Abstract
Introduction:
Antimicrobial resistance in Gram-negative pathogens is a significant threat to global health. β-Lactams (BL) are one of the safest and most-prescribed classes of antibiotics on the market today. The acquisition of β-lactamases, especially those which hydrolyze carbapenems, is eroding the efficacy of BLs for the treatment of serious infections. During the past decade, significant advances were made in the development of novel BL-β-lactamase inhibitor (BLI) combinations to target β-lactamasemediated resistant Gram-negatives.
Areas covered:
The latest progress in 20 different approved, developing, and preclinical BL-BLI combinations to target serine β-lactamases produced by Gram-negatives are reviewed based on primary literature, conference abstracts (when available), and US clinical trial searches within the last 5 years. The majority of the compounds that are discussed are being evaluated as part of a BL-BLI combination.
Expert opinion:
The current trajectory in BLI development is promising; however, a significant challenge resides in the selection of an appropriate BL partner as well as the development of resistance linked to the BL partner. In addition, dosing regimens for these BL-BLI combinations need to be critically evaluated. A revolution in bacterial diagnostics is essential to aid clinicians in the appropriate selection of novel BL-BLI combinations for the treatment of serious infections.
Keywords: Antimicrobial resistance, β-lactam, β-lactamase, β-lactamase inhibitor, Gram-negative
1. Introduction
β-Lactams (BLs) are the largest class of antibiotics. Their mechanism of action is to inhibit bacterial cell-wall synthesis by forming a stable adduct with the peptidase domain of penicillin-binding proteins (PBPs), thus stalling peptide crosslinking and resulting in cell death. There are four major classes of BLs: penicillins, cephalosporins, monobactams, and carbapenems. The most common BL resistance mechanism in Gram-negative bacteria is the production of β-lactamases or enzymes that hydrolyze the amide bond of β-lactams inactivating the antibiotic and its ability to inhibit PBPs. The most problematic and difficult-to-treat β-lactamase-producing Gram negatives include extended-spectrum β-lactamases (ESBL)-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae (CRE) that produce KPC- or OXA-48-like carbapenemases, Pseudomonas aeruginosa, and Acinetobacter spp. Based on their tertiary structures, four main groups of β-lactamases (classes A, B, C, and D) are circulating around the world [1–5]. Classes A, C, and D enzymes possess a nucleophilic serine residue that is required for BL hydrolysis; class B enzymes are metallo-β-lactamases that require Zn2+ for activity.
To evade the production of β-lactamases, β-lactamase inhibitors (BLIs) were discovered, and these molecules are given in combination with a partner BL, as most BLIs do not possess significant PBP inhibition on their own. BL-BLI combinations are referred to as β-lactam combination drugs by the Clinical Laboratory Standards Institute (CLSI). Clavulanic acid, sulbactam, and tazobactam were the first BLIs approved for use in the clinic; however, there BLI profiles are largely limited to class A serine penicillinases (e.g. TEM-1, SHV-1) and ESBLs (e.g. CTX-M-15) as well as some class C and D β-lactamases (e.g. AmpC and OXA-1) [6]. Correspondingly, due to the limited spectrum of these former BLIs as well as the spread of antimicrobial resistance in Gram-negatives due to the production of β-lactamases, novel BL-BLI combinations with expanded profiles were sought.
Three major chemical BLI scaffolds are represented in approved and developing BL-BLI combinations. β-Lactambased BLIs (sulfones and oxapenems) continue to have a presence. After decades of research, boronic acid BLIs (e.g., vaborbactam and taniborbactam) have reached the spotlight. New to the BLI space are diazabicyclooctane (DBO) BLIs (e.g., avibactam and relebactam), including DBOs with enhanced chemistries such that they can also target PBPs (e.g., durlobactam, zidebactam, and nacubactam). In addition, some older BL-BLI combinations were revamped as the partner BL was replaced (i.e., ceftolozane-tazobactam, cefepime-tazobactam, and ceftibuten-clavulanic acid). Another advancement in the BL-BLI field is a renewed focus on pharmacokinetics/pharmacodynamics – this topic will not be discussed in this review; thus, the reader is referred to two excellent contemporary reviews on this topic [7,8].
2. Recently-approved β-lactam-β-lactamase inhibitor combinations
Four BL-BLI combinations entered clinical use in the last 5 years. Ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-cilastatin-relebactam were approved by the Food and Drug Administration (FDA) to treat specific infections by certain Gram-negative pathogens (Table 1).
Table 1.
Indications and usage for FDA-approved BL-BLI combinations.
| Drug | Approved Indication | Covered Gram-negatives | Population |
|---|---|---|---|
| Ceftolozane-tazobactam | cIAI in combination with metronidazole | Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, P. aeruginosa, Bacteroides fragilis | ≥18 years |
| Ceftolozane-tazobactam | cUTI, pyelonephritis | E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa | ≥18 years |
| Ceftolozane-tazobactam | HABP/VABP | E. cloacae, E. coli, Haemophilus influenzae, K. oxytoca, K. pneumoniae, P. mirabilis, P. aeruginosa, Serratia marcescens | ≥18 years |
| Ceftazidime-avibactam | cIAI in combination with metronidazole | E. coli, K. pneumoniae, P. mirabilis, E. cloacae, K. oxytoca, Citrobacter freundii complex, P. aeruginosa | ≥3 months |
| Ceftazidime-avibactam | cUTI, pyelonephritis | E. coli, K. pneumoniae, E. cloacae, C. freundii complex, P. mirabilis, P. aeruginosa | ≥3 months |
| Ceftazidime-avibactam | HABP/VABP | K. pneumoniae, E. cloacae, E. coli, S. marcescens, P. mirabilis, P. aeruginosa, H. influenzae | ≥18 years |
| Meropenem-vaborbactam | cUTI, pyelonephritis | E.coli, Klebsiella. pneumoniae, E. cloacae species complex | ≥18 years |
| Imipenem-cilastatin-relebactam | cUTI, pyelonephritis | E. cloacae, E. coli, Klebsiella aerogenes, K. pneumoniae, P. aeruginosa | ≥18 years |
| Imipenem-cilastatin-relebactam | cIAI | Bacteroides caccae, B. fragilis, Bacteroides ovatus, Bacteroides stercoris, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, C. freundii, E. cloacae, E. coli, Fusobacterium nucleatum, K. aerogenes, K. oxytoca, K. pneumoniae, Parabacteroides distasonis, P. aeruginosa | ≥18 years |
2.1. Ceftolozane-tazobactam
Ceftolozane-tazobactam was approved by the FDA for the treatment of complicated urinary tract infections (cUTI), including acute pyelonephritis and complicated intra-abdominal infections (cIAI) when combined with metronidazole in December 2014 in adults ≥ 18 years of age (Table 1) [9]. The combination was also approved for hospital-acquired bacterial pneumonia (HABP), and ventilator-associated bacterial pneumonia (VABP) for those ≥ 18 years of age and is being further evaluated for the treatment of infections in persons with cystic fibrosis and burns (clinicaltrials.gov identifiers: , and ). Thus, the indications for treatment with ceftolozane-tazobactam may expand. Detailed reviews of ceftolozane-tazobactam are available [10,11].
