Abstract
Numerous postural sway metrics have been shown to be sensitive to balance impairment and fall risk in individuals with MS. Yet, there are no guidelines concerning the most appropriate postural sway metrics to monitor impairment. This investigation implemented a machine learning approach to assess the accuracy and feature importance of various postural sway metrics to differentiate individuals with MS from healthy controls as a function of physiological fall risk. 153 participants (50 controls and 103 individuals with MS) underwent a static posturography assessment and a physiological fall risk assessment. Participants were further classified into four subgroups based on fall risk: controls, low-risk MS (n = 34), moderate-risk MS (n = 27), high-risk MS (n = 42). Twenty common sway metrics were derived following standard procedures and subsequently used to train a machine learning algorithm (random forest – RF, with 10-fold cross validation) to predict individuals’ fall risk grouping. The sway-metric based RF classifier had high accuracy in discriminating controls from MS individuals (>86%). Sway sample entropy was identified as the strongest feature for classification of low-risk MS individuals from healthy controls. Whereas for all other comparisons, mediolateral sway amplitude was identified as the strongest predictor for fall risk groupings.
Subject terms: Multiple sclerosis, Biomedical engineering
Introduction
Multiple Sclerosis (MS) is a chronic, inflammatory-mediated neurological disorder affecting more than 2.3 million people worldwide1. MS is characterized by inflammatory demyelination and axonal damage in the central nervous system2. The neuronal damage alters a wide range of cognitive, sensory and motor functions, which contribute to impairment in balance2. Balance impairment affects over 75% of persons with MS (PwMS) during the disease course3, and it is associated with an elevated risk of falls and declines in quality of life4. Given its importance, identifying and treating balance impairment is often a focus of MS rehabilitation and research.
Traditionally, functional assessment and self-report questionnaires have been utilized to evaluate balance impairment in PwMS. Functional tests such as Berg Balance Scale (BBS)5 measures static and dynamic balance ability, whereas self-administered questionnaires such as Activity-Specific Balance Confidence Scale (ABC)6 and Falls Efficacy Scale - International (FES-I)7 measure individuals’ balance confidence and fear of falling during daily life. However, these measures rely on subjective scoring and have poor sensitivity due to ceiling or floor effects8. On the other hand, instrumented measures through posturography, which utilizes a force platform to quantify Center of Pressure (COP) movement (an indicator for the neuromuscular control of maintaining an upright stance9), provides objective and quantitative measures of postural stability and often is considered the gold standard for balance assessment10. An advantage of posturography is that it provides precise measurement of movement over fast time scales which affords measurement of subtle alterations in the control of balance.
A number of investigations have analyzed postural impairment in PwMS utilizing posturography. Different variables of postural sway are often utilized because they are thought to reflect different underlying physiological control mechanisms9,11. For example, sway velocity has been reported to be reflective on proprioception function12, whereas sample entropy, a nonlinear sway regularity/complexity measure, indirectly reflects the ability to adapt to the environment13, such as postural perturbations14. However, due to the large number of outcome variables, it is difficult to compare results across investigations. Indeed, a recent systematic review15 focusing on postural impairment in PwMS identified over 100 different variables as outcome measures of posturography. Furthermore, the choices of measures selected is rarely rationalized and results in a lack of consensus in determine the appropriate measure. Consequently, when measuring balance impairment in PwMS, it is difficult to determine which measure to use in order to provide clinical meaningful insights for treatment planning15.
In a previous attempt to quantify the diagnostic accuracy of static posturography in fall incidence prediction among PwMS, Prosperini et al.10 utilized various time domain sway measures (i.e. sway velocity, sway path, and sway area) to identify PwMS at risk of falls. They achieved over 70% of classification accuracy using a stepwise logistic regression and identified COP sway path as the only significant predictor of the fall occurrence. However, they provided minimal rational for choice of COP sway metrics and suggested the need for consensus on which sway measures to use when evaluating balance impairment in PwMS.
One solution to identify appropriate measures to differentiate between PwMS from healthy individuals is through the use of machine learning techniques. Indeed, various machine learning approach have been recently utilized to classify individuals with clinical pathology from controls, such as support vector machine (SVM), random forest (RF), k-nearest neighbor (KNN) and neural network16–19. Among those approaches, the RF algorithm20 has its unique advantage over others as it can not only construct a prediction rule to classify outcomes such as balance impairment, but it can also assess and rank variables with respect to their ability to predict the classification outcome21. The RF variable importance is determined by the Gini Importance, which is defined as the times a feature is used to split a node, normalized by the number of samples it splits21. The RF classification algorithm has also shown excellent accuracy in discriminating Parkinson’s disease participants based on gait and postural measures17 and has been used to identify important neuroimaging feature for Alzheimer’s disease diagnosis22. For more details on random forests approach, see20.
