Abstract
The link of diabetes with co-occurring disorders in the brain involves complex and multifactorial pathways. Genetically engineered rodents that express familial Alzheimer's disease-associated mutant forms of amyloid precursor protein and presenilin 1 (PSEN1) genes provided invaluable insights into the mechanisms and consequences of amyloid deposition in the brain. Adding diabetes factors (obesity, insulin impairment) to these animal models to predict success in translation to clinic have proven useful at some extent only. Here, we focus on contributing factors to diabetic brain injury with the aim of identifying appropriate animal models that can be used to mechanistically dissect the pathophysiology of diabetes-associated cognitive dysfunction and how diabetes medications may influence the development and progression of cognitive decline in humans with diabetes.
Keywords: Dementia, Diabetes mellitus, Obesity
INTRODUCTION
Type 2 diabetes mellitus accelerates age-related cognitive decline [1,2] and increases the risk for dementia [3,4,5,6,7]. Pathophysiological processes causing diabetic brain injury and cognitive decline begin years before actual manifestation of symptoms occurs [8,9]. Compared to cognitive impairment in individuals without diabetes, cognitive decline in humans with diabetes can be influenced by the type of diabetes (type 1 diabetes mellitus or type 2 diabetes mellitus), duration of diabetes, antidiabetic medications and presence of other diabetic-related complications [10,11]. Here, we focus on contributing factors to diabetic brain injury with the aim of identifying appropriate animal models that can be used to mechanistically dissect the pathophysiology of diabetes-associated cognitive dysfunction and how diabetes medications may influence the development and progression of cognitive decline in humans with diabetes.
CONTRIBUTING FACTORS TO DIABETIC BRAIN INJURY IN HUMANS
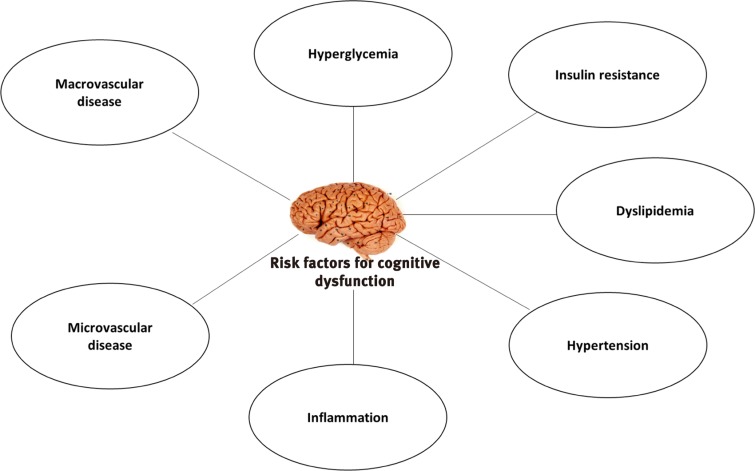
Common diabetes-associated pathological processes and complications that can have deleterious impact on brain function are summarized in Fig. 1.
Fig. 1. Risk factors for cognitive dysfunctions in diabetes. Figure showing main risk factors involved in cognitive dysfunction in diabetes. These risk factors may be associated with different types of cognitive dysfunction in diabetes.
Hyperglycemia
Efficient delivery of glucose to brain cells is critical for brain function [12]. Glucose uptake by the brain cells appears to be independent of insulin; however neurons are known to express receptors for insulin and insulin related peptide (insulin-like growth factor 1 [IGF-1]) [12]. Both insulin and IGF-1 play important roles in neuronal development and affect cognition [12]. Insulin resistance and consequent abnormal blood glucose levels as observed in type 2 diabetes mellitus can have detrimental effects on brain and cognitive function [13,14]. Fluctuation in glucose levels or peaks, which is common in diabetes, increases the risk for cognitive decline. Targeting glucose peaks has been proposed as therapeutic strategy for prevention of diabetes-associated cognitive dysfunction [14]. In laboratory animals, glucose lowering compounds such as metformin, thiazolidinediones and compounds targeting the glucagon like peptide-1 receptor have beneficial effects on cognition [10]. It was reported that agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a ligand-activated transcriptional factor, improved brain function in a rat model of streptozotocin-induced diabetes [15]. Decreased activity of PPARγ and its cofactor peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) causes mitochondrial dysfunction and oxidative stress in the settings of type 2 diabetes mellitus and in brain disorders. Glucose lowering thiazolidinediones, such as rosiglitazone and pioglitazone, act as agonist for PPARγ and improve cognitive function. Glucagon-like peptide 1 (GLP-1) receptor agonists are known elicit neuroprotective effects in animal models of stroke, Alzheimer's disease (AD) and Parkinson's disease [16]. GLP-1 receptor agonists reduced neuro-inflammation and increased neuro-survival [16]. Metformin reduces tau hyper-phosphorylation by inducing protein phosphatase 2A (PP2A) activity, a major tau phosphatase [17].