Ceftolozane is a novel cephalosporin that was designed to be more stable to the class C Pseudomonas-derived cephalosporinase (PDC, also referred to as Pseudomonas AmpC) [12]. Ceftolozane possesses a reduced affinity for PDC and is not hydrolyzed (Figure 1 and Table 2). The BLI partner, tazobactam is a sulfonebased inhibitor with limited inhibitory activity against class A carbapenemases and class D oxacillinases [13,14]. Tazobactam was previously paired with the penicillin, piperacillin; ceftolozane-tazobactam appears to be more cost effective than piperacillin tazobactam due to improved quality-adjusted life years [15,16].
Figure 1.
FDA-approved BL-BLI combinations.
Table 2.
Latest advances in β-lactam/β-lactam inhibitor combination for treatment of Gram-negative bacterial infections.
| Combination | Type of BLI | Potential pathogens covered | Unique features | Development Phase |
|---|---|---|---|---|
| Ceftolozane-tazobactam | Sulfone | P. aeruginosa, Enterobacteriaceae-producing ESBLs | Targets P. aeruginosa, including MDR strains, carbapenem-sparing | FDA approved (2014) |
| Ceftazidime-avibactam | DBO | P. aeruginosa, Enterobacteriaceae-producing carbapenemases and ESBLs | First-in-class BLI, targets KPC and OXA-48-producing Enterobacteriaceae | FDA approved (2015) |
| Meropenem-vaborbactam | Boronate | Enterobacteriaceae-producing carbapenemases and ESBLs | First-in-class BLI, targets KPC-producing Enterobacteriaceae | FDA approved (2017) |
| Imipenem-cilastatin-relebactam | DBO | P. aeruginosa, Enterobacteriaceae-producing carbapenemases and ESBLs | Targets KPC-producing Enterobacteriaceae and P. aeruginosa | FDA approved (2019) |
| Aztreonam-avibactam | DBO | Enterobacteriaceae-producing carbapenemases, including metallo-β-lactamases | Activity against metallo-β-lactamase-producing Enterobacteriaceae | Phase 3 |
| Cefepime-enmetazobactam | Sulfone | Enterobacteriaceae-producing ESBLs | Zwitterionic pair and carbapenem-sparing | Phase 3 |
| Sulbactam-durlobactam | DBO | A. baumannii-calcoaceticus complex | Niche agent, targets Acinetobacter spp., and BLI possesses enhanced reactivity, is a β-lactam ‘enhancer’, and inhibits OXAs | Phase 3 |
| Cefepime-tazobactam | Sulfone | Enterobacteriaceae-producing carbapenemases and ESBLs | High dose, extended infusion, carbapenem-sparing | Phase 3 |
| Cefepime-taniborbactam | Boronate | Enterobacteriaceae-producing ESBLs, carbapenemases, including metallo-β-lactamases (except IMP) | Bicyclic boronate, activity against metallo-β-lactamases | Phase 3 |
| Cefepime-zidebactam | DBO | P. aeruginosa, Enterobacteriaceae-producing carbapenemases, including metallo-β-lactamases and ESBLs. Acinetobacter spp. | Inhibits KPC- and metallo-β-lactamase- producing Enterobacteriaceae, BLI is a β-lactam ‘enhancer’ and bicyclo-acyl hydrazide | Phase 1 |
| Meropenem-nacubactam | DBO | Enterobacteriaceae-producing carbapenemases, including metallo-β-lactamases and ESBLs | Inhibits KPC- and metallo-β-lactamase- producing Enterobacteriaceae, BLI is a β-lactam ‘enhancer’ | Phase 1 |
| Cefpodoxime-proxetil-ETX0282 | DBO | Enterobacteriaceae-producing carbapenemases and ESBLs | Oral stepdown, and BLI possesses enhanced reactivity, is β-lactam ‘enhancer’, and inhibits OXAs | Phase 1 |
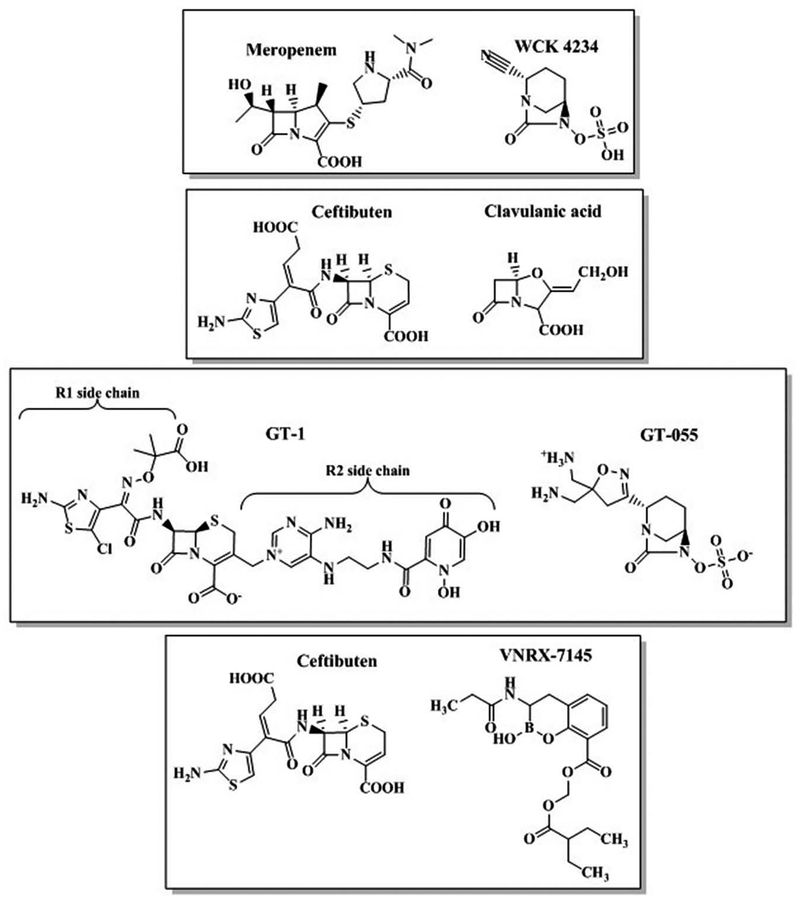
| Meropenem-WCK 4234 | DBO | Enterobacteriaceae-producing carbapenemases and ESBLs and A. baumannii | Inhibits KPC and OXA-48-producing Enterobacteriaceae and Acinetobacter spp., BLI inhibits OXAs | Preclinical |
| Ceftibuten-clavulanate | Oxapenem | Enterobacteriaceae-producing ESBLs | Oral stepdown, carbapenem-sparing | Preclinical |
| Ceftibuten-VNRX-7145 | Boronate | Enterobacteriaceae-producing carbapenemases and ESBLs | Oral stepdown, inhibits KPC and OXA-48-producing Enterobacteriaceae | Preclinical |
| GT-1-GT-055 | DBO | P. aeruginosa, Enterobacteriaceae-producing carbapenemases, metallo-β-lactamases, and ESBLs and A. baumannii | Siderophore-cephem and broad spectrum activity; inhibits KPC- and metallo-β-lactamase- producing Enterobacteriaceae, | Preclinical |
Ceftolozane-tazobactam’s strength resides in its potent activity against P. aeruginosa, including multi-drug resistant (MDR) strains with 90–98% of contemporary isolates testing susceptible [17–26]. Moreover, ceftolozane-tazobactam is a potential carbapenem-sparing treatment regimen against ESBL-producing Enterobacteriaceae [18–22,24]. Ceftolozane-tazobactam’s activity against ESBL-producing K. pneumoniae as revealed through susceptibility testing is largely lacking [20,21,24,27]; however, in spite of these in vitro results, clinical cure rates are high [28]. Conversely, the combination is mostly ineffective against Enterobacteriaceae with serine carbapenemases [29–31].
