Abstract
Bone marrow preconditioning using cyclophosphamide (CP) is generally used for bone marrow transplantation (BMT). However, because of CP’s hepatotoxicity and nephrotoxicity, additional fludarabine (FDR) administration and a reduced dose of CP are used for reduced-intensity preconditioning. Recently, preclinical studies using non-human primates (NHPs) were performed to induce immune tolerance after solid organ transplantation by conducting BMT simultaneously. However, dose optimization of CP and FDR for BMT preconditioning in cynomolgus monkeys has not been conducted. Therefore, the objective of this study was to evaluate the efficacy and tolerability of induction protocols using different doses of CP and FDR. Our results showed that relatively low-dose CP (30 mg/kg×2) combined with additional high-dose FDR (60 mg/m2×4) was associated with sufficient suppression in periphery as well as in bone marrow compared with high-dose CP (60 mg/kg×2) combined with low-dose FDR (30 mg/m2×4) and did not show hepatic or renal toxicity. CD34+ stem cells were also well suppressed with both doses. Therefore, we concluded that the combination of 60 mg/kg of CP with 240 mg/m2 of FDR can be used effectively and safely for non-myeloablative preconditioning for BMT in cynomolgus monkeys.
Keywords: Cyclophosphamide, fludarabine monophosphate, induction therapy, bone marrow ablation, cynomolgus monkey
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) and bone marrow transplantation (BMT) are commonly performed in a variety of hematological malignancies [1,2]. Recently, bone marrow transplantation combined with kidney transplantation has been attempted to induce of tolerance through mixed chimerism [3-5]. When performing bone marrow transplantation, conditioning drugs have been used for creating a ‘space’ in the recipient bone marrow for the transplant achieve successful hematopoietic chimerism [6,7].
Cyclophosphamide (CP), an alkylating agent, is classically used in a conditioning procedure before BMT as well as in many hematological disorders [8]. However, CP-based therapy is associated with liver toxicity because it is converted to toxic metabolites in the liver [9-12]. To maintain suppressive efficacy and reduce these toxicities, conditioning regimens of fludarabine monophosphate (FDR) instead of a relatively high dose of CP alone are widely used [13]. Even though total body irradiation (TBI) also could be used for non-myeloablative preconditioning in primates and has shown superior results to CP in terms of toxicity and efficacy as a preconditioning modality [14], it is available in only a few laboratories. Therefore, we analyzed the addition of FDR and reduced dose of CP.
However, in preclinical transplantation in non-human primates (NHPs), the optimal dosage of the combination of CP and FDR has not been determined for achieving a non-myeloablative condition without toxicity. Therefore, we performed a dose-optimizing study for the combination of CP and FDR in NHPs for chimerism induction through bone marrow transplantation.
Materials and methods
Animals
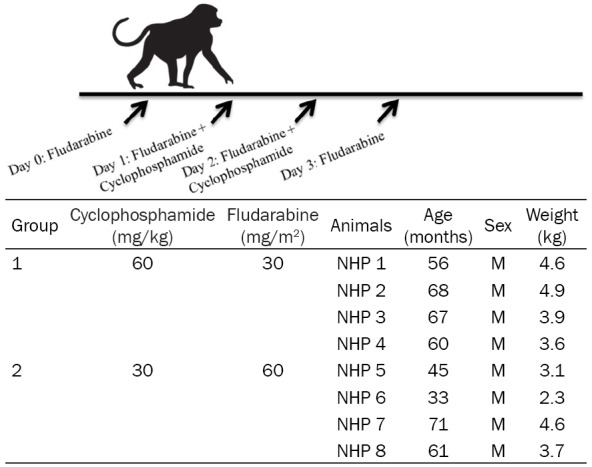
Eight male cynomolgus monkeys (Macaca fascicularis) were obtained from Orient Bio Co. Ltd. (Seongnam, Korea) and had a mean age of 57.6 months (range, 33-71 months) and mean body weight of 3.84 kg (range, 2.3-4.9 kg). These eight monkeys were included in the study group. 25 male monkeys were also obtained; their ages ranged 46 to 81 months (mean: 62.4 months), and their body weights ranged from 3.37 to 6.84 kg (mean: 4.64 kg) for collecting normal bone marrow cell data. All NHPs were screened and found to be negative for tuberculosis, herpes B virus, simian T cell leukemia virus, simian retrovirus, simian immunodeficiency virus, measles, cynomolgus cytomegalovirus, and simian varicella virus by Zoologix Inc. (Chatsworth, CA, USA).
All monkeys were housed separately in squeeze back cages (700×800×800 mm2 sized or 650×730×850 mm2 sized) at Orient Bio Co. Ltd. (Seongnam, Korea). The environment of the housing facility was controlled (temperature: 25 ± 2°C, humidity: 50 ± 10%, pressure: -5-+5 mmAq, light: 950 lux (12 hrs dark and 12 hrs light), ventilation: 10-15 times/hr, and sound: under 60 decibels). Water was supplied ad libitum. Biscuits were given to the monkeys twice daily (Certified Primate Diet 5048*, LabDiet, St Louis, MO, USA) along with fresh fruits, vegetables, and nuts. Various toys, music, and visual entertainment (DVDs) were provided as part of an enrichment program.
