Abstract
Cirrhosis is morbid and increasingly prevalent – yet the U.S. healthcare system lacks enough physicians and specialists to adequately manage patients with cirrhosis. While advanced practice providers (APPs) can expand access to cirrhosis-related care, their impact on quality of care remains unknown. We sought to determine the effect on care-quality and outcomes for patients managed by APP using a retrospective analysis of a nationally representative American commercial claims database (Optum) which included 389,257 unique adults with cirrhosis. We evaluated a complication of process measures - rates of hepatocellular carcinoma (HCC) screening, endoscopic varices screening, and use of rifaximin after hospitalization for hepatic encephalopathy (HE) – and outcomes (30-day readmissions and survival). Compared to patients without APP-care, patients with APP-care had higher rates of HCC screening (adjusted odds ratio (OR) 1.23 95%CI[1.19,1.27]), varices screening (OR 1.20 [1.13,1.27]), use of rifaximin after a discharge for HE (OR 2.09[1.80,2.43]), and reduced risk of 30-day readmission (OR 0.68[0.66,0.70]). Gastroenterology/hepatology consultation was also associated with improved quality metric performance compared to primary care, however shared visits between gastroenterologist/hepatologists and APP were associated with the best performance and lower 30-day readmissions compared to subspecialty consultation without APP (OR 0.91 [0.87, 0.95]. Multivariate analysis adjusting for comorbidities, liver disease severity and other factors including gastroenterology/hepatology consultation showed that patients seen by APPs were more likely to receive consistent HCC and varices screening over time, less likely to experience 30-day readmissions, and had lower mortality (adjusted hazard ratio 0.57 95%CI[0.55,0.60]).
Conclusion:
APPs, particularly when working with gastroenterologists/hepatologists, are associated with improved quality of care and outcomes for patients with cirrhosis.
Keywords: Liver disease, Hepatocellular Carcinoma, Readmissions, Hepatic Encephalopathy, Quality Improvement
Background
Cirrhosis is common, affecting up to 5 million Americans, and its prevalence is increasing.(1–4) It is characterized by poor quality of life and life-limiting complications such as variceal hemorrhage, ascites, hepatic encephalopathy (HE) and hepatocellular carcinoma (HCC).(5–7) There are standard measures that can be undertaken to forestall many of these complications and improve patient outcomes. In 2010, Kanwal and colleagues translated many key standards for the care of patients with liver disease into measurable quality indicators.(8) These indicators include imaging-based screening for HCC,(9) endoscopic screening for varices,(10) immunization against viral hepatitis, and optimal therapy for HE.(11) Both the American Association for the Study of Liver Diseases and advanced liver disease workgroups in the Veterans Affairs (VA) have adopted these measures into practice guidelines. Unfortunately, substantial gaps in implementation persist.(12)
A major barrier to optimal care for cirrhosis is limited access to subspecialty care. Most patients with cirrhosis are not co-managed by a gastroenterology or hepatology trained specialist.(13, 14) Previous studies have demonstrated that advanced practice providers (APPs) provide care that is equivalent in quality to medical doctors (MDs) in both primary care and specialty care when their practice is focused on one condition.(15–18) However, data are lacking regarding quality metric performance for APP in patients with cirrhosis. Herein, we examine a large commercial claims database to assess the quality of APP care and the impact on outcomes in patients with cirrhosis with or without subspecialty consultation.
Methods
We analyzed the 2001–2015 Optum Clinformatics™ DataMart (Eden Prairie, MN, USA) which includes nationally representative information for 77,883,541 unique patients covered with private insurance, including Medicare Advantage. Enrollees are followed longitudinally across inpatient and outpatient settings. Data for the present study were limited to adults with ≥2 cirrhosis claims. All coding definitions are provided in the Supplementary Table 1.(19–21) This study was exempted from review by the University of Michigan Institutional Review Board.
Quality indicators and Outcomes
We chose 4 quality indicators that were easily abstracted from administrative data: screening for HCC, screening for varices, and prescription of rifaximin following a hospitalization for HE. We then examined two clinical outcomes: 30-day readmissions and mortality. The sources and definitions of denominators and numerators for quality metrics are detailed in Supplementary Table 2. Finally, we examined healthcare expenditures by combining the charges associated with all procedures and visits (CPT and HCPCS codes) incurred by each patient per person-year. We excluded non-liver surgical procedures, non-liver oncologic therapies, and cardiac or electrophysiological procedures.