Since the introduction of the BL-BLI combination, resistance to the ceftolozane-tazobactam was reported during treatment and these resistance mechanisms were extensively explored [32–38]. The predominant resistance mechanism described is the acquisition of amino acid substitutions in PDC; these changes in select residues allow PDC to hydrolyze ceftolozane.
2.2. Ceftazidime-avibactam
In February 2015, ceftazidime-avibactam was approved by the FDA for the treatment of cUTIs, including acute pyelonephritis and cIAIs in combination with metronidazole for individuals ≥ 3 months of age (Table 1) [39]. Another clinical indication for the treatment of HABP/VABP was subsequently added for adults ≥ 18 years old [39]. The reader is directed to several recent ceftazidime-avibactam reviews with more in-depth information on the combination [40,41].
Ceftazidime is also a cephalosporin with an R1 side chain similar in structure to ceftolozane, but an aminothiazole replaces the aminothiadiazole (Figure 1 and Table 2). Avibactam is the first diazabicyclooctane (DBO) BLI to reach the clinic. Ceftazidime-avibactam is highly potent against Enterobacteriaceae carrying blaKPC and blaOXA-48; the MIC90 values reported include 0.5 μg/mL for 24,750 isolates of Enterobacteriaceae and 2 μg/mL for a panel of >500 strains of CRE [42–45]. The activity of this combination extends to P. aeruginosa with a comparable percentage of isolates testing susceptible (96.8%) as with ceftolozane-tazobactam (99%) [46]. The spectrum of activity of ceftazidimeavibactam is attributable to avibactam’s ability to inhibit class A, C, and some D β-lactamases, including KPC and OXA-48 carbapenemases [47,48].
Resistance to the ceftazidime-avibactam was observed during therapy as well as through in vitro screening, and the mechanisms leading to resistance were explored [49–53]. The principal mechanism described is the acquisition of amino acid substitutions (e.g. D179Y, V240G) in the KPC carbapenemase leading to enhanced catalytic efficiency (e.g. lower affinity or increased hydrolysis) toward ceftazidime. Other resistance mechanisms including membrane permeability and drug efflux were also found to influence ceftazidime-avibactam resistance.
2.3. Meropenem-vaborbactam
In August 2017, meropenem-vaborbactam was approved by the FDA for treatment of cUTI, including pyelonephritis in adults ≥ 18 years of age (Table 1) [54]. A Phase 1 clinical trial to test meropenem-vaborbactam in pediatric populations with bacterial infections is currently recruiting patients (clinicaltrials.gov identifier: ). In addition, the combination completed Phase 3 clinical trials for the treatment of serious bacterial infections (e.g. HABP, VABP, and bacteremia) due to CRE in adults ≥ 18 years old (clinicaltrials.gov identifier: ) [55]. Consequently, the indications for use of meropenem-vaborbactam may expand. Additional information on meropenem-vaborbactam is available in the following review articles [56–59].
Meropenem is a carbapenem and vaborbactam is a novel monocyclic boronic acid-based BLI and the first BL-boronate BLI combination to reach the market, which was highly anticipated due to the decades of research by many scientists on boronic acids and serine β-lactamases (Figure 1 and Table 2) [6]. Vaborbactam is an inhibitor of many class A and C β-lactamases, including KPC carbapenemases. Intriguingly, vaborbactam also demonstrates some inhibitory activity (IC50 values = 136–631 μM) against class B metallo-β-lactamases, including all three subclasses, B1, B2, and B3 [60]. The combination of meropenem-vaborbactam demonstrates potent antimicrobial activity against CRE with an MIC90 value of 1 μg/mL for meropenem when vaborbactam is maintained at 8 μg/mL [61,62]. Against a large panel (10,426 strains) of contemporary Enterobacteriaceae, meropenem and meropenem-vaborbactam possessed an MIC90 value of 0.06 μg/mL; however, against KPC producers the MIC90 values for meropenem and meropenem-vaborbactam differentiated to >32 μg/mL and 0.5 μg/mL, respectively [63]. Unlike ceftazidime-avibactam, vaborbactam does not potentiate the activity of meropenem against P. aeruginosa in vitro [64]. However, in a neutropenic murine thigh infection model with P. aeruginosa, meropenem-vaborbactam did reduce bacterial load; thus, the combination may have some utility against other Gram negatives [65].
In Enterobacteriaceae, resistance to meropenem-vaborbactam in vitro was attributed to the loss of expression of porins as well as increased expression of blaKPC [66–68]. Ceftazidimeavibactam-resistant KPC-3 variants (e.g. V240G and D179Y) remained susceptible to meropenem-vaborbactam [68].
2.4. Imipenem-cilastatin-relebactam
In July 2019, imipenem-cilastatin-relebactam was approved by the FDA to treat of cUTIs and cIAIs caused by certain susceptible Gram-negative bacteria, in adults with limited or no alternative therapies available (Table 1) [69]. The combination is being further evaluated in Phase 3 trials for the treatment of infections in persons with HABP and VABP (clinicaltrials.gov identifier: ). This carbapenem-DBO combination possesses antimicrobial activity against Enterobacteriaceae (MIC90 value = 0.5–1 μg/mL) and P. aeruginosa (MIC90 value = 2 μg/mL) producing class A and C β-lactamases (Figure 1 and Table 2) [70–72]. Relebactam is a potent inhibitor of KPC-2 and AmpC β-lactamases with Ki app values of 2.3 μM and 3.4 μM respectively [73,74]. The imipenem-relebactam combination is effective at reducing bacterial load in neutropenic murine disseminated and pulmonary infection models caused by P. aeruginosa and Enterobacteriaceae [75]. Unlike ceftazidime-avibactam, the MICs of imipenem-relebactam are not effected by D179 variants of the KPC-2 carbapenemase [76]. However, loss of antimicrobial activity for imipenem-relebactam was reported in Enterobacteriaceae due to lack of expression of porins [77–80]. Interestingly, oprD mutants in P. aeruginosa are more susceptible to imipenem-relebactam; this result is likely due to the essentiality of blaampC expression in the oprD null background [80–82].