Therefore, the aims of this study were: (1) to identify which postural sway measures differentiate between PwMS and healthy controls, as a function of physiological fall risk; (2) to determine the discriminative ability of postural sway measures for fall risk classification (low, moderate, or high) in PwMS. To achieve these aims, we utilized the random forest algorithm to classify individuals’ fall risk based on a comprehensive set of postural sway parameters that contain time-domain, frequency-domain, and non-linear dynamics measures, and calculated the diagnostic accuracy (sensitivity, specificity, and accuracy) and feature importance of the postural sway measures.
Methods
This study is secondary analysis of previously published and unpublished data focusing on mobility in MS23–28. All data were sampled from baseline assessments prior to any interventions.
Participants
Data from 153 participants (50 healthy controls and 103 individuals with MS) were included in the analysis. The inclusion criteria for MS participants included a previously neurologist-confirmed diagnosis of MS and the ability to stand upright for 30 s without aid. Self-reported disability was accessed with the self-reported expanded disability status scale (EDSSSR)29, with higher scores indicating higher functional impairment. Inclusion criteria for healthy controls required no history of neurological or orthopedic pathology that might influence balance or mobility, and the ability to stand upright for 30 s without aid. The experimental protocol was approved by and performed in accordance with the relevant guidelines and regulations of the University of Illinois at Urbana-Champaign Institutional Review Board, and all participants provided written informed consent prior to participation.
Procedure
On a single visit, participants were instructed to stand upright for 30 s on a force platform (FP4060-05-PT-1000, Bertec Corp, Columbus, OH) with their feet shoulder-width apart and eyes open, fixating at a target 2 m away. Individual’s risk of falls was assessed using the short form of the Physiological Profile Assessment30 (PPA, Neuroscience Research Australia, Sydney), which consist of five validated measures of physiological function (visual contrast sensitivity, proprioception, quadriceps strength, reaction time, and postural sway). The individual fall risk index score was derived from the PPA normative database (Z-scores adjusted) for comparison with population norms, and MS participants were further categorized as low risk (<1), moderate risk (1–2), and high risk (>2)30,31.
Participants also provided demographic information and completed questionnaires on balance confidence (ABC or FES-I). The ABC and FES are both validated measures for fear of falling, and have been shown to be highly correlated (r = 0.88)32. The FES-I contains 16 items scored on a four-point scale (1 = not at all concerned to 4 = very concerned), which assesses the degree of perceived self-efficacy at avoiding a fall during basic activities of daily living (ADL)7. The total score of FES-I ranged from 16–64, with lower score indicating higher perceived self-efficacy at avoiding a fall. The ABC balance scale (a 16-item scale) measures individual’s confidence in maintaining balance while performing ADL6. The total score of ABC ranges from 0–100%, and higher score indicate higher confidence in maintaining balance. Due to differences in research procedure across multiple studies, 37 participants only completed the ABC scale, 27 participants only completed the FES-I scale, and 89 participants completed both ABC and FES-I scale. In order to compare the self-reported balance confidence across multiple studies, the FES-I score was converted to a percentage score (0–100%, with higher score indicate higher confidence) similar to the ABC score using the following formula: .
The self-reported Balance Confidence was derived as the average of ABC and FESp. If only ABC or FES was available, then that available measure was used.
All participants also completed the Berg Balance Scale assessment5, which consists of 14 physical tasks, such as transfer from sitting to standing position, standing with eyes closed, and picking up an object from the floor, all of which are part of normal daily activities. Each task performance is assigned 0–4 points by a trained personnel to give a total score of 0–56.
