Abstract
Background
This study aimed to evaluate the efficacy and safety of sodium-glucose cotransporter-2 (SGLT2) inhibitors in Korean patients who had inadequately controlled type 2 diabetes mellitus (T2DM) in real-world clinical practice.
Methods
We included 410 patients who started SGLT2 inhibitors (empagliflozin or dapagliflozin) as add-on therapy or switch therapy between February 2015 and June 2017. The primary efficacy endpoint was a change in glycosylated hemoglobin (HbA1c) from baseline to week 12. The secondary endpoints were patients achieving HbA1c <7.0% and changes in the fasting plasma glucose (FPG), lipid profiles, body weight, and blood pressure (BP).
Results
The mean HbA1c at baseline was 8.5% (8.6% in the add-on group and 8.4% in the switch group). At week 12, the mean adjusted HbA1c decreased by −0.68% in the overall patients (P<0.001), by −0.94% in the add-on group, and by −0.42% in the switch group. Significant reductions in FPG were also observed both in the add-on group and switch group (−30.3 and −19.8 mg/dL, respectively). Serum triglyceride (−16.5 mg/dL), body weight (−2.1 kg), systolic BP (−4.7 mm Hg), and diastolic BP (−1.3 mm Hg) were significantly improved in the overall patients. Approximately 18.3% of the patients achieved HbA1c <7.0% at week 12. A low incidence of hypoglycemia and genital tract infection was observed (6.3% and 2.2%, respectively).
Conclusion
SGLT2 inhibitors can be a suitable option as either add-on or switch therapy for Korean patients with inadequately controlled T2DM.
Keywords: Blood glucose; Diabetes mellitus, type 2; Hypoglycemia; Sodium-glucose transporter 2
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is considerably increasing worldwide [1]. In Korea, approximately 5.0 million individuals (14.4%) aged 30 years or older have T2DM [2]. Given the progressive nature of the disease, combinations of various glucose-lowering agents are often needed to achieve glycemic targets [3,4]. To overcome unmet needs for glycemic control, several new classes of anti-diabetic agents have been recently introduced for better management of T2DM.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors act by inhibiting renal glucose reabsorption, thereby enhancing glycosuria and reducing blood glucose levels [5,6]. The efficacy and safety of SGLT2 inhibitors have been reported in several randomized controlled trials (RCTs), which demonstrated improved glycemic control and significant reductions in body weight and blood pressure (BP) with a low risk of hypoglycemia [6,7,8]. The recent Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG) and Canagliflozin Cardiovascular Assessment Study (CANVAS) showed that the SGLT2 inhibitors empagliflozin and canagliflozin have additional benefits of reducing adverse cardiovascular outcomes compared with placebo [9,10]. According to the position statement published by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes, SGLT2 inhibitors are recommended as second or third-line agents in patients who failed to achieve glycemic target with one or more oral anti-diabetic drugs (OADs) [3]. In addition, because their mechanism of action is independent of insulin secretion or action, SGLT2 inhibitors can be safely combined with insulin therapy and are even effective in patients with long-standing diabetes with β-cell dysfunction [11,12].
Recently, several real-world data from clinical practice settings were reported which showed similar glucose-lowering efficacy of SGLT2 inhibitors as add-on therapy to those from previous RCTs and meta-analyses [13,14,15]. These findings were consistently observed in Korean patients with T2DM [16,17,18]. In actual clinical practice, it may also be considered to change an OAD to other class of OAD if glycemic goal is not achieved, which means switch therapy. Here, we aimed to evaluate the efficacy and safety of SGLT2 inhibitors as add-on therapy and as switch therapy from a real clinical practice perspective in Korean patients with T2DM who exhibited inadequate glycemic control.
METHODS
Study population
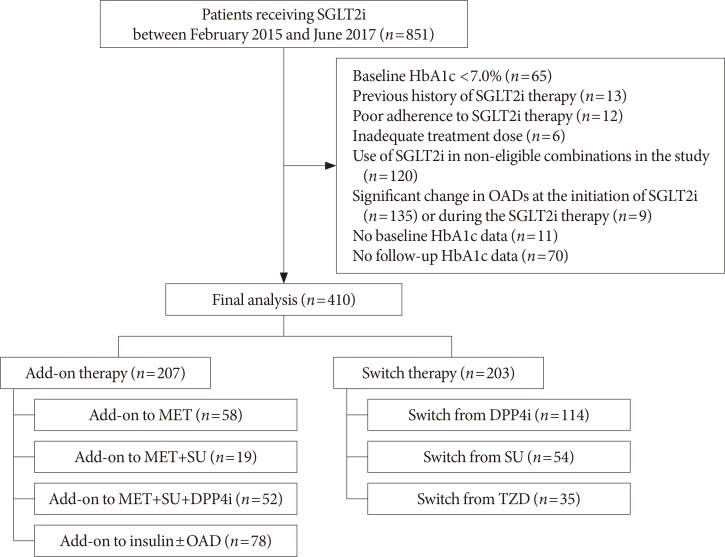
This was a single-center retrospective study conducted at Seoul Metropolitan Government Seoul National University Boramae Medical Center. The eligible study participants were T2DM patients with inadequate glycemic control (glycosylated hemoglobin [HbA1c] ≥7.0%) who started SGLT2 inhibitors (dapagliflozin or empagliflozin) between February 2015 and June 2017. Given the patients' visit schedule to the outpatient clinic, we regarded the 4 weeks before and after 12 weeks of treatment as an acceptable window for the follow-up HbA1c assessments. Among the 851 initially screened patients, we excluded patients with baseline HbA1c <7.0%, those who had previously used SGLT2 inhibitor, those who took a low dose of SGLT2 inhibitor (dapagliflozin 5 mg) or with poor treatment adherence for primary analysis, had considerable change in OAD class or dose at the initiation of SGLT2 inhibitor or during the treatment, and were not available for baseline or follow-up HbA1c test. We further excluded patients who received SGLT2 inhibitor as monotherapy or received it in combination with other treatments deemed ineligible for the analysis in the present study. The patients were divided into two groups according to their use of SGLT2 inhibitor: the add-on group (i.e., those who used SGLT2 inhibitors in addition to other OADs) and the switch group (i.e., those who switched from other OADs to SGLT2 inhibitors). The add-on group was further stratified by background therapy as add-on to metformin (MET), MET+sulfonylurea (SU), MET+SU+dipeptidyl peptidase 4 (DPP4) inhibitor, and insulin±OAD. Meanwhile, the switch group was further stratified by previously received OADs as switch from DPP4 inhibitor, SU, and thiazolidinedione (TZD). Finally, 410 patients (207 patients in the add-on group and 203 patients in the switch group) were included for analysis. The flow diagram for patient selection is shown in Fig. 1.
