Abstract
Background:
Given the potential utility of frailty, a clinical phenotype of decreased physiologic reserve and resistance to stressors, to predict post-kidney transplant (KT) outcomes, we sought to understand the perceptions and practices regarding frailty measurement in US KT programs.
Methods:
Surveys were emailed to American Society of Transplantation Kidney/Pancreas Community of Practice members and 202 US transplant programs (11/2017–4/2018). Program characteristics were gleaned from SRTR.
Results:
The 133 responding programs (response rate=66%) represented 77% of adult KTs and 79% of adult KT candidates in the US. Respondents considered frailty to be a useful concept in evaluating candidacy (99%) and endorsed a need to develop a frailty measurement specific to KT (92%). Frailty measurement was more common during candidacy evaluation (69%) than during KT admission (28%). Of the 202 programs, 38% performed frailty assessments in all candidates while 23% performed assessments only for older candidates. There was heterogeneity in the frailty assessment method; 18 different tools were utilized to measure frailty. The most common tool was a timed walk test (19%); 67% reported performing >1 tool. Among programs that measure frailty, 53% reported being less likely to list frail patients for KT.
Conclusions:
Among US KT programs, frailty is recognized as a clinically relevant construct and is commonly measured at evaluation. However, there is considerably heterogeneity in the tools used to measure frailty. Efforts to identify optimal measurement of frailty using either an existing or novel tool and subsequent standardization of its measurement and application across KT programs should be considered.
INTRODUCTION
Frailty is a clinical syndrome of decreased physiologic reserve, characterized by multisystem dysregulation with a deep biological basis of vulnerability that becomes evident when individuals are confronted with a stressor.1 In the United States, 15% of community-dwelling older adults are frail2 based on the phenotype developed by Fried and colleagues.3 While initially identified and characterized in community-dwelling older adults, frailty is now thought to be an important risk factor for adverse outcomes among individuals of all ages with end-stage kidney disease (ESKD).4 Among patients undergoing hemodialysis, frailty is associated with falls,5 hospitalizations,6–8 poor cognitive function,9 decreased health-related quality of life,10 and mortality.6,8 Additionally, among kidney transplant (KT) candidates, frailty is associated with lower likelihood of listing,11 higher waitlist mortality,12 and lower KT rates.11 Among KT recipients, frailty is associated with delayed graft function,13 post-operative delirium,14 longer length of stay,15 early hospital readmission,16 immunosuppression intolerance,17 lower health-related quality of life,18 impaired functioning,19 poor cognitive function,20 and higher mortality.21 Although research from up to 7,078 KT candidates and 893 KT recipients supports the importance of frailty as a prognosticator in KT candidates and recipients, it is unclear how these findings are impacting clinical perceptions of frailty and clinical practices regarding the assessment of frailty.
In a Delphi study (n=41 clinicians caring for older ESKD patients), a consensus building study, of clinicians treating patients with ESKD, 98% of respondents reported that frailty is an important construct among ESKD patients.22 Yet, this study demonstrated clinicians are unable to identify which patients with ESKD meet the criteria for frailty, and patients themselves cannot recognize if they are frail.23 Although frailty is widely recognized as a construct of vulnerability, clinicians and patients may not agree on how to measure frailty because there are over 67 assessment tools available.24 Given the potential utility of frailty evaluation as a prognosticator among KT candidates, it is important to understand the global perceptions and practices regarding frailty at transplant programs in the United States.
Therefore, the American Society of Transplantation (AST) Kidney/Pancreas Community of Practice (KPCOP) convened a workgroup to better understand the landscape of frailty evaluation in KT candidates and recipients through a survey of US KT programs. The goals of this study were to characterize the national perceptions of frailty and to describe how frailty was currently being used in clinical practice at US adult KT programs.
MATERIALS AND METHODS
Survey Source Population
Adult transplant programs were identified using data from the Scientific Registry of Transplant Recipients (SRTR) external release. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US submitted by members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, United States Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. We included all transplant programs that performed adult KT in the US in 2017. These national registry datasets were also used to determine program characteristics including: total adult KT volume, median wait time, 1-year mortality and graft loss, mean age of adult recipients, % of recipients who were older (age>65) at the time of KT, % of recipients who were female, % of recipients who were Hispanic, % of recipients who were African American, % of recipients who received deceased donor KT, and % of recipients who had less than a high school education.
Survey Administration
Surveys about perceptions and practices related to frailty in KT were distributed via email to members of the AST KPCOP and to US transplant programs between 11/2017 and 4/2018. Adult transplant programs were identified in SRTR and a list of transplant program medical directors was collated by the workgroup and AST Consensus Conference Organizers. All surveys were conducted using Qualtrics Survey Software. Representatives from programs without a respondent were sent a minimum of 1 and a maximum of 4 emails about the survey. One response per program was recorded and the first complete response was considered for this analysis; no significant discordance was noted when multiple responses were returned from the same program.