Data processing and feature extraction
COP data were sampled at 1000 Hz and low pass filtered (4th order Butterworth) at 10 Hz for further analysis. A comprehensive set of common postural sway measures that includes time and frequency domain measures and non-linear dynamics were derived from the COP sway data using established procedures9,33 and a customized MATLAB program (Mathworks, Inc., Natick, MA): Sway path length (Anterior-Posterior-AP, Medial-Lateral-ML, and Resultant Distance-RD); Mean sway velocity (AP, ML, and RD), 95% confidence ellipse sway area, Sway range (AP, ML direction), Root mean squared sway amplitude (AP, ML); total power, centroidal frequency, frequency dispersion, 95% power frequency; Sample Entropy (AP, ML), and Approximate Entropy (AP, ML). For both Sample Entropy and Approximate Entropy calculation, the input parameter were chosen as m = 3, r = 0.2, according to Rhea et al.33.
Model training and performance evaluation
To provide clinical meaningful output for MS fall risk diagnosis, Random Forest algorithms were employed to classify individuals’ fall risk categories (i.e. low, moderate, high) based on postural sway metrics. The RF algorithm is an ensemble of random decision trees (in our case, 1000 trees, chosen by examining the convergence of classification accuracy as additional trees were added34), in which the final predicted class for a test example is obtained by combining the predictions of all individual trees. This classifier has been shown to improve the generalization performance of individual decision trees20.
Binary classification between healthy controls and MS groups (HC vs MSLow, HC vs MSMod, HC vs MSHigh, MSLow vs MSMod, MSLow vs MSHigh, MSMod vs MSHigh) were performed based on sway measures and on clinical balance measures (BBS and Balance Confidence) separately. All classification algorithms were implemented using the open-source machine learning library Scikit-learn in Python35.
All algorithms were trained and tested through a 10-fold cross validation (CV) scheme36, and the classification performance was evaluated using standard classification metrics (accuracy, sensitivity, and specificity) derived from the confusion matrix. Briefly, sensitivity was calculated as true-positive/(true-positive + false-negative), specificity as true-negative/(true-negative + false-positive), and accuracy as true-positive + true negative/(true-positive + false-negative + true-negative + false-positive). Since no hyper-parameter tuning was performed in this work (as a proof-of-concept, all algorithms are used in their default settings) and with a limited sample size, data split into training and testing sets was not performed.
In order to identify the importance of each variable in detecting altered balance control in PwMS, a feature selection algorithm was subsequently performed based on the RF classification model. The RF classifier identifies the importance of each input features by calculating the Mean Decrease Impurity (MDI), defined as the number of times a feature is used to split a node, weighted by the number of sample it splits37. In other word, if a feature is used multiple times to split a large amount of data, it is identified as a substantial feature.
Ethical approval
All procedures were approved by the University of Illinois at Urbana-Champaign Institutional Review Board
Preprint
A pre-print of this publication has been deposited in bioRxiv: https://doi.org/10.1101/410704.
Results
Table 1 summarizes the characteristics of the 153 participants (108 Female, 45 Male) in this study. All 50 healthy controls had low fall risk (PPA < 1). Participants with MS were further categorized to low risk (n = 34, PPA < 1), moderate risk (n = 27, PPA 1–2), and high risk (n = 42, PPA > 2) of falls based on PPA. Overall, significant group difference (p < 0.05) in age, EDSS, BBS, PPA, and self-reported balance confidence scale were observed within the MS group. MS individuals with increased fall risk also exhibited higher disability (EDSSSR), lower balance confidence, and lower functional performance (BBS).
Table 1.
Participant characteristics.
| Controls | MSLow | MSMod | MSHigh | |
|---|---|---|---|---|
| N (M/F) | 50 (15/35) | 34 (10/24) | 27(7/20) | 42 (13/29) |
| Age* | 64.94 (4.91) | 54.00 (13.12) | 58.26 (8.28) | 56.76 (9.71) |
| EDSS* (Median & IQR) | NA | 4.0 (3.0) | 6.0 (2.0) | 6.0 (0.5) |
| BBS* | 55.12 (2.03) | 50.68 (4.80) | 45.22 (7.82) | 40.05 (8.36) |
| PPA* | −0.08 (0.62) | 0.28 (0.51) | 1.50 (0.31) | 3.01 (0.91) |
| Balance Confidence* | 93.12 (7.05) | 74.67 (19.87) | 58.90 (25.06) | 55.92 (17.19) |
All values were presented with mean and standard deviation, unless otherwise listed. * Significant group difference was observed (p < 0.05).
Table 2 provides a summary of the RF classification performance based on sway measures. Sway-based classifier can consistently differentiate healthy controls and MS individuals with high classification accuracy (86.3–92.3%), sensitivity (76.5–85.7%) and specificity (92.0–96.0%). However, sway-based classifier achieved relatively low accuracy in differentiating MS subgroups (Accuracy: 49.5–71.2%, Sensitivity: 48.1–71.4%, Specificity: 22.2–73.5%).
