Abstract
Recent studies suggest that Bioactive Dietary Polyphenol Preparation (BDPP) and individual polyphenolic compounds ameliorate stress-induced depression-like behaviors, but the underlying molecular mechanisms are incompletely understood. VGF (non-acronymic) in dorsal hippocampus (dHc) has been shown to play a role in depression-like behavior and antidepressant efficacy, and the VGF-derived peptide TLQP-62 (named by the N-terminal 4 amino acids and length) infused into dHc has been shown to have antidepressant efficacy that is BDNF/TrkB dependent. Here, we investigated whether BDPP influences VGF expression in dHc, and whether dHc VGF is required for BDPP antidepressant efficacy. We found that BDPP produced antidepressant-like effects in naive mice and reversed the depression-like behaviors induced by chronic variable stress (CVS). In addition, we found that BDPP had no detectable antidepressant efficacy in floxed mice with prior knockdown in dHc of either VGF or BDNF, achieved by adeno- associated virus (AAV)-Cre infusion. Our data indicate that dHc VGF and BDNF expression are required for the antidepressant actions of BDPP, and therefore suggest that a VGF(TLQP-62)/BDNF/TrkB autoregulatory feedback loop could play a role in the regulation of BDPP antidepressant efficacy, much as it has been suggested to function in the antidepressant efficacies of ketamine and TLQP-62.
Keywords: Antidepressant, Brain-Derived Neurotophic Factor (BDNF), Chronic Variable Stress (CVS), Grape Seed Polyphenolic Extract (GSPE), Major Depressive Disorder (MDD), Polyphenol
INTRODUCTION
Major depressive disorder (MDD) is a highly prevalent mental illness 1 with limited effective treatment options available. The delayed onset of action 2 and unpleasant side effects 3 of conventional antidepressant drugs make development of safer, more effective and tolerable treatments an urgent need.
Polyphenols are a group of chemical compounds with antioxidant property that naturally exist in our diet 4,5. Polyphenols has been shown to contribute to the prevention of cardiovascular diseases, cancers, diabetes and osteoporosis 6. Among them are grape-derived polyphenols, which have been shown to promote cognitive functions in preclinical Alzheimer’s disease (AD) models 7,8 potentially by improving synaptic plasticity9. In addition, grape-derived polyphenols such as proanthocyanidin and resveratrol produce antidepressant effects in animal models of depression 10,11. Recently, a novel polyphenol preparation - Bioactive Dietary Polyphenol Preparation (BDPP) - which consists of grape seed polyphenol extract (GSPE), resveratrol and Concord grape juice (CGJ) has been reported to prevent cognitive deficits induced by sleep deprivation 12, protect against amyloid accumulation in a mouse AD model 13, and reverse social avoidance behaviors induced by chronic social defeat stress 14
VGF (non-acronymic) is a neuropeptide precursor that is robustly regulated by neurotrophic factors 15,16. VGF expression is reduced in human postmortem hippocampus and prefrontal cortex while it is increased in nucleus accumbens by MDD 17,18. VGF has antidepressant efficacy in hippocampus and prefrontal cortex while it is pro-depressant in nucleus accumbens 17,18. Treatment with antidepressants, such as ketamine and imipramine, or exercise increases VGF expression in hippocampus and prefrontal cortex 17–20. Moreover, VGF expression in hippocampus and prefrontal cortex is required for the actions of antidepressant agents 17,18. VGF and its C-terminal peptides regulate depression 17–20 and memory formation 21, 22 potentially by influencing synaptic plasticity and neurogenesis 16-23. In addition, published evidence suggests that α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1 is associated with depression-like behaviors 24 and response to stress 25 as well as antidepressant treatments 26,27. In the present study, we investigated whether VGF is associated with the antidepressant actions of BDPP and explored its potential underlying molecular mechanisms.
MATERIALS AND METHODS
Animals
Generation of loxp-flanked (floxed) VGF mice and breeding to remove the FRT-flanked neomycin selection cassette, generating Vgfflpflox/flpflox mice (abbreviated here Vgfff/ff), has been previously described 22. Male C57BL/6J, Vgfff/ff BDNF floxed 28 (designated Bdnfflox/flox BL6/sv129 background; generously provided by Dr. Eric Nestler) at 2 – 3 months of age were housed on a 12 h light-dark cycle with ad libitum access to food and water. All mice were single housed at least 3 days prior to the start of behavioral experiments. All mouse studies were conducted in accordance with the U.S. National Institutes of Health Guidelines for the Care and Use of Experimental Animals, using protocols approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai.
General experimental methods
Experimental sample sizes were estimated on the basis of published studies and/or power analysis. Values were excluded from completed experiments and analyses only if they were detected as outliers by Grubb’s test. This criterion was pre-established. Mice of the same gender were randomly assigned to different experimental groups with age and weight matched as closely as possible.