Dyslipidemia
Elevated levels of plasma triglycerides and cholesterol may play a role in diabetes-associated risk for poor cognition function [18,19]. Pharmacological interventions to ameliorate dyslipidemia may influence the progression of cognitive decline in individuals with diabetes and cerebrovascular disease [18,19]. Cholesterol modulates the activity of enzymes involved (β and γ-secretases) in amyloid precursor protein (APP) processing and thus it can affect production of amyloid β (Aβ) protein. Because β and γ-secretases enzymes are membrane bound enzymes, the high cholesterol content in membrane lipid rafts could facilitate clustering of these enzymes with their substrate, thereby promoting cleavage of precursors of Aβ protein into amyloidogenic forms [20].
Hypertension
Cerebrovascular complications in individuals with diabetes are frequently linked to the presence of hypertension [21,22]. The use of anti-hypertensive medications may diminish the risk of dementia in diabetic patients by 4% to 24% [23]; however, the underlying molecular mechanisms remain unknown [24].
Inflammation and blood-brain barrier injury
Cerebrovascular accumulation of toxic lipids, advanced glycation end products (AGEs) and aggregated proteins trigger inflammatory responses and secretion of inflammatory mediators in the circulation [25,26,27,28]. Inflammatory responses are associated with blood-brain barrier (BBB) breakdown [29]. BBB injury further exposes brain parenchyma to neurotoxic blood proteins, thrombin, fibrin, plasmin, hemoglobin, and iron from lysed red blood cells. A leaky BBB causes abnormal neuronal activity [29] that plays a role in diabetes-associated neurological deficits [30].
Chronically elevated levels of C-reactive proteins, interleukin 6 (IL-6), fibrinogen, and tumor necrosis factor α are strongly correlated with cognitive decrement in diabetic patients [31,32,33,34]. Reducing systemic inflammation promotes brain health [31,32,33,34].
Vascular contributions to cognitive impairment and dementia
Macrovascular disease such as myocardial infarction or stroke negatively affects cognitive performance [8,35]. Microvascular abnormalities are also frequent in patients with type 2 diabetes mellitus [8,35]. Patients with diabetes and individuals with normal metabolic function showing retinal microvascular abnormalities are at high risk of cognitive decline [36]. Due to similarities between retina cells and cerebrovascular cells, retinopathy can be used as a marker and therapeutic target for microangiopathy and cerebral small vessel disease [37]. It is speculated that cerebral small vessel disease might be due to long term endothelium dysfunction, capillary loss and subsequent ischemia leading to white matter disease and neurological deficits [25,28,29]. White matter disease of vascular origin is commonly associated with vascular contributions to cognitive impairment and dementia (VCID) [25,28,29].
Vascular endothelial cell dysfunction is linked to vascular accumulation of toxic lipids [26], AGEs [27] and aggregated proteins [25]. Interaction of AGEs with endothelial cells increases generation of reactive oxygen species (ROS) [27] and impairs the production of vasodilatory substances resulting in a reduced cerebral blood flow [38]. Elevated ROS damage cellular structures and activate matrix metalloproteinases enzyme, which further induce cytoskeletal reorganization and vascular remodeling [29]. Cytoskeletal reorganization increases the vascular permeability by disrupting tight junction proteins in endothelium, which increases energy depletion and alters neural viability [29,38].