Table 2.
Diagnostic performance of RF algorithm using posturography measures.
| MSLow | MSMod | MSHigh | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ACC | SEN | SPC | ACC | SEN | SPC | ACC | SEN | SPC | |
| Controls | 86.3 | 76.5 | 92.0 | 92.3 | 85.2 | 96.0 | 89.3 | 85.7 | 92.0 |
| MSLow | 50.0 | 48.1 | 52.9 | 71.2 | 71.4 | 73.5 | |||
| MSMod | 49.5 | 66.7 | 22.2 | ||||||
ACC-accuracy, SEN - sensitivity, SPC – Specificity.
Table 3 provides a summary of the RF classification performance based on the BBS and self-reported balance confidence scale. Good classification performance for differentiating controls and MS individuals were observed (Accuracy: 73.5–95.6%, Sensitivity: 66.7–95.0%, Specificity: 78.0–96.0%). With moderate classification performance observed in differentiating MS subgroups (Accuracy: 61.4–76.7%, Sensitivity: 51.9–87.5%, Specificity: 59.3–72.7%). It is worth noting that this clinical measure-based classifier achieved lower accuracy (73.5%) than the sway-based classifier (86.3%) in differentiating healthy controls and low risk PwMS, but improved the accuracy in differentiating MS subgroups (61.4–76.7%), especially in differentiating PwMS with high fall risk among other subgroups (75.9–76.7% in comparison to 49.5–71.2% using only sway measures).
Table 3.
Diagnostic performance of RF algorithm using BBS and balance confidence measures.
| MSLow | MSMod | MSHigh | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ACC | SEN | SPC | ACC | SEN | SPC | ACC | SEN | SPC | |
| Controls | 73.5 | 66.7 | 78.0 | 92.1 | 88.9 | 94.0 | 95.6 | 95.0 | 96.0 |
| MSLow | 61.4 | 51.9 | 69.7 | 75.9 | 80.0 | 72.7 | |||
| MSMod | 76.7 | 87.5 | 59.3 | ||||||
ACC-accuracy, SEN - sensitivity, SPC – Specificity.
The importance of sway measures for differentiating the fall risk categories in PwMS and controls were extracted using the weighted percentage (MDI) of each RF classifier. Figure 1A shows the top-five sway measures for differentiating Low-risk MS individuals from Controls, with Sample Entropy (AP), Sway Range (ML), and Sway Area identified as the top predictors from this model with similar weight (~15%). Figure 1B shows the top sway measures for differentiating Mod-risk MS individuals from Controls. Sway Range (ML) was identified as the governing predictor from this model (62.3% of total importance measure). Figure 1C shows the top sway measures for differentiating High-risk MS individuals from Controls, in which Sway Range (ML), Sway Path Length (ML), and Mean Velocity (ML) were identified as the top predictors from the model (ML sway amplitude account for 71.1% of total importance).
Figure 1.
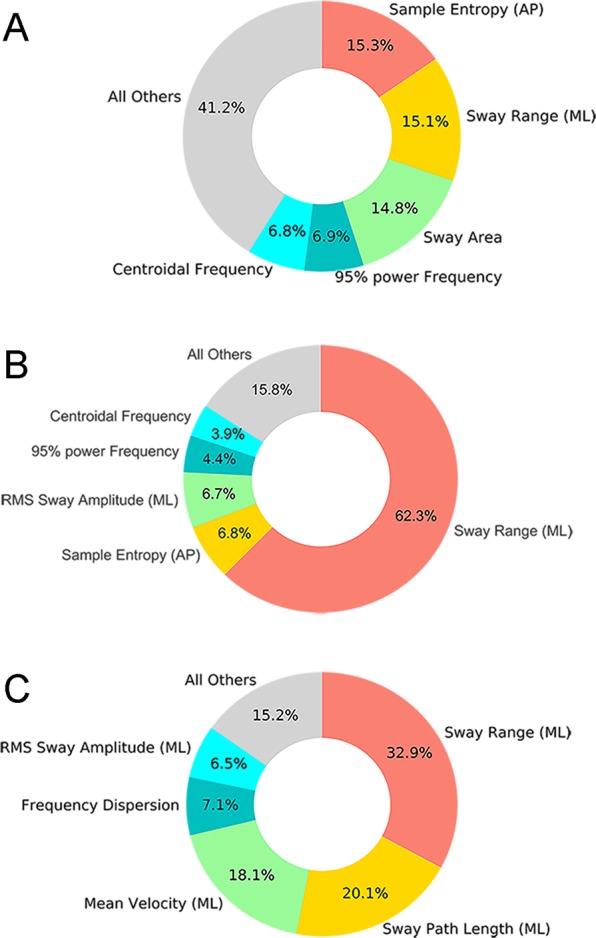
Sway metrics importance as measure by the Mean Decrease Impurity (MDI) of each RF classifier. (A) Controls vs MSLow; (B) Controls vs MSMod; (C) Controls vs MSHigh.