Special diets and BDPP treatment
AIN-93M purified rodent diet (D10012M) was obtained from Research Diets, Inc. (New Brunswick, NJ, USA). Resveratrol (ChromaDex, Irvine, CA, USA), GSPE (Warehouse, UPC: 603573579173), CGJ (Welch) were dissolved or diluted in water. The calculated daily intake of GSPE was 200 mg/kg body weight (BW), Resveratrol was 200 mg/kg BW and CGJ was 1 ml/day. All mice were acclimated for a week on the AIN-93M diet before BDPP treatment.
Stereotaxic viral infusion
Vgfff/ff or BDNFflox/flox mice at 2 – 3 months of age were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Thirty-three gauge syringe needles (Hamilton, Reno, Nevada) were used to bilaterally infuse 1.0 μl of AAV virus into mouse dorsal hippocampus (dHc) (AP = −2.0, ML = ±1.5, and DV = −2.0 from Bregma (mm)) at a rate of 0.2 μl per min, and the needle was allowed to remain in place for 5 min before removal to prevent backflow. AAV-CreGFP and AAV-GFP (AAV2 genotype, AAV5 serotype) were prepared by the Vector Core at the University of North Carolina at Chapel Hill. AAV-injected mice were allowed to recover for 21 days before treatment.
Chronic variable stress
Chronic variable stress (CVS) was performed as described previously. Briefly, it consisted of three different stressors (foot shock, tail suspension and restraint stress) that were alternated over 21 days to avoid habituation. On day 1, foot shock consisted of 100 random mild electric shocks at 0.45 mA over 1 hour. On day 2, tail suspension stress lasted 1 hour. On day 3, restraint stress was applied by placing mice into 50 ml conical tubes for 1 hour within their home cages. The same stressors were repeated for the next 18 days in the same order.
Forced swim test
Forced swim test (FST) was performed under bright light. Mice were placed in 4L beakers containing ~ 3L of tap water at a temperature of 25 ± 1 °C for 6 min. Behavior was recorded and immobility time, defined as the absence of any movement except that necessary for the mice to keep their heads above water, was manually counted over the last 4 minutes.
Open field test
The open field test (OFT) was performed under red light. Mice were placed in an open field arena (44 × 44 cm), and video tracking software (Ethovision 3.0, Noldus Information Technology, Leesburg, Virginia) was used to measure total movement of mice over 10 minutes.
Sucrose splash test
The sucrose splash test (SST) was performed as described previously 29. Briefly, mice were sprayed with 10% sucrose solution onto their dorsal coat in their home cages under red light. After the sucrose solution was applied, behavior was recorded for 5 minutes. The time spent grooming was manually counted for the entire 5 minutes.
Protein sample preparation and western blotting
To prepare total homogenate, mouse dorsal hippocampus (dHc) was homogenized in ice-cold protein lysis buffer containing 50 mM Tris-HCl (pH 7.5), 140 mM NaCl, 1% TritonX100, 0.5% Na Deoxycholate, 0.1% SDS and 2mM EDTA with 1x Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, Massachusetts). To quantify total GluR1 level, crude synaptosomes were prepared by minor modification of an established protocol30. Briefly, dissected dHc was homogenized in ice-cold solution containing 320 mM sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA with 1x Halt Protease and Phosphatase Inhibitor Cocktail. The homogenate was centrifuged at 1000 ×g for 10 min at 4°C, generating a pellet (nuclear fraction) and supernatant. The supernatant was then centrifuged at 10,000 ×g for 10 min at 4°C, the supernatant (cytosolic fraction) was removed, and the pellet (crude synaptosomal fraction) was resuspended in protein lysis buffer described above. Protein concentration was determined by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, California), and equal protein amounts (10 μg per lane) were resolved on denaturing 10% SDS-PAGE gels and transferred by electroblotting to Hybond-P PVDF membranes (Millipore, Billerica, Massachusetts). Membranes were blocked in 10% non-fat milk/PBS for 1 h at room temperature, and then were incubated with either anti-VGF C-terminal (1:2000, rabbit polyclonal), anti-β-actin (1:3000, # MAB1501, Millipore, Billerica, Massachusetts), or anti-total-GluR1 (1:1000, # 13185, Cell Signaling Technology, Danvers, Massachusetts) in 3% BSA in PBS at 4°C overnight. Membranes were washed in PBST (0.2% Tween-20 in PBS), incubated with a secondary horseradish peroxidase- labelled donkey anti-rabbit or donkey anti-mouse antibody (1:6000, #NA934 and # NA931, GE Healthcare, Piscataway, New Jersey) for 1 h, washed again in PBST and incubated with ECL detection reagents (Millipore, Billerica, Massachusetts). Densitometric analysis was performed using NIH ImageJ software, and protein levels were normalized to β-actin.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 7. Comparisons for four groups were made using two-way analysis of variance (ANOVA) followed by Fisher’s Least Significant Difference (LSD) test as indicated in the Figure Legends. All tests are two-sided. All data are presented as mean ± s.e.m.
