Abstract
The enteric nervous system (ENS) resides within the gut wall and autonomously controls gut functions through coordinated activation of sensory, inter and motor neurons. Its activity is modulated by the enteric immune and endocrine system as well as by afferent and efferent nerves of the parasympathetic and sympathetic nervous system. The ENS is often referred to as the second brain and hence is able to perform sophisticated tasks. We review the evidence that the “smartness” of the ENS may even extend to its ability to learn and to memorize. Examples for habituation, sensitization, conditioned behavior and long term facilitation are evidence for various forms of implicit learning. Moreover, we discuss how this may change not only basic Neurogastroenterology but also our understanding of development of gut diseases and chronic disorders in gut functions. At the same time we identify open questions and future challenges to confirm learning, memory and memory deficits in the gut. Despite some remaining experimental challenges, we are convinced that the gut is able to learn and are tempted to answer the question with: Yes, the gut is smart.
Keywords: enteric nervous system, implicit learning, plasticity, memory. functional gastrointestinal diseases
1. Motivation to discuss learning in the gut - the “brain in the gut”
Why is it important to discuss learning in the gut? It seems odd to propose for an organ which produces waste products to perform such a delicate and sophisticated task. It is important to realize that the gut carries its own truly autonomic nervous system.1–4 This enteric nervous system (ENS) is in its complexity comparable to the brain, hence the alias “second brain” or “little brain in the gut”. The ENS consists of ganglionated networks within the gut wall, which add up to several 100 million neurons in the human gut. These networks are the myenteric plexus and, at least in larger mammals, several submucosal plexi. Broadly speaking, neurons in the myenteric plexus control muscle functions, those in the submucosal plexus regulate epithelial functions. The task allocation is not that strict as, for example in larger animals, also neurons in the submucosal plexus innervate circular muscle or act as sensory neurons to initiate muscle reflexes. Both plexi also modulate immune functions, microcirculation and cell proliferation and there are reciprocal connections between them. Jackie D. Wood from the Ohio State University in Columbus used to educate students who just joined his research team in the lab by the dictum: “The little brain in the gut is pretty smart”. This statement reflects the complexity of its structure and at the same time recognizes the ability to control complex organ functions. Everyone shares the excitement once they see how an isolated piece of intestine is able to perform as it would in the intact animal. For example, the generation of the neurally driven peristalsis works for days even if the intestinal segment is placed in a petri dish. All what is required is a sterile medium and some oxygen. The basis for peristalsis is a hard-wired circuit in the ENS which, at the level of the motor output, consists of excitatory motor neurons which project for about 1cm up the gut and inhibitory neurons that project for about 1cm down the gut. In this context, “hard-wired” refers to the fixed projection preferences of excitatory and inhibitory motor neurons and not to the synaptic connections between enteric neurons. The activity of the motor neurons is coordinated by sensory neurons and modulated by interneurons. Similar to our “master brain” in our head, the gut carries its own “belly brain”.
With all the understandable excitement, we feel that some crucial questions remain unanswered, such as: what makes the ENS so smart; is it clever enough to learn and to memorize? This is not only important for basic ENS neurophysiology but it may also change our understanding of gut diseases. As discussed further below, some of the diseases which cause clinicians quite a headache may be reflections of memory disorders in the gut.
1.1. Much simpler systems than the gut are able to learn
Since all organisms are forced to adapt during evolution and therefore have to cope with changing environmental challenges on a shorter time-scale, much simpler biological systems than the gastrointestinal tract must be able to learn. The most intriguing examples in which learning occurs are plants and monocellular organisms, both of which lack specialized sensory and motor neurons.
Garden peas can learn and remember
“let’ get used to it”.5 Plants often grow in the direction of the light source (called phototropism) for photosynthetic energy production and growth. This mechanism was used in ingenious experiments by Gagliano and colleagues with the garden pea (Pisum sativum)6. They used a Y-maze in which pea seedlings could grow after having been trained (on an 8-h light:16-h dark cycle) to associate a light source (unconditioned stimulus) together with an airflow produced by a fan (conditioned stimulus), while in control plants both light and airflow were presented unassociated. Testing in complete darkness revealed that plants preferred the fan as predictor of the light direction, even in the absence of light or when the last light exposure during training was from the opposite maze arm. In contrast, control plants always grew in the direction of the last light exposure. Additional experiments prove that this associative (Pavlovian conditioned) learning depends on daytime cycle, and is ineffective when training and testing are done at different phases of the circadian cycle. And they learn faster and forget slower in environments where it matters.7
Even the slime mold can do it
In elegant and fascinating experiments, Reid and colleagues show that amoeboid organisms such as the slime mold (Physarum polycephalum) are able to learn.8 They used a classical decision making problem (the two-armed bandit) and exposed the amoeboid to situations with increased complexity when exploring unknown territory for food. They were given the choice between two differentially rewarding environments where food was provided on each of the two arms but with either similar (control) or variable intervals between food places, using mathematical models of distribution. As it turns out, molds do not decide to exploit one arm over the other without information suggesting they differ in quality, and when forced to chose one environment, they make a rational choice to do so. They are also sensitive to relative and not necessarily to absolute reward differences, and can calculate and predict the arm with the most rewarding food quantity even when food is randomly distributed. As molds do not have specialized memory cells, they rely on an externalized spatial memory to navigate in complex environments, similar to pheromone trails used by ants9. Thus, using a Y-maze where chemotaxis (detection of previous exploration of the environment) is prevented by masking the mold’s own trail results in poorer discrimination of food sources, thus proving the externalization of memory. In fact, external memory is therefore the older version of memory formation, internalization comes in later.
1.2. The belly brain and the head brain – what was first?
Before we discuss the more sophisticated nitty-gritty aspects of learning and memory let us start with a more trivial approach. All animals that have a central nervous system exhibit an ENS, but not all animals with enteric neurons have a brain or central ganglia. For example, Hydra has no central nervous system but it contains enteric neurons that control gut movement very much like they do in vertebrates.10 The implication is that the brain develops as an encephalised ENS which by definition is then the first brain which Mother Nature has established.3,11
Learning in the ENS has not been the focus of many studies. Actually, there are only the very few excellent studies by John Furness and colleagues (cited below). We will discuss some thought provoking aspects and hope this review will motivate others to immerse further into the subject matter. Most of our thoughts are based on studies which were motivated by questions totally unrelated to learning but, in our opinion, still allow conclusions on memory and learning in the gut. We are aware of the fact that the ENS is bombarded with inputs from other sources and we do not question the influence of luminal factors including microbiota, blood borne factors, immune cells or components of the brain-gut-axis. For the sake of staying focused we only discuss different forms of learning and memory in the ENS.
2. The various ways to learn and to memorize
2.1. General principles of learning
According to the Hebbian theory synaptic plasticity is the basis for learning and memory.12 As Hebb formulated in his seminal book: “When an axon of cell A is near enough to excite cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased.” The principle “what fires together - wires together” is commonly referred to as Hebb’s law and requires altered synaptic transmission. These principles are well documented and established in invertebrates as well as in the mammalian brain.