All NHPs procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Orient Bio Laboratories (Permit Number: ORIENT-IACUC-14194) for this study.
Conditioning regimen and management
To investigate the immunosuppressive effects of CP and FDR monophosphate on cynomolgus monkeys, the experimental regimes consisted of two dosage combinations of the two drugs (Table 1). Eight animals were treated with CP twice (on day 1 and 2) and FDR 4 times (on days 0, 1, 2, and 3). Each group contained four cynomolgus monkeys. These animals were categorized according to drug dosage and combination: 1) Group 1, cyclophosphamide 120 mg/kg (60 mg/kg, two times) and FDR 120 mg/m2 (30 mg/kg, four times), and 2) Group 2, CP 60 mg/kg (30 mg/kg, two times), FDR 240 mg/m2 (60 mg/kg, four times). CP and FDR were injected as scheduled (Table 1). The pretreatment regimen for CP was dexamethasone 2 mg IV and granisetron 1 mg mixed in 10 ml of normal saline and administered through intravenous (IV) injection over 10 minutes. On the day of CP treatment, 10 mg/kg of mesna mixed in 10 ml of normal saline was administrated at three time points (immediately after CP injection, 4 hours later, and 8 hours later) to prevent hemorrhagic cystitis. FDR (30 mg/kg) mixed in 20 ml of normal saline was given over 30 minutes [15]. A prophylactic antibiotic (cefazolin 20 mg/kg) was given twice daily during the four days of conditioning.
Table 1.
Conditioning regimen and management
|
The animals were closely monitored with daily physical examinations and measurements of body weight, intake, urine output, stool output, and overall activity. The following blood hematological parameters were assessed regularly: white blood cell count (WBC) with differential count, hemoglobin, hematocrit and platelet counts, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, blood urea nitrogen (BUN), creatinine, albumin, globulin, sodium, potassium, chloride, calcium, inorganic phosphorus, cholesterol, triglycerides, amylase, and C-reactive protein levels.
Sample collection and preparation
Peripheral blood samples were obtained periodically from saphenous veins and collected in EDTA tubes. These samples were subjected to flow cytometry or stored in freezing media (90% fetal bovine serum and 10% dimethyl sulfoxide) after red blood cell lysis. Approximately 9 ml of bone marrow were collected from the humerus head into a syringe containing 1 ml of heparin. The bone marrow sample was layered over Ficoll-Paque PLUS (GE Healthcare, Sweden) by dilution in 10% PBS to avoid red blood cell contamination. The layered samples were centrifuged at 2000 g for 30 minutes at 24°C, leaving a mononuclear cell layer at the interface. After washing twice in PBS containing 2 mM EDTA, suspended cells were used for flow cytometric analysis.
Flow cytometric analysis
For flow cytometric analysis, the following monoclonal antibodies that had cross-reactivity with NHP species were used; FITC CD20 (clone: 2H7), PE CD28 (clone: CD28.2), PE CD10 (clone: HI10a) PerCP-cy5.5 CD4 (clone: L200), PerCP-cy5.5 CD7 (clone: M-T701), PE-cy7 CD3 (clone: SP34-2), PE-cy7 CD20 (clone: 2H7), PE-cy7 CD14 (clone: M5E2), APC CD95 (clone: DX2), APC CD14 (clone: M5E2), APC CD34 (clone: 563), and APC-H7 CD8 (clone: SK1). They were all purchased from BD Pharmingen™ (San Diego, CA, USA). V450 CD16 (clone: 3G8), and BV510 NHP-CD45 (clone: D058-1283) were obtained from BD Biosciences (San Jose, CA, USA). FITC CD38 (clone AT-1) was purchased from Stemcell Technologies (Vancouver, British Columbia, Canada).
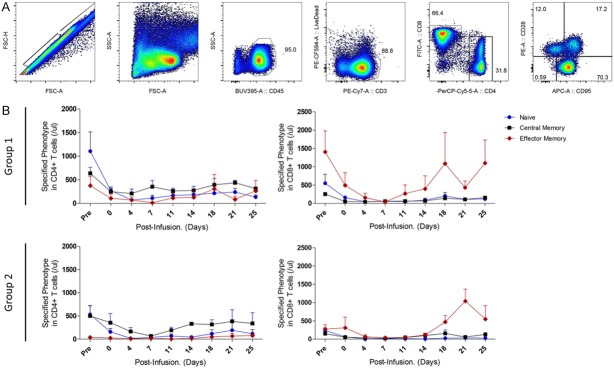
T-lymphocyte subsets in the macaque peripheral blood were analyzed based on the surface expression levels of NHP-CD45, CD3, CD4, and CD8. Both CD4+ helper T cells and CD8+ cytotoxic T cells can be divided into two major subsets: naïve and memory cells. We have used cell surface expression levels of CD28 and CD95 to delineate naïve, central memory, and effector memory T cell subpopulations in cynomolgus monkeys [16,17]. We analyzed the B cell subset in cynomolgus monkeys based on the expression level of CD20 as a definitive B cell marker.