Exposures
Provider type was our principal exposure variable. Specifically, we evaluated the impact of APP visits (nurse practitioners (NP) or physician’s assistants (PA)). We included APP visits that occurred in either primary care or gastroenterology/hepatology clinics, some of which occurred as ‘shared visits’ with gastroenterology/hepatology specialists on the same day of service. We collected additional exposure variables for complete description of the cohort and risk-adjustment. These included age, sex, race, education, Charlson Comorbidity Index (modified to exclude liver disease),(22) etiology of liver disease, complications of cirrhosis, number of outpatient visits, and evaluation by a provider associated with a transplant-facility. Liver disease severity was captured using a combination of diagnosis and procedure codes (cirrhosis complications such as HE and procedures such as paracentesis and portosystemic shunt placement). Transplant facility was defined as any center which performed a liver transplant within the same year of service
Statistical analyses
To analyze outcomes, we employed 4 strategies. First, we performed logistic regression analyses to determine the relative impact of APP evaluation on practice metrics, limiting the cohort for evaluation to those with at least 12 months’ follow-up (Figure 1). To account for the effect of gastroenterology/hepatology consultation, we evaluated multiple scenarios:
Scenario 1: Patients with any APP visit vs no APP visits. Analyses adjusted for gastroenterology/hepatology consultation if it occurred.
Scenario 2: Patients with neither APP nor gastroenterology/hepatology consultation vs gastroenterology/hepatology consultation without any APP visits.
Scenario 3: Patients with APP and gastroenterology/hepatology consultation vs gastroenterology/hepatology consultation but no APP
Scenario 4: Patients with Gastroenterology/hepatology consultation but no APP vs neither APP nor gastroenterology/hepatology consultation
Figure 1. Association of Provider Type with Process Measures and 30-day Readmissions.
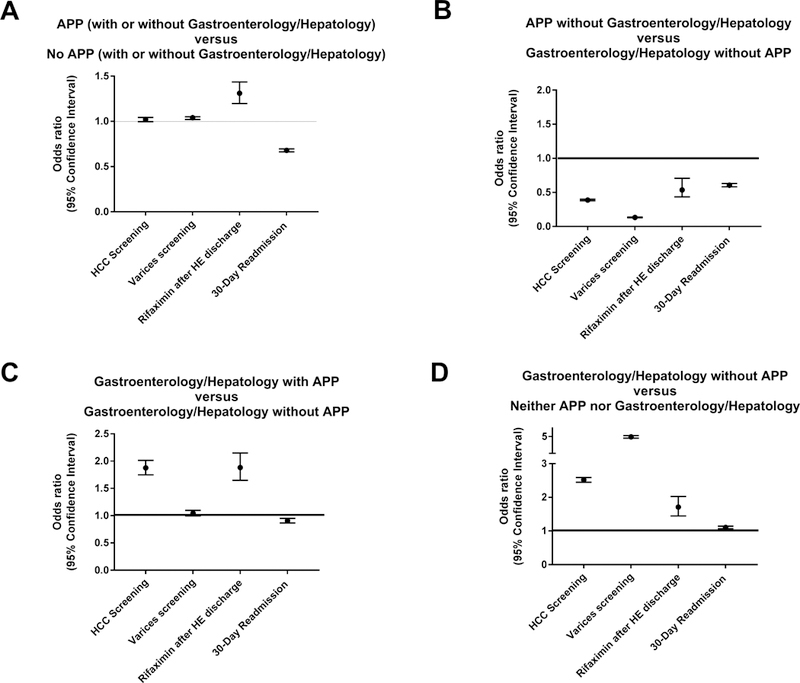
Adjusted odds ratios for multiple metrics are presented to show the quality of care provided by various healthcare providers. Raw data including unadjusted estimates are presented in the online supplement (Supplementary Tables 3a–3d).
A: Compared to patients who were never seen by an advanced practice provider (APP), those who were seen by an APP, regardless of presence or absence of gastroenterologist/hepatologist consultation, were marginally more likely to receive screening for hepatocellular carcinoma (HCC) and varices, more likely to receive rifaximin after discharge for hepatic encephalopathy (HE), and less likely to experience a 30-day readmission.
B: Patients seen by APP without gastroenterologists/hepatologists were less likely to receive care that satisfies the practice metrics but also less likely to experience a 30-day readmission compared to patients seen by gastroenterologists/hepatologists without APPs.
C: Patients seen by gastroenterologists/hepatologists with assistance from APPs received better care and had less readmissions compared to those seen by gastroenterologists/hepatologists alone except for varices screening where there was no difference.
D: Patients seen by gastroenterologists/hepatologists alone received better care for all measures and had less readmissions compared to those not seen by gastroenterologists/hepatologists or APPs.
Second, we analyzed two ‘pre-post’ cohorts to assess the impact of APP involvement (Table 2). In the first cohort, we included all patients with 6 months of coverage before and after a first visit with an APP. For the outcome of endoscopic screening we excluded patients who had an endoscopy examination prior to the 1-year period analyzed given the recommended interval for screening (Supplementary Table 2). For this cohort, we adjusted for the impact of gastroenterology/hepatology consultation as a time-varying covariate in the event that a patient received such a consultation. In the second pre-post cohort, we included all patients with 6 months of coverage before and after a shared-visit with a gastroenterologist/hepatologist MD and an APP (their first visit with either provider type in the database).