RESULTS
BDPP treatment produces antidepressant effects in naive mice and prevents the development of depression-like behaviors following chronic variable stress
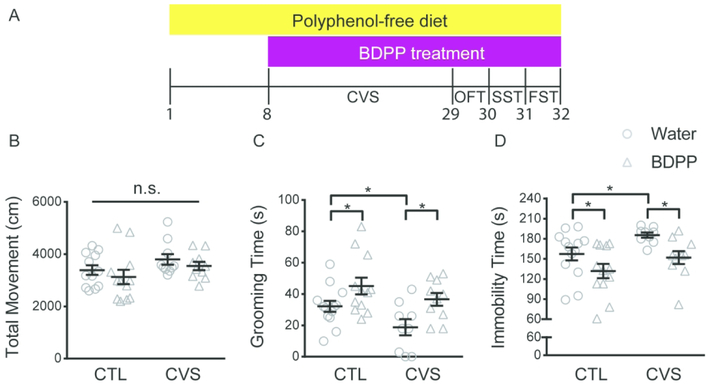
To determine the antidepressant effects of BDPP administration, we treated male wild- type mice with BDPP or water (vehicle) throughout the 21-day chronic variable stress (CVS) protocol and behaviorally assessed them subsequently (Figure 1A). Another cohort of mice was subjected to the same BDPP treatment without exposure to CVS to serve as a naive control. BDPP treatment did not affect locomotor activity in both naive mice and CVS-exposed mice (Figure 1B). BDPP-treated naive mice showed increased grooming time in the SST (Figure 1C) and decreased immobility time in the FST (Figure 1D) compared to control mice. CVS exposure reduced grooming time in the SST and increased immobility time in the FST in water-treated, but not BDPP-treated mice (Figure 1C, D).
Figure 1.
BDPP treatment produces antidepressant effects in both naive and stressed mice. (A) Timeline of chronic BDPP treatment, chronic variable stress and behavioral tests. (B) BDPP treatment did not affect the locomotor activity in naive and stressed mice (C) BDPP treatment significantly increased grooming time in the SST in naive mice and averted reduction in grooming time in stressed. (D) BDPP treatment significantly decreased immobility time in the FST in naive mice and averted increase in immobility time in stressed mice, n = 9 ~ 13 per group. All data are presented as mean ± s.e.m. Two- way analysis of variance followed by Fisher’s LSD test for B-D (* P < 0.05). OFT open field test, SST sucrose splash test, FST forced swim test.
BDPP treatment up-regulates VGF expression in dorsal hippocampus
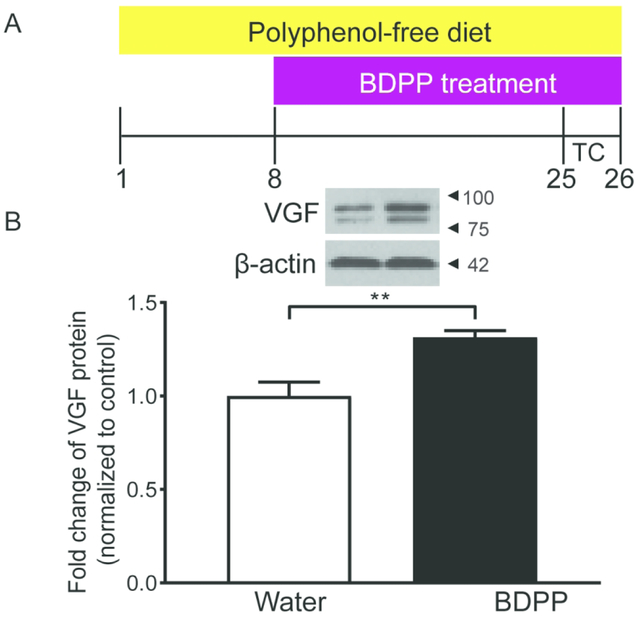
To investigate whether VGF is regulated in the dHc by BDPP, dHc from an independent cohort of naive mice (without behavioral assessment) was dissected after 17 days of BDPP treatment for western blot analysis (Figure 2A). VGF protein levels were significantly increased in dHc total homogenate of BDPP-treated mice compared to water-treated controls (Figure 2B).
Figure 2.
BDPP treatment increases VGF expression in dorsal hippocampus of naive mice (A) Timeline of chronic BDPP treatment and tissue collection time points. (B) VGF protein levels were significantly up-regulated by BDPP treatment, n = 5 per group. All data are presented as mean ± s.e.m.. Student’s t test for B (** P < 0.01). TC tissue collection.