Study Design
The survey instrument was developed using an iterative process based on a thorough review of the literature surrounding frailty in KT and discussions with the AST KPCOP Frailty workgroup and the members of the AST Frailty in Solid Organ Transplantation Consensus Conference. The final survey was approved by the AST KPCOP Educational Committee and the AST Board and consisted of 2 screening questions, 2 questions about the respondent, and included 10 core questions applicable to all solid organ transplant programs and 16 questions developed specifically for kidney transplant programs. All questions were multiple choice or open text (See Table S1, SDC, http://links.lww.com/TP/B746). The survey was reviewed and acknowledged to be exempt by the Johns Hopkins School of Medicine Institutional Review Board (9/2017); this research is in adherence with the Declaration of Helsinki and the Declaration of Istanbul.
Analysis
First, we used individual, recipient-level data from 2017 to estimate program characteristics (mean age of adult recipients, % of recipients who were older at the time of KT, % of recipients who were female, % of recipients who were Hispanic, % of recipients who were African American, % of recipients who received deceased donor KT, and % of recipients who had less than a high school education) for each program. We then compared the distribution of these program characteristics and assessed whether they responded to the survey using weighted means and standard deviations; the weights were based on program volume in 2017.
The frequency of responses for all survey questions was calculated. Additionally, we quantified the associations between program characteristics and whether the program assessed frailty at evaluation and at admission for KT using modified Poisson regression to directly estimate the prevalence ratios (as opposed to logistic regression to estimate the odds ratio); all program characteristics were included in a single model. For surveys with partial responses, each question was treated as a complete case analysis. For all analyses, a p-value <0.05 was considered significant. All analyses were performed using Stata 14.0 (College Station, Texas).
RESULTS
Participating Program Characteristics
Of the 202 adult KT programs in the US, 133 responded (66%) to this national survey. Respondents were most commonly transplant nephrologists (61%) or transplant surgeons (28%). In 2017, the programs that responded to the survey listed 79% of the total KT candidates and performed 77% of the total adult KTs in the US. The transplant programs that responded to the survey had a higher mean volume in 2017 (110.2 vs. 62.6, p<0.001) but were similar otherwise (Table 1).
Table 1. Comparison of Adult KT Programs that Responded and Did Not Respond to the Frailty Practice and Perceptions Survey (n = 202).
Program characteristics were gleaned from SRTR data on adult KTs performed in 2017. 77.3% of all adult KTs in the US are represented by the responses to this survey. The table shows the mean (SD) program characteristics across programs that responded and those that did not, including characteristics that are percentages.
| Adult KT programs | ||
|---|---|---|
| Responded (N = 133) |
Did not respond (N = 69) |
|
| Adult KT volume | 110.2 (76.3) | 62.6 (64.8) |
| Age in years | 52.0 (2.5) | 52.2 (5.7) |
| Waitlist time (years) | 1.6 (0.6) | 1.3 (0.7) |
| % Female | 38.9 (8.4) | 36.7 (12.1) |
| % African American | 27.0 (18.8) | 26.1 (20.3) |
| % Hispanic | 16.2 (19.6) | 21.0 (23.8) |
| % Living donor | 28.6 (13.4) | 23.1 (16.5) |
| % older (>65y) | 19.6 (7.1) | 21.1 (10.7) |
| % Working for income | 31.0 (13.0) | 28.1 (15.8) |
| % Less than high school | 44.3 (12.7) | 47.9 (13.7) |
All values are presented as mean (SD) across transplant programs.
KT: Kidney transplantation
Perceptions of Frailty in KT
Among the 133 responding adult KT programs, 99% agreed that frailty is potentially a useful concept in evaluating KT candidacy because frailty was viewed as a risk factor for adverse outcomes both before (98%) and after (98%) KT (Table 2). Functional limitations (eg, walking speed, grip strength, and sarcopenia) were the most important feature of frailty with 65% of programs ranking this the most important facet; functional limitations were followed by morbidity (16% of programs ranked most important) and cognitive ability (14% of programs ranked most important) (Figure 1). When asked about the essential components of frailty, the need for activities of daily living (ADL) assistance was reported by 95% of programs (Figure 1); the next most commonly reported components included sit to stand test (89%), unintentional weight loss (82%), and low physical activity (82%). The least commonly reported components were mood (30%) and laboratory markers (39%).
Table 2.
Perceptions on Frailty Among Kidney Transplant (KT) Programs (n = 133).
| % Yes | |
|---|---|
| In your view, is frailty a useful component in evaluating candidacy for KT? | 99.2 |
| Do you think the results of a frailty assessment should be used to influence decisions regarding candidate selection for transplantation? | 96.0 |
| Do you think frailty in transplant candidates is a risk factor for adverse outcomes before transplantation? | 98.4 |
| Should frailty assessment be used to influence decision for the timing of KT? | 61.3 |
| Do you think frailty in transplant candidates is a risk factor for adverse outcomes after transplantation? | 98.4 |
| Is there a distinction between irreversible and reversible frailty? | 84.2 |
| Is there a pathophysiological distinction between biological aging and frailty? | 92.5 |
| Should biological age be considered in assessing frailty? | 61.3 |
| Is there a need to develop frailty score in the setting of KT? | 91.6 |
| If you do not measure frailty, are you interested in measuring frailty among your KT candidates and recipients? | 97.3 |
Figure 1. Perceptions of the essential components of frailty by kidney transplant programs (n = 133).
