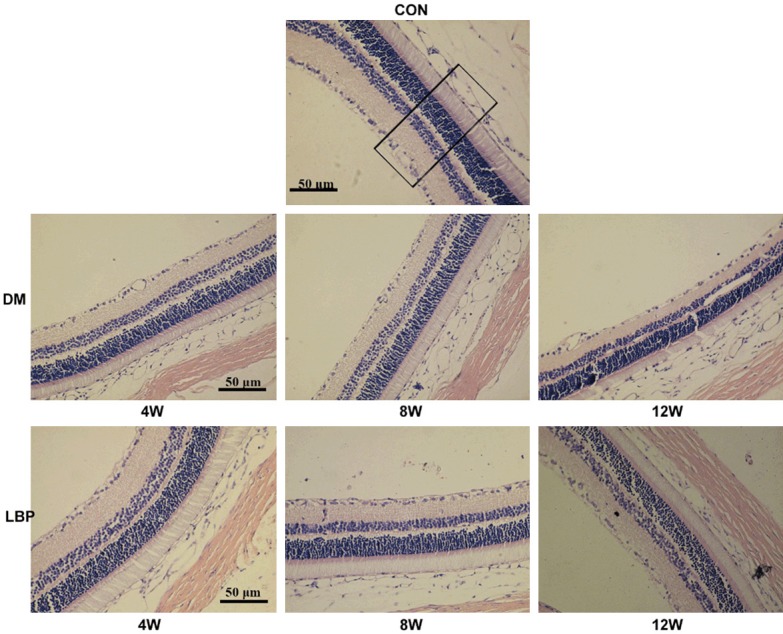
Effects of LBP on morphological changes of blood-retinal barrier in diabetic rats. Morphological changes were shown in HE-staining at 4, 8 and 12 weeks after administering LBP. CON: control group treated with normal saline; DM: model group treated with normal saline; LBP: model group treated with LBP. Overall retina was shown in frame.
In Figure 4, the results suggested the twisted capillary in DM group at 12 weeks. The branch point of DM group significantly increased compared with control, and this tendency could be reversed via adding LBP, especially at 12 weeks. LBP significantly decrease the angiogenesis induced by DM status. In addition, we could observe the obvious increase or decrease of VEGF expression in DM or LBP group indirectly and qualitatively, indicating that LBP might protect BRB by inhibiting the increase of VEGF expression.
Figure 4.
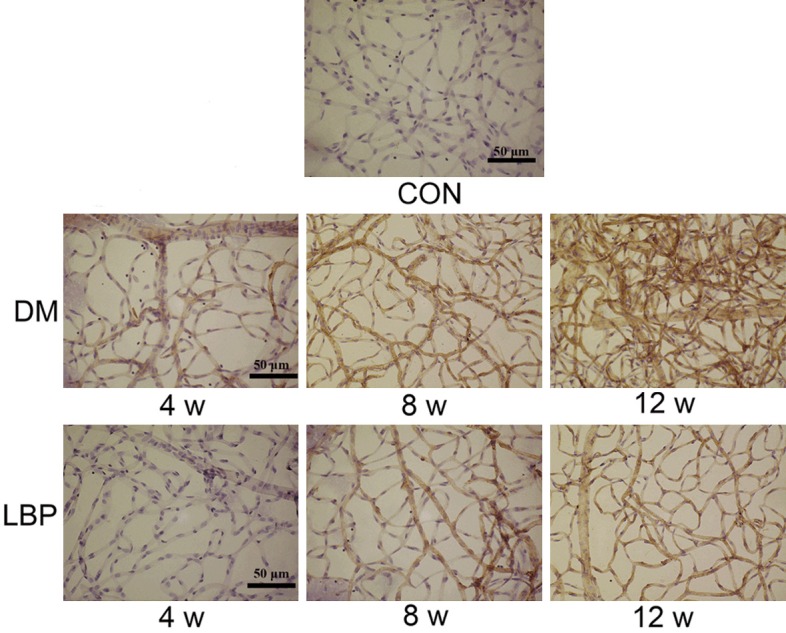
Immunohistochemistry of retinal vessel and VEGF expression. VEGF expression was observed at 4, 8 and 12 weeks after administering LBP. CON: control group treated with normal saline; DM: model group treated with normal saline; LBP: model group treated with LBP.