As shown in Aplysia, elementary forms of learning have distinct short and long-term stages of memory storage and in parallel synaptic plasticity.13,14 Basically, habituation, dishabituation and sensitization represent synaptic plasticity and structural changes that underlie short and long term stages of memory storage. While habituation is associated with decreased neurotransmitter release, sensitization is associated with enhanced synaptic strength and transmitter release. The gill- and siphon-withdrawal reflex of Aplysia has been used to detect several forms of learning including habituation, dishabituation, sensitization, classical and operant conditioning.13 We will later pose the question of whether similar processes exist in the gut and whether the gut is able to exhibit Hebbian learning.
2.2. Different complexities of learning
Learning is the change of the behavior of an organism following - temporal - alterations of the environment, that persists beyond the altered environmental challenge and becomes part of the behavioral inventory of the organism for at least some time, if not permanently. It requires that the organism is able to store the novel behavior (or is biological equivalents) in some type of memory. Different forms of learning can be conceptualized in a pyramidal model (Figure 1) with increasing complexity but decreasing relevance for intestinal functions.
Figure 1.
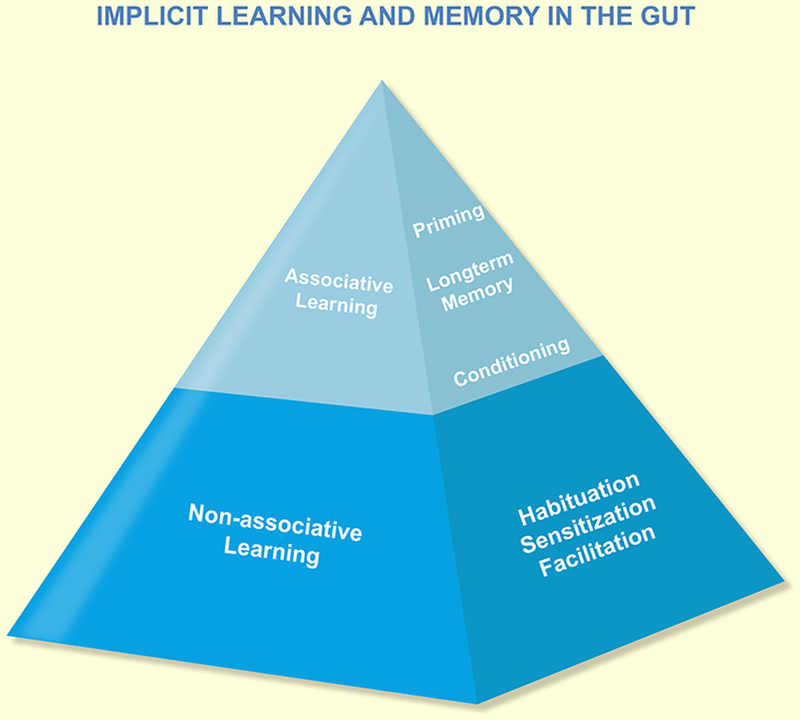
Hypothetical model for learning and memory in the gut. The pyramid in A illustrates the types of learning and the general processes involved. Note that there is no evidence for explicit learning in the gut.
Adaptation, non-associative implicit learning (Habituation, Sensitization):
The simplest form of accommodation is adaptation, i.e. the behavioral change occurs within a well-defined interval after a novel environmental stimulus is perceived by the organism, and is related to the stimulus intensity in a dose-dependent fashion: stronger stimuli cause stronger responses either in time (frequency) or in amplitude or both. Learning is more than simple adaptation.13,15 Repetitive stimulation of the organ can result in either sensitization (less stimulus intensity needed for the same response or stronger response to the same stimulus), or in habituation (higher stimulation intensity needed for the same response or less response to the same stimulus). Habituation and sensitization - as adaptive mechanisms - are mostly in-built and “hard-wired” reflex responses that allow the organism to respond to fast and often occurring environmental challenges without wasting energy to find new solutions (behaviors); examples from clinical Neurogastroenterology may be the post-operative ileus, gastroparesis following colonic obstruction (constipation), or retrograde peristalsis following food poisoning. This non-declarative or implicit learning is learning in an incidental manner, without awareness of what has been learned. It assures performance of reflexive tasks - automatically and unconsciously - involves memory functions and learned behaviors such as sensitization and habituation (non-associative learning) or conditioning (non-associative and associative learning). Most will relate these features to higher centers, either central ganglia or brain; but it involves in simplest cases just motor and sensory pathways.
Slowly occurring but longer-lasting environmental challenges, e.g. changes in nutritional supply may require adaptation towards more complex signals that are not necessarily pre-installed but may require novel behaviors of the organisms. A switch from an omnivore diet to a pure vegan, plant-based diet, or in the opposite direction, may force the intestinal ecological system (microbiome) as well as the gut of the host to adapt its secretory and motor functions; a change in feeding schedule (night shift or time-shift) will not only require the immuno-endocrine system of the gut to alter circadian hormone release, but also the ENS to respond.16 While still within the normal range of the system’s behavior, these changes may elicit longer lasting sensitization and/or inhibition within the ENS. Longer lasting facilitation in Aplysia is associated with either the rapid filling of synapses and thereby the recruitment of previously silent synapses or formation of new synapses.17 Both contribute to long term facilitation (potentiation).
Associative learning: Conditioning, Stimulus-Response (S-R) learning
More complex forms of learning are summarized under the term “associative learning”, and can be subdivided, according to their rising complexity, into Pavlovian conditioning, and S-R-learning (operant or instrumental conditioning).
Pavlovian conditioning is a specific variant of general conditioning. The model of Pavlovian (or classical) conditioning is often used to experimentally demonstrate the effectiveness of associative learning under specific circumstances: by choosing a conditioning stimulus (CS) that under normal circumstances does not elicit a response of the organism, e.g. a light or acoustic stimulus not affecting the motor system, and combining it with an unconditioned stimulus (US) that elicits a specific motor reflex (UR), e.g. a tactile stimulus to elicit the withdrawal reflex in Kandeĺs Aplysia model.13 After repetitive pairing of both stimuli, the CS alone will be able to elicit the motor response.
This type of associative learning is not restricted to experimental situations where both the CS and the US can be well controlled, it may happen in “real life” much more often but is difficult to prove, e.g. for anticipatory nausea in cancer patients.18 In a more global sense, complex circumstances (rather than specific CS) can take over control of specific functions, and the detailed underlying series of biological mechanisms may become less visible. Asthma attacks when seeing a flower, nausea with the smell of a specific food than once has been sickening, or diarrhea at the thought of or in advance of an oral examination may serve as examples for associative learning as a conditioning procedure. It has yet to be shown whether exposure of the gut (and the ENS) to novel stimuli only perceivable to the gut sensory system is able to induce such associative learning. Post-infectious sensitization may provide a model to test this, but also surgical alterations of the gut anatomy (bowel resection, anastomosis, short bowel, gastric bypass, ileoanal pouch) may induce the ENS to learn.
Of quite different nature is S-R-learning via instrumental (or operant) conditioning. Here, instead of an accidental or intentional association of a conditioning stimulus with a hard-wired reflex pathways, the consequence of a novel, adaptive behavior of the organism is reinforcing its future occurrence (positive) or suppression (negative). This may occur as “trial and error” learning at the CNS level, while the ENS provides merely feedback of positive and negative consequences. If, in case of lactose intolerance, minimal amounts of lactose induce diarrhea and abdominal pain, the avoidance of such food is positively reinforced. Although avoidance of such food is certainly a conscious decision involving CNS centers which control eating behavior, it remains unknown to what degree the gut participates in the decision to prefer or avoid a particular food component.