Dorsal hippocampal VGF expression is required for the antidepressant efficacy of BDPP treatment
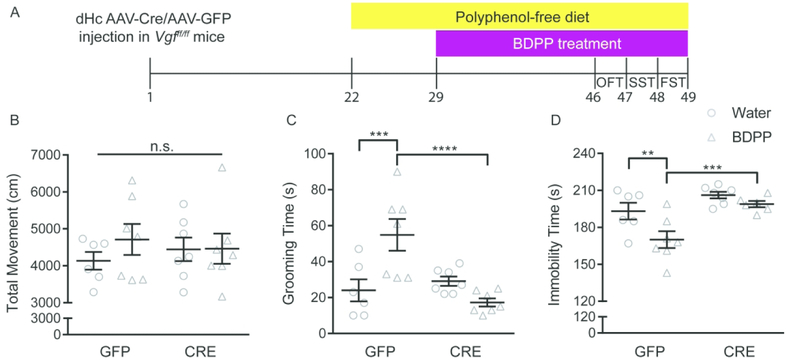
To determine whether VGF expression in dHc is required for the antidepressant effects of BDPP, we treated AAV-Cre or AAV-GFP-injected loxp-flanked (floxed) VGF mice (with the FRT-flanked neomycin selection cassette removed, these homozygous mice are designated Vgfff/ff) with BDPP or water for 17 days, and then we subsequently assessed their behavioral phenotypes (Figure 3A). BDPP did not affect locomotor activity (Figure 3B), but induced antidepressant effects in dHc AAV-GFP-injected Vgfff/ff mice in the SST (Figure 3C) and FST (Figure 3D). However, these effects were completely absent in dHc AAV-Cre-injected Vgfff/ff mice, suggesting that dHc VGF is required for the antidepressant actions of BDPP. As we have seen in past studies 17, AAV-Cre-mediated ablation of VGF in dHc alone did not significantly affect immobility time in the FST following other behavioral tests or experimental procedures (i.e. in AAV-Cre-injected Vgfff/ff mice treated with water in Fig. 3, or injected with saline in previous experiments 17).
Figure 3.
Dorsal hippocampal VGF expression is required for the antidepressant actions of BDPP. (A) Timeline of stereotaxic surgery, chronic BDPP treatment and behavioral tests. (B) BDPP treatment did not affect the locomotor activity in dHc-AAV-GFP- and dHc-AAV-Cre-injected Vgfff/ff mice. (C) BDPP treatment significantly increased grooming time in the SST in dHc-AAV-GFP- but not dHc-AAV-Cre-injected Vgfff/ff mice(D) BDPP treatment significantly reduced immobility time in the FST in dHc-AAV-GFP- but not dHc-AAV-Cre-injected Vgfff/ff mice, n = 6 ~ 7 per group. All data are presented as mean ± s.e.m.. Two-way analysis of variance followed by Fisher’s LSD test for B-D (**P < 0.01, *** P < 0.001, **** P < 0.0001). OFT open field test, SST sucrose splash test, FST forced swim test.
BDPP treatment up-regulates GluR1 levels in dorsal hippocampal synaptosomes in a VGF dependent manner
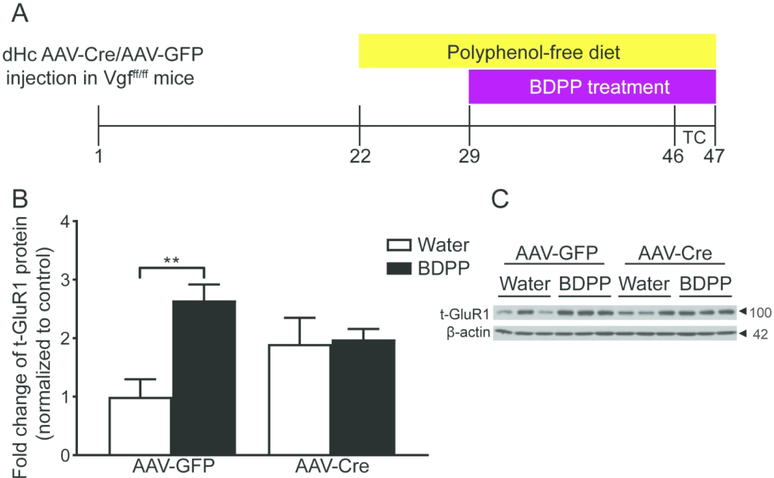
To explore the molecular mechanism underlying BDPP’s antidepressant actions, we examined abundance of GluR1 in dHc synaptosomes obtained from an independent cohort of naive AAV-GFP- or AAV-Cre-injected Vgfff/ff mice (without behavioral assessment) 17 days after the initiation of BDPP or water treatment (Figure 4A). BDPP treatment up-regulated GluR1 levels in dHc synaptosomes in AAV-GFP-injected mice but this effect was blocked in AAV-Cre-injected mice (Figure 4B, C).
Figure 4.
Dorsal hippocampal VGF expression is required for increased total GluR1 abundance in synaptosome induced by BDPP. (A) Timeline of chronic BDPP treatment and tissue collection time points. (B) The total abundance of GluR1 was significantly increased in synaptosomes of AAV-GFP-injected but not AAV-Cre-injected Vgfff/ff receiving BDPP treatment. (C) Representative western blot images of data shown in B. n = 3 ~ 4 per group. All data are presented as mean ± s.e.m.. Two-way analysis of variance followed by Fisher’s LSD test for B (** P < 0.01). TC tissue collection.