Discussion
This work aimed to identify postural sway parameters that best differentiate between PwMS and healthy controls, as a function of physiological fall risk utilizing a machine learning approach. This work also investigated the discriminative ability of using postural sway measures for fall risk classification in PwMS. Utilizing a machine learning approach (random forest classifier), we demonstrated that postural sway measures can discriminate low-risk PwMS from healthy controls, with over 86% of classification accuracy. In contrast, clinical balance measures (BBS) and self-report balance confidence measures achieved equal to superior discriminative ability in separating MS individuals with moderate and high risk of falls. These findings suggest that posturography measures are sensitive to subtle change in the balance control among MS individuals with minimal fall risk, whereas the balance impairment in moderate and high-risk MS individuals can be assessed with traditional clinical measures with high accuracy.
Overall, the observations further confirm that individuals with MS across the disability spectrum have postural control deficits15. The novel contribution of this investigation is identifying distinct COP parameters that are sensitive to balance impairment in MS as a function of fall risk. Indeed, ML sway amplitude parameters including sway range, sway path length, mean sway velocity were the strongest predictors for discriminating moderate and high-risk PwMS from healthy controls. This observation is consistent with Morrison and colleagues38 who demonstrated that a sample of 22 persons with MS had greater fall risk which coincided with deficits in mediolateral postural control. On the other hand, Sample Entropy, a measure of movement complexity (lower value indicating reduced signal complexity) and potential reduced adaptability to small perturbations13,14, was further identified as a key predictor for discriminating low-risk PwMS from healthy controls. This finding is consistent with previous research by Roeing et al.26 and Huisinga et al.39 that proposed reduced complexity in postural control as a biomarker for balance impairment in individuals with MS.
Several previous reports have attempted to predict fall risk in PwMS using postural sway measures. For example, Prosperini et al.10 used time-domain sway measures and logistic regression that achieved over 70% accuracy in discriminating faller from non-faller in PwMS. Furthermore, Kasser et al.40 utilized a logistic regression to achieve 81% accuracy in discriminating faller and non-faller with differences in the amount of body sway under different sensory conditions (i.e., eyes open to eyes closed). Hoang et al.31 also utilized logistic regression and achieved 71.2% accuracy (area under the Receiving Operating Characteristic curve) in discriminating faller from non-faller with sway range during eyes closed balance task. It is important to note that these previous reports predicted future falls while the current investigation is based on physiological fall risk. Consequently, direct comparisons between investigations may not be appropriate.
One limitation of the present study is that only a single postural control condition was included. Altered sensory test conditions which are common in balance assessments (i.e., eyes closed, standing on compliance surface) were not included in the data analysis. It has been reported that static posturography under altered sensory condition may yield better discriminative ability between PwMS and healthy controls41, and thus could further improve the classification performance. Another limitation of this work is the relative small sample size (n = 30–50 per group), which may limit generalization. It is also important to note that the current observations relate to postural assessment at one point in time. Further research is needed to determine which posturography metrics are most sensitive to rehabilitation. Lastly, the present work did not measure the fall occurrence, thus how the results relate to prediction of future falls is not clear. However, findings from this work may set the foundation for the development of guidelines for accurate reporting of balance impairment in PwMS.