Associative learning in particular, in the gut or elsewhere, requires neural plasticity in that newly developed neural circuits integrate the new behavior into the existing repertoire; the underlying biology or memory and retrieval is widely unknown for the gut, but well described in models such as Aplysia.13 The persistent utilization of these pathways determines the long-term stability and reliability of the connectivity. If, in case of Pavlovian conditioning, the CS is no longer associated with the CR, but occurs independent of it, the learned behavior is extinguished (“forgotten”) but not erased: Successful Pavlovian learning is demonstrated, if in a further conditioning procedure, the CR will occur much faster than the first time, indicative of persistence of the neural connectivity. The same holds true for associative learning of S-R-type.
Model learning, learning by insight and reflection/anticipation
Clearly, these forms of learning require an external instance that allows to judge and decide the appropriateness of a social model to be followed, or the anticipation of the putative consequences of future behaviors before their execution. Evidently, the CNS and ENS have specialized to share this responsibility, as the CNS is much better equipped for this task due to its multiple additional sensory inputs and motor control functions.
Increasing complexity of the learning mechanism makes it less likely to be executed by “lower” organisms, but as Pavlovian learning has been demonstrated even in monocellular organisms (without specialized neuronal cells) and in plants, we can reasonable assume that at least “simple to medium-complex” learning forms may as well be present in the gut.
3. Learning in the gut
3.1. Basic principles of learning in the gut
In a series of elegant studies, Terry Smith and colleagues investigated ascending muscle excitation and descending muscle inhibition after distension or distortion of mucosal villi as well as chemical stimulation of the mucosa.19–21 They observed a decline in the excitatory as well as inhibitory muscle responses if distension or mucosal distortion was repeated at intervals less than 10sec. Additionally, the number of fast excitatory postsynaptic potentials (EPSPs) in excitatory muscle motor neuron dropped to almost 0, however, it is not known how long this habituation lasts. The motor neurons still respond to mucosal deformation even after they stop responding to muscle distension which shows that motor fatigue cannot explain the habituation. Likewise, motor neurons still respond normally to muscle distension even after they failed to respond to successive mucosal distortion. This is different to the habituation of the gill withdrawal reflex observed in Aplysia.13 In Aplysia the response of the motor neurons declined gradually while the adaptation to muscle distension evoked reflexes in the gut is due to declined responsiveness of sensory neurons. Separate sets of mechanosensitive sensory neurons, which converge on common motor neurons guarantee that the gut still responds to mucosal stimulation even when successive muscle distensions fail to excite motor neurons. A run-down of the response to mucosal distortion is prevented when the stimuli are applied at 2min intervals. Under these conditions, mucosal distortion causes an enhanced response of motor neurons to muscle distension. Similar to the case of habituation, it is not known how long this sensitization lasts. This cross-sensitization is not only a phenomenon of mechanosensitive circuits but also occurs if chemical stimulation of the mucosa by acid is combined with muscle distension.
Conclusions:
The basic principles of learning - sensitization and habituation - described in Aplysia also apply to the ENS. This suggests that the ENS contains the necessary networks and wiring to learn and to memorize.
Open questions:
As it is not known how long the behavioral changes last one may argue that these observations are just short-lived adaptations rather than learning. However, even short-term habituation or sensitization are considered learned behavioral changes in Aplysia.13 It remains to be studied whether retraction of synapses from the sensory neurons to motor neurons underlie habituation or whether growth of dendritic spines occur after sensitization. Studies on long-term memory in the isolated gut for days and weeks are a challenge. While the muscle reflexes can be recorded for days, one loses the input of epithelial cells as the mucosa will stop working within one day.
3.2. Conditional learning in the gut.
An experiment by John Furness and colleagues convincingly demonstrated long lasting changes in reflex evoked muscle responses and sustained hyperexcitabiliy in enteric sensory neurons.22 This study shows evidence for conditioned nerve triggered muscle reflex responses in isolated intestinal segments. The experimental protocol started with a 1.5g distension, which produced reproducible contractile responses above and below. Multiple stretches with a 3 g weight evoked a stronger muscle response and conditioned the intestine, leading to a sustained increase in responses to a 1.5g stretch for at least 40min. Important for the interpretation is the finding that the muscle response to a direct stimulation with the muscarinic agonist carbachol is not sensitized. This argues against a merely enhanced muscle responsiveness.
The stomach actively relaxes in response to increases in intragastric pressure. This adaptive relaxation, which is important to accommodate food, is a neural reflex controlled by the ENS because it is also observed in the isolated stomach.23 Repeated runs of gastric distensions at 5–10 mmHg increased the relaxatory response and thereby enhanced the accomodation reflex.24 This facilitation remained after bilateral vagotomy or coeliac ganglionectomy confirming that neither vagal nor sympathetic nerves but enteric nerves were responsible for the sensitization. The enhanced response to repeated distensions was present even if the second distension was 60 min later. This was the longest time period in between the distensions tested in this study. Thus, we do not know whether the sensitization would last for hours. The fast EPSPs in the gastric ENS are very robust and do not show any signs of run-down even when stimulated at frequencies of 80 Hz.25
The same phenomenon occurs in humans. Single distension of a balloon in the jejunum elicits relaxation at the site of distension. Consecutive distensions of the same region causes habituation in that the relaxation becomes smaller. In contrast, sensitization occurs if an adjacent region orally is distended at the same time.26
Conclusion:
Gut reflexes may be conditioned.
Open question:
Is this conditional learning (see also the following paragraph)? It remains to be shown that synaptic plasticity rather than a sensitized sensor parallel the conditioned reflex. Adaptations in the sensitivity of enteric sensory networks controlling muscle reflexes are long known.27
3.3. Long term potentiation in the ENS.
John Furness and colleagues went further to ask which neurons remain hyperexcitable after the conditional stimulus and may thereby act as the “memory storage”.28–31 In the ENS, a rather low frequency (1 Hz) of electrical stimulation of interganglionic fiber tracts for 4–30 min induces a sustained slow postsynaptic excitation (SSPE). The SSPE consists of an increased spike discharge which lasts up to 4 hours. It is noteworthy that SSPE is only observed in neurons with a long lasting postspike afterhyperpolarisation (AH neurons, most of which do not receive fast EPSPs) but not in S neurons which do receive fast EPSPs. Many of the AH neurons function as mechano- or chemosensors whereas most S neurons are motor neurons or (sensory) interneurons. This is an important finding in several aspects. First, it clearly shows that SSPE is not a generalised hyperexcitability of all enteric neurons but a characteristic feature of sensory neurons. Second, SSPE in the ENS and thereby memory storage is restricted to sensory and not motor networks while LTP is a feature of motor neurons in Aplysia.13 As we see later, there are also stimuli which cause long-term synaptic plasticity in S neurons which may be indicative of memory storage in motor pathways. John Furness and colleagues also reveal the mechanism behind SSPE. They find that blockade of proteinkinase C suppresses SSPE.
Conclusion:
The ENS shows electrophysiological properties reminiscent of long-term potentiation in the brain (LTP). SSPE in sensory networks may be responsible for memorizing altered sensitivity of neurons to a stimulus to adapt to different digestive needs or to evoke responses under pathological conditions.