Dorsal hippocampal BDNF expression is required for the antidepressant efficacy of BDPP treatment
To determine whether BDNF expression in dorsal hippocampus (dHc) is required for the antidepressant effects of BDPP, we treated AAV-Cre or AAV-GFP-injected floxed BDNF mice (homozygotes designated Bdnfflox/flox) with BDPP or water for 17 days and subsequently assessed their behavioral phenotypes (Figure 5A). BDPP did not affect locomotor activity (Figure 5B), but induced antidepressant effects in dHc AAV-GFP- injected Bdnfflox/flox mice in the SST (Figure 5C) and FST (Figure 5D). However, these effects were completely absent in dHc AAV-Cre-injected Bdnfflox/flox mice, suggesting that dHc BDNF is required for the antidepressant actions of BDPP.
Figure 5.
Dorsal hippocampal BDNF expression is required for the antidepressant actions of BDPP (A) Timeline of stereotaxic surgery, chronic BDPP treatment and behavioral tests. (B) BDPP treatment did not affect the locomotor activity in dHc-AAV- GFP- and dHc-AAV-Cre-injected Vgfff/ff mice. (C) BDPP treatment significantly increased grooming time in the SST in dHc-AAV-GFP- but not dHc-AAV-Cre-injected Bdnfflox/flox mice. (D) BDPP treatment significantly reduced immobility time in the FST in dHc-AAV-GFP- but not dHc-AAV-Cre-injected Bdnfflox/flox mice, n = 11 ~ 15 per group. All data are presented as mean ± s.e.m.. Two-way analysis of variance followed by Fisher’s LSD test for B-D (# P = 0.0693, * P < 0.05, *** P < 0.001). OFT open field test, SST sucrose splash test, FST forced swim test.
DISCUSSION
In the present study, we demonstrate that chronic BDPP treatment produces behavioral phenotypes that mimic those induced by antidepressant drugs31 in naive mice. In addition, our results show that chronic BDPP treatment protects the mice from the detrimental effects of CVS. Many dietary polyphenols have been demonstrated to produce antidepressant effects 12. Most recently, one study reported that chronic BDPP treatment alleviates chronic social defeat stress-induced social avoidance and two BDPP-derived metabolites, dihydrocaffeic acid (DHCA) and malvidin-3-O-glucoside (Malgluc) promote resilience by modulating synaptic plasticity and peripheral inflammation 14 With BDPP treatment administered in parallel with stress exposure, our current results suggest a preventive effect of BDPP on stress-induced depression. However, further investigation will be required to determine whether BDPP treatment initiated after the end of stress exposure has the same beneficial effects as parallel treatment.
Dorsal hippocampus has been demonstrated to be sensitive to stress and antidepressant treatments. Various stress paradigms have been shown to cause synaptic dysfunction and decrease BDNF levels 32,33 while antidepressant treatments reverse these changes in dorsal hippocampus 34 Here, we show that BDPP treatment upregulates VGF expression in dorsal hippocampus of naive mice. Our current results are consistent with previous studies demonstrating that hippocampal VGF is upregulated by antidepressant treatments such as imipramine, exercise and ketamine17,19,20. Once ingested, polyphenols in BDPP are metabolized by microbiota in the intestine, producing an extensive array of phenolic metabolites 13,35. Many of these metabolites could be found in brain, where they could activate cAMP response element-binding protein (CREB) signaling in hippocampus 12. The vgf promoter contains a CREB binding site 15, suggesting that BDPP-induced VGF expression could be potentially regulated by CREB signaling. Polyphenols have been reported to render their beneficial effects by modulating neuroplasticity such as synaptogenesis and neurogenesis 36,37, which could be potentially mediated by the induction of VGF. Indeed, VGF C-terminal peptides, such as TLQP-62 and AQEE-30, have been demonstrated to facilitate dendritic maturation 38, enhance synaptic transmission 21, upregulate synaptic plasticity-related genes 19 and promote proliferation of neural progenitor cells 23.
We recently reported that antidepressant agents such as ketamine increase GluR1 levels and produce antidepressant-like phenotypes, and that these effects are blocked by VGF ablation in dorsal hippocampus 17 Our current results show that dorsal hippocampal VGF knockdown abolishes the antidepressant effects of BDPP, indicating that VGF expression in dorsal hippocampus is required for the actions of BDPP. GluR1 expression is downregulated in dorsal hippocampus by chronic stress 25 and GluR1 knockout mice manifest depression-like phenotypes 24. Conventional antidepressant drugs 39,40, the rapidacting antidepressant agent ketamine 17 and dietary polyphenols 41 have been shown to promote GluR1 phosphorylation at Ser845. As phosphorylation of GluR1 at Ser845 controls the synaptic trafficking and incorporation of GluR1-containing AMPA receptor 42, many of these antidepressant agents increase GluR1 levels in the membrane, in synaptosomal preparations 18,26,27. Our results show that BDPP increases GluR1 protein levels in dorsal hippocampal synaptosomes of controls but not in those mice with VGF ablated in this region, indicating that BDPP-induced synaptic GluR1 accumulation requires VGF.