Peripheral whole blood cells were stained with relevant antibody mixtures at room temperature for 25 min. The blood samples were then treated with FACS Lysing Solution (BD Biosciences, San Jose, CA) to lyse the red blood cells for 15 minutes prior to flow cytometry.
We performed multicolor flow cytometric analysis for hematopoietic stem cell populations in bone marrow after infusion of the drug combinations. Hematopoietic stem cells that were negative for the lineage mixture (CD3, CD20, and CD14) were used to determine the expression of CD34 and CD38. Bone marrow mononuclear cells were incubated with these antibodies at 4°C for 20 min followed by washing and flow cytometric analysis. Percentages of cell subpopulations determined from the flow cytometric analysis were converted to absolute numbers using lymphocyte number per micro-liter of whole blood based on complete blood analysis data. Stained cells were analyzed on an LSRFortessaTM cell analyzer (Becton Dickinson, USA) equipped with four lasers and FACSDiva software (BD Biosciences).
Histological analysis
After sacrificing the animals, histologic samples of the liver and kidney were obtained, fixed in 10% formalin, embedded in paraffin, sectioned to a thickness of 4 μm, and stained with hematoxylin and eosin. All histologic slides were examined by a pathologist.
Statistical analysis
Statistical analyses were performed using Prism software. We compared suppressed patterns between the groups using the paired t test or the Mann-Whitney U-test for non-parametric comparisons. A P-value of <0.05 was considered statistically significant.
Results
Immunosuppressive effect of CP combined with FDR on white blood cell, neutrophil, and lymphocyte number in whole blood components
To evaluate the immunosuppressive effects of CP and FDR in various dosage combinations, WBC, neutrophil, lymphocyte, hemoglobin, and platelet levels were observed at the indicated time points. The absolute numbers of WBC, neutrophils, and lymphocytes initially decreased and remained so for approximately 2 weeks after drug infusion in all groups (Figure 1). Despite the dosage variation of CP and FDR, the profiles of peripheral cell suppression were very similar between groups. After non-myeloablative conditioning, Group 2 (CP 60 mg/kg and FDR 240 mg/m2) showed no significant changes in hemoglobin or platelet level. However, in Group 1 (CP 120 mg/kg and FDR 120 mg/m2), the platelet level significantly decreased compared to that of Group 2 approximately 1 week after the drug infusion (Figure 1E, P<0.05).
Figure 1.
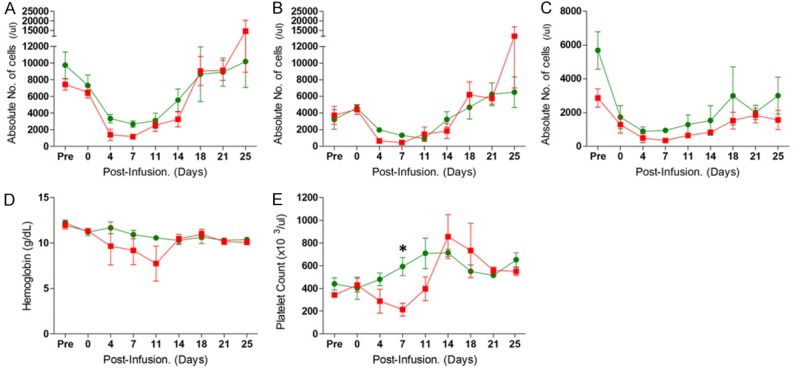
Whole blood cell monitoring in the experimental groups after induction therapy with cyclophosphamide and fludarabine. The absolute numbers of white blood cells (A), neutrophils (B), and lymphocytes (C) were counted after induction in Groups 1 and 2. Hemoglobin (D) and platelet counts (E) are shown. Red squares represent the kinetics of Group 1 with 120 mg/kg CP and 120 mg/m2 FDR. Green circles represent the kinetics of Group 2 with 60 mg/kg CP and 240 mg/m2 FDR. These results are expressed as average ± SEM. *P<0.05 versus the two groups at same time point.
The suppressive effects of CP combined with FDR on T cell subset and B cell subset frequencies
We investigated the effects of the immunosuppressive drug combination of CP and FDR on all circulating lymphocyte subsets in peripheral blood and measured the frequencies of T-lymphocyte subpopulations; the naïve, central memory, and effector memory cells within the CD4+ and CD8+ T cell populations.