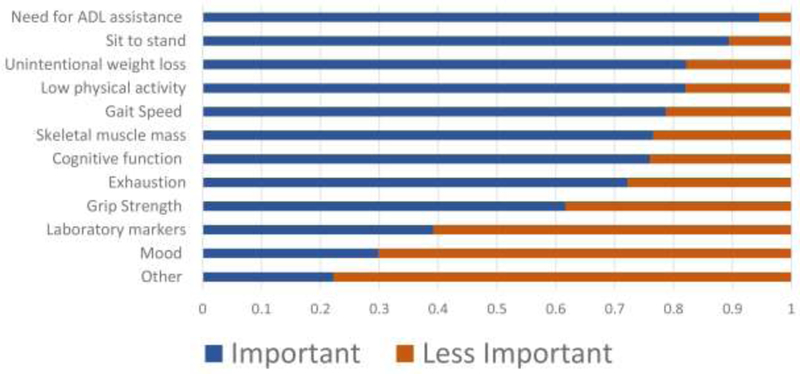
Participants were asked, “In your view, what components are essential in assessing frailty?” and were asked to select either important or less important for each component.
While 96% of programs agreed that the results of a frailty assessment should be used in decisions regarding candidate selection for KT, only 61% of programs reported that frailty assessments should be used in decisions for the timing of KT (Table 2). There was support for a distinction between irreversible and reversible frailty (84%) and a distinction between biological aging and frailty (93%); only 61% of programs considered biological age when assessing frailty in KT candidates and recipients. As such, 92% of programs reported that there is a need to develop a frailty score in the setting of KT.
Practices Related to Frailty in KT
In practice, 31% of programs never measured, 44% of programs sometimes, and 25% always measured frailty during KT evaluation (Figure 2). No program characteristics were associated with measuring frailty at the time of KT evaluation (Table 3). Fewer programs measured frailty at the time of admission for KT (never: 72%; sometimes: 19%; and always: 9%). Of those programs that measured frailty at evaluation, 34% also measured frailty at admission for KT. KT programs with longer wait times were less likely to perform frailty assessments at KT evaluation (Table 3); for example, compared to centers with a wait time between 1 month to 9.6 months, those with a wait time of over 1 year and 8 months were 22% less likely to measure frailty at KT evaluation. KT programs that had a higher percentage of male recipients (PR for a 10% increase: 1.54, 95% CI: 1.05–2.24; p=0.03) and older recipients (PR for a 10% increase: 1.91, 95% CI: 1.10–3.32; p=0.02) were more likely to perform frailty assessments than not perform frailty assessments at the time of admission for KT (Table 3). Of those who did not measure frailty, 97% were interested in measuring frailty in KT candidates and recipients. Frailty assessments were most often performed by a nurse (16%) or doctor (32%) during evaluation. Finally, transplant centers that measured frailty at KT had 1-year patient mortality (mean: 2.1% [SD=1.6] vs. 2.7% [SD=1.9]) and 1-year graft loss (mean: 0.5% [SD=0.6] vs. 0.7% [SD=1.0]) that were less than those center who did not measure frailty at admission for KT.
Figure 2. Frequency of frailty assessments conducted by transplant programs at evaluation for kidney transplantation (KT) and at admission for KT (n = 133).
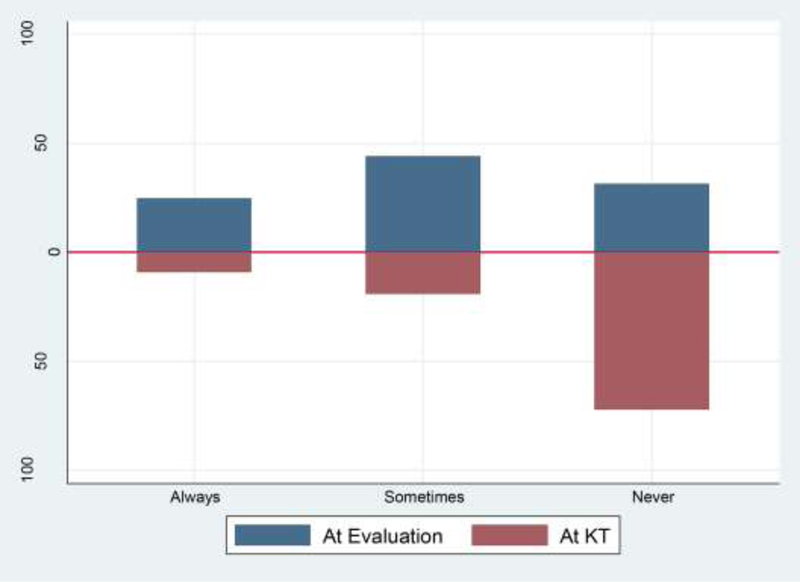
Participants were asked, “Do you currently perform a standardized frailty assessment as part of evaluation for kidney transplant candidacy [for kidney transplant recipients at the time of transplantation] in your practice?”