Systemic amylin dyshomeostasis
Amylin, or islet APP, is a pancreatic hormone that is synthesized and co-secreted with insulin by pancreatic β-cells and participates in the central regulation of satiety [39]. Individuals with pre-diabetic insulin resistance have hypersecretion of amylin (and insulin) leading to amylin oligomerization and pancreatic amylin amyloid. Amylin dyshomeostasis plays an important role in the development and progression of type 2 diabetes mellitus [39,40,41]. Deposits of amylin amyloid were detected in extra-pancreatic tissues, including the heart [42], kidneys [43] and the brain [44,45,46,47,48]. Because amylin amyloid is toxic [39,40,41], the presence of amylin deposition in brains of individuals with AD suggests that the development of drugs that could limit amylin deposition in the brain may provide benefit to patients with AD or mild cognitive impairment.
Calcium dysregulation in diabetes and dementia
Altered Ca2+ signaling contributes in brain dysfunction through multiple mechanisms [49]. Erickson et al. [50], showed that diabetes causes Ca2+/calmodulin-dependent protein kinase II (CaMKII) modification at Ser279 through o-linked N-acetylglucosamine (O-GlcNAc). This modification of CaMKII has been detected both in hearts and brains of humans with type 2 diabetes mellitus [50].
DIABETIC INJURY IN LABORATORY ANIMALS
Mouse and rat models for diabetes and AD are generated by gene manipulation or/and pharmacological intervention (Table 1). In mice genetically modified to develop parenchymal deposition of Aβ and intraneuronal accumulation of hyperphosphorylated tau, brain pathology and behavior changes are accelerated by diabetic states induced by streptozotocin injection or by diet interventions [51,52,53,54,55,56,57]. Mice generated by crossing obese db/db mice with AD mice develop microhemorrhages and accelerated behavior changes compared to AD mice [55].
Table 1. Diabetes and Alzheimer's disease rodent models.
| Intervention | Pathophysiology |
|---|---|
| Non-AD mouse and rat models [50,51,52,53] | |
| Sterptozotocin | Increase pTau pathology and altered hippocampal synaptic plasticity |
| Diet | Mild effect on central nervous system |
| Amylin dyshomeostasis | Vascular amylin oligomer deposition |
| Microhaemorrhages | |
| Brain inflammation | |
| Brain atrophy | |
| Microglia activation | |
| Parenchymal amylin plaques | |
| Impaired synthesis of neurotransmitters | |
| Leptin deficiency | Increased amyloid-β generation |
| Mouse and rat models of AD [50-53] | |
| Sterptozotocin | Exacerbated brain amyloidosis |
| Neuro-inflammation and injury | |
| Diet | Increased amyloid-β pathology |
| Leptin deficiency | Amyloid-β |
| Aneurisms | |
| Small strokes |
AD, Alzheimer's disease; pTau, phosphorylated tau.
Brain insulin resistance
Brain insulin resistance increases the activity of β-secretase and γ-secretase [54,55] promoting cerebral Aβ deposition and tau hyperphosphorylation [51,52,53,54,55,56,57]. Intracerebroventricular administration of insulin increased learning ability in normal mice [58,59]; however, a similar treatment showed no significant effect on brain function in diabetic mice [59]. Cerebral accumulation of Aβ and phosphorylation of tau in mice were reduced by pharmacological interventions that ameliorate insulin resistance [60].
White matter disease
In a rat model for type 2 diabetes mellitus transgenic for human islet amyloid polypeptide (the HIP rat) [25], the development of diabetes is associated with amylin-mediated vascular endothelial dysfunction leading to axonal demyelination, white matter rarefaction and neurological deficits [25]. Phenylalanine and tyrosine, two main precursors of neurotransmitters, were greatly decreased in brains of diabetic HIP rats compared to non-diabetic littermates [61]. These results [25,61] indicate amylin dyshomeostasis as a direct molecular link between pancreatic pathology in diabetes and VCID.