CP and FDR treatment resulted in broad suppression of T and B cells at approximately 2 weeks (Figure 2A and 2B). The combination of these drugs also resulted in reduction of CD4+ and CD8+ T cells, which consequently influenced the ratio of CD4+/CD8+ (Figure 2C). The CD4+/CD8+ ratios demonstrated that CD8+ T cells were more susceptible to CP and FDR than CD4+ T cells in the initial suppression at 1 week (Figure 2C). The CD4+/CD8+ ratio gradually decreased due to an increase in CD8+ T cells following reconstitution of T cell subpopulations. This kinetic pattern was similar between groups.
Figure 2.
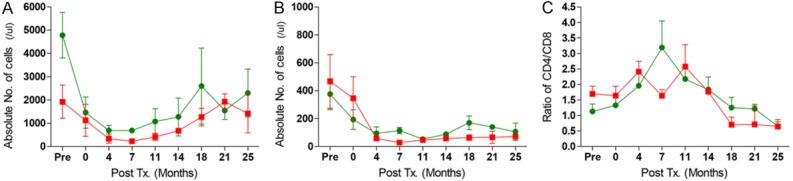
The kinetics of T and B cell counts in the periphery after CP and FDR treatment. The counts of CD3+ Pan-T cells (A), and CD20+ Pan-B cell (B) were measured by flow cytometry after CP and FDR treatment. The graph of the CD4+/CD8+ T cell ratio (C) was also calculated by flow cytometric results. Red squares represent the kinetics of Group 1, and green circles show the kinetics of Group 2. These results are shown average ± SEM.
The phenotype of the three functional T cell compartments in the peripheral blood was assessed by analyzing the patterns of CD95 and/or CD28 expression within CD4+ and CD8+ T cells (Figure 3A). All subpopulations of T cells decreased after infusion of alkylating agents. In both groups, the majority of recovering T cells had the memory phenotype rather than the naïve phenotype (Figure 3B). The dominant recovered T cell subsets in the CD4+ T cell compartment were enriched in cells co-expressing CD28 and CD95 (central memory). However, these cell counts did not differ between the two groups. After lymphopenia due to infusion with CP and FDR, the CD28-CD95+ effector memory T cells largely consisted of CD8+ T cells. However, those patterns were not significantly different between the two groups.
Figure 3.
The kinetics of T cell subpopulations after CP and FDR administration. A gating strategy of naïve and memory phenotype T-cells in periphery is presented (A). The kinetics of T cell subsets were chronologically measured by flow cytometric analysis (B). Blue circles represent naïve (CD28+CD95-) T cells. Black squares represent central memory (CD28+CD95+) cells, and red diamonds represent effector memory (CD28-CD95+) cells. Data are expressed as average ± SEM.
The suppressive effect on bone marrow after administration of the combination of CP and FDR
To investigate the effect of combined CP and FDR on bone marrow cells, we measured changes in the absolute number of collected bone marrow cells in response to drug infusion (Figure 4A). The normal control means number of bone marrow cells estimated from the 25 healthy cynomolgus monkeys was 4652.86 cells/µl (SEM=691.7 cells/µl). This mean was chronologically compared to the absolute number of bone marrow cells in each group. At 1 week after administration, the bone marrow cell count in Group 1 was significantly suppressed by 13.10% (mean: 609.80 cells/µl) compared to the normal range (Figure 4A, P-value <0.005). The counts in this group were less than 200 cells/µl at 1-week post-drug administration, except in one of the monkeys (NHP 4: 1800 cells/µl). Even though Group 2 at the same time point showed less depletion in bone marrow cell count (mean: 1704.135 cells/µl) compared to Group 1, there was no significant difference. The bone marrow cell count of Group 2 was also significantly suppressed, by 36.6%, in comparison with normal BM-MNCs (P<0.05). In one of the monkeys, this drug combination was insufficient to suppress bone marrow cells (NHP 8: 4473 cells/µl), although the peripheral blood cells were affected. At the subsequent time points, the reconstituted counts of bone marrow mononuclear cells increased above normal basal counts. The suppressive effect of the combined dose treatment on bone marrow was highest by 7-days after drug infusion and gradually recovered thereafter (Figure 4A). Also, administration of CP and FDR affected the types of hematopoietic stem cells (Figure 4B). In both groups, after drug infusion, lineage-negative populations of total BM MNCs decreased, as did CD34+ hematopoietic stem cells. The counts of the most primitive type, Lineage-CD34+CD38- cells, were also suppressed post-drug infusion.
Figure 4.