Table 2:
Pre-Post ‘Experiments’ – Relative Practice Metric Performance After an Advanced Practice Provider (APP) Visit that is (or is not) shared with a Gastroenterologist/Hepatologist
| Screening for HCC | Endoscopy screening for varices | On rifaximin after discharge for HE | ||
|---|---|---|---|---|
| Before and after an APP visit | Denominator | 97013 | 84138 | 5082 |
| Metric Satisfied Prior to APP visit | 26.1% | 7.2% | 7.9% | |
| Metric Satisfied After APP visit | 30.1% | 8.9% | 14.8% | |
| Odds ratio (OR) (95% CI) | 1.27 (1.19, 1.24) | 1.19 (1.15, 1.24) | 2.02 (1.78, 2.30) | |
| Adjusted OR (95% CI) | 1.23 (1.19, 1.27) | 1.20 (1.13, 1.27) | 2.09 (1.80, 2.43) | |
| Before and after visit with both GI/Hepatology MD and APP | Denominator | 4830 | 3593 | 572 |
| Metric Satisfied Prior to GI/Hep and APP visit | 44.8% | 12.4% | 9.3% | |
| Metric Satisfied After GI/Hep and APP visit | 55.6% | 27.2% | 27.4% | |
| OR (95% CI) | 1.54 (1.42, 1.67) | 2.66 (2.35, 3.01) | 3.70 (2.64, 5.19) | |
| Adjusted OR (95% CI) | 1.58 (1.45, 1.73) | 2.84 (2.48, 3.24) | 4.08 (2.34, 7.13) |
Patients in both cohorts were seen by primary care MD’s prior to APP involvement; in the first they could have seen a gastroenterologist/hepatologist but in the second, gastroenterology/hepatology consultation first occurred in a shared visit with an advanced practice provider (APP). Patients were censored from the evaluation of HCC screening at the time of an HCC diagnosis. Patients were excluded from the denominator for endoscopic screening if they experienced variceal bleeding at any point or if they had an endoscopy within 1 year prior to the period evaluated. All adjustments included age, sex, race, education, Charlson Comorbidity Index, etiology of liver disease, complications of cirrhosis, number of outpatient visits, and evaluation by gastroenterologists or hepatologists. HCC = hepatocellular carcinoma. GI=gastroenterology
Third, we determined the incidence rate (per person-year) of each practice metric. Practice metric events were compared between groups of patients with and without APP involvement using incidence rate ratios derived from negative binomial regressions (Table 3).
Table 3:
Cumulative Adherence to Practice Metrics Associated with Visits to Advanced Practice Providers (APP)
| Screening for HCC (screens per person year) | Screening for varices (endoscopy per person year) | 30-day readmissions per discharge | |
|---|---|---|---|
| Number of events in patients seen by APP per person year, median (IQR) | 0.75 (0.40 – 1.46) | 0.35 (0.21 – 0.59) | 0 (0–0.46) |
| Number of events in patients never seen by APP per person year, median (IQR) | 0.45 (0.22 – 0.89) | 0.24 (0.13 – 0.44) | 0 (0.0 – 0.5) |
| Incidence rate ratio (95% Confidence Interval) | 1.73 [1.72, 1.74] | 1.48 [1.47, 1.49] | 0.90 [0.89, 0.91] |
| Adjusted incidence rate ratio (95% Confidence Interval) | 1.61 [1.60, 1.63] | 1.51 [1.49, 1.54] | 0.88 [0.87, 0.90] |
The incidence rate ratios present a measure of the relative rate of events between patients managed (at least in part) by APPs accounting for the time under evaluation. The adjusted measures account for demographics, clinical factors (see Table 1), consultation by gastroenterologists/hepatologists, number of outpatient visits and interaction terms for APP and gastroenterology/hepatology. Patients were censored from all analyses at the time of death or loss-of-coverage. Patients were censored from hepatocellular carcinoma (HCC) screening metrics at the time of HCC diagnosis and from screening varices metrics at the time of variceal hemorrhage.