Fig. 1. Flow diagram of study subjects. SGLT2i, sodium-glucose cotransporter-2 inhibitor; HbA1c, glycosylated hemoglobin; OAD, oral anti-diabetic drug; MET, metformin; SU, sulfonylurea; DPP4i, dipeptidyl peptidase 4 inhibitor; TZD, thiazolidinedione.
Patient characteristics including age, height, body weight, BP, duration of diabetes, comorbidities, and concomitant medications for hypertension, dyslipidemia, and cardiovascular disease were examined at baseline.
This study was approved by the Institutional Review Board of Seoul Metropolitan Government Seoul National University Boramae Medical Center (No. 10-2017-27) and was conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective nature of the study.
Efficacy and safety assessments
The primary efficacy endpoint was a change in HbA1c between baseline and week 12 (±4 week). The secondary efficacy endpoints were changes in fasting plasma glucose (FPG), lipid profiles, BP, and body weight after 12 weeks of treatment. The proportion of patients who achieved the glycemic target of HbA1c <7.0% was also evaluated following ADA 2017 recommendation [19]. We further investigated clinical characteristics of patients who have shown good response to SGLT2 inhibitor therapy. We defined good responders as those with HbA1c <7.0% or a decrease in HbA1c of more than 1.0% at 12 weeks after SGLT2 inhibitor therapy [20].
The safety evaluations included self-monitored hypoglycemia and adverse event of hepatic and renal function based on medical records during the treatment with SGLT2 inhibitors. Hepatic adverse event was defined as over 3-fold increase of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) of the patients' baseline levels. Renal adverse event was considered as a ≥30% decline of estimated glomerular filtration rate (eGFR) from baseline levels as calculated using the Modification of Diet in Renal Disease-equation (mL/min/1.73 m2) [21].
Laboratory measurements
HbA1c level was determined using high-performance liquid chromatography (SST; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). FPG was measured in the 12-hour fasted state using the glucose oxidase method (Hitachi 747 chemistry analyzer; Hitachi, Tokyo, Japan). Fasting insulin level was measured using an immunoradiometric assay (DIAsource ImmunoAssays, Nivelles, Belgium). The homeostasis model assessment (HOMA) was used to evaluate pancreatic β-cell function (HOMA-β) and insulin resistance (HOMA-IR) [22]. The fasting total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglyceride (TG) levels were measured using an enzymatic colorimetric method (Toshiba Medical System Co. Ltd., Tokyo, Japan). The serum concentrations of AST, ALT, and creatinine were measured using a Hitachi 747.
Statistical analysis
All measurements were expressed as the mean±standard deviation (SD) or number (%). Categorical variables were compared using the chi-square test. The changes in HbA1c, FPG, body weight, and lipid profiles between baseline and week 12 within each treatment group were analyzed by via paired t-test. Comparisons across the treatment groups were performed using analysis of covariance after adjusting for their baseline levels. The changes in each parameter were presented as adjusted least square (LS) means and standard error. The predictive factors for SGLT2 inhibitor response were obtained using univariate and multivariate logistic regression analyses. Risk was reported with odds ratios and 95% confidence intervals (CIs). A P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistics version 21 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics of study subjects
The baseline demographic and clinical characteristics of study subjects are presented in Table 1. The mean age was 59.6 years, and the mean duration of diabetes was 12.0 years. Approximately 37.6% of patients were older than 65 years. The mean body weight was 71.6 kg, and the mean body mass index (BMI) was 27.3 kg/m2, with 46.3% of patients classified as obese (BMI ≥25 kg/m2) according to the definition of obesity for Asians [23]. Approximately 37.6% of the patients were treated with insulin in overall patients. The proportion of patients with titration for insulin doses (>10% of the baseline) was 20.1% [24]. The patients' clinical characteristics were comparable between the two groups except for the type of SGLT2 inhibitor therapy, in which a higher proportion of patients were receiving dapagliflozin as add-on therapy than switch therapy (P=0.001).
Table 1. Baseline demographic and clinical characteristics.