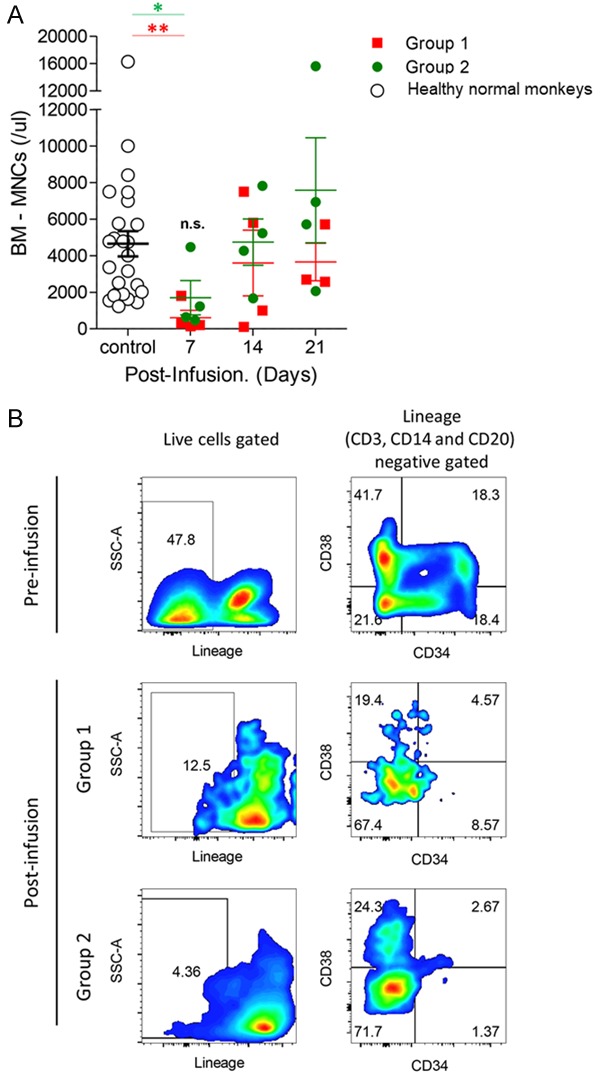
The suppressive effect on bone marrow cells in the experimental regimens. To evaluate the suppressive effects of a combination drug of CP and FDR in bone marrow, bone marrow mononuclear cell (BM-MNC) count (A) was measured at the indicated time points. Red squares represent the BM-MNC of Group 1, green circles represent the BM-MNC of Group 2, and white circles represent the BM-MNC of healthy normal cynomolgus monkeys (n=25, mean: 4652.86 cells/µl, SEM: 691.786 cells/µl). Representative FACS dot plots of hematopoietic stem cells of expression of lineage (CD3, CD14, and CD20), CD34, and CD38 pre- and post-infusion (B). *P<0.05, and **P<0.005 versus the group compared as healthy normal monkeys.
Hepatotoxicity and nephrotoxicity of experimental regimens of CP and FDR
The liver enzymes AST and ALT and albumin level were not affected by the conditioning regimen used in Group 2 (Figure 5A-C). However, in Group 1, AST and ALT levels were elevated immediately following administration of the conditioning regimens, especially in NHP 1 and NHP 2. These levels normalized within 1 week except in NHP 2 (Figure 5A and 5B), which showed severe elevation of liver enzymes (above 800 U/L) on day 13 and had to be sacrificed. In histology of this animal, hepatocytes showed vacuolar degeneration and necrosis around central veins (Figure 6A).
Figure 5.
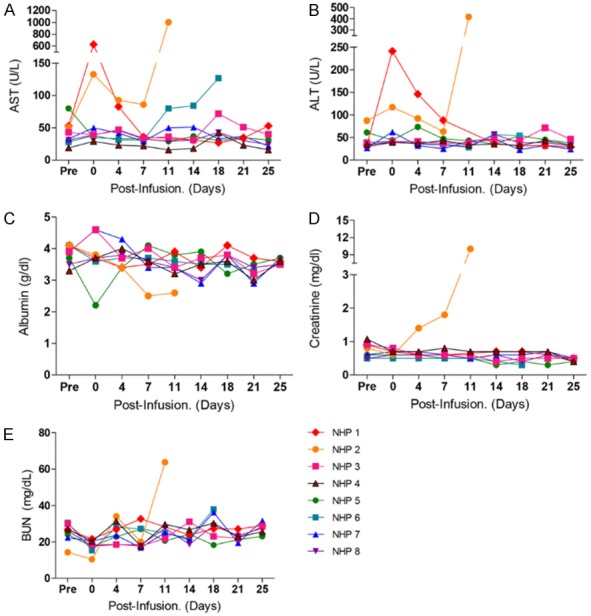
Hepatotoxicity and nephrotoxicity after a combination drug treatment in each group. The levels of liver enzymes AST (A) and ALT (B) and of albumin (C) were monitored in all experimental regimens. As indicators of nephrotoxicity, creatinine (D) and BUN (E) were also monitored individually.
Figure 6.