Open Questions:
LTP is widely believed to be one cellular correlate of memory formation. In the seminal paper by Bliss and Lomo electrical pulses to perforant pathways at 10–15 Hz for 10–15sec facilitate synaptic transmission and increase postsynaptic spiking in granule cells ranging from 30min to 10hrs.32 Since then numerous protocols with differing stimulus frequencies are used to demonstrate long-term potentiation. Central nervous system neurons can express multiple forms of LTP that may differ in their synaptic locus, molecular mechanisms, timescale, and role in learning and memory.13 With this in mind and the conclusion that there seems to be no uniformly accepted stimulus protocol for LTP, it seems fair to assume that SSPE in the ENS is one form of LTP. The reservations of Furness and colleagues are probably related to the fact that LTP is commonly believed to require higher stimulus frequencies although there is no rational for them to be a necessary precondition. However, it may very well be that higher frequency stimulation, which targets a functionally identified pathway would also lead to “classical” LTP in the ENS. Unlike in the brain, where functionally different regions and nuclei are connected by well-defined pathways, individual enteric ganglia contain functionally different neurons. It is therefore not possible to stimulate a defined pathway; instead, electrical pulses activate all types of inhibitory as well as excitatory synapses that converge onto the impaled neuron.
3.4. Long term memory in the gut after an inflammatory insult.
Gary Mawe, Keith Sharkey and colleagues published seminal papers on post-inflammatory plasticity in the enteric nervous system.33–37 They first discovered neuroplasticity at various levels in the ENS during trinitrobenzene sulphonic acid (TNBS) induced colitis in guinea-pig. The synaptic strength and efficacy increased during acute inflammation as the fast EPSP amplitudes increased, and the fast EPSPs lacked the run down phenomenon at higher stimulus frequencies. The paired pulse ratio revealed that the second fast EPSP evoked 50 msec after the first one exhibited an even larger amplitude in inflamed tissue. The synaptic facilitation is due to presynaptic increase in protein kinase A which is linked to inhibition of BK (big potassium) channels. Besides altered presynaptic transmission there was also postsynaptic plasticity in the inflamed gut. AH neurons exhibited an increased excitability whereas the electrical and synaptic properties of S neurons remained unchanged. This result again points to the relevance of changes in sensory and/or interneuronal circuits rather than in motor neuron pathways for memory formation in the ENS. Interestingly, AH neurons, which hardly received any fast EPSPs in non-inflamed tissue, now prominently exhibited fast EPSPs. The probability to record fast EPSPs in AH neurons increased by a factor of 5. This means that there is recruitment of either new or previously silent synapses (Figure 2). A simpler explanation could be that increased postsynaptic excitability helps to reveal previously undetectable fast EPSPs. This seems unlikely as fast EPSPs amplitude also increased in S neurons despite their unchanged postsynaptic excitability.
Figure 2.
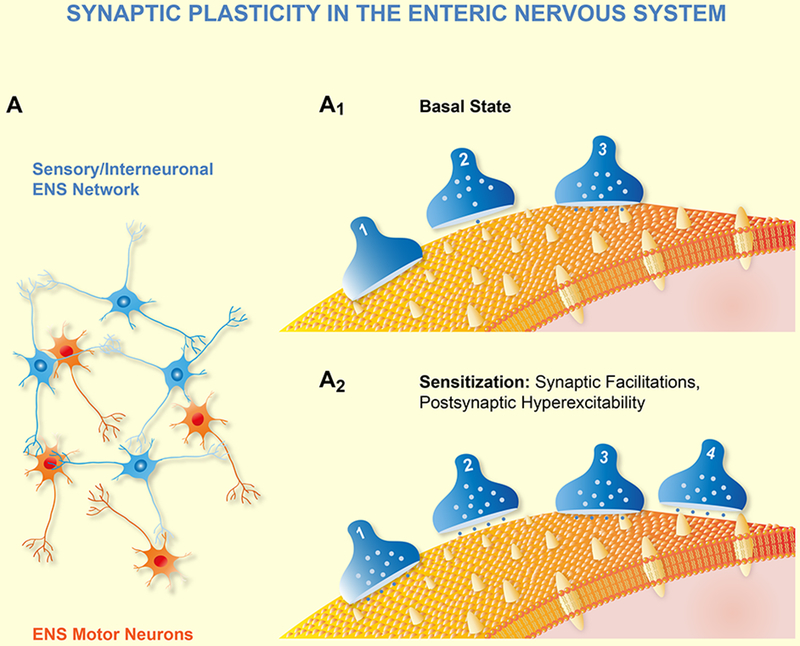
A shows a simplified diagram of an ENS circuitry consisting of a network of sensory neurons, interneurons together with motor neurons. A1 is an expanded synapse within the circuitry with 3 synapses and one postsynaptic neuron expressing various receptors (yellow). Under basal conditions synapse 2 and 3 release transmitter once activated. A2 illustrates synaptic plasticity during learning and memory. In this example only facilitation is shown which can be induced by three mechanisms. First, recruitment of previously silent synapses (presynaptic terminal 1) by transporting transmitter loaded vesicles into the terminal. Second, increase of readily available synaptic vesicles (presynaptic terminal 2); and three, by formation of new synapses (presynaptic terminal 4).
The above changes all happened in acutely inflamed tissue, although they persisted during in vitro recordings in preparations containing longitudinal muscle and myenteric plexus but lacking the inflamed mucosa. It is noteworthy, that the hyperexcitability in AH neurons and the facilitation of fast EPSPs in S neurons remained for at least 8 weeks after full remission and recovery from the TNBS induced flare.
Besides enhancement of fast EPSPs there is also evidence for inflammation-associated increase in slow EPSP induced excitability.38 Brief tetanic stimulation (20 Hz for 1 sec) induced slow EPSPs in AH neurons which lasted for about 4 min; the excitability of the neuron quickly returned back to the pre-stimulus state. Only in the inflamed intestine (TNBS-induced inflammation), there was a maintained, enhanced excitability which outlasted the stimulus for up to 3 hrs.
Conclusion.
An inflammatory insult causes neuroplasticity and motility changes in enteric neurons. The pre- and postsynaptic changes persist several weeks after the inflammation resolves and must therefore require memory storage in the ENS.
Open questions.
The plastic changes in the ENS during and after inflammation affect AH neurons which, at least in the guinea pig ENS, are one population of sensory neurons. Synaptic plasticity is also recorded in S neurons which are commonly considered (sensory) interneurons or motor neurons. Future studies need to address the question whether the S neurons which receive stronger fast EPSPs belong to the class of interneurons or motor neurons.
Furthermore, it appears that the rise time of the fast EPSPs also increased, at least this is our impression when interpreting the figures.35,37 This requires re-analysis of the data as quantification of LTP in the brain is often illustrated as an increase in fast EPSP rise time.
The synaptic facilitation which occurred in inflamed gut tissue was not associated with increased synaptic density and therefore likely due to increase in the readily releasable pool of vesicles. This has not been studied under post-inflammatory conditions where memory storage seems to be responsible for the long-term changes.