| Characteristic | Overall (n=410) | Add-on (n=207) | Switch (n=203) | P value |
|---|---|---|---|---|
| Age, yr | 59.6±11.8 | 60.1±10.9 | 59.1±12.6 | 0.398 |
| ≥65 | 154 (37.6) | 77 (37.2) | 77 (37.9) | 0.878 |
| Female sex | 236 (57.6) | 126 (60.9) | 110 (54.2) | 0.171 |
| Height, cm | 161.6±8.5 | 161.3±7.9 | 161.8±9.1 | 0.612 |
| Body weight, kg | 71.6±14.9 | 71.0±13.6 | 72.3±16.4 | 0.437 |
| Body mass index, kg/m2 | 27.3±4.7 | 27.0±4.1 | 27.6±5.3 | 0.272 |
| Systolic blood pressure, mm Hg | 130.3±13.5 | 131.5±13.5 | 129.0±13.4 | 0.075 |
| Diastolic blood pressure, mm Hg | 77.7±9.8 | 78.7±9.6 | 76.6±9.8 | 0.052 |
| Duration of diabetes, yr | 12.0±8.2 | 12.5±8.6 | 11.4±7.7 | 0.200 |
| Insulin therapy | 154 (37.6) | 78 (37.7) | 76 (37.4) | 0.960 |
| Titration of insulin dose (>10% of baseline) during SGLT2 inhibitor therapy | 31 (20.1) | 15 (19.2) | 16 (21.1) | 0.778 |
| Subtype of SGLT2 inhibitor | 0.001 | |||
| Dapagliflozin | 327 (79.8) | 179 (86.5) | 148 (72.9) | |
| Empagliflozin | 83 (20.2) | 28 (13.5) | 55 (27.1) | |
| HbA1c, % | 8.5±1.2 | 8.6±1.1 | 8.4±1.2 | 0.099 |
| ≥7.0% and <8.0% | 158 (38.5) | 70 (33.8) | 88 (44.4) | 0.047 |
| ≥8.0% and <9.0% | 137 (33.4) | 75 (36.2) | 62 (30.5) | 0.222 |
| ≥9.0% | 115 (28.0) | 62 (30.0) | 53 (26.1) | 0.386 |
| Fasting plasma glucose, mg/dL | 156.6±42.6 | 164.8±42.4 | 148.4±41.3 | <0.001 |
| Total cholesterol, mg/dL | 152.6±29.2 | 153.4±29.2 | 151.8±29.2 | 0.571 |
| Triglyceride, mg/dL | 156.4±96.6 | 161.1±110.5 | 151.7±80.0 | 0.330 |
| HDL-C, mg/dL | 45.3±10.7 | 45.7±11.1 | 45.0±10.3 | 0.509 |
| LDL-C, mg/dL | 82.1±24.2 | 81.6±22.5 | 82.7±25.9 | 0.664 |
| Fasting C-peptide, ng/mL | 2.5±1.5 | 2.5±1.5 | 2.4±1.6 | 0.649 |
| Fasting insulin, mIU/L | 14.7±7.7 | 15.8±8.5 | 12.6±6.1 | 0.341 |
| HOMA-β | 65.0±37.5 | 64.6±40.1 | 65.7±34.6 | 0.948 |
| HOMA-IR | 5.7±3.0 | 6.2±3.1 | 4.6±2.7 | 0.238 |
| Aspartate aminotransferase, U/L | 31.0±18.3 | 30.4±17.7 | 31.6±19.0 | 0.506 |
| Alanine aminotransferase, U/L | 35.8±27.4 | 35.6±28.6 | 36.0±26.1 | 0.907 |
| Serum creatinine, mg/dL | 0.78±0.19 | 0.77±0.18 | 0.80±0.21 | 0.146 |
| eGFR, mL/min/1.73 m2 | 91.1±22.8 | 91.8±22.9 | 90.5±22.8 | 0.582 |
| Hypertension | 297 (72.4) | 151 (72.9) | 146 (71.9) | 0.816 |
| Dyslipidemia | 348 (84.9) | 175 (84.5) | 173 (85.2) | 0.847 |
| Cardiovascular disease | 94 (22.9) | 43 (20.8) | 51 (25.1) | 0.295 |
| Medication | ||||
| Statin | 243 (83.7) | 173 (83.6) | 170 (83.7) | 0.963 |
| Fenofibrate | 25 (6.1) | 13 (6.3) | 12 (5.9) | 0.876 |
| Omega-3 fatty acid | 6 (1.5) | 4 (1.9) | 2 (1.0) | 0.425 |
| ACEi/ARB | 246 (60.0) | 126 (60.9) | 120 (59.1) | 0.717 |
| Anti-platelet agent | 223 (54.4) | 118 (57.0) | 105 (51.7) | 0.283 |
Values are presented as mean±standard deviation or number (%). Hypertension is defined as a systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg, or taking anti-hypertensive medication. Dyslipidemia is defined as a total cholesterol ≥240 mg/dL or taking lipid-lowering agents [2].
SGLT2, sodium-glucose cotransporter-2; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration rate; ACEi, ACE inhibitor; ARB, angiotensin II receptor blocker.
At baseline, the average HbA1c was 8.6% in the add-on group and 8.4% in the switch group. The mean concentration of FPG was significantly higher in the add-on group than that in the switch group (164.8 mg/dL vs. 148.4 mg/dL, P<0.001). Meanwhile, BP and lipid profiles did not differ between the two groups. In addition, there were also no significant differences in the baseline AST, ALT, serum creatinine, and eGFR levels. The add-on and switch therapy groups were also similar in terms of the prevalence of hypertension, dyslipidemia, or cardiovascular disease and use of concurrent medications including statins, fenofibrates, omega-3 fatty acids, angiotensin-converting enzyme inhibitors (or angiotensin II receptor blocker), or anti-platelet agents.
Efficacy
At 12 weeks, SGLT2 inhibitors exhibited a significant reduction in HbA1c levels of −0.68% (95% CI, −0.78 to −0.58) in the overall patients (P<0.001). The mean adjusted HbA1c decreased by −0.94% in the add-on group, and by −0.42% in the switch group (both P<0.001). The between-treatment difference in the LS mean change was −0.52% (95% CI, −0.68 to −0.37; P<0.001) (Table 2). There was a significant reduction of −25.1 mg/dL (95% CI, −29.5 to −20.8) in the FPG level in the overall patients (P<0.001). The add-on group and switch group exhibited −30.3 and −19.8 mg/dL of reduction in FPG levels, respectively (both P<0.001). The between-treatment difference in the LS mean change was −10.5 mg/dL (95% CI, −16.6 to −4.4; P=0.001). In the overall patients, there were also considerable reductions in serum TG level, body weight, systolic BP, and diastolic BP of −16.5 mg/dL (95% CI, −24.2 to −8.7), −2.1 kg (95% CI, −2.4 to −1.7), −4.7 mm Hg (95% CI, −6.1 to −3.2), and −1.3 mm Hg (95% CI, −2.4 to −0.2), respectively. Serum HDL-C, LDL-C, and eGFR levels did not differ between baseline and week 12.