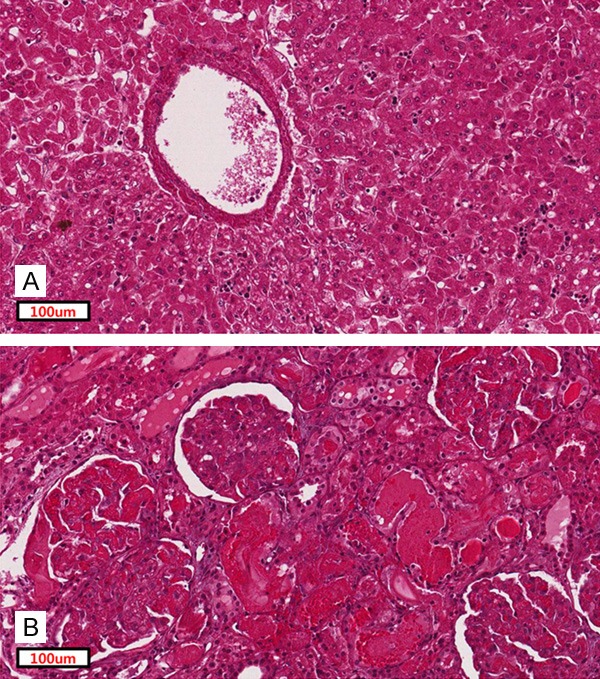
Histologic changes of liver and kidney in Group 1 with CP 120 mg/kg and FDR 120 mg/m2. (A) Representative image of hematoxylin-and-eosin staining of hepatocytes of NHP2 (A, magnification ×400). An image of damaged glomeruli of NHP 2 (B, magnification ×400).
Creatinine and BUN levels were stable after administration of the conditioning regimens in Group 2 (Figure 5D and 5E). One monkey in Group 1 (NHP 2) had acute elevation of creatinine level on the fourth day after conditioning (Figure 5D). In histology on day 13, glomeruli showed severe congestion of capillaries and tubules showed acute damage with intraluminal hyaline casts (Figure 6B).
Discussion
A preconditioning regimen with a combination of CP and FDR is commonly used for clinical HSCT [18,19] and the immunosuppressive mechanism of these drugs is also well known. However, in NHP, the efficacy of this combination has not been sufficiently studied.
Preclinical studies on allogeneic HSCT in NHPs have been conducted for immune tolerance induction. Hiroshi et al have reported that CP induction is less effective but more toxic than TBI as non-myeloablative conditioning for combined kidney and bone marrow transplantation in cynomolgus monkeys [14]. However, the optimal dose for conditioning with the regimen used in clinical setting has not been reported in NHPs.
Total body irradiation (TBI) has been used as a conditioning regimen in allogeneic HSCT. However, toxicities related to TBI such as pulmonary edema, secondary malignancy, growth retardation, and hypothyroidism have been reported [20-22]. More recently, CP with or without anti-thymocyte globulin (ATG) has been used and showed acceptable engraftment rates. However, several cases of hepatic sinusoidal endothelial cell toxicity due to CP have been reported [23,24]. High levels of CP metabolites can lead to liver toxicity [11], cardiac toxicity [25,26], and renal toxicity [27]. Thus, FDR, a critical component of reduced intensity conditioning regimens, has been added to decrease the dose of CP. For conditioning regimens containing low doses of CP, FDR with or without ATG has yielded good engraftment results [28,29].
This study, for the first time, reported the optimal dose of the CP and FDR combination for effective bone marrow suppression with minimal toxicity in cynomolgus monkeys. Our conditioning protocol in cynomolgus monkeys initially used 60×2 mg/kg of CP based upon previous studies in humans [30], and primates [14]. In this study, although TBI and other chemotherapies were used, a total dose of 120 mg/kg of CP permitted mixed chimerism with donor-derived cells in recipients. Also, we investigated the optimal combined dose of CP by adding FDR to the preconditioning protocol, except for case of TBI. We used combinations of CP at 30, or 60 mg/kg two times and FDR at 30, or 60 mg/m2 four times. The dose of FDR was based on several publications on use of this drug in the transplantation field [31-34].
In this study, transient hepatotoxicity related to CP was observed in two cases in Group 1 (CP, 120 mg/kg; FDR, 120 mg/m2). The hepatotoxicity was too severe to continue the experiment in one case, and that monkey also showed elevated serum creatinine and azotemia. Hepatotoxicity seemed to be correlated with CP dose but was unrelated to dose of FDR. In rhesus monkeys, pharmacokinetic and clinical effective dosage studies of FDR showed no clinical, or hematological toxicity beyond a 300 mg/m2 total dose, despite additional administration of busulfan [35]. The range of dosages of FDR used in this study was clinically tolerated, while that of CP was not.
Our results revealed that a total dose of 120 mg/kg of CP increased the toxicity parameters and produced tissue toxicities, although the combinations of CP and FDR in Group 1 appeared efficacious for periphery and bone marrow. The results of Group 2, using FDR at a higher dose and CP at a lower dose, were not significantly different from those of Group 1 and showed no toxic signs and symptoms. Therefore, we concluded that the combination used in Group 2 (60 mg/kg of CP and 240 mg/m2 of FDR) is appropriate for bone marrow ablation and depletion of whole blood cells in the periphery without toxicity in cynomolgus monkeys.