In Figure 5, the photoreceptor cell membranous disks showed orderliness, and their structure was discernible in control group. In DM group, the photoreceptor cell membranous disks appeared irregularity, frizzy and loose at 12 weeks. Nevertheless, LBP group improved the structure disturbance of photoreceptor cell membranous disks.
Figure 5.
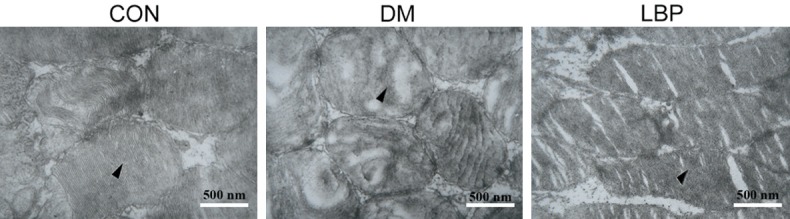
The structure of retina under electron microscope. After treatment for 12 week, the structure of retina was observed using electron microscope. CON: control group treated with normal saline; DM: model group treated with normal saline; LBP: model group treated with LBP.
Effect of LBP on Rho/ROCK1 pathway in diabetic rats
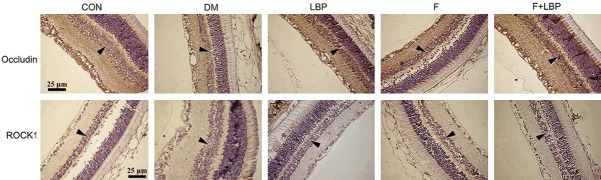
Followed by part 2, the immunohistochemistry assay was conducted to evaluate the expression of P-Occludin and ROCK1 in inner nuclear layer. Our results were shown decreased expression of P-Occludin and increased expression of ROCK1 during the progression of diabetes after 8 weeks (Figure 6). However, LBP induced the significant increase of P-Occludin and decrease of ROCK1 in LBP group compared with DM group. Additionally, the expression of P-Occludin and ROCK1 in LBP group was similar to Fasudil group.
Figure 6.
Immunohistochemistry (IHC) assay for P-Occludin and ROCK1 protein expression. The IHC assay was performed at 4, 8 and 12 weeks after administration. CON: control group treated with normal saline; DM: model group treated with normal saline; LBP: model group treated with LBP; F: model group treated with fasudil; F + LBP: model group treated with fasudil and LBP.
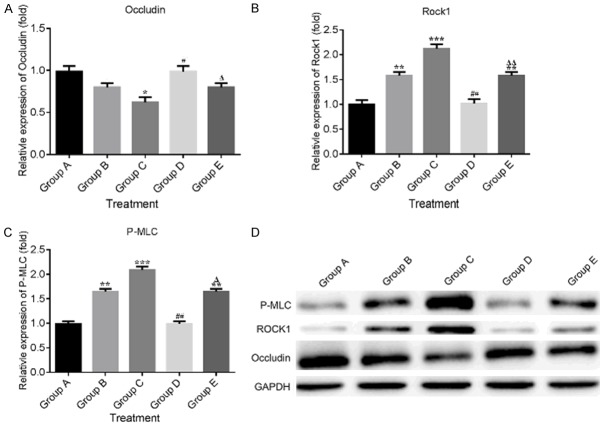
The expression of P-Occludin, ROCK1 and P-MLC was determined by Western blot after 4, 8 and 12 weeks incubation. In Figure 7A, the results demonstrated that the P-Occludin expression was declined in DM group compared with control group. However, the decreased P-Occludin was significantly rescued by LBP and/or Fasudil compared with DM group at 8 weeks. For ROCK1 expression, the results in Figure 7B indicated that the expression of ROCK1 was increased in DM group compared with control group and the increased ROCK1 was reduced by LBP and/or Fasudil. As shown in Figure 7C, the expression of P-MLC was largely increased in DM group compared with control group. While in LBP and/or Fasudil treated group, the decreased expression of P-MLC was observed at 8 weeks compared with DM group.