Table 2. Changes in measurements after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors.
| Variable | Baseline | Week 12 | LS mean change from baseline (95% CI) | Difference in LS mean change (95% CI)a |
|---|---|---|---|---|
| HbA1c, % | ||||
| Overall (n=410) | 8.5±0.1 | 7.8±0.1 | −0.68 (−0.78 to −0.58)b | −0.52 (−0.68 to −0.37)c |
| Add-on (n=207) | 8.6±0.1 | 7.6±0.1 | −0.94 (−1.05 to −0.83)b | |
| Switch (n=203) | 8.4±0.1 | 8.0±0.1 | −0.42 (−0.53 to −0.30)b | |
| FPG, mg/dL | ||||
| Overall (n=410) | 156.6±2.1 | 131.5±1.6 | −25.1 (−29.5 to −20.8)b | −10.5 (−16.6 to −4.4)c |
| Add-on (n=207) | 164.8±2.9 | 128.5±2.2 | −30.3 (−34.6 to −26.1)b | |
| Switch (n=203) | 148.4±2.9 | 134.5±2.3 | −19.8 (−24.1 to −15.6)b | |
| TG, mg/dL | ||||
| Overall (n=410) | 157.0±5.1 | 140.6±3.7 | −16.5 (−24.2 to −8.7)b | −2.7 (−14.0 to 8.5) |
| Add-on (n=207) | 160.8±8.1 | 141.0±5.3 | −17.8 (−25.6 to −9.9)b | |
| Switch (n=203) | 153.1±6.0 | 140.1±5.3 | −15.1 (−23.1 to −7.1)b | |
| HDL-C, mg/dL | ||||
| Overall (n=410) | 45.4±0.6 | 45.8±0.5 | 0.5 (−0.3 to −1.2) | 0.6 (−0.8 to 2.0) |
| Add-on (n=207) | 45.7±0.8 | 46.4±0.7 | 0.8 (−0.2 to 1.7) | |
| Switch (n=203) | 45.0±0.8 | 45.2±0.8 | 0.1 (−0.8 to 1.1) | |
| LDL-C, mg/dL | ||||
| Overall (n=410) | 82.4±1.2 | 80.5±1.3 | −1.9 (−4.1 to 0.3) | 2.0 (−2.0 to 6.0) |
| Add-on (n=207) | 81.6±1.6 | 81.0±1.7 | −0.9 (−3.7 to 1.9) | |
| Switch (n=203) | 83.2±1.9 | 80.0±1.9 | −2.9 (−5.8 to 0.1) | |
| Body weight, kg | ||||
| Overall (n=410) | 74.6±1.2 | 72.5±1.1 | −2.1 (−2.4 to −1.7)b | 0.5 (−0.3 to 1.1) |
| Add-on (n=207) | 74.1±1.5 | 72.3±1.5 | −1.9 (−2.4 to −1.5)b | |
| Switch (n=203) | 75.2±1.8 | 72.8±1.7 | −2.4 (−2.8 to −1.9)b | |
| SBP, mm Hg | ||||
| Overall (n=410) | 130.1±0.7 | 125.4±0.7 | −4.7 (−6.1 to −3.3)b | 1.1 (−1.3 to 3.5) |
| Add-on (n=207) | 131.3±1.0 | 126.5±1.0 | −4.2 (−5.9 to −2.6)b | |
| Switch (n=203) | 128.8±1.0 | 124.2±1.0 | −5.3 (−7.0 to −3.6)b | |
| DBP, mm Hg | ||||
| Overall (n=410) | 78.7±0.7 | 77.3±1.0 | −1.3 (−2.4 to −0.2)b | 0.6 (−1.4 to 2.7) |
| Add-on (n=207) | 78.4±0.7 | 77.1±1.0 | −1.0 (−2.5 to 0.4) | |
| Switch (n=203) | 76.5±0.7 | 75.3±0.7 | −1.7 (−3.2 to −0.2) | |
| eGFR, mL/min/1.73 m2 | ||||
| Overall (n=410) | 91.4±1.2 | 91.8±1.2 | 0.4 (−1.0 to 1.8) | −1.9 (−4.7 to 0.8) |
| Add-on (n=207) | 91.9±1.6 | 91.3±1.6 | −0.6 (−2.6 to 1.4) | |
| Switch (n=203) | 90.8±1.7 | 92.3±1.8 | 1.5 (−0.4 to 3.5) |
Values are presented as mean±standard error.
LS, least square; CI, confidence interval; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
aDifference in the LS mean change, which was calculated as add-on therapy minus switch therapy, bP<0.05 from baseline in overall patients and each treatment group, cP<0.05 for the between-treatment group difference.
We further compared changes in cardiometabolic parameters in each treatment group according to the background glucose-lowering therapy. In the add-on group, the glucose-lowering effect did not differ between add-on to MET, MET+SU, and MET+SU+DPP4 inhibitor (LS mean change of −1.20%, −1.16%, and −1.06%, respectively), whereas, insulin add-on showed a smaller reduction of HbA1c than that in other regimens (LS mean change of −0.71%; between-treatment difference from add-on to MET of −0.49% [95% CI, −0.84 to −0.15; P=0.001]). The magnitude of HbA1c reduction did not differ among previous OADs in the switch group (Table 3). We observed an increase in serum TG level for switching from TZD compared with switching from DPP4 inhibitor (P=0.002). There were no between-treatment differences in FPG, LDL-C, body weight, systolic BP, and diastolic BP in both treatment groups according to the background therapy (Supplementary Tables 1 and 2).