Peroxidative neuronal injury and neuroinflammation
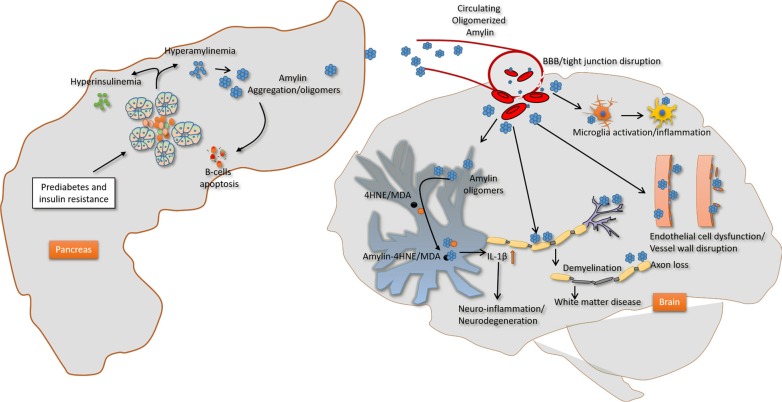
In addition to amylin plaques and mixed amylin-Aβ deposits, brains of diabetic patients with AD show amylin immunoreactive deposits inside the neurons [62]. Neuronal amylin formed adducts with 4-hydroxynonenal (4HNE), a marker of peroxidative membrane injury, and increased synthesis of the proinflammatory cytokine IL-1β [62]. These pathological changes were mirrored in rats expressing human amylin in pancreatic islets (HIP rats) and mice intravenously injected with aggregated human amylin, but not in hyperglycemic rats secreting wild-type non-amyloidogenic rat amylin [62]. In cultured primary hippocampal rat neurons, aggregated amylin increased IL-1β synthesis via membrane destabilization and subsequent generation of 4HNE [62]. These effects were blocked by membrane stabilizers and lipid peroxidation inhibitors [62]. Thus, elevated circulating levels of aggregated amylin negatively affect the neurons causing peroxidative membrane injury and aberrant inflammatory responses independent of other confounding factors of diabetes. Schematic view of effect of amylin dyshomeostasis on brain is shown in Fig. 2.
Fig. 2. Proposed mechanism underlying the impact of amylin dyshomeostasis on the brain. Prediabetic hyperamylinemia in humans promotes amylin oligomerization within the pancreatic secretory pathway and consequent secretion of oligomerized amylin in the blood (amylin dyshomeostasis), which causes brain microhemorrhages leading to neuroinflammation and hypoxic-ischemic brain injury. BBB, blood-brain barrier; 4HNE, 4 hydroxynonenal; MDA, malondialdehyde; IL-1β, interleukin 1β.
CONCLUSIONS
In conclusion, preclinical data and epidemiological studies show a consistent association of diabetes with cognitive decline. Since some diabetes-associated factors (hyperglycemia, insulin resistance) are also extending in non-diabetic populations, the association of diabetes with cognitive impairment should also be studies in non-diabetic populations.
The link of diabetes with co-occurring disorders in the brain involves complex and multifactorial pathways. Laboratory animals can help to better understand the relationship between diabetes and cognitive impairments, which can further be used to develop new treatment strategies to slow the progression of pathological processes and cognitive decline. Since diabetes-associated amylin dyshomeostasis appears central to white matter injury in AD [25], we propose that an appropriate combination of human amylin-expressing non-AD rats and AD rats have the potential to uncover: (1) cellular and molecular mechanisms that underlie the effects of amylin dyshomeostasis on small blood vessels and white matter; (2) phenotypical characteristics of the interaction between amylin dyshomeostasis and Aβ pathology; and (3) the interplay between mixed VCID-AD and known amylin-mediated vascular injury [63]. Such knowledge is critical for designing novel interventions based on our hypothesis of the central role of amylin dyshomeostasis in the development of mixed VCID-AD.