Gary Mawe put forward a provocative interpretation of the altered ENS functions in inflamed tissue.33 He suggests that neuroplasticity in acutely inflamed tissue may be a form of attention deficit disorder in the ENS. Thus, hyperexcitability together with downregulated inhibitory responses may cause motility disorders as the hyperactivity causes chaos in the circuitry and thereby prevents coordinated peristaltic activity. We need to wait for dedicated studies, which confirm or refute this fascinating hypothesis.
Learning is commonly associated with behavioral changes for the better. In many cases we do not know whether altered gut functions associated with neuroplasticity in the ENS are part of an acute pathophysiology or protective. We also lack studies on whether a putatively learned response occurs faster if the stimulus, in this case the inflammatory insult, is repeated.
3.5. Memory in the gut after an extra-intestinal stress stimulus.
Wolfgang Kunze and colleagues reported an exciting finding which at first sight seems unrelated to the topic of this review.39 Restraint stress for 1hr induced dysmotility in mouse small and large intestine. This of course is well documented in the literature. However, the fascinating aspect of this study is that the dysmotility persisted in the ex vivo isolated intestine. We suggest that SSPE and hypersensitivity in enteric sensory networks may be responsible for memorizing the stress-induced alterations. This is supported by long term activation of cholinergic myenteric neurons after water avoidance stress as demonstrated by increased c-fos expression.40
Conclusion:
An extraintestinal insult is memorised in the ENS and the behavioral changes persist even in isolated intestinal segments for hours after stress application.
Open questions:
Motility is regulated by the ENS. It therefore seems plausible to suggest that the ENS stores the memory that initiates motor disorder after a centrally acting stressor. We need studies which record stress-induced gut function as well as electrophysiology of enteric neurons. No information is available whether a stressor may induce long-term plasticity for weeks or even years. It is intriguing to speculate that chronic functional gut diseases are a consequence of such long-term memory. This also means that we need to broaden our mind when it comes to interpretation of changes in gut functions. We usually assume that neuroplasticity in the ENS and the altered motor functions represent a disorder and thereby a pathological factor. What if plasticity is a loss of memory or a newly learned protective response?
3.6. Re-programming the ENS
A high-fat diet can change how the ENS processes information.41–42 Enteric neurons in obese mice are more sensitive to acetylcholine and serotonin, both neurotransmitters of fast synaptic excitation. There is a strong correlation between body weight and the numbers of neurons responding to a nicotinic and 5-HT3 receptor agonist, or to the tissue availability of the two neurotransmitters. Importantly, those changes occur without inflammation or leaky epithelium. They occur together with faster colonic transit after 12, but not 4 weeks of a high fat diet. This suggests that the neuronal changes take time to develop. Diet induced obesity also increases gastric emptying in mice together with a stronger response to electrical stimulation of myenteric synapses. Surprisingly, obesity is rather neuroprotective in the stomach but not in the intestine. The neuronal loss, which starts in mice shortly after birth is prevented in mice receiving a high fat diet by leptin induced increase in glia-derived neurotrophic factor (GDNF).
Conclusion:
The gut adapts to diets even without noticeable metabolic disorders and in the absence of immune imbalance.
Open questions:
Are the effects seen on neuronal survival, synaptic transmission, intestinal transit and neuronal excitability learning by reprogramming of the ENS? Are these effects reversible? How does the gut react if it is exposed a second time to a high fat diet after recovery from the consequences of an obesogenic diet? Does it memorize the previous “experience” and handle excess calories differently?
3.7. Learning in the gut: consequences and shift in paradigms
Under physiological conditions, adaptations to different digestive functions need to be rather fast. It therefore seems unlikely that the ENS utilizes LTP lasting for days or even longer for such purposes. The necessity for long-term storage of hard-wired programs such as motor patterns initiated during digestive and interdigestive periods remains untouched thereby. However, these are already present at time of birth and may experience some fine-tuning shortly after but also suffer from loss of function due to a pathological insult or with age.43 Such programs are like pre-installed Apps on a smart phone – a comparison inspired by Jackie D. Wood. To stay with this descriptive image, altered connectivity, responsiveness and excitability in the ENS are like an update of a particular App, download of new ones or deletion of unwanted or not anymore useful ones.
If the ENS is able to learn and to memorize it seems plausible that it may also forget. The simplest mechanism behind memory deficit is loss of neurons. The ENS clearly experiences a loss of myenteric neurons with age, i.e. these neurons that control muscle reflexes die with progressive age (Figure 3).44 This may explain the impaired motility in elderly. In contrast, there is no significant loss of neurons in the submucosal plexus in the aged gut, which may explain why nerve triggered epithelial secretion is comparable between patients of different age.45
Figure 3.
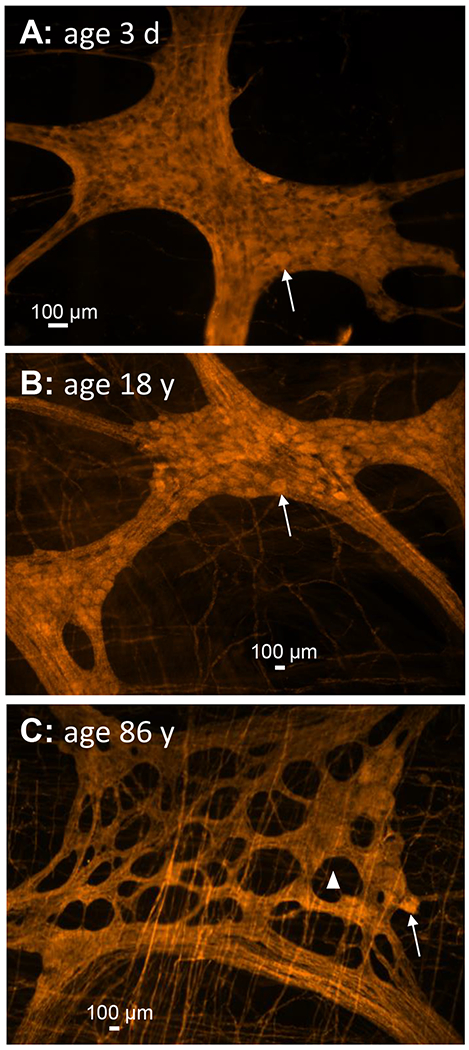
Representative pictures of myenteric ganglia from patients with different age. The ENS is stained with the pan-neuronal marker PGP9.5 labelled with Cy3. A shows myenteric plexus ganglion densely packed with neurons in a 3 day old baby. B is the same staining in an 18 year old patient still showing intact ganglia. In C the ganglion of an 86 year old patient showing large holes in the ganglion where there used to be neurons. This is associated with a dramatic loss of neurons. The arrows point to one neuronal cell body. The triangle in C is marking one of the numerous holes in the ganglion. Note that there is no hole or notable degeneration in the ganglia of the younger patient.
3.8. Molecular basis of learning in the ENS: similarities between ENS and established learning models.
Some of the electrophysiological features and behavioral changes associated with learning in invertebrates or mammals are present in the gut and reflected by altered ENS neurobiology. Some, however, are difficult or may be even impossible to confirm in the gut. We must realize that, due to the specialized reflex behavior, memory and learning in the ENS may follow others principles than in the brain. For example, behavioral changes indicative for learning are easy to study in invertebrates or mammals as they may be observed over days and weeks. Although the gut survives in isolation for several days and performs surprisingly well with ongoing nerve-mediated reflex activity, this is not true for all layers of the gut. For example, currently it is still not possible to keep the mucosa alive and functionally operative for more than one day. In addition, all extrinsic nerves will degenerate well before the ENS as their terminals are separated from the cell bodies. Last but not least, while learning in animal models often results in reflexes to control different organs, reflexes in the gut are directed to control different functions of a particular region.