In reconstitution of T cells after induction with FDR, it has been reported that CD4+ T cells are less susceptible to FDR than are CD8+ T cells in vitro [36] and in vivo [37]. Consequently, the ratio of CD4+/CD8+ T cells increased in circulating peripheral cells after FDR treatment. In our results, the CD4+/CD8+ T cells ratio gradually increased within one month after administration of CP and FDR. However, the ratio of CD4+/CD8+ T cells decreased after a second month. It has been reported that CD8+ T cells tend to be represent a major proportion of reconstituted T cells as a result of homeostatic proliferation following lymphopenia-induced treatment with immunosuppressive drugs [38].
In summary, we evaluated the optimal dose combination for the conditioning regimen in allogeneic HSCT for induction of immune tolerance in NHPs. Future studies should focus on the outcomes of allogeneic HCT in NHPs including rate of engraftment, mixed chimerism induction, and long-term data on infection and GVHD. We conclude that the combination of 60 mg/kg of CP and 240 mg/m2 of FDR is optimal for bone marrow ablation and depletion of whole blood cells in the periphery without inducing toxicity in cynomolgus monkeys.
Acknowledgements
This research was supported by a grant (HI13C1263) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea.
Disclosure of conflict of interest
None.
References
- 1.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, Wadleigh M, DeAngelo DJ, Stone RM, Sakamaki H, Appelbaum FR, Dohner H, Antin JH, Soiffer RJ, Cutler C. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, Duarte RF, Dufour C, Kuball J, Farge-Bancel D, Gennery A, Kroger N, Lanza F, Nagler A, Sureda A, Mohty M. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51:786–792. doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Curr Opin Organ Transplant. 2013;18:402–407. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]
- 4.Elias N, Cosimi AB, Kawai T. Clinical trials for induction of renal allograft tolerance. Curr Opin Organ Transplant. 2015;20:406–411. doi: 10.1097/MOT.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 5.LoCascio SA, Morokata T, Chittenden M, Preffer FI, Dombkowski DM, Andreola G, Crisalli K, Kawai T, Saidman SL, Spitzer TR, Tolkoff-Rubin N, Cosimi AB, Sachs DH, Sykes M. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90:1607–1615. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Le-Rademacher J, Artz A, McCarthy PL, Logan BR, Pasquini MC. Comparison of non-myeloablative conditioning regimens for lymphoproliferative disorders. Bone Marrow Transplant. 2015;50:367–374. doi: 10.1038/bmt.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto N, Ludeman SM, Dolan ME. Drug focus: pharmacogenetic studies related to cyclophosphamide-based therapy. Pharmacogenomics. 2009;10:1897–1903. doi: 10.2217/pgs.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg JW, Lidsky MD. Cyclophosphamide-associated hepatotoxicity. South Med J. 1985;78:222–223. doi: 10.1097/00007611-198502000-00034. [DOI] [PubMed] [Google Scholar]
- 10.Cleland BD, Pokorny CS. Cyclophosphamide related hepatotoxicity. Aust N Z J Med. 1993;23:408. doi: 10.1111/j.1445-5994.1993.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C, Gooley T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 12.McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B, Schoch HG, McDonald GB. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, Lindemans CA, Bierings MB, Boelens JJ. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–353. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Sogawa H, Boskovic S, Nadazdin O, Abrahamian G, Colvin RB, Sachs DH, Cosimi AB, Kawai T. Limited efficacy and unacceptable toxicity of cyclophosphamide for the induction of mixed chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 2008;86:615–619. doi: 10.1097/TP.0b013e3181821bac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CT, Higbee GA. Determination of body surface area in the rhesus monkey. J Appl Physiol. 1976;40:101–104. doi: 10.1152/jappl.1976.40.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 18.Laport GG, Wu J, Logan B, Bachanova V, Hosing C, Fenske T, Longo W, Devine SM, Nademanee A, Gersten I, Horowitz M, Lazarus HM, Riches ML Blood and Marrow Transplant Clinical Trials Network. Reduced-intensity conditioning with fludarabine, cyclophosphamide, and high-dose rituximab for allogeneic hematopoietic cell transplantation for follicular lymphoma: a phase two multicenter trial from the blood and marrow transplant clinical trials network. Biol Blood Marrow Transplant. 2016;22:1440–1448. doi: 10.1016/j.bbmt.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanghavi K, Wiseman A, Kirstein MN, Cao Q, Brundage R, Jensen K, Rogosheske J, Kurtzweil A, Long-Boyle J, Wagner J, Warlick ED, Brunstein CG, Weisdorf DJ, Jacobson PA. Personalized fludarabine dosing to reduce nonrelapse mortality in hematopoietic stem-cell transplant recipients receiving reduced intensity conditioning. Transl Res. 2016;175:103–115. e4. doi: 10.1016/j.trsl.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeg HJ, O’Donnell M, Tolar J, Agarwal R, Harris RE, Feig SA, Territo MC, Collins RH, McSweeney PA, Copelan EA, Khan SP, Woolfrey A, Storer B. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108:1485–1491. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A, Sakamaki H, Ikuta K, Tsuchida M, Hoshi Y, Morishima Y, Kodera Y. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan marrow donor program. Blood. 2002;100:799–803. doi: 10.1182/blood.v100.3.799. [DOI] [PubMed] [Google Scholar]