Table 3. Changes in glycosylated hemoglobin after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors as add-on therapy and switch therapy.
| Variable | Baseline | Week 12 | LS mean change from baseline (95% CI) | Difference in LS mean change (95% CI)a |
|---|---|---|---|---|
| Add-on therapy | ||||
| Overall (n=207) | 8.6±0.1 | 7.6±0.1 | −0.94 (−1.05 to −0.83)b | |
| MET (n=58) | 7.8±0.1 | 7.0±0.1 | −1.20 (−1.39 to −1.02)b | - |
| MET+SU (n=19) | 8.1±0.2 | 7.1±0.1 | −1.16 (−1.46 to −0.86)b | −0.05 (−0.51 to 0.42) |
| MET+SU+DPP4i (n=52) | 8.9±0.2 | 7.7±0.1 | −1.06 (−1.25 to −0.88)b | −0.14 (−0.50 to 0.22) |
| Insulin (n=78) | 9.0±0.1 | 8.1±0.1 | −0.71 (−0.86 to −0.56)b | −0.49 (−0.84 to −0.15)c |
| Switch therapy | ||||
| Overall (n=203) | 8.4±0.1 | 8.0±0.1 | −0.42 (−0.53 to −0.30)b | |
| DPP4i (n=114) | 8.2±0.1 | 8.0±0.1 | −0.33 (−0.50 to −0.16)b | - |
| SU (n=54) | 8.7±0.2 | 8.2±0.2 | −0.42 (−0.67 to −0.18)b | 0.09 (−0.28 to 0.46) |
| TZD (n=35) | 8.3±0.1 | 7.9±0.2 | −0.44 (−0.74 to −0.13)b | 0.11 (−0.32 to 0.53) |
Values are presented as mean±standard error.
LS, least square; CI, confidence interval; MET, metformin; SU, sulfonylurea; DPP4i, dipeptidyl peptidase 4 inhibitor; TZD, thiazolidinedione.
aDifference in the LS mean change, which was calculated as compared to adding MET in add-on group and switching from DPP4i in switch group, bP<0.05 from baseline in overall patients and each treatment subgroup, cP<0.05 for the between-treatment group difference.
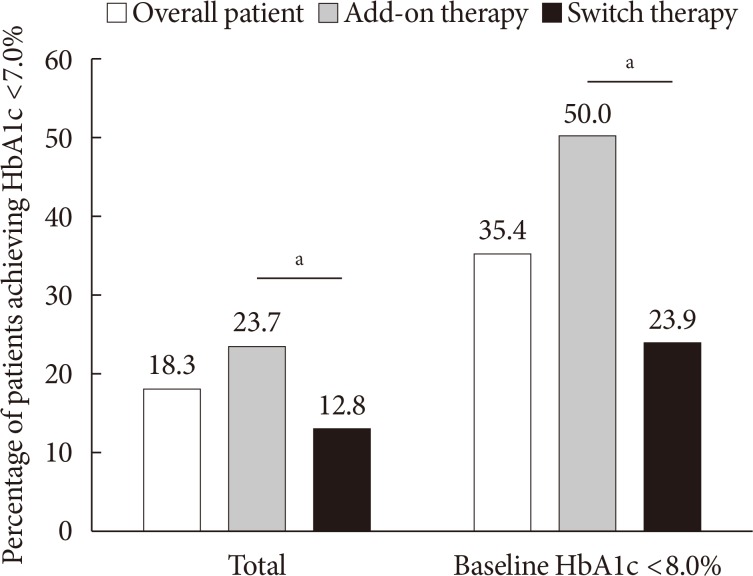
The proportion of patients who achieved HbA1c <7.0% was 18.3% in overall patients, 23.7% in the add-on group, and 12.8% in the switch group (P=0.004). Among the patients with baseline HbA1c levels <8.0% (n=158), 50.0% in the add-on group and 23.9% in the switch group achieved HbA1c levels <7.0% at week 12 (P=0.001) (Fig. 2). In the add-on group, 46.6%, 47.4%, 11.5%, and 9.0% of the patients in the add-on to MET, MET+SU, MET+SU+DPP4 inhibitor, and insulin±OAD subgroups, respectively, exhibited HbA1c <7.0% at week 12 (P<0.001). In the switch group, 15.8%, 7.4%, and 11.4% of the patients in the DPP4 inhibitor, SU, and TZD subgroups, respectively, exhibited HbA1c <7.0% (P=0.305).
Fig. 2. Percentage of patients who achieved glycosylated hemoglobin (HbA1c) <7.0% in overall patients and those with baseline HbA1c <8.0%. aP<0.05 between add-on therapy and switch therapy.
In overall, 171 patients (41.7%) experienced HbA1c <7.0% or a decrease in HbA1c >1.0% and were categorized as good responders. Higher baseline glycemic parameters (HbA1c and FPG) and higher HOMA-IR were associated with a SGLT2 inhibitor response. Patients receiving insulin therapy showed a poor response to SGLT2 inhibitors (Table 4). To identify predictive factors for good responders, we performed logistic regression analyses. Using univariate analysis, higher baseline HbA1c and FPG levels, and no insulin therapy were associated with a good response to SGLT2 inhibitors. Even after multivariate analysis, higher baseline HbA1c and no insulin therapy had significant associations with good responders (Supplementary Table 3). In subgroup analysis, higher baseline HbA1c and no insulin therapy were significantly associated with good responders in the add-on group, whereas only higher HbA1c level remained significant in the switch group using multivariate analysis (Supplementary Tables 4 and 5).
Table 4. Comparison of clinical characteristics between the good responders and the poor responders in overall patients.
| Characteristic | Good responder (n=171) | Poor responder (n=239) | P value |
|---|---|---|---|
| Age, yr | 58.6±12.1 | 60.4±11.6 | 0.125 |
| Female, sex | 97 (56.7) | 139 (58.2) | 0.839 |
| Body mass index, kg/m2 | 27.6±4.9 | 27.0±4.5 | 0.264 |
| Duration of diabetes, yr | 11.1±8.2 | 12.5±8.2 | 0.091 |
| Subtype of SGLT2 inhibitor, dapagliflozin:empagliflozin | 137:34 | 190:49 | 0.901 |
| Hypertension | 121 (70.8) | 176 (73.6) | 0.575 |
| Dyslipidemia | 145 (84.8) | 203 (84.9) | 0.968 |
| HbA1c, % | 8.8±1.3 | 8.3±1.0 | <0.001 |
| Fasting plasma glucose, mg/dL | 162.7±42.3 | 152.3±42.4 | 0.015 |
| Fasting C-peptide, ng/mL | 2.6±1.6 | 2.5±1.4 | 0.609 |
| Fasting insulin, mIU/L | 17.2±10.0 | 12.7±5.0 | 0.216 |
| HOMA-β | 58.8±49.0 | 62.1±27.4 | 0.682 |
| HOMA-IR | 7.1±3.6 | 4.6±2.0 | 0.044 |
| eGFR, mL/min/1.73 m2 | 93.1±23.9 | 89.7±4.5 | 0.137 |
| Insulin therapy | 54 (31.6) | 100 (41.8) | 0.039 |
Values are presented as mean±standard deviation or number (%).