It will be a challenge to replicate the cellular and molecular features of learning in the central nervous system within the ENS. Unlike the brain, the ENS is not that strictly separated from exogenous inputs. The ENS is heavily influenced by mediators released from immune cells, enteroendocrine cells, adipocytes as well as from blood-borne factors or components of the gut lumen. In this respect, the brain lives more in a protected comfort zone and primarily deals with inputs from other nerves. On the other hand, the brain is substantially more complex regarding structure, wiring and functions. It may therefore be naive to believe that whatever happens during learning and memory formation in the brain directly translates to the ENS.
In principle, synaptogenesis also occurs in the ENS, but morphometric changes in synaptic density or new formation of synapses have not been studied in relation to learning in the gut and not even as a consequence of electrical stimulation. However, there is evidence that structural changes of synapses may also happen in the ENS because axonal sprouting, formation of new synapses and expression of proteins involved in synaptic transmission has been reported. At least in cultured enteric neurons synaptogenesis has been observed as GDNF increases expression of the synaptic vesicle markers SNAP-25, synaptobrevin as well as synaptophysin and enhances the numbers of synaptic vesicles.46–48 Interestingly, in patients with diverticular disease SNAP-25 expression is decreased.46 Brain derived neurotrophic factor (BDNF), although not directly stimulating myenteric neurons, increased circuitry activity and synaptic vesicle clusters.49 This enhanced synaptic transmission is also reflected by FM1–43-labelled vesicle destaining in enteric terminals during burst-type electrical stimulation of synaptic release, very similar to what is observed in the brain after BDNF.50 Mechanical or functional stenosis of intestinal segment causes dendritic arborisation.51 Enteric glia profoundly affect formation of synapses.51 Intestinal biopsies of patients with irritable bowel syndrome reveal increased neuronal density and enhanced expression of nerve growth factor along with its receptor tyrosine kinase receptor A (NTRK1).53 Most importantly, this study also revealed enteric neuritogenesis together with sprouting of nerve processes, hence increased synaptic density, after cultured enteric neruons were exposed to mucosal biopsy supernatants from these patients. TNBS-induced inflammation caused increased nerve fiber density in the mucosa, which was due to neuronal sprouting as the number of nerve cell bodies did not increase.54 Last but not least. nutrients in the intestinal lumen induced long-term changes in neurotransmitter expression, excitability, and neuronal survival.55
5-HT, which is important for learning in Aplysia, contributes to neurite outgrowth in the ENS, through 5-HT4 receptor activation.56 At the same time 5-HT4 agonists enhance synaptic transmission by increasing the amplitude of cholinergic fast EPSPs and promotes cAMP response element-binding protein (CREB) phosphorylation. The switch from short to long-term memory requires the synthesis of new proteins.57 There are numerous factors involved in this process such as protein kinases or the transcription factor CREB-1 which then acts on genes to activate the synthesis of proteins and stimulate formation of new synapses.
4. Learning in the gut: Clinical examples and relevance
“A good reliable set of bowels is worth more to a man than any quantity of brains” (attributed to the American writer Josh Billings) seems a good choice to start this chapter as it lifts the gut to a level usually reserved for the brain. Wouldńt it be intriguing if some diseases, at least in part, would be caused by loss of memory or learning something that was originally meant to be protective but turns out to also contribute to a serious disorder? Some of the readers may by now say: “That’s stretching it too far”. We openly admit that a link between learning and clinical Neurogastroenterology is highly speculative. However, we believe that we have at least to admit the thought by looking at functional changes in patients with irritable bowel syndrome (IBS) or functional dyspepsia (FD). These diseases go along with sensorimotor disorders of the gut leading to abnormal transit, altered reflex activity and abdominal pain.58,59 Although this is a chronic disease the symptoms come and go. The reasons are not fully understood but there is a consensus that there are numerous causes that can be subsumed under disorders of the little brain in the gut, the big brain in the head or the communication routes between the two. With a prevalence of approximately 10 – 15%, it is the most frequent reason for visits to the doctors in western countries.58
Synaptic plasticity occurs in the human gastrointestinal tract under pathological conditions without saying that this is proof of learning. Long-term morphometric and biomechanical changes for the remaining intestine have been described following extensive small bowel resection in rats.60 The density of mucosal innervation increases in patients with abdominal pain.61 A similar increase in addition to enhanced expression of nerve growth factor and its receptor NTRK1 (neurotrophic receptor tyrosine kinase 1) occurs in IBS.54 This is likely due to signaling between ENS and epithelial or subepithelial cells, because supernatants released from IBS mucosal biopsies induce neurite sprouting in ENS cell cultures.54 Although the contribution of these changes in IBS pathophysiology remains unknown, they may be the results of altered memory formation in the ENS. This would require demonstration of long-term changes in the ENS of IBS patients. Indeed, this is the case as both ENS sensitization or desensitization occur involving histamine - TRPV1 or protease - PAR1 interactions, respectively.62,63 This extends to FD patients in which the ENS shows decreased responses to synaptic activation.64
An important trigger of IBS is an infectious gastroenteritis which increases the risk of developing the so called postinfectious IBS (PI-IBS) even years after the infection resolved. It is tempting to discuss this particular time line and the late-developing symptoms in relation to learning and look at the initial insult as a kind of priming in the gut, which thereby may involve implicit learning. This would require alterations that persists for a long time after the infection. Indeed this occurs in nociceptors with terminal endings in the gut wall.65 Whether this is a learned protective processes or an unlearned existing process is open to discussion. Interestingly, the symptoms associated with PI-IBS disappear within 5 years, suggesting that if there is altered memory, the infection-triggered learned behavior is extinguished, similar to the Pavlovian conditioning where CS and CR uncouple with time. Seventy-two of the 669 participants who experienced episodes of diarrhea prior to or during their journey developed new-onset IBS after 7 months.66 This corresponds to a rate of post-infectious IBS of 10.7% (95 % CI=8.4 vs. 13.4 %). In contrast, only 13 of 514 participants who did not experience episodes of diarrhea prior to or during their journey developed new-onset IBS after 7 months, corresponding to a rate of 2.5% (95%CI=1.3 to 4.0%). However, it would be interesting to know the rate of PI-IBS in those patients who developed diarrhea before and during travel. Unfortunately, this was not looked into. Additionally, it was also missed to check whether patients that experienced an acute GI infection without post-infectious IBS symptoms at one point in time (and we all do) may have done so after a second or third infection much later, and whether these additional episodes were more severe or not. This would be indicative of a stored response pattern based on long-term neural plasticity. In this respect it may also be asked whether the very early-life experience of infant colic 67 when the immune system and the ESN first learn to handle foreign antigens, predisposes the gut to respond stronger to gastrointestinal infections later in life, and finally determines the occurrence of IBS.
Conclusion:
Some gut disorders may be the result of altered memory in the ENS and along the brain-gut axis.