3. β-lactam-β-lactamase inhibitor combinations in development
Eight BL-BLI combinations are in various stages (Phase 1–3) of clinical development. These combinations include two BL-sulfone BLI combinations (i.e., cefepime-enmetazobactam and cefepime-tazobactam), several BL-DBO-BLI combinations (i.e., aztreonamavibactam, sulbactam-durlobactam, cefepime-zidebactam, meropenem-nacubactam, and cefpodoxime proxetil-ETX0282), and a BL-boronate-BLI combination (i.e., cefepime-taniborbactam). Multiple unique features exist for the BL-DBO-BLI combinations: dual-action, increased reactivity, and oral bioavailability.
Durlobactam, zidebactam, nacubactam, and ETX0282 possess dual PBP and β-lactamase inhibitor activity. These BLIs with β- lactam activity are occasionally referred to as β-lactam ‘enhancers’. When given in combination with a β-lactam partner, these DBOs not only inhibit the β-lactamases, but also target PBPs. These combinations work synergistically by targeting different PBPs at the same time, thus these DBOs ‘enhance’ the activity of the partner β-lactam. The chosen partner β-lactams for these DBOs, sulbactam, cefepime, meropenem, and cefpodoxime are potent PBP3 inhibitors resulting in the characteristic filamentation of the bacteria cell upon inhibition; conversely the DBOs inhibit PBP2 thus resulting in the formation of spheroplasts [83–85]. Together these BL-DBO combinations produced ‘spindle-shaped’ cells.
Using innovative chemistry, diazabicyclooctenone DBOs, durlobactam and ETX0282 were engineered with a double bond between C3 and C4 and methyl groups at the C3 position [85]. These modifications increased their reactivity as well as enhanced binding to β-lactamases. Predecessor DBOs, such as avibactam, lacked inhibitor activity against most class D β-lactamases (e.g., OXA-23 and OXA-24/40). Consequently, this subclass of DBOs was rationally-designed using in silico approaches to expand the inhibition profile of DBOs to include class D β-lactamases.
Due to poor oral bioavailability, most BL-BLI combinations in development are only available in an intravenous formulation. IV drug administration is a critical route for drug delivery, but has associated risks and caveats (e.g., variable venous access, phlebitis, thrombophlebitis, infiltration, extravasation, infections, higher costs) [86,87]. Oral step-down therapy is beneficial as it eliminates the risks associated with IV administration and has the potential to decrease the length of hospital stay as well as improve quality of life for patients [88–94]. Cefopodoxime-ETX0282 is a novel oral BL-BLI combination in clinical trials.
3.1. Aztreonam-avibactam
Pfizer is developing the combination of aztreonam-avibactam, which will be entering Phase 3 clinical trials for the treatment of serious bacterial infections due to metallo-β-lactamase producing Gram negatives (clinicaltrials.gov identifier: ) (Figure 2 and Table 2). Aztreonam, the only monobactam β-lactam approved for clinical use in the US, is stable to metallo-β-lactamases and by adding avibactam, the combination demonstrates antimicrobial activity against Enterobacteriaceae co-producing class B and A or C β-lactamases [95–97]. In neutropenic murine thigh infection models caused by metallo-β-lactamase producing Enterobacteriaceae and P. aeruginosa, aztreonam-avibactam lower the bacterial load [98]. Resistance to aztreonam-avibactam was reported in a panel of clinical isolates of E. coli producing blaNDM-1 [99]. The mechanism of resistance was a four amino acid insertion into PBP3 abrogating the activity of aztreonam.
Figure 2.
BL-BLI combinations in clinical development.
3.2. Cefepime-enmetazobactam
The cefepime-enmetazobactam combination is being developed by Allecra Therapeutics and is in Phase 3 clinical trials for cUTI (clinicaltrials.gov identifier: ). The combination possesses potent activity against Enterobacteriaceae producing class A ESBLs and is a potential carbapenem-sparing treatment regimen [100,101]. Enmetazobactam is a penicillanic acid sulfone β-lactamase inhibitor, similar in structure to tazobactam (Figure 2 and Table 2). However, enmetazobactam possesses a methyl group on the triazole moiety that gives the molecule a neutral charge and is predicted to enhance entry into the bacterial cell as well as interactions with β-lactamases [102]. Enmetazobactam inhibits class A β-lactamases, including KPC carbapenemases with IC50 values ≤ 0.52 μM [102]. In murine neutropenic thigh infection and immunocompetent septicemia models using cefepime-resistant Enterobacteriaceae, the cefepime-enmetazobactam combination significantly reduced bacterial burdens [102,103]. According to the developer’s website, enmetazobactam will likely be paired with piperacillin as well.
3.3. Sulbactam-durlobactam
Entasis Therapeutics is a pioneer in the development of antimicrobial niche therapy, their sulbactam-durlobactam (ETX2514) combination is slated to target MDR Acinetobacter spp. (Figure 2 and Table 2). This BL-BLI is in Phase 3 clinical trials for A. baumannii-calcoaceticus complex HABP, VABP, and bacteremia (clinicaltrials.gov identifier: ). This is the only BL-BLI combination in development that demonstrates potent antimicrobial activity against Acinetobacter spp., a formidable threat to public health [85,104]. Sulbactam is traditionally known as a BLI, however due to sulbactam’s strong affinity for PBP3 in Acinetobacter spp., this BLI behaves as a BL [105]. Durlobactam inhibits class A, C, and D β-lactamases, thus is able to target the AmpC of Acinetobacter spp. (Acinetobacter-derived cephalosporinase, ADC) as well as the major groups of acquired oxacillinases (i.e., OXA-23-, OXA-24/40-, and OXA-58-families) in Acinetobacter spp [85,104]. Durlobactam also possesses β-lactam properties as it can inhibit PBP2 [85]. The sulbactam-durlobactam combination is effective in neutropenic murine thigh and lung infections models caused by MDR Acinetobacter spp. [85,104]. To identify potential resistance mechanisms to sulbactam-durlobactam, the combination and each drug alone were used to select for resistant mutants. With sulbactam, mutations in pbp3 were identified that result in amino acid substitutions to the PBP3 active site and affect sulbactam binding [106]. Moreover, alterations in the bacterial stringent response occurred, which were correlated with durlobactam exposure [106].
3.4. Cefepime-tazobactam
The cefepime-tazobactam combination at a 1:1 ratio in development by Wockhardt Ltd will be entering Phase 3 clinical trials for cUTI and acute pyelonephritis (clinicaltrials.gov identifier: ) (Figure 2 and Table 2). CLSI established susceptibility dose dependent (SDD) breakpoints (≤ 2 μg/mL up to ≤ 8 μg/mL) for cefepime that vary based on the chosen dose and infusion (0.5–2 grams every 8–12 hours) [107]. The addition of tazobactam will help cefepime cover isolates producing ESBLs that are resistant to piperacillin-tazobactam as well as strains with derepressed AmpCs [108,109]. Within the combination, cefepime is set at maximum dosage of 2 grams with 2 grams of tazobactam and is suggested to be administered every 8 hours as an extended infusion (90 min), thus allowing for broader coverage of isolates with higher cefepime-tazobactam MICs (8–16 μg/mL) [107,109]. Cefepimetazobactam demonstrates potent antimicrobial activity against Enterobacteriaceae, including those producing ESBLs with MIC90 values of 0.25 and 0.5 μg/mL when tazobactam if fixed at 4 and 8 μg/mL, respectively [107]. In addition, high-dose extended-infusion cefepime-tazobactam has potential applicability against KPC-producing Enterobacteriaceae [108,109].