BDNF plays an important role in regulating response to antidepressant treatments 43. Forebrain BDNF conditional knockout and virus-mediated BDNF knockdown in hippocampus impair responses to conventional antidepressant drugs and/or ketamine 44–46 Our results show that BDNF knockdown in dorsal hippocampus attenuates antidepressant effects of BDPP, indicating that dorsal hippocampal BDNF expression is required for the actions of BDPP. Treatment with a number of botanical compounds is associated with increased levels of BDNF, phosphorylation of its receptor TrkB, and phosphorylation/activation of the transcription factor CREB 12,47–50. Importantly, activated CREB is a known transcriptional regulator of VGF 51–53 and BDNF 54,55. Although speculative based on our current data, and clearly requiring additional experimentation, this suggests the possibility that the VGF/BDNF autoregulatory feedback loop might play a more generalized role in polyphenol antidepressant and procognitive efficacy. It remains possible that in addition to effects on signaling molecules downstream of TrkB, including ERK, p70S6K, and mTOR (Fig. 6A), specific polyphenols could potentially induce epigenetic modifications to increase VGF and/or BDNF expression in specific brain regions, thereby initiating the VGF/BDNF autoregulatory cascade (Fig. 6B). For example, polyphenol administration to naive mice and those susceptible to repeated episodes of social defeat stress alters patterns of global DNA methylation in the central nervous system and periphery, and thus may promote resiliency to stress 56. Of note, recent studies demonstrate that histone crotonylation and H3K27 trimethylation on the VGF promoter, driven by chromodomain Y-like protein (CDYL), are associated with transcriptional repression of VGF in mPFC and increased depression-like behavior 57.
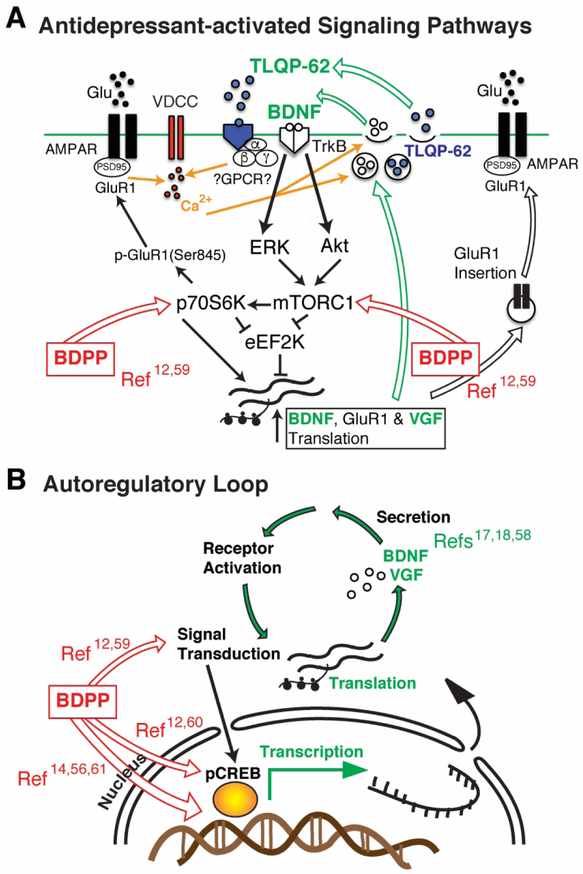
Figure 6.
Potential involvement of a VGF/BDNF/TrkB autoregulatory feedback loop in the antidepressant actions of BDPP. In panel A, BDNF/TrkB and VGF signaling pathways that hypothetically could contribute to the antidepressant responses to BDPP are shown, based on our results in Figs. 3 and 5 that demonstrate requirements for VGF and BDNF expression (highlighted in green), respectively, in dHc, in BDPP antidepressant efficacy. As has been suggested in previous studies of rapid-acting antidepressants (e.g. ketamine)17,18,58, secretion of TLQP-62, shown binding to a putative, currently unidentified GPCR, is postulated to trigger BDNF secretion, increasing BDNF/TrkB signaling and relieving translational suppression via mTOR and/or eEF2 pathways, as does ketamine 46. BDPP has previously been shown to activate mTOR and p70S6K signaling pathways (indicated by red arrows)59, and to increase levels of activated CREB 12, a critical transcriptional regulator of VGF 51–53 and BDNF 54,55. Panel B illustrates potential mechanism(s) underlying BDPP antidepressant actions (indicated by the red arrows), which initially could involve modulation of signal transduction pathways, particularly those that regulate translation (e.g. ERK, p70S6K, mTOR, and 4E-BP1)12,59, activation of transcription factors (e.g. pCREB and c-Fos)12,60, and epigenetic regulation (e.g. DNA methylation)14,56,61. We postulate that BDPP treatment impacts one or more of these pathways, resulting in activation of the VGF/BDNF/TrkB autoregulatory feedback loop (highlighted in green), leading to increased transcription, synthesis and/or secretion of VGF and BDNF, increased synaptic plasticity, and antidepressant behavioral effects.