Fourth, we used a time-dependent Cox proportional hazards model to determine the impact of APP evaluation on survival using time-varying covariates to adjust the hazard ratio (HR).(Table 4) Patients were censored if they received a liver transplant or were lost-to-follow-up due to loss of insurance coverage. The reasons for loss-of-coverage are not known but generally include changes in employment (and thus insurer) or transition to a public insurer (Medicare/Medicaid). This dataset is linked to the social security death index and thus mortality data was complete in the dataset’s ‘death view’. For those who died within 3 months after loss of coverage, death rather than loss-of-coverage was considered the outcome. Gastroenterologists and hepatologists were considered separately because hepatology consultation may reflect management at a transplant facility. In order to adjust as fully as possible for severity of illness, we added co-variates for common infections in patients with cirrhosis which are linked with adverse outcomes.(23)(Supplementary Table 1) We addressed biases in multiple ways. First, we employed Fine-Gray modeling to account for the competing risk of liver transplantation.(24) Second, we addressed the risk of residual immortal time bias despite the use of time-dependent Cox modelling using a Landmark analysis,(25) setting cohort entry for those seen by APP or not as the time of cirrhosis diagnosis. Third, to further address confounding by indication, we performed a 1:1 propensity score matching for exposure to APP-care.
Table 4:
Association of Provider Type with Mortality
| Variable | Univariate Hazard Ratio (95% Confidence Interval) | Multivariate Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Age (per year) | 1.05 [1.05,1.05] | 1.04 [1.04, 1.04] |
| Female Sex | 0.76 [0.75,0.77] | 0.91 [0.89, 0.93] |
| Asian Race | 0.79 [0.75, 0.83] | 0.75 [0.71, 0.79] |
| African American Race | 1.10 [1.07, 1.13] | 1.07 [1.04, 1.09] |
| Hispanic | 0.80 [0.78, 0.82] | 0.67 [0.65, 0.69] |
| Charlson comorbidity Index (per point) | 1.20 [1.19, 1.20] | 1.10 [1.09, 1.11] |
| Alcoholic Cirrhosis | 1.32 [1.30,1.34] | 1.09 [1.05, 1.12] |
| Hepatitis C | 0.83 [0.81,0.85] | 0.98 [0.95, 1.01] |
| Non-alcoholic, non-viral Cirrhosis | 0.90 [0.88, 0.92] | 1.01 [0.97, 1.02] |
| Ascites | 2.30 [2.26, 2.34] | 1.80 [1.76, 1.84] |
| Paracentesis | 2.42 [2.37, 2.47] | 1.75 [1.70, 1.79] |
| Spontaneous Bacterial Peritonitis | 2.30 [2.20, 2.40] | 1.07 [1.02, 1.13] |
| Varices | 1.12 [1.10, 1.15] | 1.00 [0.97, 1.02] |
| Transjugular Intrahepatic Portosystemic Shunt | 1.75 [1.63, 1.87] | 1.01 [0.93, 1.10] |
| Hepatic Encephalopathy | 2.05 [2.02, 2.09] | 1.54 [1.51, 1.58] |
| Hepatocellular Carcinoma | 2.33 [2.27, 2.40] | 1.78 [1.72, 1.84] |
| Dialysis | 1.69 [1.64, 1.75] | 1.09 [1.05, 1.13] |
| Advanced Practice Provider (APP) visit | 0.70 [0.69, 0.71] | 0.57 [0.55, 0.60] |
| Gastroenterology visit (non-Hepatology) | 0.99 [0.97,1.00] | 1.39 [1.36, 1.43] |
| Hepatology visit | 0.78 [0.75, 0.80] | 0.93 [0.89, 0.98] |
| Transplant Facility | 0.84 [0.83, 0.86] | 0.83 [0.81, 0.84] |
Patients were censored for transplant or loss-of-coverage > 3 months prior to date of death. For adjusted models we also included adjustment for number of outpatient visits, shared visits between MDs and APPs as well as interaction terms for APP and gastroenterology as well as APP and hepatology. The variable for APP visits included any APP visit (alone or in conjunction with an MD). To adjust further for illness severity, we included diagnosis codes for sepsis, bacteremia, urinary tract infection, pneumonia, clostridium difficile infection, cellulitis and cholangitis.
Descriptive statistics are presented as median and interquartile range (IQR) for continuous variables and as number and percent for categorical variables. Comparisons of continuous variables were performed using Student’s T test and Wilcoxon Rank Sums tests for parametric and non-parametric variables, respectively. Categorical variables were compared using a Chi-Squared test. All analyses were performed using R (packages dplyr, plyr, stringr, comorbidity, tidyr, survival, survminer, cmprsk2, knit, MatchIt, haven).
Results
Population Characteristics
Descriptive statistics for our population are shown in Table 1. Of the 389,257 patients included, 57% never had a visit with an APP. Although the differences in characteristics of the cohorts with and without APP visits were statistically significant in many aspects, they were small and not clinically meaningful except for medical comorbidities which were more common in the cohort with APP visits than those without, 70% vs. 53% with ≥3 comorbidities (p<0.001).