3.5. Cefepime-taniborbactam
A novel cephem-bicyclic-boronate-BLI combination, cefepime-taniborbactam (VNRX-5133), which is entering in Phase 3 clinical trials for cUTI and acute pyelonephritis is in development by VenatoRx Pharmaceuticals (clinicaltrials.gov identifier: ) (Figure 2 and Table 2). Taniborbactam potentiates the activity of cefepime against groups of Enterobacteriaceae producing KPC, VIM, NDM, ESBLs, and AmpCs, but not strains carrying IMP metallo-β-lactamases [110,111]. Moreover, cefepime-taniborbactam was found to be more potent than ceftolozane-tazobactam against a panel of P. aeruginosa, 70% vs. 56% of isolates tested susceptible, respectively [112]. Taniborbactam is a potent inhibitor of class A, B, C, and D β-lactamases with a Ki of 21.6 nM for the VIM-2 metallo-β-lactamase [113]. In addition, ceftazidime-avibactam-resistant KPC-3 variants and ceftolozane-tazobactam-resistant PDC variants (except the E221K variant in the parent background) were susceptible to cefepime-taniborbactam [114]. The combination was found to be efficacious in a neutropenic murine lung infection model caused by cephalosporin-resistant K. pneumoniae and murine bacteremia and neutropenic-thigh infection models caused by carbapenem-resistant Enterobacteriaceae, including metallo-β-lactamase producers [115–117]. In Enterobacteriaceae, loss of porin production resulted in increased MICs to cefepime-taniborbactam; thus alterations in permeability impacts resistance to this combination [118].
3.6. Cefepime-zidebactam
Wockhardt, Ltd is developing the cephem-DBO combination of cefepime-zidebactam that has completed Phase 1 clinical trials (clinicaltrials.gov identifiers: , , , ) (Figure 2 and Table 2). Cefepime-zidebactam at a 1:1 ratio demonstrated antimicrobial activity against 5946 strains of Enterobacteriaceae and 1291 isolates of P. aeruginosa with reported MIC90 values of 0.12 and 4 μg/mL, respectively [119]. As zidebactam is a β-lactam ‘enhancer’, the cefepime-zidebactam combination also possesses activity against Enterobacteriaceae producing metallo-β-lactamases and class D oxacillinases and P. aeruginosa with metallo-β-lactamases [120–122]. Zidebactam, referred to as a bicyclo-acyl hydrazide on the basis of its chemical scaffold, inhibits class A and C β-lactamases and PBP-2 of K. pneumoniae, P. aeruginosa, and A. baumannii [84,123–125]. Despite poor in vitro activity (MICs 16–64 μg/mL) against A. baumannii, cefepime-zidebactam reduced bacterial burdens in neutropenic murine lung and thigh infection models with cefepime-resistant A. baumannii isolates [126,127]. The discrepancy in the in vitro and in vivo observations was likely due to the β-lactam ‘enhancer’ properties of zidebactam that alter the PK/PD properties of cefepime [128]. A similar observation was obtained with P. aeruginosa [129]. The eventual cefepime-zidebactam MIC susceptibility breakpoints will likely need to take these disagreements into account [130].
3.7. Meropenem-nacubactam
Meropenem-nacubactam is a carbapenem-DBO-BLI combination that completed Phase 1 clinical trials (clinicaltrials. gov identifier: ) and is being developed by NacuGen Therapeutics, a joint venture between Fedora Pharmaceuticals and Meiji Seika Pharma (Figure 2 and Table 2). The addition of nacubactam restored meropenem susceptibility to Enterobacteriaceae including an isogenic panel of E. coli producing ceftazidime-avibactam-resistant KPC-3 variants [131–134]. As a result of meropenem-nacubactam’s dual action, the combination also demonstrates reasonable activity (e.g. 71.2% of 309 isolates possessed an MIC of ≤1 μg/mL meropenem with 4 μg/mL of nacubactam) against metallo-β-lactamase producing Enterobacteriaceae [132,133,135]. Nacubactam inhibits PBP2 of Enterobacteriaceae and is a potent inhibitor of class A and C β-lactamases (IC50 < 1 μM) [83,136]. In neutropenic murine lung and cUTI infection models, meropenem-nacubactam was efficacious against Enterobacteriaceae-producing class A carbapenemases and Enterobacteriaceae-producing class A, B, C, or D β-lactamases, respectively [137,138]. Resistance to the nacubactam alone was attributable in most cases to global stringent response signal with induction of RpoS [139].
3.8. Cefpodoxime proxetil-etx0282
Cefpodoxime proxetil-ETX0282 is a novel oral cephem-diazabicyclooctenone combination in development by Entasis Therapeutics and is the first novel oral BL-BLI combination to reach Phase 1 clinical trials (clinicaltrials.gov identifier: ) (Figure 2 and Table 2). The active components, cefpodoxime and ETX1317 demonstrate activity against Enterobacteriaceae and isogenic E. coli producing class A, C, and D β-lactamases [140–142]. In addition, ceftazidime-avibactam-resistant KPC-3 variants (V240G, D179Y, and D179Y/T243M) were susceptible to the combination [143]. ETX1317 inhibits class A, C and D β-lactamase with an IC50 values <0.54 μM and also binds to E. coli PBP2 [142]. The addition of ETX0282 to cefpodoxime reduced bacterial burdens in murine UTI and thigh infection models using ESBL-producing E. coli and KPC-2-producing K. pneumoniae [142,144].
4. Preclinical β-lactam-β-lactamase inhibitor combinations
Four novel BL-BLI combinations are in preclinical stages of development. These include a carbapenem-DBO combination, two oral-stepdown BL-BLIs, and a novel siderophore-cephem-DBO combination.
4.1. Meropenem-WCK 4234
WCK 4234 is a BLI with a DBO scaffold and a nitrile side chain and is in preclinical testing by Wockhardt Ltd in combination with meropenem under the name WCK 5999 (Figure 3 and Table 2). WCK 4234 is unique as it is the first molecule in the DBO class reported to meaningfully inhibit class D oxacillinases [123,145]. Correspondingly, at the time of its unveiling, WCK 4234 possessed superior inhibitory kinetic constants compared to avibactam and relebactam against class A and D β-lactamases [123]. Combined with meropenem, WCK 4234 lowered MICs against A. baumannii producing OXA-23, OXA-24/40 and OXA-51 carbapenemases [145]. Meropenem-WCK 4234 also demonstrated activity against Enterobacteriaceae producing KPCs and OXA-48-like β-lactamases [123,145]. The meropenem-WCK 4234 combination was not listed in the 2017–2018 Annual Report available on the developer’s website; the status is uncertain.
Figure 3.
BL-BLI combinations in preclinical testing.