Open questions:
Do the late-developing symptoms involve altered memory? If this is the case, one would expect a much faster onset of IBS symptoms after a second infection. The answer may be out there but requires re-analysis of data. Diarrhea 4 months prior to a long distance travel or travelers’ diarrhea during the journey significantly predicts IBS development post-travel.66 As expected the study reports an increased risk to develop PI-IBS in both populations. The crucial question is whether the risk further increases in the population which has infectious diarrhea before and during the travel and whether the onset of IBS symptoms occurs faster.
Post-infectious IBS and FD are frequently associated with immune imbalance resembling inflammatory processes.58,59 It remains to be shown whether the post-inflammatory plasticity observed in animal models (see above) is involved in the gastrointestinal symptoms of post-infectious gut diseases.
The fact that only a small proportion of patients develops PI-IBS relates to particular features of the enteric immune system.58 It is tempting to speculate that robust memory circuits may in addition help to avoid neuroplasticity favoring sensorimotor disorders.
5. Concluding Remarks
This review discusses the provocative hypothesis that the gut is able to learn behavior. Furthermore, we propose the idea that the ENS truly acts like a little brain in the gut. Synaptic plasticity and altered neuronal sensitivity as well as structural changes in the ENS support the notion for implicit learning (for summary see Tabel 1). While those simple ways of learning may occur, the gut does not seem able to perform complex learning. However, the possibility for memory formation and alterations open new ways to interpret altered gut behavior under pathological conditions and may change our approach to gut disease. We require dedicated studies to distinguish true learning and memory from proxies associated with them. Moreover, studies must investigate how the gut profits from learning and memory and how much of a learned behavior is used for the better. Despite some fascinating aspects, there is a need for specially designed studies using those protocols and paradigms that are well-established in other learning models – in keeping with the Star Trek motto: “…to boldly go where no man has gone before”.
Table 1.
Indications of learned gut behavior and the neurophysiological proxis (see text for detailed discussion)
| Function | Stimulus | Induced, “learned” gut behavior | Putative neural correlate |
|---|---|---|---|
| Motility | Muscle response induced by: • Repetitive distension (<10 sec) • Repetitive distortion or chemical stimulation of mucosa • Muscle distension after mucosal distortion (≥2 min) • Distension of adjacent regions • Conditoned distension (repetitive stretch of 1.5g→3g→1.5g) • Repetitive gastric step-wise distension (5-20 mmHg) • Stress • High fat diet |
• Attenuated peristaltic reflex • Attenuated peristaltic reflex • Enhanced peristaltic reflex (cross-sensitization) • Enhanced relaxation • Enhanced intestinal contraction after conditioning • Enhanced adaptive relaxation • Increased colonic motility • Increased gastric emptying |
• Synaptic depression in sensory circuits • Synaptic depression in sensory circuits • Synaptic facilitation in sensory circuits • ? • Sustained postsynaptic excitation (LTP), hyperexcitability of sensory neurons • Synaptic facilitation in sensorimotor circuits • LTP and hyperexcitability of sensory neurons • Synaptic facilitation |
| ENS activity | • Acute inflammatory insult • Post-inflammatory conditions |
• Decreased propulsive motility “Attention deficit disorder” • Decreased colonic propulsion “Attention deficit disorder” |
• Synaptic facilitation, hyperexcitability of sensory neurons, • Hyperexcitability of sensory neurons and synaptic facilitation remains |
| Motility disorders | • Postinfectious irritable bowel syndrome • Postinfectious functional dyspepsia |
• Dysmotility • Dysmotility |
• Postsynaptic sensitization and desensitization • Hyporesponsiveness to synaptic activation |
Acknowledgements:
M.S. wishes to thank Deutsche Forschungsgemeinschaft for continuous support and US National Institute of Health SPARC 1OT2OD024899-01 for ongoing support. P.E. received the German-Norwegian Günther Jantschek Research Stipend.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest
We confirm that the material submitted is conform with Good Publishing Practice in Physiology
References:
- 1.Gershon M The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine. 1999; HarperCollins Publishers, New York, NY, USA. [Google Scholar]
- 2.Furness JB. The Enteric Nervous System. 2006, Blackwell Publishing Ltd, London, UK [Google Scholar]
- 3.Enck P, Frieling T, Schemann M. Darm an Hirn! Der geheime Dialog unserer beiden Nervensysteme und sein Einfluss auf unser Leben. 2017, Verlag Herder GmbH, Freiburg, Germany. [Google Scholar]
- 4.Grundy D, Schemann M, Wood J. A tale of two brains, one little and one big. Neurogastroenterol Motil. 2000;12:105–111. [DOI] [PubMed] [Google Scholar]
- 5.Gagliano M, Abramson CI, Depczynski M. Plants learn and remember: lets get used to it. Oecologia. 2018. January;186(1):29–31. [DOI] [PubMed] [Google Scholar]
- 6.Gagliano M, Vyazovskiy VV, Borbély AA, Grimonprez M, Depczynski M. Learning by Association in Plants. Sci Rep. 2016;6:38427. doi: 10.1038/srep38427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagliano M, Renton M, Depczynski M, Mancuso S. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia. 2014. May;175(1):63–72. [DOI] [PubMed] [Google Scholar]
- 8.Reid CR, MacDonald H, Mann RP, et al. Decision-making without a brain: how an amoeboid organism solves the two-armed bandit. J R Soc Interface. 2016;13: pii: 20160030. doi: 10.1098/rsif.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid CR, Latty T, Dussutour A, Beekman M. Slime mold uses an externalized spatial “memory” to navigate in complex environments. Proc Natl Acad Sci U S A. 2012;109:17490–17494. doi: 10.1073/pnas.1215037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu H, Koizumi O, Fujisawa T. Three digestive movements in Hydra regulated by the diffuse nerve net in the body column. J Comp Physiol. 2004;190:623–630. [DOI] [PubMed] [Google Scholar]
- 11.Furness JB, Stebbing MJ. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil. 2018;30: doi: 10.1111/nmo.13234. [DOI] [PubMed] [Google Scholar]
- 12.Hebb DO (1949). The Organization of Behavior: A Neuropsychological Theory. New York: Wiley and Sons. [Google Scholar]
- 13.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. [DOI] [PubMed] [Google Scholar]
- 14.Pinsker H, Kupfermann I, Castellucci V, Kandel ER. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 1970;167:1740–1742 [DOI] [PubMed] [Google Scholar]
- 15.Reber PJ. The neural basis of implicit learning and memory: a review of neuropsychological and neuroimaging research. Neuropsychologia. 2013;51:2026–42. doi: 10.1016/j.neuropsychologia.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Roosen L1, Boesmans W, Dondeyne M, et al. Specific hunger- and satiety-induced tuning of guinea pig enteric nerve activity. J Physiol. 2012;590:4321–33. doi: 10.1113/jphysiol.2012.231134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockhorst U, Enck P, Klosterhalfen S. Role of classical conditioning in learning gastrointestinal symptoms. World J Gastroenterol. 2007;13:3430–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith TK, Bornsteim JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea pig ileum, J Auton Nerv Syst. 1991;34:69–76. [DOI] [PubMed] [Google Scholar]