Table 3. Program Characteristics Associated with Measuring Frailty at the Time of Kidney Transplant (KT) Evaluation and Admission for KT (n = 133).
Bolded results indicate statistical significance (P < 0.05). In practice, 31% of programs never measured, 44% of programs sometimes, and 25% always measured frailty during KT evaluation and 72% of programs never measured, 19% of programs sometimes, and 9% always measured frailty at admission for KT. For this analysis “sometimes” and “always” measured frailty were combined. This table identifies the independent associations between transplant center characteristics and the chance that they measure frailty at either evaluation or at admission for KT, separately; two separate models are presented below.
| Measurement of Frailty | ||
|---|---|---|
| At Evaluation for KT PR (95% CI) |
At Admission for KT PR (95% CI) |
|
| Adult KT volume | 0.99 (0.95–1.03) | 1.05 (0.95–1.16) |
| Age in years | 0.73 (0.51–1.06) | 0.44 (0.17–1.09) |
| Mean wait time | ||
| Quartile 1 (1 to 9.6 months) | Reference | Reference |
| Quartile 2 (9.7 months to 1 year and 2 months) | 0.85 (0.65–1.10) | 0.95 (0.24–3.75) |
| Quartile 3 (1 year and 2 months to 1 year and 8 months) | 0.73 (0.54–0.98) | 0.56 (0.14–2.21) |
| Quartile 4 (>1 year and 8 months) | 0.78 (0.62–0.98) | 0.81 (0.21–3.15) |
| % Male | 1.15 (0.98–1.35) | 1.54 (1.05–2.24) |
| % African American | 0.95 (0.87–1.04) | 0.99 (0.81–1.22) |
| % Hispanic | 1.03 (0.96–1.11) | 0.97 (0.78–1.20) |
| % Living donor | 1.03 (0.95–1.03) | 1.02 (0.79–1.32) |
| % older (>65y) | 1.13 (0.89–1.45) | 1.91 (1.10–3.32) |
| % Working for income | 1.00 (0.90–1.12) | 0.99 (0.74–1.34) |
| % Less than high school | 0.95 (0.84–1.06) | 1.15 (0.85–1.54) |
Note: All program characteristics are scaled by 10%, except volume which was scaled by 25 transplants, and age which was scaled by 5 years. PR=Prevalence Ratio. Wait times are the time between evaluation and KT for both deceased and live donor recipients.
Programs reported using a variety of tools to measure frailty in KT (Figure 3); 18 out of 20 tools were used by more than one program and 10% of programs reported a tool outside of the 20 frailty tools that were listed. The most commonly used tool to assess frailty in practice was a timed walk test which was reported by 19% of programs. The next most commonly reported tools were measuring body mass index (BMI) (15%), assessing functional status (12%), testing stair climbing (11%), conducting a timed up and go test (11%), and counting the number of hospitalizations (10%). Sarcopenia, Montreal Cognitive Assessment (MoCA), and the Fried frailty phenotype were assessed by 8% of programs. Body mass index as tool to quantify wasting was used to measure frailty at 15% of centers. No programs reported using the Winograd Screening Instrument or the Rockwood Clinical Frailty Scale. Most programs (67%) reported assessing frailty using more than 1 tool.
Figure 3. The most commonly used tools to assess frailty among kidney transplant programs (n = 133).
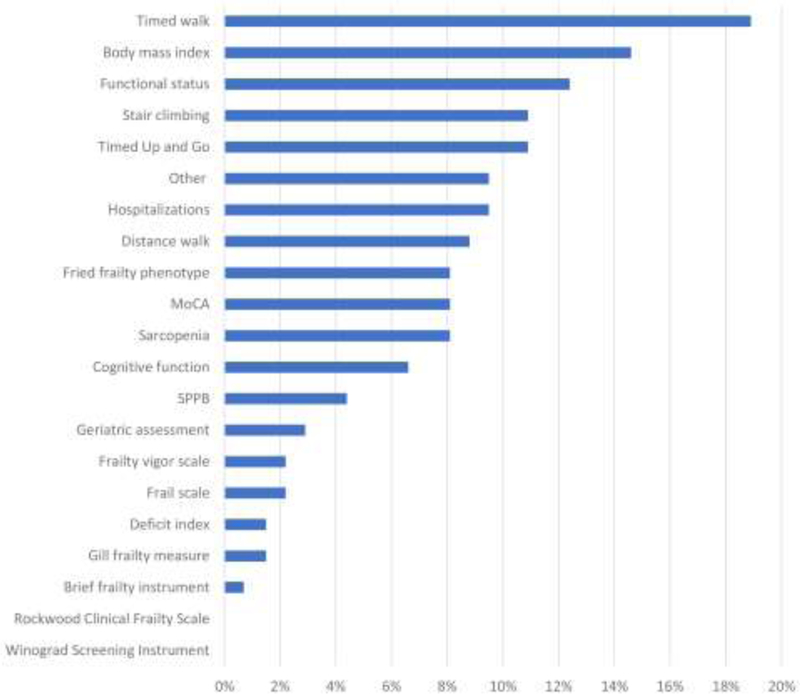
Participants were asked, “What tool for the assessment of frailty do you currently use routinely?” and were able to select all that applied.