SGLT2, sodium-glucose cotransporter-2; HbA1c, glycosylated hemoglobin; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration rate.
Safety
In the present study, two patients showed treatment-emergent ≥3-fold increase in ALT or ALT compared with baseline levels. However, the actual AST and ALT levels were within normal limits, indicating no clinically relevant hepatic adverse event. Four patients showed more than 30% decrease in eGFR levels after initiation of SGLT2 inhibitor. All four patients had baseline eGFR levels of ≥60 mL/min/1.73 m2, and only one patient had eGFR <60 mL/min/1.73 m2 after starting SGLT2 inhibitor treatment. No patient required renal replacement therapy during the treatment period.
Overall, hypoglycemia and genital tract infection was observed in 26 (6.3%) and nine patients (2.2%), respectively. The frequency of hypoglycemia was higher in the patients receiving insulin therapy than those receiving OADs (10.4% vs. 3.9%, P=0.009). Diabetic ketoacidosis did not occur during the treatment period.
DISCUSSION
In this real-world study, we demonstrated that SGLT2 inhibitors significantly improved glycemic control in Korean patients with inadequately controlled T2DM (change in HbA1c of −0.68% in the overall study population; −0.94% in the add-on group; and −0.42% in the switch group). Treatment with SGLT2 inhibitors also exhibited significant improvements in FPG, TG, body weight, systolic BP, and diastolic BP. Our findings are consistent with previous RCTs that support the efficacy of SGLT2 inhibitors in patients with T2DM in a real-world setting.
Because previous RCT data were limited to analyses for add-on therapy, we separately examined the efficacy SGLT2 inhibitors as add-on and switch therapy. SGLT2 inhibitors when used in combination with OADs or insulin were shown to be significantly effective in reducing HbA1c, and this finding was comparable to the results from RCTs [7] and those from the real-world setting [25,26]. The glucose-lowering efficacy of SGLT2 inhibitors did not differ between its combination with MET, MET+SU, and MET+SU+DPP4; however, the efficacy was greater than that in the combination with insulin. Meanwhile, the adjusted mean change of HbA1c with add-on to MET (−1.20%) was greater in the current study than those from RCTs (−0.94% to −0.56%) [27,28,29,30,31]. The efficacy of SGLT2 inhibitors as an add-on to MET+SU in HbA1c reduction (−1.16% vs. −1.06% to −0.82%) was better in the current study than that reported in RCTs [32,33,34]. Meanwhile, the observed reductions of HbA1c in the other add-on subgroups (Table 3) were comparable to those obtained from RCTs, and these findings seem to be attributed to higher baseline HbA1c level in our study than those in previous studies.
The additive glucose-lowering effect of SGLT2 inhibitors to insulin was greater than those reported in a meta-analysis (HbA1c reduction, −0.71% vs. −0.56%) [12]. In our study, insulin doses were self-titrated for the individual patient during the treatment period. Along with the insulin-independent mechanism of action, SLGT2 inhibitors may also improve insulin sensitivity and thereby preserve β-cell function [11,35,36]. It is difficult to determine why our study showed better glucose-lowering efficacy than previous reports. However, it may be partly attributed to the different pathophysiology of diabetes between Caucasian and Asians patients, including Koreans. Compared with Caucasian patients, Asian patients with T2DM have reduced β-cell function and higher insulin sensitivity [37,38]. There might be also some differences in the pharmacokinetic concentration and pharmacodynamic response of SGLT2 inhibitors between Asians and Caucasian T2DM populations caused by body size difference, similar to those observed in DPP4 inhibitors [39,40]. Therefore, differences in ethnic group and characteristics of the study population seem to influence the findings of our study. In addition, our study participants may have greater β-cell deterioration due to the long disease duration (>10 years), which may result in higher baseline HbA1c than those in the previous RCTs. This could further contribute to a greater reduction to SGLT2 inhibitors in the present study.
In addition to HbA1c, we observed improvements in FPG (−25.1 mg/dL), body weight (−2.1 kg), systolic BP (−4.7 mm Hg), and diastolic BP (−1.3 mm Hg). These findings were comparable to meta-analysis data of RCTs that showed significant reduction in FPG of 19.8 to 34.2 mg/dL, body weight of 1.6 to 2.5 kg, systolic BP of 2.8 to 4.9 mm Hg, and diastolic BP of 1.5 to 2.0 mm Hg [7]. Further, SGLT2 inhibitors significantly improved serum TG levels (−16.5 mg/dL) in the current study, which is consistent with the findings of RCTs, whereas, HDL-C and LDL-C did not differ between baseline and 12 weeks after treatment. Because majority of patients were already receiving statin therapy, treatment with SGLT2 inhibitors may not result in meaningful changes in lipid profiles. However, given the limited number of our study participants, further studies are needed to investigate this issue. Although we could not evaluate the incidence of major adverse cardiovascular events due to the short treatment period, the observed significant improvements in cardiometabolic parameters suggest a potential microvascular and macrovascular benefit with SGLT2 inhibitors.