Table 1:
Baseline Demographics and Clinical Characteristics
| Seen by Advanced Practice Provider |
Never seen by Advanced Practice Provider |
P value | |
|---|---|---|---|
| (n = 166708) | (n = 222549) | ||
| Age (median IQR) | 60 (48 – 70) | 58 (46 – 69) | <0.001 |
| Female | 54.20% | 50.27% | <0.001 |
| Race | <0.001 | ||
| Asian | 1.93% | 3.49% | |
| African American | 10.30% | 9.73% | |
| Hispanic | 9.47% | 12.14% | |
| White (non-Hispanic) | 69.58% | 62.26% | |
| Unknown | 8.72% | 12.39% | |
| Education | <0.001 | ||
| < 12th Grade | 0.83% | 1.34% | |
| High School | 32.62% | 31.38% | |
| Less than Bachelor Degree | 50.94% | 46.49% | |
| Bachelor Degree or greater | 11.40% | 12.07% | |
| Unknown | 3.49% | 9.26% | |
| Charlson comorbidity index | <0.001 | ||
| 0 | 4.49% | 6.86% | |
| 1–2 | 25.82% | 39.60% | |
| 3–4 | 28.96% | 28.85% | |
| 5+ | 41.05% | 24.45% | |
| Alcoholic cirrhosis | 33.94% | 30.98% | <0.001 |
| Hepatitis C | 13.64% | 16.31% | <0.001 |
| Nonalcoholic, non-viral cirrhosis | 62.43% | 61.06% | <0.001 |
| Hepatic Encephalopathy | 18.31% | 16.33% | <0.001 |
| Ascites | 31.19% | 41.71% | <0.001 |
| Varices | 13.19% | 14.00% | <0.001 |
| Hepatocellular Carcinoma | 4.42% | 4.53% | 0.12 |
| Gastroenterology/hepatology Consultation | 70.31% | 57.41% | <0.001 |
| Hepatology consultation (subset of above) | 7.81% | 6.47% | <0.001 |
Overall, patients were followed for a median of 5.00 (IQR 2.33–9.00) person-years; 5.92 (3.00–9.58) and 4.41 (2.00–8.33) for those with >1 and 0 APP visits, respectively. The median number of outpatient visits per person-year for patients seen by APP and those who were not was 8.8 (4.8–14.4) and 4.7 (2.3–8.5), respectively. The most common endpoint was censoring (303,139; 77.8%; 134,936 censored patients were alive at the study end-date), occurring within 5.25 (2.50 – 9.33) person-years. Overall, 83,647 (21.5%) died within 4.17 (1.91–7.58) person-years, and 2,471 (0.63%) received a liver transplant within 2.64 (1.11–5.34) person-years. We counted death and not loss of coverage as an outcome in 6,872 patients who lost coverage within 3 months prior to mortality; 9,366 patients died within 6 months and 13,874 died within 1 year of loss of coverage.
Association of APP Involvement with Practice Metrics in the Context of Gastroenterology/Hepatology Consultation
The quality of care provided by APP in the presence and absence of gastroenterology/ hepatology consultation is depicted in Figure 1. In panel A (Scenario 1), we show that, compared to patients never seen by an APP, management by an APP with or without gastroenterologists/hepatologists was not different regarding rates of screening for HCC but APP management was associated with slightly higher rate of varices screening and much higher rates of rifaximin use after discharge for HE (OR 1.31 [1.20, 1.44]) and lower 30-day readmissions (OR 0.68 [0.66, 0.70]). When we compared APP management without gastroenterology/hepatology consultants to management by gastroenterologists/hepatologists without assistance by APPs (scenario 2, panel B), APP management alone was inferior for all measures except 30-day readmissions (OR 0.61 [0.58, 0.63]). In panel C (scenario 3), we show that management by gastroenterologists/hepatologists with APP was superior to management by gastroenterologists/hepatologists without APP in all practice metrics as well as 30-day readmissions (save for endoscopic screening). Finally, panel D (scenario 4) shows that patients seen by gastroenterologists/hepatologists without APP received much higher quality management in all practice metrics but also slightly higher 30-day readmissions than patients seen by neither gastroenterologist/hepatologist nor APP.
Practice Metrics Before and After Evaluation by APPs: Natural Experiments
In Table 2, we demonstrate the temporal association with practice metric completion in two pre-post analyses of patients evaluated by an APP with or without gastroenterology/hepatology consultation. In the first analysis, APP evaluation was associated with improved HCC screening (adjusted odds ratio (OR) 1.23 [1.19, 1.27]), varices screening (OR 1.20 [1.13, 1.27]), and use of rifaximin after a discharge for HE (OR 2.09 [1.80, 2.43]). In the second analysis, a similar and stronger relationship was observed for patients whose first APP visit was a shared visit with a gastroenterologist/hepatologist. Notably, the baseline rate of metric completion was higher for patients in the second analysis.