The application for frailty assessments varied. Twenty-three percent of programs performed these assessments only for older patients (which was most commonly defined as age 65 and older) and 38% for all candidates. In addition, 89% of program reported comorbidities other than age were considered when deciding who should undergo frailty measurement; the most common of these comorbidities was history of a stroke and 7% of centers considered a patients’ obesity status when considering whether to measure frailty for that patient. Among programs that measured frailty, 91% indicated that information on frailty is presented at selection committee. When a patient was identified as being frail, frailty was an absolute contraindication to listing at 75% of centers, and to deceased donor transplantation at 33% of centers. Additionally, programs reported that when a patient was identified as being frail, the program was more likely to determine the amount of social/home support prior to listing (89%), prescribe prehabilitation (97%), and tailor immunosuppression (92%). No program reported using a candidate’s frailty status in isolation to decide whether to proceed with a living donor transplant or to accept a high kidney donor profile index (KDPI) organ offer.
DISCUSSION
In this national survey of KT programs, representing 79% of all KT candidates and 77% of all KTs performed in the US, we found that 99% of programs agreed that frailty is a useful concept in evaluating candidacy for KT. The majority of these programs (95%) identified the inability to perform ADLs as the most essential component of frailty. However, we found that US KT programs use a variety of tools to measure frailty, and that no single tool was used by more than 20% of programs. The most commonly reported tool was a timed walk test and 67% of programs used more than one tool. Frailty was more likely to be measured at KT evaluation than at admission for KT, and programs that had a higher percentage of male and older KT recipients were more likely to measure frailty at the time of KT. Over half of programs reported that they would be less likely to list a KT candidate who is frail and the programs favored the use of interventions such as prehabilitation for frail candidates. Our survey results representing majority of the US Transplant programs highlight the heterogeneity in practice patterns for frailty measurements and the importance of standardizing tools and practices to improve the care of frail KT candidates.
The results from this survey extend the previous findings on the importance of frailty from a single center Delphi study of clinicians who care for older patients with ESKD,22 showing that 99% of transplant programs consider frailty as a useful concept in evaluating candidacy for KT. The Delphi study found that there was consensus about the need for an ESKD-specific measure of frailty. In the current survey, our survey found that 92% of programs reported the need to develop a frailty score in the setting of KT. Furthermore, we found that 96% of programs felt that the results of a frailty assessment should be used to influence decisions regarding candidate selection for KT.
We have extended the findings of an international survey of geriatricians that suggested that there is a wide range of tools to measure frailty in clinical practice25 and in research;24 our survey findings confirmed that KT programs use many different tools and often more than one to assess frailty. Similar to geriatric practices25, walk speed, as measured by a timed walk test, was the most commonly used tool to measure frailty. Many tools that were used by transplant programs are included in the hypothesized cycle of frailty by Fried and colleagues (gait speed, disability, and sarcopenia) or tools to measure geriatric syndromes that are associated with frailty (the Short Physical Performance Battery [SPPB] as a measure of lower extremity impairment, MoCA as a measure of cognitive function, and self-reported functional status as a measure of dependence).3 However, it is important to consider that from a geriatrics and gerontology stand point, comorbidity, cognitive impairment, physical function, and frailty are all separate geriatric syndromes.9,19,26–29 However, previous research in KT recipients suggests that different tools to assess vulnerability will identify different recipients as being frail,19 with prevalence rates among KT recipients ranging from 3% for cognitive impairment measured by the Modified Mini Mental Status Exam30 to 53% for the Short Physical Performance Battery.19,31
As transplant centers consider the potential trade-offs of incorporating frailty measures into their practices, it is important to note that not all frailty assessments have been studied in the context of KT, and not all measures of vulnerability are necessarily associated with adverse outcomes among KT candidates or recipients. For example, the timed-up-and-go test is not associated with waitlist outcomes or post-KT hospitalizations among KT candidates.32 A previous study of KT recipients suggests that among the 5 components of the Fried frailty phenotype, poor grip strength, exhaustion, and slowed walking speed was the combination of components that was most strongly associated with post-KT mortality.29 Finally, previous studies have identified specific subgroups of patients in whom frailty is most strongly associated with adverse outcomes,33–35 suggesting that targeted screening for frailty may be warranted. For example, a study of 2,086 KT candidates on the waitlist at Johns Hopkins or University of Michigan found that frailty was more strongly associated with waitlist mortality among candidates with a low comorbidity burden; this suggests that among high-risk patients knowing a candidates frailty status provides less information about their mortality risk than among those who are lower risk such as those without a high comorbidity burden.33