Due to retrospective nature of this study, we examined relative reduction in HbA1c after switching from several OADs to SGLT2 inhibitors, instead of direct comparison of glucose-lowering efficacy between SGLT2 inhibitors and other OADs. Switching to SGLT2 inhibitors demonstrated significant reduction in HbA1c of −0.42% regardless of previous OAD classes, including DPP4 inhibitor, SU, and TZD. A total of 122 patients (60.1%) showed reduction in HbA1c with SGLT2 inhibitors, and 105 (51.7%) showed more than 0.3% reduction in HbA1c in the switch group. FPG and serum TG were also substantially reduced after 12-week treatment with SLGT2 inhibitors. Furthermore, switch therapy to SGLT2 inhibitors showed additional benefits for reduction of body weight and systolic BP. Because all switch and add-on regimens showed significant glycemic improvement with SGLT2 inhibitor, we considered the possibility of selection bias in our study participants; if patients who stopped SGLT2 inhibitors early due to poor treatment response were excluded from the analysis, the glycemic efficacy may be overestimated. Hence, we thoroughly reviewed 12 patients who were excluded in the final analysis due to poor adherence to SGLT2 inhibitors (Fig. 1). There was only one patient who stopped the medication because of poor glycemic response, and she took high-dose glucocorticoid during the period when she was receiving dapagliflozin. Considering the degree of HbA1c reduction after switching to SGLT2 inhibitors, it is possible that altering OAD itself may improve the patients' lifestyle in a positive direction, which may further enhance glycemic and cardiometabolic parameters. Collectively, our findings suggest that switching to SGLT2 inhibitors may be an attractive regimen in patients with inadequately controlled T2DM in the real-clinical practice.
We analyzed the clinical predictors of a good response to SGLT2 inhibitors. Higher glycemic parameters and no treatment with insulin therapy were linked with a SGLT2 inhibitor response. A previous study showed that younger age, male sex, higher glycemic parameter, lower BMI, short duration of T2DM, and higher eGFR were linked to a good response to dapagliflozin in Korean patients [16]. This study defined the good responder as a ≥10% reduction in HbA1c value after 12 weeks of dapagliflozin, and add-on and switch group were not separately analyzed.
In the current study, the overall incidence of self-reported hypoglycemia and genital tract infections was similar or slightly lower than those reported in RCTs (6.3% in hypoglycemia; 2.2% in genital tract infection) [32,41,42,43,44,45,46,47]. When we considered the cases who discontinued SGLT2 inhibitor early due to these adverse events—mild vaginitis (n=1) and hypoglycemia (n=1)—their overall incidences were still low. All reported cases of hypoglycemia and genital symptoms were mild in our study. No clinically relevant adverse hepatic or renal event and diabetic ketoacidosis were observed during the treatment period. This further supports the safety profiles of SGLT2 inhibitors shown by RCTs in a real-world setting.
There are several limitations of our study to be considered. First, because this was a retrospective study, there was substantial heterogeneity across the background glucose-lowering therapy for individual patients. In addition, we could not systematically collect information about adverse events. In the present study, the occurrence of adverse events including hypoglycemia and genital tract infection were determined by self-report from patients. Hence, there may be missing data and misdiagnosis for the safety profiles related to SGLT2 inhibitors. For example, in the case of genital infection, it is possible that confusion with urinary tract infection has occurred. Second, because a substantial proportion of patients was receiving dapagliflozin (10 mg), direct comparisons of glucose-lowering efficacy and drug-related safety profiles according to the dose and type of SGLT2 inhibitor were not possible. Third, because canagliflozin is not yet commercially available in South Korea, and dapagliflozin and empagliflozin are the only available SGLT2 inhibitors in our institution, we collected data for these two drugs. Fourth, this study was conducted over a relatively short 12-week period. Thus, further studies are needed to investigate long-term effect and tolerability of SGLT2 inhibitors in real clinical practice.
Despite these limitations, the present study is valuable to investigate the real-world efficacy and safety profiles of SGLT2 inhibitors in Korean patients and to comparatively assess them based on the various combination regimens of SGLT2 inhibitors. There have been few real-world studies of SGLT2 inhibitors reported in Koreans. Previous two studies evaluated the efficacy and safety of dapagliflozin or empagliflozin-based quadruple therapy in Koreans [17,18]. Another Korean study examined the efficacy of dapagliflozin and clinical characteristics of good responders to dapagliflozin [16]. Our study is the first Korean study that analyzed the efficacy and safety of dapagliflozin and empagliflozin as add-on or switch therapy in various combinations of background glucose-lowering therapy.
In conclusion, SGLT2 inhibitors as add-on or switch therapy resulted in a greater reduction in HbA1c in Korean patients with inadequately controlled T2DM in a real-world setting. SGLT2 inhibitors also demonstrated substantial reductions in body weight and BP and improvement of lipid profiles. These findings indicate that SGLT2 inhibitors as add-on or switch regimen may be applicable in real clinical practice, particularly in patients with inadequately controlled T2DM under treatment with dual or triple drug combinations.
ACKNOWLEDGMENTS
None
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Conception or design: M.K.M.
- Acquisition, analysis, or interpretation of data: A.R.H., M.K.M.
- Drafting the work or revising: A.R.H., M.K.M.
- Final approval of the manuscript: A.R.H., B.K.K., S.W.K., K.H.Y., M.K.M.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2018.0134.
Changes in FPG, lipid profiles, body weight, and blood pressure after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors as add-on therapy
Changes in FPG, lipid profiles, body weight, and blood pressure after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors as switch therapy
Logistic regression analysis to determine variables associated with good responders to SGLT2 inhibitors in overall patients
Comparison of clinical characteristics between the good responders and the poor responders in the add-on and switch group
Logistic regression analysis to determine variables associated with good responders to SGLT2 inhibitors in the add-on and switch group
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korean Diabetes Association. Diabetes fact sheet in Korea 2018. [cited 2019 Jan 12]. http://www.diabetes.or.kr/bbs/skin/dianews/download.php?code=admin&number=1694.