- 22.Deeg HJ, Seidel K, Casper J, Anasetti C, Davies S, Gajeweski JL, Territo M, Ramsay N, Harris RE, Catro-Malaspina H, Collins R, Champlin R, Schoch G, King R, Howe C. Marrow transplantation from unrelated donors for patients with severe aplastic anemia who have failed immunosuppressive therapy. Biol Blood Marrow Transplant. 1999;5:243–252. doi: 10.1053/bbmt.1999.v5.pm10465104. [DOI] [PubMed] [Google Scholar]
- 23.DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837. doi: 10.1002/hep.510240414. [DOI] [PubMed] [Google Scholar]
- 24.Modzelewski JR Jr, Daeschner C, Joshi VV, Mullick FG, Ishak KG. Veno-occlusive disease of the liver induced by low-dose cyclophosphamide. Mod Pathol. 1994;7:967–972. [PubMed] [Google Scholar]
- 25.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J. Clin. Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 26.Ayash LJ, Wright JE, Tretyakov O, Gonin R, Elias A, Wheeler C, Eder JP, Rosowsky A, Antman K, Frei E 3rd. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J. Clin. Oncol. 1992;10:995–1000. doi: 10.1200/JCO.1992.10.6.995. [DOI] [PubMed] [Google Scholar]
- 27.Hamsa TP, Kuttan G. Protective role of Ipomoea obscura (L.) on cyclophosphamide-induced uro- and nephrotoxicities by modulating antioxidant status and pro-inflammatory cytokine levels. Inflammopharmacology. 2011;19:155–167. doi: 10.1007/s10787-010-0055-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Lee JH, Joo YD, Bae SH, Hyun MS, Lee JH, Kim DY, Lee WS, Ryoo HM, Kim MK, Park JH, Lee KH Cooperative Study Group A for Hematology (COSAH) A randomized comparison of cyclophosphamide vs. reduced dose cyclophosphamide plus fludarabine for allogeneic hematopoietic cell transplantation in patients with aplastic anemia and hypoplastic myelodysplastic syndrome. Ann Hematol. 2012;91:1459–1469. doi: 10.1007/s00277-012-1462-x. [DOI] [PubMed] [Google Scholar]
- 29.Anderlini P, Acholonu SA, Okoroji GJ, Bassett RE, Shpall EJ, Qazilbash MH, Popat UR, Worth LL, Giralt SA, Champlin RE. Fludarabine, cyclophosphamide, and antithymocyte globulin for matched related and unrelated allogeneic stem cell transplant in severe aplastic anemia. Leuk Lymphoma. 2011;52:137–141. doi: 10.3109/10428194.2010.524328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YB, Kawai T, Spitzer TR. Combined bone marrow and kidney transplantation for the induction of specific tolerance. Adv Hematol. 2016;2016:6471901. doi: 10.1155/2016/6471901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwitz ME, Spasojevic I, Morris A, Telen M, Essell J, Gasparetto C, Sullivan K, Long G, Chute J, Chao N, Rizzieri D. Fludarabine-based nonmyeloablative stem cell transplantation for sickle cell disease with and without renal failure: clinical outcome and pharmacokinetics. Biol Blood Marrow Transplant. 2007;13:1422–1426. doi: 10.1016/j.bbmt.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtman SM, Etcubanas E, Budman DR, Eisenberg P, Zervos G, D’Amico P, O’Mara V, Musgrave K, Cascella P, Melikian A, Hinderling PH, Ferrer JM, Williams GJ. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest. 2002;20:904–913. doi: 10.1081/cnv-120005903. [DOI] [PubMed] [Google Scholar]
- 34.Tarantal AF, Giannoni F, Lee CC, Wherley J, Sumiyoshi T, Martinez M, Kahl CA, Elashoff D, Louie SG, Kohn DB. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol Ther. 2012;20:1033–1045. doi: 10.1038/mt.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarantal AF, Giannoni F, Lee CC, Wherley J, Sumiyoshi T, Martinez M, Kahl CA, Elashoff D, Louie SG, Kohn DB. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol Ther. 2012;20:1033–1045. doi: 10.1038/mt.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gamberale R, Galmarini CM, Fernandez-Calotti P, Jordheim L, Sanchez-Avalos J, Dumontet C, Geffner J, Giordano M. In vitro susceptibility of CD4+ and CD8+ T cell subsets to fludarabine. Biochem Pharmacol. 2003;66:2185–2191. doi: 10.1016/j.bcp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 37.McCune JS, Mager DE, Bemer MJ, Sandmaier BM, Storer BE, Heimfeld S. Association of fludarabine pharmacokinetic/dynamic biomarkers with donor chimerism in nonmyeloablative HCT recipients. Cancer Chemother Pharmacol. 2015;76:85–96. doi: 10.1007/s00280-015-2768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12:1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]