Although centers agree that frailty is important, there are several potential barriers to more widespread adoption of frailty assessments by transplant programs. One very substantial barrier is that there are a multitude of ways to identify vulnerable patients, as evidenced by the numerous frailty assessments that were reported in our survey study. Centers are already beginning to adopt specific measures for waitlist management;36 for example, Mayo clinic has reported that as part of routine practice all high-risk KT candidates undergo both physical frailty phenotype and SPPB at their center.37 Furthermore, new research suggests that the timing of frailty measurement matters with respect to post-KT risk stratification because frailty is dynamic while awaiting KT.38 To address the current lack in consistency in transplant center practices, future studies should establish and validate a single ESKD-specific tool to measure frailty in both KT candidates and recipients,39 build off the prior research on possible interventions to reverse frailty,40,41 and clarify whether all KT candidates and recipients, regardless of age, should have frailty assessed.41,42 Given that much of the evidence on the association between frailty and KT candidate and recipient outcomes has been derived from just a few centers (ie, 7,078 KT candidates and 893 KT recipients from the University of Michigan, Johns Hopkins, and Methodist [KT candidates only]), future studies should include a broader spectrum of transplant centers across the US and identify the ideal components of frailty metric for kidney transplant candidates as suggested by the recent AST sponsored consensus conference on frailty in solid organ transplantation. Finally, an additional next step may be to initiate an implementation science study to promote the systematic expansion of measuring frailty among KT candidates and recipients pre and post rehabilitative programs to improve physiological processes and physical function.43–46
This survey has limitations. We were unable to obtain surveys from all US KT programs. The response rate, or percentage of all adult KT programs that responded to the survey, was 66%. However, most national surveys of transplant programs have response rates less than 60%,47–49 and our results reflect perceptions and practices of programs that impact 77% of all KT recipients and 79% of all KT candidates in the US. Furthermore, we did not obtain information about perceptions and practices regarding frailty from dialysis units or during waitlist clinic evaluations. There are a number of strengths of this study including a wide range of questions on the perceptions and practices regarding frailty, the geographic diversity of the participating programs, and the high percentage of US KT recipients that are reflected by the results of this survey.
In conclusion, this national survey of KT programs suggests that frailty is widely recognized as an important and clinically useful tool for evaluation of candidates. However, there was no consistent tool used to measure frailty and assess candidacy for KT across US transplant programs. Our data suggest an inconsistency of programs assessing KT candidates; thus, concerns arise that ambiguity regarding the most appropriate choice of frailty tools to be used in KT evaluations may adversely impact access to KT, particularly for older KT candidates.50 There is a need for education of transplant providers surrounding the number of tools to measure frailty. It is important for the field of transplantation to identify an existing tool or develop a novel tool to measure frailty51 that is valid, reliable, and easy to implement in clinical practice to determine if a frail candidate will benefit from KT.
ACKNOWLEDGMENTS
This manuscript is a work product of the American Society of Transplantation’s Kidney Pancreas Community of Practice and has been endorsed by the American Society of Transplantation.
We thank the study participants for their contributions to this study.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, OPTN/UNOS, or the US Government. The authors are members of the Frailty Consensus workgroup formed by the American Society of Transplantation (AST) Kidney-Pancreas Community of Practice; KLL is the American Society of Nephrology (ASN) Quality Committee representative to the workgroup.
Funding: This study was supported by NIH grant R01AG042504 (PI: Dorry Segev), R01AG055781 (PI: McAdams-DeMarco) and K24DK101828 (PI: Dorry Segev). Mara McAdams-DeMarco was also supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30AG021334) and NIH grant (K01AG043501). Raymond Lynch was supported by NIH grant (MDO11682). Meera Harhay was supported by NIH grant (K23DK105207). Jane Tan was supported by the John M. Sobrato Foundation. Stefan G. Tullius was supported by NIH grant (R01/R56: AG039449). Maya K. Rao was supported by NIH grant (R03AG053294).
ABBREVIATIONS:
- ADL
activities of daily living
- AST
American Society of Transplantation
- BMI
Body mass index
- ESKD
end-stage kidney disease
- KDPI
kidney donor profile index
- KT
kidney transplant(ation)
- KPCOP
Kidney/Pancreas Community of Practice
- MoCA
Montreal Cognitive Assessment
- OPTN
Organ Procurement and Transplantation Network
- PR
prevalence ratio
- SPPB
Short Physical Performance Battery
- SRTR
Scientific Registry of Transplant Recipients
Appendix Table 1:
AST Frailty Working Group Survey of US Adult KT Centers
| Survey Questions | Response | |
|---|---|---|
| Format | Options | |
| Please indicate your provider type | Select one from drop-down | Transplant
Nephrologist Transplant Surgeon Transplant Coordinator Nurse/PA/NP Pharmacist Social Worker Other (open text) |
| What is your transplant center’s UNOS code? Please use the following link to look up your code if you do not know it. | Free text | 4 digit UNOS Code |
| Frailty Perception Questions | ||
| In your view, is frailty a useful concept in evaluating candidacy for kidney transplantation? | Select one | Yes No |
| In defining frailty, which of the following features are important to consider: | Rank with “1” being most important | Functional limitation (e.g. walking
speed, grip strength, sarcopenia) Morbidity (e.g. diabetes mellitus) Psychosocial status (e.g. depression or anxiety) Cognitive ability (e.g. memory or attention) |
| In your view, what components are
essential in assessing
frailty? • Grip strength • Gait speed • Sit to stand • Exhaustion • Unintentional weight loss • Low physical activity • Cognitive function • Mood • Skeletal muscle mass • Laboratory markers • Need for ADL assistance • Other (please specify) |
Select one | Important Less Important |