Figure 7.
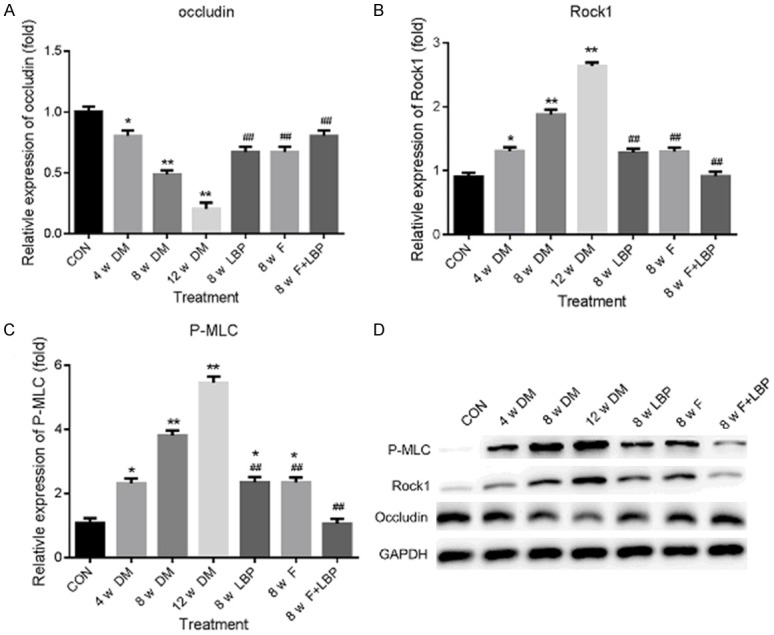
Western blot assay for P-Occludin, ROCK1 and P-MLC protein expression. The Western blot was performed at 4, 8 and 12 weeks after administration. The relative expression of P-Occludin, ROCK1 and P-MLC was displayed in (A-C) respectively. (D) Western blot assay. *P<0.05, **P<0.01 vs. CON group; #P<0.05, ##P<0.01 vs. 8 w DM group.
Effect of LBP on Rho/ROCK1 pathway in high glucose induced RF/6A cells
As it was shown in part 3, after incubation with high glucose and LBP in RF/6A cells, morphological observation was demonstrated. As shown in Figure 8A, the normal RF/6A in control group (Group A) displayed in the shape of the flattened monolayer epithelium. While in groups A and B, the cell number was decreased and the cells were contracted with long pseudopods, blurring the border at 7 day, and indicated that high glucose condition contribute to cell permeability and apoptosis. However, the cells in LBP-treated group (Group D-E) only showed the slightly rounded shape and less decreased cell number at 7 day. According to Figure 8B, the permeability of RF/6A cells in high glucose treated group (Group B-C) suggested the higher OD values compared with control group at 3, 5 and 7 days, especially in group C. However, the increased permeability of RF/6A cells was reversed by LBP treatment (Groups D-E).
Figure 8.