Additional abbreviations: cAMP-response element-binding protein (CREB); eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1); mammalian target of rapamycin (mTOR); ribosomal protein S6 kinase β-1 (p70S6K); voltage-dependent calcium channel (VDCC). Numbered references in the figure refer to published studies we cite that identify specific pathways that are activated by BDPP.
In conclusion, our study confirms the antidepressant efficacy of BDPP. We propose that VGF-mediated synaptic GluR1 accumulation in dHc could potentially be the molecular mechanism underlying BDPP’s actions.
ACKNOWLEDGEMENTS AND CONTRIBUTIONS
This study was supported in part by: NIH grants MH086499 (SRS), MH083496 (SRS), AG062355 (SRS), pilot grant to SRS from the P50 AT008661-01 Center grant from the NCCIH and the ODS to GMP, and P50AT008661 (GMP); Hope for Depression Research Foundation (SRS); BrightFocus Foundation (SRS); and Brain and Behavior Research Foundation (SRS). We acknowledge that the contents of this study do not represent the views of the NCCIH, the ODS, the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Footnotes
CJ, JW, GMP and SRS designed research; CJ, ES and WJL performed research; CJ analyzed data; JW and GMP provided reagents; CJ and SRS wrote the paper.
COMPETING INTERESTS
The authors have no competing interests to disclose.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. 2005. Lifetime Prevalence and Age- of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62: 593. [DOI] [PubMed] [Google Scholar]
- 2.Gelenberg AJ & Chesen CL. 2000. How fast are antidepressants? J. Clin. Psychiatry 61: 712–21. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson JM 2001. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 3: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manach C, Scalbert A, Morand C, et al. 2004. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr 79: 727–47. [DOI] [PubMed] [Google Scholar]
- 5.Scalbert A, Johnson IT & Saltmarsh M. 2005. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 81: 215S–217S. [DOI] [PubMed] [Google Scholar]
- 6.Scalbert A, Manach C, Morand C, et al. 2005. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 45: 287–306. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Ho L, Zhao W, et al. 2008. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 28: 6388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho L, Ferruzzi MG, Janle EM, et al. 2013. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 27: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Ferruzzi MG, Ho L, et al. 2012. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 32: 5144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Li S, Chen R, et al. 2010. Antidepressant-like effect of low molecular proanthocyanidin in mice: Involvement of monoaminergic system. Pharmacol. Biochem. Behav. 94: 447–453. [DOI] [PubMed] [Google Scholar]
- 11.Hurley LL, Akinfiresoye L, Kalejaiye O, et al. 2014. Antidepressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 268: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Wang J, Bi W, et al. 2015. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 89: 191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Bi W, Cheng A, et al. 2014. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Hodes GE, Zhang H, et al. 2018. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 9: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salton SR, Fischberg DJ & Dong KW. 1991. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol. Cell. Biol. 11: 2335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alder J, Thakker-Varia S, Bangasser DA, et al. 2003. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J. Neurosci. 23: 10800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Lin W-J, Sadahiro M, et al. 2018. VGF function in depression and antidepressant efficacy. Mol. Psychiatry 23: 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang C, Lin W-J, Labonté B, et al. 2018. VGF and its C-terminal peptide TLQP-62 in ventromedial prefrontal cortex regulate depression-related behaviors and the response to ketamine. Neuropsychopharmacology. doi: 10.1038/s41386-018-0277-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunsberger JG, Newton SS, Bennett AH, et al. 2007. Antidepressant actions of the exercise-regulated gene VGF. Nat. Med. 13: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 20.Thakker-Varia S, Krol JJ, Nettleton J, et al. 2007. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J. Neurosci. 27: 12156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozdagi O, Rich E, Tronel S, et al. 2008. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci. 28: 9857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W-J, Jiang C, Sadahiro M, et al. 2015. VGF and Its C-Terminal Peptide TLQP-62 Regulate Memory Formation in Hippocampus via a BDNF-TrkB- Dependent Mechanism. J. Neurosci. 35: 10343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakker-Varia S, Behnke J, Doobin D, et al. 2014. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res. 12: 762–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chourbaji S, Vogt MA, Fumagalli F, et al. 2008. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 22:3129–3134. [DOI] [PubMed] [Google Scholar]
- 25.Toth E, Gersner R, Wilf-Yarkoni A, et al. 2008. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 107: 522–532. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Turrillas R, Frechilla D & Del Río J. 2002. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology 43: 1230–7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Xu T, Yuan Z, et al. 2016. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci. Signal. 9: ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios M, Fan G, Fekete C, et al. 2001. Conditional Deletion Of Brain-Derived Neurotrophic Factor in the Postnatal Brain Leads to Obesity and Hyperactivity. Mol. Endocrinol. 15: 1748–1757. [DOI] [PubMed] [Google Scholar]