- 3.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl 1):S64–S74. doi: 10.2337/dc17-S011. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, DeFronzo RA. Lowering plasma glucose concentration by inhibiting renal sodium-glucose cotransport. J Intern Med. 2014;276:352–363. doi: 10.1111/joim.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 7.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 8.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Singh R. Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin: rationale and evidences. Expert Rev Clin Pharmacol. 2016;9:409–418. doi: 10.1586/17512433.2016.1131121. [DOI] [PubMed] [Google Scholar]
- 12.Tang H, Cui W, Li D, Wang T, Zhang J, Zhai S, Song Y. Sodium-glucose co-transporter 2 inhibitors in addition to insulin therapy for management of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:142–147. doi: 10.1111/dom.12785. [DOI] [PubMed] [Google Scholar]
- 13.Tamez-Perez HE, Delgadillo-Esteban E, Soni-Duque D, Hernandez-Coria MI, Tamez-Pena AL. SGLT2 inhibitors as add on therapy in type 2 diabetes: a real world study. J Diabetes Metab Disord. 2017;16:27. doi: 10.1186/s40200-017-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RE, Gupta N, Aronson R. Effect of dapagliflozin on glycemic control, weight, and blood pressure in patients with type 2 diabetes attending a specialist endocrinology practice in Canada: a retrospective cohort analysis. Diabetes Technol Ther. 2017;19:685–691. doi: 10.1089/dia.2017.0134. [DOI] [PubMed] [Google Scholar]
- 15.Blonde L, Patel C, Bookhart B, Pfeifer M, Chen YW, Wu B. A real-world analysis of glycemic control among patients with type 2 diabetes treated with canagliflozin versus dapagliflozin. Curr Med Res Opin. 2018;34:1143–1152. doi: 10.1080/03007995.2018.1458709. [DOI] [PubMed] [Google Scholar]
- 16.Han E, Kim A, Lee SJ, Kim JY, Kim JH, Lee WJ, Lee BW. Characteristics of dapagliflozin responders: a longitudinal, prospective, nationwide dapagliflozin surveillance study in Korea. Diabetes Ther. 2018;9:1689–1701. doi: 10.1007/s13300-018-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku EJ, Lee DH, Jeon HJ, Oh TK. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine-based therapy in patients with inadequately controlled type 2 diabetes: an observational study in clinical practice. Diabetes Obes Metab. 2019;21:173–177. doi: 10.1111/dom.13476. [DOI] [PubMed] [Google Scholar]
- 18.Jeon HJ, Ku EJ, Oh TK. Dapagliflozin improves blood glucose in diabetes on triple oral hypoglycemic agents having inadequate glucose control. Diabetes Res Clin Pract. 2018;142:188–194. doi: 10.1016/j.diabres.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Standards of medical care in diabetes: 2017. Summary of revisions. Diabetes Care. 2017;40(Suppl 1):S4–S5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 20.Horie I, Haraguchi A, Sako A, Akeshima J, Niri T, Shigeno R, Ito A, Nozaki A, Natsuda S, Akazawa S, Mori Y, Ando T, Kawakami A, Abiru N. Predictive factors of efficacy of combination therapy with basal insulin and liraglutide in type 2 diabetes when switched from longstanding basal-bolus insulin: association between the responses of β- and α-cells to GLP-1 stimulation and the glycaemic control at 6 months after switching therapy. Diabetes Res Clin Pract. 2018;144:161–170. doi: 10.1016/j.diabres.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. [cited 2019 Jan 12]. Available from: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf.
- 24.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–136. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 25.Chow W, Miyasato G, Kokkotos FK, Bailey RA, Buysman EK, Henk HJ. Real-world canagliflozin utilization: glycemic control among patients with type 2 diabetes mellitus: a multi-database synthesis. Clin Ther. 2016;38:2071–2082. doi: 10.1016/j.clinthera.2016.07.168. [DOI] [PubMed] [Google Scholar]
- 26.Scheerer MF, Rist R, Proske O, Meng A, Kostev K. Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diabetes Metab Syndr Obes. 2016;9:337–345. doi: 10.2147/DMSO.S116243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–1160. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 29.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–1659. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Erratum. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–393. 1173. doi: 10.2337/dc14-2364. [DOI] [PubMed] [Google Scholar]
- 31.Ross S, Thamer C, Cescutti J, Meinicke T, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17:699–702. doi: 10.1111/dom.12469. [DOI] [PubMed] [Google Scholar]
- 32.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S Study 05 Group. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial. Diabetes Care. 2015;38:365–372. doi: 10.2337/dc14-0666. [DOI] [PubMed] [Google Scholar]
- 34.Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern M, Kloting N, Mark M, Mayoux E, Klein T, Bluher M. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism. 2016;65:114–123. doi: 10.1016/j.metabol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Ahn CH, Oh TJ, Kwak SH, Cho YM. Sodium-glucose cotransporter-2 inhibition improves incretin sensitivity of pancreatic β-cells in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:370–377. doi: 10.1111/dom.13081. [DOI] [PubMed] [Google Scholar]
- 37.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 39.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411–427. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Hu P, Yin Q, Deckert F, Jiang J, Liu D, Kjems L, Dole WP, He YL. Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J Clin Pharmacol. 2009;49:39–49. doi: 10.1177/0091270008325152. [DOI] [PubMed] [Google Scholar]
- 41.Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–628. doi: 10.1111/dom.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–728. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 43.Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40:201–209. doi: 10.2337/dc16-1347. [DOI] [PubMed] [Google Scholar]
- 44.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 45.Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, Desai M, Shaw W, Capuano G, Alba M, Jiang J, Vercruysse F, Meininger G, Matthews D CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 47.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in FPG, lipid profiles, body weight, and blood pressure after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors as add-on therapy
Changes in FPG, lipid profiles, body weight, and blood pressure after 12 weeks of treatment with sodium-glucose cotransporter-2 inhibitors as switch therapy
Logistic regression analysis to determine variables associated with good responders to SGLT2 inhibitors in overall patients
Comparison of clinical characteristics between the good responders and the poor responders in the add-on and switch group
Logistic regression analysis to determine variables associated with good responders to SGLT2 inhibitors in the add-on and switch group