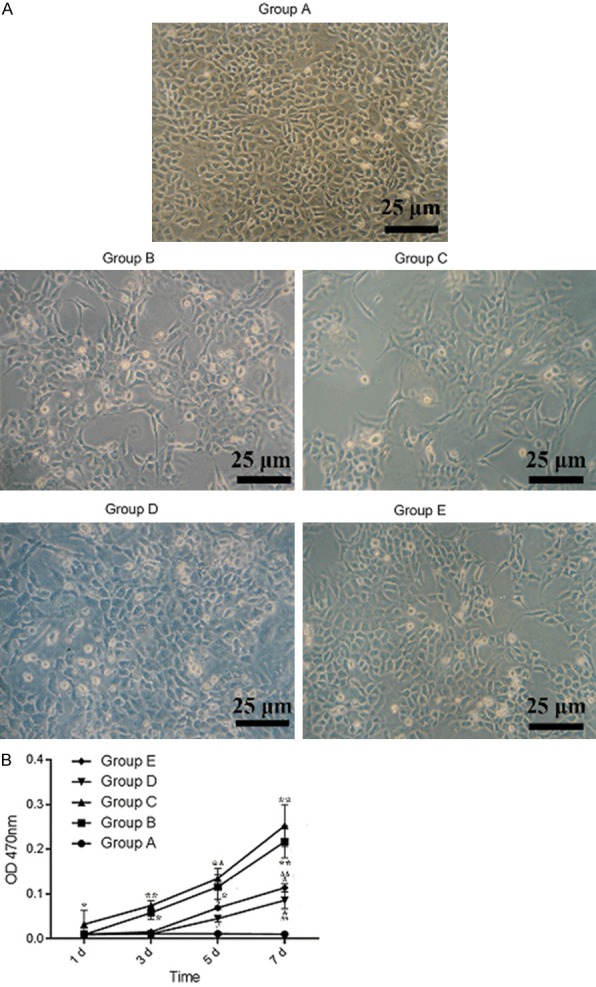
Morphology and permeability of RF/6A monolayer. After administration of LBP to RF/6A cells, the experiments were conducted at 1, 3, 5 and 7 days. A: The results of RF/6A monolayer after horse radish peroxidase stain at 7 day. B: OD value of each group at 1, 3, 5 and 7 days under the 470 mm. *P<0.05, **P<0.01 vs. 5.5 mM Glu; #P<0.05, ##P<0.01 vs. 30 mM Glu; ΔP<0.05, ΔΔP<0.01 vs. 40 mM Glu.
The expression of occudin, ROCK1 and P-MLC protein was evaluated by Western blot after 7 d incubation. The results implied that the expression of P-Occludin was decreased according to the increasing concentration of glucose in Figure 9A. Furthermore, the decrease of P-Occludin in high glucose condition was dramatically inhibited by LBP (Groups D-E) compared with high glucose induced groups B-C. In addition, as shown in Figure 9B, the expression of ROCK1 was increased along with the increased glucose levels while the increased ROCK1 expression was significantly reversed by LBP (Groups D-E). For P-MLC, the expression of P-MLC was increased due to the increasing levels of glucose, while LBP markedly suppressed the increasing of P-MLC in groups D and E (Figure 9C).
Figure 9.
Western blot assay for P-Occludin, ROCK1 and P-MLC protein expression. The expression of Occudin, ROCK1 and P-MLC protein was evaluated by Western blot after 7 day incubation. A-C: Displayed the expression profile of P-Occludin, ROCK1 and P-MLC respectively. D: The photograph of Western blot band. *P<0.05, **P<0.01 vs. 5.5 mM Glu; #P<0.05, ##P<0.01 vs. 30 mM Glu; ΔP<0.05, ΔΔP<0.01 vs. 40 mM Glu.
Discussion
Lycium barbarum has acted as a traditional herbal medicine for thousands of years in China. It was reported that LBP could prevent or postpone the development of diabetic complications [20]. A previous clinical study indicated that LBP exerts a remarkable protective effect in patients with type 2 diabetes, significantly decreasing serum glucose, increasing the insulinogenic index, and increasing high density lipoprotein (HDL) levels [21]. In present study, we reported the diabetic rats treated by LBP could increase weight of rats, decrease the levels of blood glucose and blood lipids after 12 weeks. This study implied that long-term treatment with LBP may be beneficial for controlling blood glucose in diabetic patient.