- 29.Santarelli L, Saxe M, Gross C, et al. 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–9. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Lee B, Liu R-J, et al. 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David DJ, Samuels BA, Rainer Q, et al. 2009. Neurogenesis-dependent and - independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS, Nasca C & Gray JD. 2016. Stress Effects on Neuronal Structure: Hippocampus, Amygdala and Prefrontal Cortex. Neuropsychopharmacology 41: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duman RS & Aghajanian GK. 2012. Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duman RS, Aghajanian GK, Sanacora G, et al. 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22: 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Ho L, Faith J, et al. 2015. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 59: 1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias GP, Cavegn N, Nix A, et al. 2012. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell. Longer. 2012: 541971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy T, Dias GP & Thuret S. 2014. Effects of diet on brain plasticity in animal and human studies: mind the gap. NeuralPlast. 2014: 563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behnke J, Cheedalla A, Bhatt V, et al. 2017. Neuropeptide VGF Promotes Maturation of Hippocampal Dendrites That Is Reduced by Single Nucleotide Polymorphisms. Int.J. Mol. Sci 18:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Turrillas R, Frechilla D & Del Río J. 2002. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology 43: 1230–7. [DOI] [PubMed] [Google Scholar]
- 40.Du J, Suzuki K, Wei Y, et al. 2007. The Anticonvulsants Lamotrigine, Riluzole, and Valproate Differentially Regulate AMPA Receptor Membrane Localization: Relationship to Clinical Effects in Mood Disorders. Neuropsychopharmacology 32: 793–802. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki K, Miyazaki Kenichi, Sakai S, et al. 2008. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 578: 194–200. [DOI] [PubMed] [Google Scholar]
- 42.Esteban JA, Shi S-H, Wilson C, et al. 2003. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 6: 136–143. [DOI] [PubMed] [Google Scholar]
- 43.Castrén E & Antila H. 2017. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 22: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteggia LM, Barrot M, Powell CM, et al. 2004. Essential role of brain- derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. 101: 10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adachi M, Barrot M, Autry AE, et al. 2008. Selective Loss of Brain-Derived Neurotrophic Factor in the Dentate Gyrus Attenuates Antidepressant Efficacy. Biol. Psychiatry 63: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Autry AE, Adachi M, Nosyreva E, et al. 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Xie Y, Zhang T, et al. 2016. Resveratrol reverses chronic restraint stress-induced depression-like behaviour: Involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res. Bull. 125: 134–143. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Fang Y, Xu Y, et al. 2015. Curcumin Improves Amyloid β-Peptide (1- 42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoSOne 10: e0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D, Wang Z, Gao Z, et al. 2014. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 271: 116–121. [DOI] [PubMed] [Google Scholar]
- 50.Cimini A, Gentile R, D’Angelo B, et al. 2013. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 114: 2209–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawley RJ, Scheibe RJ & Wagner JA. 1992. NGF induces the expression of the VGF gene through a cAMP response element. J. Neurosci. 12: 2573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Arcangelo G, Habas R, Wang S, et al. 1996. Activation of codependent transcription factors is required for transcriptional induction of the vgf gene by nerve growth factor and Ras. Mol. Cell. Biol. 16: 4621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonni A, Ginty DD, Dudek H, et al. 1995. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol. Cell. Neurosci. 6: 168–83. [DOI] [PubMed] [Google Scholar]
- 54.Shieh PB, Hu SC, Bobb K, et al. 1998. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20: 727–40. [DOI] [PubMed] [Google Scholar]
- 55.Tao X, Finkbeiner S, Arnold DB, et al. 1998. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–26. [DOI] [PubMed] [Google Scholar]
- 56.Blaze J, Wang J, Ho L, et al. 2018. Polyphenolic Compounds Alter Stress- Induced Patterns of Global DNA Methylation in Brain and Blood. Mol. Nutr. Food Res. 62: e1700722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Li M, Fan M, et al. 2018. Chromodomain Y-like Protein-Mediated Histone Crotonylation Regulates Stress-Induced Depressive Behaviors. Biol. Psychiatry. doi: 10.1016/j.biopsych.2018.11.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Jiang C, Lin W-J & Salton SR. 2018. Role of a V GF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy. J. Mol. Neurosci. doi: 10.1007/s12031-018-1124-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frolinger T, Smith C, Cobo CF, et al. 2018. Dietary polyphenols promote resilience against sleep deprivation-induced cognitive impairment by activating protein translation. FASEB J. 32: 5390–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith C, Frolinger T, Brathwaite J, et al. 2018. Dietary polyphenols enhance optogenetic recall of fear memory in hippocampal dentate gyrus granule neuron subpopulations. Commun. Biol. 1: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frolinger T, Herman F, Sharma A, et al. 2018. Epigenetic modifications by polyphenolic compounds alter gene expression in the hippocampus. Biol. Open 7: bio035196. [DOI] [PMC free article] [PubMed] [Google Scholar]