Association between APP Involvement and Quality Care and Outcomes Over Time
In order to evaluate the consistency of effect as well as adjust for the time each patient is under evaluation, we analyzed the impact of APP visits on average rate of quality metrics over time (Table 3). For all metrics, APP involvement was associated with improved delivery of care: increased HCC screening (adjusted incidence rate ratio 1.61 [1.60, 1.63]), increased varices screening (1.51 [1.49, 1.54]), and decreased 30-day readmissions (0.88 [0.87, 0.90]).
Association between APP Care and Mortality
Table 4 details associations between clinical and demographic features and mortality. As expected, older age, patients with more medical comorbidities, and those with more advanced cirrhosis had higher hazard ratios for mortality. APP involvement was associated with reduced risk of death, adjusted hazard ratio (HR) 0.57, 95%CI (0.55, 0.60). When analyses account for the competing risks of death and transplant, the adjusted HR for death and transplant associated with APP involvement were 0.57, 95%CI (0.55, 0.60) and 0.33, 95%CI (0.21, 0.50), respectively.(Supplementary Table 3) In Supplementary Table 4, we performed a landmark analysis to further reduce the risk of immortal time bias. In this case, the adjusted HR for death associated with APP management was 0.80 95%CI( 0.75, 0.85). In Supplementary Table 5, we provide the results of a propensity-score matching procedure and show that APP-care remains inversely associated with mortality, HR 0.43 95%CI(0.41, 0.45).
Association between APP and Healthcare Expenditures
We evaluated the association between APP involvement and charges incurred per person-year (Supplementary Table 6). APPs were associated with increased charges overall, $9,619 (IQR 5,041–18,183) compared to $4,450 (IQR 2,143–9,033) for patients who were not co-managed by APP. When the analysis was restricted to outpatient charges alone, the difference was a median of $6,196 per person-year compared to $2,756 for non-APP. Adjusting for confounders including gastroenterology/Hepatology involvement and disease severity, APP were associated with increased charges, incidence rate ratio 1.79 95%CI(1.77–1.80). Overall, these charges reflect 8,858 unique procedure and visit codes. The top 20 sources of healthcare expenditure by weight (charges multiplied by frequency) included procedure codes related to transthoracic echocardiography, magnetic resonance and computed tomography, liver biopsy, laparoscopic liver resection, endoscopy, colonoscopy, and emergency department, and outpatient visits.
Discussion
The prevalence of cirrhosis in the US is increasing, outstripping the capacity of specialists to provide optimal care. This study of a large commercial claims database covering >380,000 patients with cirrhosis demonstrates that care from an APP was associated with improved quality metric adherence, reduced readmissions, and potentially decreased mortality.
APPs improve the quality of care provided to patients with cirrhosis
Quality Metrics
We chose to examine screening for HCC because it can be ordered by any provider and is associated with the receipt of curative therapy and improved overall survival,(26) yet less than 1 in 5 patients with cirrhosis receives HCC screening.(12) Efforts to improve HCC screening rates including reminders in the electronic health record, mailed invitations, and staff dedicated to facilitating screen completion have had modest effects.(12, 27) We now report strong improvements in the rate of HCC screening associated with APP management, incidence rate ratio 1.61 95%CI(1.60, 1.63). In contrast, likely because endoscopy has to be scheduled by gastroenterologists’ offices, we found only modest improvements in endoscopic screening for varices after APP management (with or without gastroenterology consultation).
Rifaximin is recommended for secondary prophylaxis of HE. We observed a marked rate of improvement in rifaximin use after discharge for HE following an APP visit. Rifaximin use often requires an extensive prior-approval process. APPs may have a more important role in tasks that require additional efforts outside of traditional clinical workflow.
Outcomes
One in every four patients with admissions for cirrhosis will be readmitted within 30 days.(12) Their individual risk is commensurate with disease severity, burden of comorbidities, and strength of social support. Few interventions aside from the use of rifaximin for HE have been linked with reduced readmissions.(12, 28) In our study, we observed a novel finding, namely a reduced rate of 30-day readmissions in patients managed by APPs. Given the morbidity and costs of repeated hospitalizations among patients with cirrhosis, further research to explore the role of APPs providing timely post-discharge clinic follow-up are warranted.
Finally, we found that care by an APP was associated with a lower risk of death adjusting for gastroenterology or hepatology involvement. The magnitude of benefit associated with APP-care was substantial and robust across landmark and competing-risk analyses. Both outcome measures evaluated (30-day readmissions and mortality) favored APPs independent of gastroenterology/hepatology consultation. Though the mechanism deserves further study, as informed by improved quality metric adherence, care provided by APP appears to be detail-oriented and effective.