ACKNOWLEDGMENTS
This research was supported by: National Institutes of Health AG057290, AG053999 and Alzheimer's Association VMF-15-363458.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Brady CC, Vannest JJ, Dolan LM, Kadis DS, Lee GR, Holland SK, Khoury JC, Shah AS. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes. 2017;18:297–303. doi: 10.1111/pedi.12383. [DOI] [PubMed] [Google Scholar]
- 2.Kadohara K, Sato I, Kawakami K. Diabetes mellitus and risk of early-onset Alzheimer's disease: a population-based case-control study. Eur J Neurol. 2017;24:944–949. doi: 10.1111/ene.13312. [DOI] [PubMed] [Google Scholar]
- 3.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 4.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exalto LG, Biessels GJ, Karter AJ, Huang ES, Katon WJ, Minkoff JR, Whitmer RA. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013;1:183–190. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 9.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 10.Patrone C, Eriksson O, Lindholm D. Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol. 2014;2:256–262. doi: 10.1016/S2213-8587(13)70125-6. [DOI] [PubMed] [Google Scholar]
- 11.Areosa Sastre A, Vernooij RW, Gonzalez-Colaco Harmand M, Martinez G. Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804. doi: 10.1002/14651858.CD003804.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 13.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3:75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 14.Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin E. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care. 2017;40:879–886. doi: 10.2337/dc16-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma B, Singh N. Behavioral and biochemical investigations to explore pharmacological potential of PPAR-gamma agonists in vascular dementia of diabetic rats. Pharmacol Biochem Behav. 2011;100:320–329. doi: 10.1016/j.pbb.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Gault VA, Holscher C. GLP-1 receptor agonists show neuroprotective effects in animal models of diabetes. Peptides. 2018;100:101–107. doi: 10.1016/j.peptides.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Kohler A, Glossmann H, Schneider R, Sutherland C, Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Cai L, Chen B, Liang J, Lin F, Li L, Lin L, Yao J, Wen J, Huang H. Serum level of endogenous secretory receptor for advanced glycation end products and other factors in type 2 diabetic patients with mild cognitive impairment. Diabetes Care. 2011;34:2586–2590. doi: 10.2337/dc11-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlmuter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. J Diabet Complications. 1988;2:210–213. doi: 10.1016/s0891-6632(88)80011-4. [DOI] [PubMed] [Google Scholar]
- 20.Di Paolo G, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Foster JK, Almeida OP, Davis TM. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241–248. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- 22.Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, Johansson B. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Parikh N, Kunik ME, Schulz PE, Patel JG, Chen H, Aparasu RR, Morgan RO. Antihypertensive drug use and the risk of dementia in patients with diabetes mellitus. Alzheimers Dement. 2012;8:437–444. doi: 10.1016/j.jalz.2011.05.2414. [DOI] [PubMed] [Google Scholar]
- 24.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 25.Ly H, Verma N, Wu F, Liu M, Saatman KE, Nelson PT, Slevin JT, Goldstein LB, Biessels GJ, Despa F. Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol. 2017;82:208–222. doi: 10.1002/ana.24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118:1771–1785. doi: 10.1161/CIRCRESAHA.115.306884. [DOI] [PubMed] [Google Scholar]
- 27.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. C-reactive protein, advanced glycation end products, and their receptor in type 2 diabetic, elderly patients with mild cognitive impairment. Front Aging Neurosci. 2015;7:209. doi: 10.3389/fnagi.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marioni RE, Deary IJ, Murray GD, Lowe GD, Strachan MW, Luciano M, Houlihan LM, Gow AJ, Harris SE, Rumley A, Stewart MC, Fowkes FG, Price JF. Genetic associations between fibrinogen and cognitive performance in three Scottish cohorts. Behav Genet. 2011;41:691–699. doi: 10.1007/s10519-011-9449-2. [DOI] [PubMed] [Google Scholar]
- 33.Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FG, Frier BM, Lee AJ, Butcher I, Rumley A, Murray GD, Deary IJ, Price JF. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:710–713. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller M, Feinkohl I, Anderson N, Deary IJ, Strachan MWJ, Price JF. Plasma fibrinogen and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabet Med. 2012;29:30–117. [Google Scholar]