4.2. Ceftibuten-clavulanate
A novel oral combination of ceftibuten-clavulanate, a cephem-oxapenem BLI was in preclinical development for ESBL-producing Enterobacteriaceae by Achaogen (Figure 3 and Table 2). Ceftibuten-clavulanate was the most active oral agent tested against a world-wide collection of 5,568 isolates of Enterobacteriaceae from 2017 [146,147]. Moreover, the pharmacodynamics of ceftibuten-clavulanate were evaluated in an in vitro chemostat model and a murine thigh infection model using ESBL-producing Enterobacteriaceae [148,149]. Unfortunately, in April 2019, Achaogen filed for bankruptcy, so the future of ceftibuten-clavulanate is unclear.
4.3. Ceftibuten-VNRX-7145
In preclinical testing by VenatoRx Pharmaceuticals, ceftibutenVNRX-7145 is an oral cephem-bicyclic boronate BLI combination that debuted at the American Chemical Society (ACS) National Meeting in 2019 (Figure 3 and Table 2) [150]. Ceftibuten combined with VNRX-5236, the active molecule, demonstrates activity against Enterobacteriaceae producing class A, C, and D β-lactamases, including KPC and OXA-48 carbapenemases [151–154]. VNRX-5236 is a potent inhibitor of serine β-lactamases with an IC50 value of <0.5 μM for all tested enzymes [155]. The addition of VNRX-5236 to ceftibuten reduced bacterial burdens in neutropenic murine UTI and thigh infection models using ESBL- and KPC-2-producing E. coli and OXA-48 and KPC-producing Enterobacteriaceae, respectively [156,157].
4.4. GT-1-GT-055
The GT-1-GT-055 combination is a joint venture between Geom Therapeutics and LegoChem Biosciences and first debuted at American Society of Microbiology’s Microbe meeting in 2018. GT-1 is a novel siderophore-based cephalosporin with an R1 side chain similar to ceftazidime, but with the addition of chloride to the aminothiazole (Figure 3 and Table 2). The introduction of the siderophore moiety enhances bacterial cell entry by allowing the drug to use the bacterial ferric iron transport system. GT-055 is a DBO-based BLI that also selectively binds to PBP2 in E. coli and K. pneumoniae (Figure 3) [158]. The GT-1-GT-055 combination possesses potent activity (MIC90 2–4 mg/L) against a panel of Enterobacteriaceae producing class A, B, and C β-lactamases [159]. Against P. aeruginosa and A. baumannii, MIC90 values were 0.5–1 mg/L and 8 mg/L, respectively for GT1-GT-055 [160]. The combination also reduced bacterial loads ~1–2 logs below stasis in a murine thigh infection model with K. pneumoniae producing KPC-2 or GES-5 [161]. In Australia, Phase 1 clinical trials were discontinued in April 2019 which suggests that the development of this combination may have stalled (Australianclinicaltrials.gov identifier: ACTRN12618001980224).
5. Promising β-lactamase inhibitors
Based on their potent β-lactamase inhibitory activity, the MK-6183, QPX7728, ARX-1798, and BOS-572 BLIs are reviewed below despite lack of definitive or proposed β-lactam partners. Each of these BLIs has distinctive traits that fuel interest in further development.
5.1. MK-6183 (CB-238,618, CB-618)
MK-6183 is a DBO-based inhibitor that completed Phase 1 clinical trials in February 2019 (clinicaltrials.gov identifier: ) (Figure 4). The clinical trial was initiated by the compound’s former developer, Cubist; however, in 2015 Cubist was acquired by Merck and MK-6183 was transferred. To date, the pharmacokinetics-pharmacodynamics (PK/PD) relationship for MK-6183 efficacy in combination with various β-lactams against β-lactamase-producing Enterobacteriaceae was determined [162,163]. Based on their PK/PD observations, the authors suggest the MK-6183 may be useful as a ‘standalone’ BLI for the clinician to pair with the ‘right’ β-lactam [162]. The status of this compound is unknown as Merck’s website does not list the drug in its pipeline.
Figure 4.
BLIs in preclinical testing.
5.2. QPX7728
In preclinical evaluation by Qpex Biopharma, QPX7728 is a novel bicyclic boronate-based BLI that was identified following in silico screening against serine and metallo-β-lactamases (Figure 4) [164]. This BLI debuted at American Society of Microbiology’s Microbe 2019 meeting in San Francisco, CA [165]. Combined with either aztreonam, ceftolozane, or meropenem, QPX7728 lowers MICs against Enterobacteriaceae, A. baumannii and P. aeruginosa producing class A, B, C, and D β-lactamases. QPX7728 also potentiated oral β-lactams, ceftibuten and tebipenem against CRE. QPX7728 possesses nM Ki values against purified class A, B, C, and D β-lactamases. In murine thigh and lung infection models, meropenem-QPX7728 lowered the bacterial load.
5.3. ARX-1796
ARX-1796 (AV-006) is an avibactam prodrug in preclinical testing by Arixa Pharmaceuticals (Figure 4). The charged sulfate moiety on avibactam limits its oral bioavailability; however, the addition of a neopentyl ester group to the sulfate enhances oral bioavailability in rats, dogs, and monkeys [166–168]. The oral β-lactams, ceftibuten, cefixime, amoxicillin, cefpodoxime, sulopenem, and tebipenem, were evaluated in combination with avibactam against a panel of Enterobacteriaceae producing ESBLs, KPCs, AmpCs, and OXA-48 and ceftibuten-avibactam demonstrated the lowest MICs overall [169,170]. Consequently, ARX-1796 may be partnered with ceftibuten.
5.4. BOS-572 (IID572)
Part of the DBO family, BOS-572 possesses a third ring that transforms this BLI into a dioxotriazatricyclohendecane (Figure 4). Originally discovered by Novartis, the compound is preclinical evaluation by Boston Pharmaceuticals. BOS-572 does not possess antibacterial activity on its own; thus, it must be combined with a β-lactam partner [171]. Combined with piperacillin, BOS-572 lowers MICs against an isogenic panel of E. coli carrying single class A, C, and D β-lactamases, including KPC-2 and OXA-48 carbapenemases [171]. The acylation rate (k2/Ki) of BOS-572 is ~32x faster compared to avibactam against CTX-M-15 [171]. Relative to piperacillin-tazobactam with an MIC90 of >64 mg/L against 190 Enterobacteriaceae, piperacillin-BOS-572 possessed an MIC90 of 16 mg/L [171].
6. Conclusion
In this review, the latest advances in BL-BLI combinations were reviewed, including approved agents, ones in various stages of development (i.e., Phases 1–4), as well as BL-BLI combinations in preclinical testing. Significant progress was made in the past decade to develop BL-BLI combinations that target some of the most formidable Gram-negatives (ESBL-producing Enterobacteriaceae, CRE, P. aeruginosa, and A. baumannii). The spectrum of inhibition for these novel BLIs include class A, B, C, and D β-lactamases. Multiple BL-BLI combinations are carbapenem-sparing treatment regimens. Three oral stepdown BL-BLI combinations are in or close to the pipeline. Within the next decade, many of these new agents are anticipated to reach clinical use.