- 20.Yuan SY, Furness JB, Bornstein JC, Smith TK. Mucosal distortion by compression elicits polarized reflexes and enhances responses of the circular muscle to distension in the small intestine. J Auton Nerv Syst. 1991;35:219–226 [DOI] [PubMed] [Google Scholar]
- 21.Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci. 1992;12:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furness JB, Kumano K, Larsson H, et al. Sensitization of enteric reflexes in the rat colon in vitro. Auton Neurosci. 2002;97:19–25. [DOI] [PubMed] [Google Scholar]
- 23.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. [DOI] [PubMed] [Google Scholar]
- 24.Römer M, Painsipp E, Schwetz I, Holzer P. Facilitation of gastric compliance and cardiovascular reaction by repeated isobaric distension of the rat stomach. Neurogastroenterol Motil. 2005;17:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schemann M, Wood JD. Electrical behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989;417:501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serra J, Azpiroz F, Malagelada JR. Perception and reflex responses to intestinal distention in humans are modified by simultaneous or previous stimulation. Gastroenterology. 1995;109:1742–1749 [DOI] [PubMed] [Google Scholar]
- 27.Trendelenburg P Physiologische und pharmakologische Versuche über die Dünndarmperistaltik. Arch. Exp. Pathol. Pharmakol. 1917;81:55–129 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TV, Stebbing MJ, Clerc N, et al. Evidence for protein kinase involvement in long-term postsynaptic excitation of intrinsic primary afferent neurons in the intestine. Auton Neurosci. 2004;115:1–6. [DOI] [PubMed] [Google Scholar]
- 29.Alex G, Clerc N, Kunze WA, Furness JB. Responses of myenteric S neurones to low frequency stimulation of their synaptic inputs. Neuroscience. 2002;110:361–373. [DOI] [PubMed] [Google Scholar]
- 30.Furness JB, Clerc N, Kunze WA. Memory in the enteric nervous system. Gut. 2000;47:iv60–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerc N, Furness JB, Kunze WA, Thomas EA, Bertrand PP. Long-term effects of synaptic activation at low frequency on excitability of myenteric AH neurons. Neuroscience. 1999;90:279–289. [DOI] [PubMed] [Google Scholar]
- 32.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949–955. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krauter EM, Strong DS, Brooks EM, et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007;19:990–1000. [DOI] [PubMed] [Google Scholar]
- 35.Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol. 2007;581:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurgali K, Nguyen TV, Thacker M, et al. Slow synaptic transmission in myenteric AH neurons from the inflamed guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2009;297:G582–93. doi: 10.1152/ajpgi.00026.2009 [DOI] [PubMed] [Google Scholar]
- 39.West C, Wu RY, Wong A, et al. Lactobacillus rhamnosus strain JB-1 reverses restraint stress-induced gut dysmotility. Neurogastroenterol Motil. 2017;29. doi: 10.1111/nmo.12903. [DOI] [PubMed] [Google Scholar]
- 40.Miampamba M, Million M, Yuan PQ, Larauche M, Taché Y. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport. 2007;18:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baudry C1, Reichardt F, Marchix J, et al. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichardt F, Baudry C, Gruber L, et al. Properties of myenteric neurones and mucosal functions in the distal colon of diet-induced obese mice. J Physiol. 2013;591:5125–5139. doi: 10.1113/jphysiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao MM, Bornstein JC, Vanden Berghe P, et al. The emergence of neural activity and its role in the development of the enteric nervous system. Dev Biol. 2013;382:365–74. doi: 10.1016/j.ydbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol & Motil 2009;21, e746–e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krueger D, Michel K, Zeller F, et al. Neural influences on human intestinal epithelium in vitro. J Physiol. 2016. ;594:357–372. doi: 10.1113/JP271493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrenschee M, Böttner M, Harde J, et al. SNAP-25 is abundantly expressed in enteric neuronal networks and upregulated by the neurotrophic factor GDNF. Histochem Cell Biol. 2015;143:611–623. doi: 10.1007/s00418-015-1310-x [DOI] [PubMed] [Google Scholar]
- 47.Böttner M, Harde J, Barrenschee M, et al. GDNF induces synaptic vesicle markers in enteric neurons. Neurosci Res. 2013;77:128–136. [DOI] [PubMed] [Google Scholar]
- 48.Zeng F, Watson RP, Nash MS. Glial cell-derived neurotrophic factor enhances synaptic communication and 5-hydroxytryptamine 3a receptor expression in enteric neurons. Gastroenterology. 2010;138:1491–1501. doi: 10.1053/j.gastro.2009.11.048 [DOI] [PubMed] [Google Scholar]
- 49.Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berghe P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2008;57:314–322 [DOI] [PubMed] [Google Scholar]
- 50.Mayford M, Siegelbaum SA, Kandel ER. Synapses and Memory Storage. Cold Spring Harb Perspect Biol. 2012;4: a005751. doi: 10.1101/cshperspect.a005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brehmer A1, Frieser M, Graf M, et al. Dendritic hypertrophy of Stach type VI neurons within experimentally altered ileum of pigs. Auton Neurosci. 2001;89:31–37. [DOI] [PubMed] [Google Scholar]
- 52.Le Berre-Scoul C, Chevalier J, Oleynikova E, et al. A novel enteric neuron-glia coculture system reveals the role of glia in neuronal development. J Physiol. 2017;595:583–598. doi: 10.1113/JP271989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e4. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 54.Nurgali K1, Qu Z, Hunne B, et al. Morphological and functional changes in guinea-pig neurons projecting to the ileal mucosa at early stages after inflammatory damage. J Physiol. 2011;589:325–339. doi: 10.1113/jphysiol.2010.197707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neunlist M, Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J Physiol. 2014;592:2959–2965. doi: 10.1113/jphysiol.2014.272948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 60.Dou Y, Lu X, Zhao J, Gregersen H. Morphometric and biomechanical remodelling in the intestine after small bowel resection in the rat. Neurogastroenterol Motil. 2002. February;14(1):43–53 [DOI] [PubMed] [Google Scholar]
- 61.Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008;57:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wouters MM, Balemans D, Van Wanrooy S et al. , Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150:875–887. e9. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 63.Ostertag D, Buhner S, Michel K, et al. Reduced Responses of Submucous Neurons from Irritable Bowel Syndrome Patients to a Cocktail Containing Histamine, Serotonin, TNFα, and Tryptase (IBS-Cocktail). Front Neurosci. 2015;9:465. doi: 10.3389/fnins.2015.00465. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cirillo C, Bessissow T, Desmet AS, et al. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol. 2015;110:1205–1215. doi: 10.1038/ajg.2015.158 [DOI] [PubMed] [Google Scholar]
- 65.Balemans D, Mondelaers SU, Cibert-Goton V, et al. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Sci Rep. 2017;7:13606. doi: 10.1038/s41598-017-12618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Löwe B, Lohse A, Andresen V, et al. The Development of Irritable Bowel Syndrome: A Prospective Community-Based Cohort Study. Am J Gastroenterol. 2016;111:1320–1329. doi: 10.1038/ajg.2016.255. [DOI] [PubMed] [Google Scholar]
- 67.Camilleri M, Park SY, Scarpato E, Staiano A. Exploring hypotheses and rationale for causes of infantile colic. Neurogastroenterol Motil. 2017;29. doi: 10.1111/nmo.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]