APPs and Healthcare Charges
Consistent with our finding that APPs are associated with increased quality metric performance which involve utilization of radiology tests and other procedures, we found that APPs were associated with a nearly two-fold increase in healthcare expenditures. This translates to an incremental $5,169 in charges per person-year. The main procedures that drove charges were radiology tests, endoscopic procedures, and liver resection. Prospective costing analyses are needed to confirm these associations as well as the appropriateness of the ordered tests/procedures. In conjunction with the associated improvement in survival, these increased charges could be cost-effective.
APPs are an Integral Component of a Model for Optimal Care
APPs are viewed as a key component of primary care delivery, where substantial data suggests that APPs provide care equivalent in quality to MDs.(15–18) A randomized controlled trial comparing NPs practicing independently vs. physicians in primary care showed no differences in mortality or other important health outcomes.(29) APP visits are associated with lower hemoglobin A1C levels and systolic blood pressure as well as longer duration of visits and frequently lower costs.(15, 30) Further, APPs are not more likely to provide low value care – such as magnetic resonance imaging for headache.(16) Beyond primary care, when APPs are experienced and the scope of practice is well defined such as care of patients infected with Human Immunodeficiency Virus, the quality of care provided is no different than that of MDs.(17)
In this study, we extend the literature on quality of APP care by examining its impact on patients with cirrhosis. Cirrhosis is a highly complex medical condition with multiple challenging needs ranging from unique indications for screening tests to frequent needs for lab monitoring and adjustment of medications such as diuretics and life-threatening complications such as variceal bleeding. Roughly half of our study cohort had decompensated cirrhosis. Even in the context of these highly specialized needs, our data show that patients with cirrhosis received higher quality care when seen by APPs. We showed that compared to patients never seen by an APP, APP visits were associated with sustained increases in quality metric adherence over time as well as reduced 30-day readmission risk, even when adjusted for co-management by gastroenterologists/hepatologists.
Overall, our data suggest that the optimal care for patients with cirrhosis may require both gastroenterologists/hepatologists and APPs. Although APP involvement was associated with higher quality care compared to no APP, the quality of care provided by APP without gastroenterologists/hepatologists was inferior to that provided by gastroenterologists/ hepatologists alone in all categories except 30-day readmissions. Our findings confirm a prior study from the VA where gastroenterology/hepatology consultation was associated with improved outcomes in patients with cirrhosis.(14) However, access to gastroenterologists/ hepatologists is limited; among Medicare and VA enrollees 55% and 67% of patients with cirrhosis, respectively, are never evaluated by a gastroenterologist/hepatologist.(13, 14) The key opportunity suggested by our data is to encourage care delivery that leverages broader availability of APPs to implement care plan co-developed with gastroenterologists/ hepatologists.
Contextual Factors
Our data must be interpreted in the context of the study design. First, we adjusted for confounding factors as thoroughly as possible given these administrative data including diagnostic codes and procedures for cirrhosis complications, however without access to laboratory values we cannot directly adjust for indices of liver disease severity such as Child classification and MELD score. Although our study period predates recommendations to consider foregoing endoscopic screening in patients with low liver stiffness and robust platelet counts, our data lack the factors needed to determine which patients could be safely excluded from the screening denominator. Second, as this is a study of a commercial insurance database, our findings may not generalize to patients with other insurance such as Medicaid and are limited by the loss of follow-up when patients lost coverage after loss of employment or transition to public insurers. Third, we cannot know the reasons for test ordering. As seen in Table 2, the pre-consultation rate of metric completion was higher for patients who were referred to gastroenterologists/hepatologists compared to those about to see an APP. It is possible that, in many cases, the ‘screening test’ may have prompted the consultation (e.g. by disclosing ascites or varices). As the number of visits performed increases the likelihood that a provider satisfies any given practice metric simply due to opportunity, we adjusted for the number of visits attended and provide pre/post cohorts to evaluate the temporal effect of the first visit with a gastroenterologist/hepatologist and/or APP. Fourth, we evaluated healthcare charges to the system which are typically inflated over true costs. Further the charges we describe reflect the sum of all tests/procedures incurred for any given patient to reflect overall expenditures associated with APP-care. Prospective studies are needed to determine the charges/costs attributed to specific providers. Finally, APPs may not be a part of the healthcare apparatus in some countries and even within the US there are variations in their scope of practice. In general, APPs often provide independent full-service healthcare but some regions/states specify whether visits must be shared with MDs or whether prescriptions must be co-signed. While we carefully evaluated the relative impact of shared (MD/APP) and independent (APP-alone) visits, future research could assess the impact of regional differences in policy as well as temporal (before and after rules to liberalize APP practice) differences.