| Do you think the results of a frailty assessment should be used to influence decisions regarding candidate selection for transplantation (i.e. determination of medical appropriateness)? | Select one | Yes (please explain) No (please explain) |
| In your area of practice, do you think frailty in transplant candidates is a risk factor for adverse outcomes before transplantation (e.g. waiting list mortality)? Please explain. | Select one | Yes (please explain) No (please explain) |
| Do you think the results of a frailty assessment should be used to influence decisions regarding the timing of transplantation (i.e. determination of medical urgency)? Please explain. | Select one | Yes (please explain) No (please explain) |
| In your area of practice, do you think frailty in transplant candidates is a risk factor for adverse outcomes after transplantation (e.g. affecting length of stay or post-transplant mortality)? Please explain. | Select one | Yes (please explain) No (please explain) |
| What interventions before transplantation do you think are useful to improve frailty? | Rank with “1” being most useful | Optimization of dialysis/fluid
status Nutrition stimulation or supplements Physical therapy Psychotherapy |
| If you consider frailty reversible, how do you monitor reversibility? | Free text | |
| In your view, should there be a distinction between “irreversible” frailty, denoting frailty that is not directly due to end-stage organ failure, and “reversible” frailty, that is likely to improve after transplantation? Please explain. | Select one | Yes (please explain) No (please explain) |
| In your view, is there a pathophysiological distinction between biological aging and frailty? Please explain. | Select one | Yes (please explain) No (please explain) |
| In your view, should biological age be considered in assessing frailty? (Is frailty in the young less concerning than frailty in those of advanced age?) Please explain. | Select one | Yes (please explain) No (please explain) |
| In your view, is there a need for the development of a frailty score in the setting of kidney transplantation? Please explain. | Select one | Yes (please explain) No (please explain) |
| Please feel free to add any additional comments. | Free text | |
| Frailty Practice Questions | ||
| Do you currently perform a standardized frailty assessment as part of evaluation for kidney transplant candidacy in your practice? Please explain. | Select one | Yes- always (please
explain) Yes- sometimes (please explain) No-never (please explain) Not sure |
| Do you currently perform a standardized frailty assessment for kidney transplant recipients at the time of transplantation in your practice? Please explain. | Select one | Yes- always (please
explain) Yes- sometimes (please explain) No-never (please explain) Not sure |
| If you do not measure frailty, are you interested in measuring frailty among your KT candidates and recipients? | Select one | Yes No |
| If you currently perform a frailty assessment as part of evaluation for kidney transplant candidacy at your center, who performs the assessment? | Select one | Nurse Nutritionist Occupational Therapist Physical Therapist Respiratory Therapist Physician We do not perform frailty assessments as part of evaluation I don’t know Other (please specify) |
| If you currently perform a frailty assessment as part of for kidney transplant recipients at the time of transplantation at your center, who performs the assessment? | Select one | Nurse Nutritionist Occupational Therapist Physical Therapist Respiratory Therapist Physician We do not perform frailty assessments as part of evaluation I don’t know Other (please specify) |
| What tool for the assessment of frailty do you currently use routinely? | Check all that apply | Fried Frailty
Phenotype Rockwood Clinical Frailty Scale Short Physical Performance Battery Deficit Index (DAI, also called Frailty Index) Montreal Cognitive Assessment Cognitive function (i.e. 3MS, MMSE, or Trail Making Test) Functional status (i.e. KDQOL, SF-36, or PCS) Level of sarcopenia Body Mass Index to quantify wasting Number of hospitalizations in the previous year Gill Frailty Measure Frailty/Vigor Assessment Brief Frailty Instrument Vulnerable Elders Survey FRAIL Scale Stair Climbing Assessment Winograd Screening Instrument Timed Up and Go Timed walk (i.e. 6-minute walk test) Distance walk test (i.e. 10-meter walk test) Geriatric Assessment Assessment by a physical therapist Other (Please specify) Don’t know |
| In which candidates do you perform these assessments? | Select one | Just older candidates (please
indicate age cut point) All candidates Other (Please explain) Don’t know |
| In addition to age, what comorbidities are considered when identifying individuals who should undergo measures of frailty? | Select one | None Cardiovascular Diabetes History of a stroke Dialysis vintage Obesity Other (Please specify) Don’t know |
| Is this information presented at selection committee? | Select one | Yes No |
| How else are these assessments used? | Check all that apply | Decisions about listing a
patient Decisions about transplanting a patient Decisions to proceed with a living donor transplant Decisions about accepting a high KDPI organ offer Decisions on whether or not to use induction therapy Decisions on which induction therapy to use Decisions to tailor immunosuppression Decisions about prehabilitation prior to KT Determination of the amount of social/home support required prior to listing Quality improvement/research Other (Please specify) |
| If a KT candidate or recipient (of
any age) is frail, how likely are you to make the following
decisions? • List for transplant • Perform deceased donor transplant • Perform living donor transplant • Accept a high KDPI organ offer • Use induction therapy • Tailor maintenance immunosuppression • Prescribe prehabilitation prior to KT • Increase amount of social/home support required prior to listing • Other |
Select one | More likely About the same Less likely Frailty is an absolute contraindication to this action |
| Is there anything else you would like us to know about assessing frailty at your center? | Free text | |
Footnotes
Disclosure: Dr. Segev reports personal fees from Sanofi-Aventis and Novartis outside the submitted work. No other disclosures were reported.