Conclusion
The current findings highlight the benefits of posturography for balance impairment and fall risk assessment among PwMS, and provide insights on the standardization of metrics. Specifically, static posturography is sensitive for discriminating PwMS with minimal fall risk from healthy controls, with sample entropy measure identified as a core predictor. Whereas for quantification of balance impairment in high-risk MS individuals, medio-lateral sway amplitude is the strongest predictor. Furthermore, clinical balance measures also achieved high discriminative ability in separating MS individuals with moderate and high risk of falls. Therefore, the assessment technique and sway metrics should be based on the target populations, i.e. utilizing posturography (sway entropy) to identify individuals with subtle balance impairment and using clinical balance measures to assess individuals with high fall risk. These findings from this work may set the foundation for the development of guidelines for accurate reporting of balance impairment in PwMS.
Supplementary information
Acknowledgements
The authors would like to thank Dr. Salma Musaad at the Illinois Biostatistics Core of the Interdisciplinary Health Sciences Institute at Illinois for the assistance and feedback in the preparation of this manuscript. Data from this work was collected in previous studies that were funded in part by the National Multiple Sclerosis Society, Consortium of MS Centers, and MC10, Inc.
Author contributions
J.S. and R.S. conceived of the study. R.S. performed the data analysis. R.S., K.H. and J.S. co-wrote the manuscript text. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-52697-2.
References
- 1.Browne P, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010;10:407–412. doi: 10.1007/s11910-010-0128-0. [DOI] [PubMed] [Google Scholar]
- 3.Kister I, et al. Disability in multiple sclerosis A reference for patients and clinicians. Neurology. 2013;80:1018–1024. doi: 10.1212/WNL.0b013e3182872855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosnoff JJ, et al. Mobility, balance and falls in persons with multiple sclerosis. Plos One. 2011;6:e28021. doi: 10.1371/journal.pone.0028021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian journal of public health = Revue canadienne de sante publique. 1992;83:S7–11. [PubMed] [Google Scholar]
- 6.Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1995;50:M28–M34. doi: 10.1093/gerona/50A.1.M28. [DOI] [PubMed] [Google Scholar]
- 7.Yardley L, et al. Development and initial validation of the Falls Efficacy Scale-International (FES-I) Age and ageing. 2005;34:614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 8.Edginton Bigelow K, Berme N. Development of a protocol for improving the clinical utility of posturography as a fall-risk screening tool. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2010;66:228–233. doi: 10.1093/gerona/glq202. [DOI] [PubMed] [Google Scholar]
- 9.Prieto TE, Myklebust J, Hoffmann R, Lovett E, Myklebust B. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Transactions on biomedical engineering. 1996;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 10.Prosperini L, Fortuna D, Giannì C, Leonardi L, Pozzilli C. The diagnostic accuracy of static posturography in predicting accidental falls in people with multiple sclerosis. Neurorehabilitation and neural repair. 2013;27:45–52. doi: 10.1177/1545968312445638. [DOI] [PubMed] [Google Scholar]
- 11.Chiari L, Rocchi L, Cappello A. Stabilometric parameters are affected by anthropometry and foot placement. Clinical biomechanics. 2002;17:666–677. doi: 10.1016/S0268-0033(02)00107-9. [DOI] [PubMed] [Google Scholar]
- 12.Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D. Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. Journal of Neurophysiology. 2003;90:3774–3782. doi: 10.1152/jn.00730.2002. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitz LA, Goldberger AL. Loss of complexity and aging. Jama. 1992;267:1806–1809. doi: 10.1001/jama.1992.03480130122036. [DOI] [PubMed] [Google Scholar]
- 14.Busa MA, van Emmerik RE. Multiscale entropy: A tool for understanding the complexity of postural control. Journal of Sport and Health Science. 2016;5:44–51. doi: 10.1016/j.jshs.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comber, L., Sosnoff, J. J., Galvin, R., & Coote, S. Postural control deficits in people with Multiple Sclerosis: A systematic review and meta-analysis. Gait & posture, 61, 445–452 (2018). [DOI] [PubMed]
- 16.Tahir NM, Manap HH. Parkinson Disease Gait Classification based on Machine Learning Approach. Journal of Applied Sciences. 2012;12:180–185. doi: 10.3923/jas.2012.180.185. [DOI] [Google Scholar]
- 17.Arora, S., Venkataraman, V., Donohue, S., Biglan, K. M., Dorsey, E. R., & Little, M. A. (2014, May). High accuracy discrimination of Parkinson's disease participants from healthy controls using smartphones. In 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) (pp. 3641–3644). IEEE.