- 35.Feinkohl I, Price JF, Strachan MW, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015;7:46. doi: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, Price JF. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol. 2008;92:1017–1025. doi: 10.1136/bjo.2008.141994. [DOI] [PubMed] [Google Scholar]
- 37.Hugenschmidt CE, Lovato JF, Ambrosius WT, Bryan RN, Gerstein HC, Horowitz KR, Launer LJ, Lazar RM, Murray AM, Chew EY, Danis RP, Williamson JD, Miller ME, Ding J. The cross-sectional and longitudinal associations of diabetic retinopathy with cognitive function and brain MRI findings: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2014;37:3244–3252. doi: 10.2337/dc14-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 40.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Bers DM, Despa F. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ Res. 2012;110:598–608. doi: 10.1161/CIRCRESAHA.111.258285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong W, Liu ZH, Zeng CH, Peng A, Chen HP, Zhou H, Li LS. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int. 2007;72:213–218. doi: 10.1038/sj.ki.5002305. [DOI] [PubMed] [Google Scholar]
- 44.Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F. Amylin deposition in the brain: a second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185:834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Schultz N, Byman E, Fex M, Wennstrom M. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017;37:1470–1482. doi: 10.1177/0271678X16657093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, Dineley KT, Kong Y, Li J, Jhamandas J, Perry G, Murray IV. Islet amyloid polypeptide (IAPP): a second amyloid in Alzheimer's disease. Curr Alzheimer Res. 2014;11:928–940. doi: 10.2174/1567205011666141107124538. [DOI] [PubMed] [Google Scholar]
- 48.Roostaei T, Nazeri A, Felsky D, De Jager PL, Schneider JA, Pollock BG, Bennett DA, Voineskos AN Alzheimer's Disease Neuroimaging Initiative (ADNI) Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer's disease. Mol Psychiatry. 2017;22:287–295. doi: 10.1038/mp.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thibault O, Anderson KL, DeMoll C, Brewer LD, Landfield PW, Porter NM. Hippocampal calcium dysregulation at the nexus of diabetes and brain aging. Eur J Pharmacol. 2013;719:34–43. doi: 10.1016/j.ejphar.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 53.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One. 2012;7:e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Liu L, Lu S, Wang D, Liu X, Xie L, Wang G. Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats. J Endocrinol Invest. 2011;34:26–31. doi: 10.1007/BF03346691. [DOI] [PubMed] [Google Scholar]
- 55.Son SM, Song H, Byun J, Park KS, Jang HC, Park YJ, Mook-Jung I. Accumulation of autophagosomes contributes to enhanced amyloidogenic APP processing under insulin-resistant conditions. Autophagy. 2012;8:1842–1844. doi: 10.4161/auto.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos-Rodriguez JJ, Ortiz-Barajas O, Gamero-Carrasco C, de la Rosa PR, Infante-Garcia C, Zopeque-Garcia N, Lechuga-Sancho AM, Garcia-Alloza M. Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology. 2014;48:123–135. doi: 10.1016/j.psyneuen.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Devi L, Alldred MJ, Ginsberg SD, Ohno M. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease. PLoS One. 2012;7:e32792. doi: 10.1371/journal.pone.0032792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell GA, Fadool DA. Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol Behav. 2017;174:104–113. doi: 10.1016/j.physbeh.2017.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 61.Ilaiwy A, Liu M, Parry TL, Bain JR, Newgard CB, Schisler JC, Muehlbauer MJ, Despa F, Willis MS. Human amylin proteotoxicity impairs protein biosynthesis, and alters major cellular signaling pathways in the heart, brain and liver of humanized diabetic rat model in vivo. Metabolomics. 2016;12:95. doi: 10.1007/s11306-016-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, Hersh LB, Despa F. Intraneuronal amylin deposition, peroxidative membrane injury and increased IL-1β synthesis in brains of Alzheimer's disease patients with type-2 diabetes and in diabetic hip rats. J Alzheimers Dis. 2016;53:259–272. doi: 10.3233/JAD-160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma N, Liu M, Ly H, Loria A, Campbell KS, Bush H, Kern PA, Jose PA, Taegtmeyer H, Bers DM, Despa S, Goldstein LB, Murray AJ, Despa F. Diabetic microcirculatory disturbances and pathologic erythropoiesis are provoked by deposition of amyloid-forming amylin in red blood cells and capillaries. Kidney Int. 2019 Sep 05; doi: 10.1016/j.kint.2019.07.028. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]