7. Expert opinion
A key finding in the field of BL-BLI development is the discovery of novel boronic acid- and DBO-based BLI scaffolds that inhibit the β-lactamases of today (e.g., KPC- and OXA- carbapenemases). This advancement was critical in order to provide clinicians with alternative therapies to treat infections caused by CRE and MDR P. aeruginosa. Prior to this achievement, treatment options were limited to toxic agents, such as colistin. Another exciting development is the bicyclic boronates that inhibit metallo-β-lactamases. Between these BL-bicyclic boronate BLI combinations and aztreonam-avibactam, hopefully, the clinician will soon have an agent to use against Enterobacteriaceae-producing metallo-β-lactamases. A major advance in BL-BLI development is also oral step-down therapy, thus removing the risks associated with IV administration, reducing costs and improving the quality of life for patients.
Dosing regimens for these BL-BLI combinations need to be critically evaluated. For example, higher-dose extended infusion cefepime-tazobactam capitalizes on the safety of these two molecules. By increasing the dosage to the maximum, the spectrum of activity for this combination expands to potentially include CRE. However, caution is warranted, and the addition of therapeutic drug monitoring to clinical practice would likely enhance the utility of the antibiotic arsenal as well as reduce emergence of resistance.
A significant challenge exists in the selection of BL partners for these BLIs. Given what has been observed to date with ceftolozane-tazobactam and ceftazidime-avibactam, the BL partner is critical. Upon release of these two new BL-BLI combinations, resistance emerged during treatment. Single amino acid substitutions in the PDC and KPC β-lactamases were the main drivers behind resistance to these BL-BLI combinations. The PDC and KPC variants were more catalytically ‘competent’ against the partner BLs, diminishing their efficacy against PBPs when in combination. Are partner-less BLIs an option? MK-6183 was suggested to be useful as a ‘standalone’ BLI that could be then partnered pro re nata by an infectious disease clinician with a select BL to target different Gram-negatives. For example, MK-6183 paired with aztreonam may be effective against metallo-β-lactamaseproducing Enterobacteriaceae vs. MK-6183 partnered with imipenem may demonstrate activity against KPC-producing Enterobacteriaceae. However, such a practice would require intricate knowledge of the bacteria responsible for the infection as well as the resistance mechanisms present.
For the future, rapid methods to identify the bacterium and resistance mechanisms produced is critical toward choosing the correct antibiotic as well as preserving the antibiotics in our armamentarium. Molecular diagnostics in other fields has exponentially advanced; however, bacterial diagnostics remain trapped in the 1940s [172]. For true advancement, additional diagnostic tools are necessary. In fact, the challenge for the current Longitude Prize, which is a £10 million prize funded by several agencies in the United Kingdom, is to design an ‘accurate, rapid, affordable and easy to use’ point of care bacterial diagnostic test that will help combat antimicrobial resistance. Nevertheless, as we wait in anticipation, the latest advances in BL-BLI combinations are promising.
Article highlights.
The development of novel BL-BLI combinations has escalated over the last decade.
The spectrum of activity for 20 different novel BL-BLI combinations, including in vitro and in vivo studies, are presented; in addition, potential resistance mechanisms are described.
The boronic acid- and diazabicyclooctane-based BLI scaffolds are likely the most outstanding advances in the field.
The pursuit of novel ‘oral-stepdown’ BL-BLI combinations is fundamental necessity in the antibiotic arsenal.
The greatest limitations in BL-BLI development are the selection of the BL partner, appropriate dosing strategies to obtain clinical cure, as well as rapid bacterial diagnostics to pinpoint the most suitable therapies.
This box summarizes key points contained in the article.
Funding
The author’s lab is supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program BX002872 to KMP-W from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U. S. Department of Veterans Affairs or the United States Government.
Footnotes
Declaration of interest
The author of this manuscript has or has had in the past two years research collaborations with Allecra, Entasis, Merck, VenatoRx, Wockhardt, Roche and Allergan. She has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee is an employee of JMI Laboratories. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321–331. [DOI] [PubMed] [Google Scholar]
- 2.Tooke CL, Hinchliffe P, Bragginton EC, et al. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol. 2019;431 (18):3472–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An engaging manuscript reviewing BLIs from a structural perspective with respect to the β-lactamases that are inhibited.
- 3.Bush K, Bradford PA. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol. 2019;17:295–306. [DOI] [PubMed] [Google Scholar]; • A notable article from prominent experts in the field that reviews interactions between β-lactamases and BLIs with a focus in β-lactamase families and nomenclature.
- 4.Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 2018;66:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Akker F, Bonomo RA. Exploring additional dimensions of complexity in inhibitor design for serine β-lactamases: mechanistic and intra- and inter-molecular chemistry approaches. Front Microbiol. 2018;9:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A comprehensive review of the classical BLIs and BLI field prior to the BL-BLI boom.
- 7.Abodakpi H, Wanger A, Tam VH. What the clinical microbiologist should know about pharmacokinetics/pharmacodynamics in the era of emerging multidrug resistance: focusing on β-lactam/β-lactamase inhibitor combinations. Clin Lab Med. 2019;39:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A detailed review that describes the essentials of PK/PD with BL-BLI combinations and provides important guidance for BL-BLI development and clinical use.
- 8.Monogue ML, Nicolau DP. Pharmacokinetics-pharmacodynamics of β-lactamase inhibitors: are we missing the target? Expert Rev Anti Infect Ther. 2019;17:571–582. [DOI] [PubMed] [Google Scholar]; • A comprehensive review that focuses on the PK/PD of BL-BLIs in development.