- 18.Howcroft J, Kofman J, Lemaire ED. Feature selection for elderly faller classification based on wearable sensors. Journal of neuroengineering and rehabilitation. 2017;14:47. doi: 10.1186/s12984-017-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun R, Sosnoff JJ. Novel sensing technology in fall risk assessment in older adults: a systematic review. BMC geriatrics. 2018;18:14. doi: 10.1186/s12877-018-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiman L. Random forests. Machine learning. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 21.Boulesteix AL, Janitza S, Kruppa J, König IR. Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery. 2012;2:493–507. [Google Scholar]
- 22.Gray KR, et al. Random forest-based similarity measures for multi-modal classification of Alzheimer’s disease. NeuroImage. 2013;65:167–175. doi: 10.1016/j.neuroimage.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon, Y., Wajda, D. A., Motl, R. W. & Sosnoff, J. J. Stride-time variability and fall risk in persons with multiple sclerosis. Multiple sclerosis international2015 (2015). [DOI] [PMC free article] [PubMed]
- 24.Roeing KL, Wajda DA, Motl RW, Sosnoff JJ. Gait termination in individuals with multiple sclerosis. Gait & posture. 2015;42:335–339. doi: 10.1016/j.gaitpost.2015.06.192. [DOI] [PubMed] [Google Scholar]
- 25.Sosnoff JJ, et al. Fall risk and incidence reduction in high risk individuals with multiple sclerosis: a pilot randomized control trial. Clinical rehabilitation. 2015;29:952–960. doi: 10.1177/0269215514564899. [DOI] [PubMed] [Google Scholar]
- 26.Roeing KL, Wajda DA, Sosnoff JJ. Time dependent structure of postural sway in individuals with multiple sclerosis. Gait & posture. 2016;48:19–23. doi: 10.1016/j.gaitpost.2016.04.023. [DOI] [Google Scholar]
- 27.Sosnoff JJ, et al. Dual task training in persons with Multiple Sclerosis: a feasability randomized controlled trial. Clinical rehabilitation. 2017;31:1322–1331. doi: 10.1177/0269215517698028. [DOI] [PubMed] [Google Scholar]
- 28.Sun R, et al. Assessment of Postural Sway in Individuals with Multiple Sclerosis Using a Novel Wearable Inertial Sensor. Digital Biomarkers. 2018;2:1–10. doi: 10.1159/000485958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1444. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 30.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical therapy. 2003;83:237–252. [PubMed] [Google Scholar]
- 31.Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Archives of physical medicine and rehabilitation. 2014;95:480–486. doi: 10.1016/j.apmr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Huang T-T, Wang W-S. Comparison of three established measures of fear of falling in community-dwelling older adults: psychometric testing. International journal of nursing studies. 2009;46:1313–1319. doi: 10.1016/j.ijnurstu.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Rhea CK, et al. Noise and complexity in human postural control: interpreting the different estimations of entropy. Plos One. 2011;6:e17696. doi: 10.1371/journal.pone.0017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz MH, Rozumalski A, Truong W, Novacheck TF. Predicting the outcome of intramuscular psoas lengthening in children with cerebral palsy using preoperative gait data and the random forest algorithm. Gait & posture. 2013;37:473–479. doi: 10.1016/j.gaitpost.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Pedregosa F, et al. Scikit-learn: Machine learning in Python. Journal of machine learning research. 2011;12:2825–2830. [Google Scholar]
- 36.Kohavi, R. (1995, August). A study of cross-validation and bootstrap for accuracy estimation and model selection. In Ijcai (Vol. 14, No. 2, pp. 1137–1145).
- 37.Archer KJ, Kimes RV. Empirical characterization of random forest variable importance measures. Computational Statistics & Data Analysis. 2008;52:2249–2260. doi: 10.1016/j.csda.2007.08.015. [DOI] [Google Scholar]
- 38.Morrison S, Rynders C, Sosnoff J. Deficits in medio-lateral balance control and the implications for falls in individuals with multiple sclerosis. Gait & posture. 2016;49:148–154. doi: 10.1016/j.gaitpost.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Huisinga JM, Yentes JM, Filipi ML, Stergiou N. Postural control strategy during standing is altered in patients with multiple sclerosis. Neuroscience letters. 2012;524:124–128. doi: 10.1016/j.neulet.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Kasser SL, Jacobs JV, Foley JT, Cardinal BJ, Maddalozzo GF. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Archives of physical medicine and rehabilitation. 2011;92:1840–1846. doi: 10.1016/j.apmr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Spain R, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait & posture. 2012;35:573–578. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