Conclusion
APPs, particularly when working in conjunction with gastroenterology/hepatology consultation, are associated with improved quality of care and outcomes for patients with cirrhosis. These findings have important implications for the design of interventions to improve the quality of care among patients with cirrhosis. The modest incremental expenditures associated with APP care appear to be related to their association with improved quality metric performance and, in the context of reduced mortality, may prove justifiable within conventional definitions of cost-effectiveness. Efforts to provide APPs with training in specialty care and to facilitate team management involving specialists and APPs to coordinate care for patients with cirrhosis appear warranted.
Supplementary Material
Acknowledgments
Financial support: Dr. Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Tapper has received research grants from Valeant.
Footnotes
Disclosure:
Tapper is the guarantor of this article
Roles
a. Concept: Tapper
b. Design, Analysis: Tapper, Hao, Lin, Parikh, McCurdy, Mafi, Lok
c. Data acquisition: Tapper, Hao
d. Writing: Tapper
e. Critical revision: Hao, Lin, Parikh, McCurdy, Mafi, Lok
Conflicts of interest. No other author has relevant conflicts of interest.
References
- 1.Wong R, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Alimentary pharmacology & therapeutics 2017;46:974–980. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482. e1475. [DOI] [PubMed] [Google Scholar]
- 4.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, Volk ML, et al. The High Burden of Alcoholic Cirrhosis in Privately Insured Persons in the United States. Hepatology 2018. [DOI] [PubMed]
- 6.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. The American journal of gastroenterology 2011;106:1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, Langa KM, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology 2012;55:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, El-Serag H, Spiegel BM, Edmundowicz S, Sanyal AJ, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol 2010;8:709–717. [DOI] [PubMed] [Google Scholar]
- 9.Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero JA, Yopp AC, Parikh ND, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. The American journal of medicine 2017;130:1099–1106. e1091. [DOI] [PubMed] [Google Scholar]
- 10.Arguedas MR, Heudebert GR, Eloubeidi MA, Abrams GA, Fallon MB. Cost-effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varices. The American journal of gastroenterology 2002;97:2441–2452. [DOI] [PubMed] [Google Scholar]
- 11.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. New England Journal of Medicine 2010;362:1071–1081. [DOI] [PubMed] [Google Scholar]
- 12.Tapper EB. Building effective quality improvement programs for liver disease: a systematic review of quality improvement initiatives. Clinical Gastroenterology and Hepatology 2016;14:1256–1265. e1253. [DOI] [PubMed] [Google Scholar]
- 13.Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clinical Gastroenterology and Hepatology 2013;11:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellinger JL, Moser S, Welsh DE, Yosef MT, Van T, McCurdy H, Rakoski MO, et al. Access to subspecialty care and survival among patients with liver disease. The American journal of gastroenterology 2016;111:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. Bmj 2002;324:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mafi JN, Wee CC, Davis RB, Landon BE. Comparing Use of Low-Value Health Care Services Among US Advanced Practice Clinicians and PhysiciansProviding Value: Advanced Practice Clinicians Versus Physicians. Annals of internal medicine 2016;165:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson IB, Landon BE, Hirschhorn LR, McInnes K, Ding L, Marsden PV, Cleary PD. Quality of HIV care provided by nurse practitioners, physician assistants, and physicians. Annals of Internal Medicine 2005;143:729–736. [DOI] [PubMed] [Google Scholar]
- 18.Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, et al. Randomised controlled trial of specialist nurse intervention in heart failure. Bmj 2001;323:715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011;154:85–93. [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, et al. Increasing Prevalence of HCC and Cirrhosis in Patients With Chronic Hepatitis C Virus Infection. Gastroenterology 2011;140:1182–1188 e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology 2015;149:1471–1482 e1475. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association 1999;94:496–509. [Google Scholar]
- 25.Anderson JR. Analysis of survival by tumor response. J Clin Oncol 1983;1:710–719. [DOI] [PubMed] [Google Scholar]
- 26.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS medicine 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, Tiro JA, Murphy CC, Marrero JA, McCallister K, Fullington H, Mejias C, et al. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients with Cirrhosis: A Randomized Clinical Trial. Hepatology [DOI] [PMC free article] [PubMed]
- 28.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clinical Gastroenterology and Hepatology 2015. [DOI] [PMC free article] [PubMed]
- 29.Mundinger MO, Kane RL, Lenz ER, Totten AM, Tsai WY, Cleary PD, Friedewald WT, et al. Primary care outcomes in patients treated by nurse practitioners or physicians: a randomized trial. JAMA 2000;283:59–68. [DOI] [PubMed] [Google Scholar]
- 30.Swan M, Ferguson S, Chang A, Larson E, Smaldone A. Quality of primary care by advanced practice nurses: a systematic review. International Journal for Quality in Health Care 2015;27:396–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