REFERENCES
- 1.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 4.Brown EA, Johansson L. Old age and frailty in the dialysis population. J Nephrol. 2010;23(5):502–507. [PubMed] [Google Scholar]
- 5.McAdams-Demarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen KL, Dalrymple LS, Delgado C, et al. Factors Associated with Frailty and Its Trajectory among Patients on Hemodialysis. Clin J Am Soc Nephrol. 2017; 12(7):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10(12):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Frailty and Health-Related Quality of Life in End Stage Renal Disease Patients of All Ages. J Frailty Aging. 2016;5(3):174–179. [PMC free article] [PubMed] [Google Scholar]
- 11.Haugen CE, Chu NM, Ying H, et al. Frailty and Access to Kidney Transplantation. Clin J Am Soc Nephrol. 2019;14(4):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients with End-Stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018;102(10):1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]
- 14.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29(6):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2016;266(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu NM, Gross AL, Shaffer AA, et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J Am Soc Nephrol. 2019;30(2):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Pilsum Rasmussen S, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salter ML, Gupta N, Massie AB, et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruyere O, Buckinx F, Beaudart C, et al. How clinical practitioners assess frailty in their daily practice: an international survey. Aging Clin Exp Res. 2017;29(5):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840–851. [DOI] [PubMed] [Google Scholar]
- 27.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 28.Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clin Transplant. 2018;32(10):e13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality in Kidney Transplant Recipients. Transplantation. 2017;101(9):2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas AG, Ruck JM, Shaffer AA, et al. Kidney Transplant Outcomes in Recipients with Cognitive Impairment: A National Registry and Prospective Cohort Study. Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nastasi AJ, Bryant TS, Le JT, et al. Pre-kidney transplant lower extremity impairment and transplant length of stay: a time-to-discharge analysis of a prospective cohort study. BMC Geriatr. 2018;18(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelson AT, Tsapepas DS, Husain SA, et al. Association between the “Timed Up and Go Test” at transplant evaluation and outcomes after kidney transplantation. Clin Transplant. 2018;32:e13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez Fernandez M, Martinez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality among Kidney Transplant Candidates of All Ages. Am J Nephrol. 2019;49(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick J, Sozio SM, Jaar BG, et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients. Am J Kid Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sy J, McCulloch CE, Johansen KL. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int. 2019;23(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng XS, Busque S, Lee J, et al. A new approach to kidney wait-list management in the kidney allocation system era: Pilot implementation and evaluation. Clin Transplant. 2018;32(11):e13406. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz EC, Cosio FG, Bernard SL, et al. The Relationship Between Frailty and Decreased Physical Performance With Death on the Kidney Transplant Waiting List. Prog Transplant. 2019:1526924819835803. [DOI] [PubMed] [Google Scholar]
- 38.Chu NM, Deng A, Ying H, et al. Dynamic Frailty Before Kidney Transplantation-Time of Measurement Matters. Transplantation. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Loon IN, Goto NA, Boereboom FTJ, et al. Frailty Screening Tools for Elderly Patients Incident to Dialysis. Clin J Am Soc Nephrol. 2017;12(9):1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson BM, Dutton M, Day E, et al. Frailty Intervention Trial iN End-Stage patientS on haemodialysis (FITNESS): study protocol for a randomised controlled trial. Trials. 2018;19(1):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clin Transplant. 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese PP, Bloom RD, Shults J, et al. Functional status and survival after kidney transplantation. Transplantation. 2014;97(2):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdulnassir L, Egas-Kitchener S, Whibley D, et al. Captivating a captive audience: a quality improvement project increasing participation in intradialytic exercise across five renal dialysis units. Clin Kidney J. 2017;10(4):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAdams-DeMarco MA, Konel J, Warsame F, et al. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int Rep. 2017;3(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;(10):CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25(4):352–364. [DOI] [PubMed] [Google Scholar]
- 47.Fleming JN, Lai JC, Te HS, et al. Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant. 2017;31(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sher L, Quintini C, Fayek SA, et al. Attitudes and barriers to the use of donation after cardiac death livers: Comparison of a United States transplant center survey to the united network for organ sharing data. Liver Transpl. 2017;23(11):1372–1383. [DOI] [PubMed] [Google Scholar]
- 49.Cote DR, Chirichella TJ, Noon KA, et al. Abdominal Organ Transplant Center Tobacco Use Policies Vary by Organ Program Type. Transplant Proc. 2016;48(6):1920–1926. [DOI] [PubMed] [Google Scholar]
- 50.Salter ML, McAdams-Demarco MA, Law A, et al. Age and sex disparities in discussions about kidney transplantation in adults undergoing dialysis. J Am Geriatr Soc. 2014;62(5):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]