As previously mentioned, DR was considered a microvascular complication of endothelial dysfunction which is characterized blood-retina barrier leakage, neovascularization, retina thinning, changes of neurocyte morphology, retinal pigment epithelium dysfunction occur in DR and result in the gradual loss of retinal function [3-5]. In this study, retina permeability was prevented by LBP treatment compared with DM group. HE-stained retinal sections showed that the overall thickness of the retina in the diabetic rats was reduced compared with the control group, while, in the LBP-treated group, the thickness of the overall retina increased compared with the diabetic group. By transmission electron microscopy, the photoreceptor cell membranous disks appeared inordinate, frizzy and loose in diabetic rats, and which could be rescued by LBP. All these results were consistent with the previous study, and LBP might play important roles in BRB protection by morphological assays.
Previous studies have shown that the expression of VEGF could induce the breakdown of blood-retinal barrier in the progress of diabetic retinopathy [22-25]. In our present study, LBP could inhibit the angiogenesis, which might associate with VEGF expression and result in the delay of DR development. More importantly, Rho/ROCK pathway play an important role in DR development [17,26]. Rho kinase activation could preserve the phosphorylated myosin light chain (P-MLC), which is associated with hypertension and vascular contractility dysfunction [27,28]. Recent studies also indicated Rho kinases (RhoK) directly phosphorylates occludin and other tight junctional proteins [29,30]. Our present study suggested that the downregulated P-Occludin and upregulated ROCK1 and P-MLC in diabetic rats was reversed by LBP treatment, which associated with the integrated morphology of retinal cells and blood-retinal barrier. Furthermore, LBP could attenuate the damage of retina cells caused by high glucose. All these results above indicate that LBP has the protective effects on blood-retinal barrier in diabetic rats by targeting Rho/ROCK1 signaling pathway.
Conclusion
LBP has the protective effects on blood-retinal barrier by regulating the Rho/ROCK signaling pathway in diabetic rats. Rho/ROCK may be promising targets for the treatment of DR.
Acknowledgements
This study was supported by the 13th “The Peak of Six Talent” of Jiangsu Province (No. WSN-242), Chinese Medicine Science and Technology Programs of Jiangsu Province (No. LZ11125), Health Science and Technology programs of Wuxi City Health Bureau (No. MD201211) and Science and Technology Plan Projects of Wuxi City (No. CSZ00N1225).
Disclosure of conflict of interest
None.
References
- 1.Khodaeian M, Enayati S, Tabatabaei-Malazy O, Amoli MM. Association between genetic variants and diabetes mellitus in iranian populations: a systematic review of observational studies. J Diabetes Res. 2015;2015:585917. doi: 10.1155/2015/585917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unnikrishnan R, Shah VN, Mohan V. Challenges in diagnosis and management of diabetes in the young. Clin Diabetes Endocrinol. 2016;2:18. doi: 10.1186/s40842-016-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res. 2016;2016:3789217. doi: 10.1155/2016/3789217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamiec-Mroczek J, Zając-Pytrus H, Misiuk-Hojło M. Caspase-dependent apoptosis of retinal ganglion cells during the development of diabetic retinopathy. Adv Clin Exp Med. 2015;24:531–535. doi: 10.17219/acem/31805. [DOI] [PubMed] [Google Scholar]
- 6.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson JT. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol. 2011;56:162–72. doi: 10.1016/j.survophthal.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–52. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du M, Hu X, Kou L, Zhang B, Zhang C. Lycium barbarum Polysaccharide mediated the antidiabetic and antinephritic effects in diet-streptozotocin-induced diabetic sprague dawley rats via regulation of NF-kappaB. Biomed Res Int. 2016;2016:3140290. doi: 10.1155/2016/3140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song MK, Roufogalis BD, Huang TH. Reversal of the caspase-dependent apoptotic cytotoxicity pathway by taurine from lycium barbarum (Goji Berry) in human retinal pigment epithelial cells: potential benefit in diabetic retinopathy. Evid Based Complement Alternat Med. 2012;2012:323784. doi: 10.1155/2012/323784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du S, Han B, Li K, Zhang X, Sha X, Gao L. Lycium barbarum polysaccharides protect rat corneal epithelial cells against ultraviolet B-Induced apoptosis by attenuating the mitochondrial pathway and inhibiting JNK phosphorylation. Biomed Res Int. 2017;2017:5806832. doi: 10.1155/2017/5806832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173:6965–6972. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- 13.Paulus YM, Sodhi A. Anti-angiogenic therapy for retinal disease. Handb Exp Pharmacol. 2017;242:271–307. doi: 10.1007/164_2016_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–47. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi M, Nakao S, Arima M, Wada I, Kaizu Y, Hao F, Yoshida S, Sonoda KH. Rho-Kinase/ROCK as a potential drug target for vitreoretinal diseases. J Ophthalmol. 2017;2017:8543592. doi: 10.1155/2017/8543592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–29. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei L, Surma M, Shi S, Lambert-Cheatham N, Shi J. Novel insights into the roles of Rho kinase in cancer. Arch Immunol Ther Exp (Warsz) 2016;64:259–78. doi: 10.1007/s00005-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H, Luo P, Li Y, Wang C, Liu X, Ye Z, Li C, Lou T. Simvastatin alleviates hyperpermeability of glomerular endothelial cells in early-stage diabetic nephropathy by inhibition of RhoA/ROCK1. PLoS One. 2013;8:e80009. doi: 10.1371/journal.pone.0080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, Taher M, Melhorn MI, Schering A, Gatti F, Tezza S, Xie F, Vergani A, Yoshida S, Ishikawa K, Yamaguchi M, Sasaki F, Schmidt-Ullrich R, Hata Y, Enaida H, Yuzawa M, Yokomizo T, Kim YB, Sweetnam P, Ishibashi T, Hafezi-Moghadam A. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015;10:1173–1186. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behl T, Kotwani A. Chinese herbal drugs for the treatment of diabetic retinopathy. J Pharm Pharmacol. 2017;69:223–235. doi: 10.1111/jphp.12683. [DOI] [PubMed] [Google Scholar]
- 21.Cai H, Liu F, Zuo P, Huang G, Song Z, Wang T, Lu H, Guo F, Han C, Sun G. Practical application of antidiabetic efficacy of lycium barbarum polysaccharide in patients with type 2 diabetes. Med Chem. 2015;11:383–390. doi: 10.2174/1573406410666141110153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Wang H, Nie J, Wang F. Protective factors in diabetic retinopathy: focus on blood-retinal barrier. Discov Med. 2014;18:105–112. [PubMed] [Google Scholar]
- 23.Deissler HL, Lang GK, Lang GE. Capacity of aflibercept to counteract VEGF-stimulated abnormal behavior of retinal microvascular endothelial cells. Exp Eye Res. 2014;122:20–31. doi: 10.1016/j.exer.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Hu ZL. Role of vascular endothelial growth factor in the progress of diabetic retinopathy. Int J Ophthalmol. 2008;8:990–993. [Google Scholar]
- 25.Xiong SQ, Jiang HB, Xu HZ, Xia XB. Effect of pyridone agent on blood-retinal barrier in diabetic mice. Int J Ophthalmol. 2017;10:890–895. doi: 10.18240/ijo.2017.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita R, Hata Y, Ishibashi T. ROCK as a therapeutic target of diabetic retinopathy. J Ophthalmol. 2010;2010:175163. doi: 10.1155/2010/175163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeng J, Sheverdin V, Shin H, Ha I, Bae SS, Yang-Yen HF, Lee K. Up-regulation of Rhoa/Rho kinase pathway by translationally controlled tumor protein in vascular smooth muscle cells. Int J Mol Sci. 2014;15:10365–76. doi: 10.3390/ijms150610365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L, Surma M, Gough G, Shi S, Lambert-Cheatham N, Chang J, Shi J. Dissecting the mechanisms of doxorubicin and oxidative stress-induced cytotoxicity: the involvement of actin cytoskeleton and ROCK1. PLoS One. 2015;10:e0131763. doi: 10.1371/journal.pone.0131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–52. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dörfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. [DOI] [PMC free article] [PubMed] [Google Scholar]