- 9.Merck & Co., Inc. ZERBAXA® (ceftolozane and tazobactam) for injection, for intravenous use. Whitehouse Station: NJ 08889 USA; 2014. [Google Scholar]
- 10.Giacobbe DR, Bassetti M, De Rosa FG, et al. Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther. 2018;16:307–320. [DOI] [PubMed] [Google Scholar]
- 11.Ceftolozane and tazobactam for the treatment of bacterial infections: a review of clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016. [PubMed] [Google Scholar]
- 12.Takeda S, Ishii Y, Hatano K, et al. Stability of FR264205 against ampC β-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:443–445. [DOI] [PubMed] [Google Scholar]
- 13.Drawz SM, Bethel CR, Doppalapudi VR, et al. Penicillin sulfone inhibitors of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papp-Wallace KM, Bethel CR, Distler AM, et al. Inhibitor resistance in the KPC-2 β-lactamase, a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother. 2010;54:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GJ, Pan SC, Foo J, et al. Comparing ceftolozane/tazobactam versus piperacillin/tazobactam as empiric therapy for complicated urinary tract infection in Taiwan: A cost-utility model focusing on gram-negative bacteria. J Microbiol Immunol Infect. 2019. DOI: 10.1016/j.jmii.2019.04.003:S1684-182(18)30315-3. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu VS, Solomkin JS, Medic G, et al. Cost-effectiveness of ceftolozane/tazobactam plus metronidazole versus piperacillin/tazobactam as initial empiric therapy for the treatment of complicated intra-abdominal infections based on pathogen distributions drawn from national surveillance data in the United States. Antimicrob Resist Infect Control. 2017;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Barrio-Tofino E, Zamorano L, Cortes-Lara S, et al. Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J Antimicrob Chemother. 2019;74:1825–1835. [DOI] [PubMed] [Google Scholar]
- 18.Shortridge D, Duncan LR, Pfaller MA, et al. Activity of ceftolozane-tazobactam and comparators when tested against Gram-negative isolates collected from paediatric patients in the USA and Europe between 2012 and 2016 as part of a global surveillance programme. Int J Antimicrob Agents. 2019;53:637–643. [DOI] [PubMed] [Google Scholar]
- 19.Carvalhaes CG, Castanheira M, Sader HS, et al. Antimicrobial activity of ceftolozane-tazobactam tested against Gram-negative contemporary (2015–2017) isolates from hospitalized patients with pneumonia in US medical centers. Diagn Microbiol Infect Dis. 2019;94:93–102. [DOI] [PubMed] [Google Scholar]
- 20.Yin D, Wu S, Yang Y, et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63:e02431–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean SS, Lu MC, Shi ZY, et al. In vitro activity of ceftazidime-avibactam, ceftolozane-tazobactam, and other comparable agents against clinically important Gram-negative bacilli: results from the 2017 Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect Drug Resist. 2018;11:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader HS, Farrell DJ, Castanheira M, et al. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011–12). J Antimicrob Chemother. 2014;69:2713–2722. [DOI] [PubMed] [Google Scholar]
- 23.Farrell DJ, Sader HS, Flamm RK, et al. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents. 2014;43:533–539. [DOI] [PubMed] [Google Scholar]
- 24.Farrell DJ, Flamm RK, Sader HS, et al. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. Hospitals (2011–2012). Antimicrob Agents Chemother. 2013;57:6305–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walkty A, Karlowsky JA, Adam H, et al. In vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012. Antimicrob Agents Chemother. 2013;57:5707–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkty A, Adam H, Baxter M, et al. In vitro activity of ceftolozane/tazobactam versus antimicrobial non-susceptible Pseudomonas aeruginosa clinical isolates including MDR and XDR isolates obtained from across Canada as part of the CANWARD study, 2008–16. J Antimicrob Chemother. 2018;73:703–708. [DOI] [PubMed] [Google Scholar]
- 27.Sader HS, Farrell DJ, Flamm RK, et al. Ceftolozane/tazobactam activity tested against aerobic Gram-negative organisms isolated from intra-abdominal and urinary tract infections in European and United States hospitals (2012). J Infect. 2014;69:266–277. [DOI] [PubMed] [Google Scholar]
- 28.Popejoy MW, Paterson DL, Cloutier D, et al. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of Phase 3 clinical trials. J Antimicrob Chemother. 2017;72:268–272. [DOI] [PubMed] [Google Scholar]
- 29.Pazzini C, Ahmad-Nejad P, Ghebremedhin B. Ceftolozane/tazobactam susceptibility testing in extended-spectrum β-lactamaseand carbapenemase-producing Gram-negative bacteria of various clonal lineages. Eur J Microbiol Immunol (Bp). 2019;9:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanheira M, Duncan LR, Mendes RE, et al. Activity of ceftolozane-tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae isolates collected from respiratory tract specimens of hospitalized patients in the United States during 2013 to 2015. Antimicrob Agents Chemother. 2018;62:e02125–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Malan SM, Mishra AJ, Mushtaq A, et al. In vitro activity of imipenem-relebactam and ceftolozane-tazobactam against resistant Gram-negative bacilli. Antimicrob Agents Chemother. 2018;62:e00533–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoglund E, Abodakpi H, Rios R, et al. In vivo resistance to ceftolozane/tazobactam in Pseudomonas aeruginosa arising by AmpC- and non-AmpC-mediated pathways. Case Rep Infect Dis. 2018;2018: 9095203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraile-Ribot PA, Cabot G, Mulet X, et al. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother. 2018;73:658–663. [DOI] [PubMed] [Google Scholar]
- 34.Barnes MD, Taracila MA, Rutter JD, et al. Deciphering the evolution of cephalosporin resistance to ceftolozane-tazobactam in Pseudomonas aeruginosa. MBio. 2018;9:e02085–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacVane SH, Pandey R, Steed LL, et al. Emergence of ceftolozane-tazobactam-resistant Pseudomonas aeruginosa during treatment is mediated by a single AmpC structural mutation. Antimicrob Agents Chemother. 2017;61:e01183–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraile-Ribot PA, Mulet X, Cabot G, et al. In vivo emergence of resistance to novel cephalosporin-β-lactamase inhibitor combinations through the duplication of amino acid D149 from OXA-2 β-lactamase (OXA-539) in sequence type 235 Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e01117–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berrazeg M, Jeannot K, Ntsogo Enguene VY, et al. Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother. 2015;59:6248–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabot G, Bruchmann S, Mulet X, et al. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother. 2014;58:3091–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An in vitro study revealing that structural substitutions and expression changes in the Pseudomonal AmpC lead to ceftolozane-tazobactam resistance.
- 39.Allergan USA, Inc. AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use. Madison: NJ 07940 USA; 2019. March. [Google Scholar]
- 40.Shirley M Ceftazidime-avibactam: a review in the treatment of serious Gram-negative bacterial infections. Drugs. 2018;78:675–692. [DOI] [PubMed] [Google Scholar]
- 41.Tuon FF, Rocha JL, Formigoni-Pinto MR. Pharmacological aspects and spectrum of action of ceftazidime-avibactam: a systematic review. Infection. 2018;46:165–181. [DOI] [PubMed] [Google Scholar]
- 42.Endimiani A, Choudhary Y, Bonomo RA. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother. 2009;53:3599–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazmierczak KM, Bradford PA, Stone GG, et al. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance program from 2012 to 2015. Antimicrob Agents Chemother. 2018;62:e00592–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sader HS, Castanheira M, Shortridge D, et al. Antimicrobial activity of ceftazidime-avibactam tested against multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa isolates from U.S. medical centers, 2013 to 2016. Antimicrob Agents Chemother. 2017;61:e01045–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis. 2016;63:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stachyra T, Levasseur P, Pechereau MC, et al. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother. 2009;64:326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehmann DE, Jahic H, Ross PL, et al. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem. 2013;288:27960–27971. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A pivotal paper in the field that established the inhibitory parameters for β-lactamases with BLIs that have become the gold standard for comparison of each new BLI.
- 49.Barnes MD, Winkler ML, Taracila MA, et al. Klebsiella pneumoniae carbapenemase-2 (KPC-2), substitutions at Ambler position Asp179, and resistance to ceftazidime-avibactam: unique antibiotic-resistant phenotypes emerge from β-lactamase protein engineering. MBio. 2017;8:e00528–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother. 2015;59:6605–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livermore DM, Warner M, Jamrozy D, et al. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother. 2015;59:5324–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61:e02097–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A manuscript detailing the initial emergence of the D179Y variant of KPC-3 in the clinic.
- 53.Winkler ML, Papp-Wallace KM, Bonomo RA. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother. 2015;70:2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An article that foreshadowed the evolution of ceftazidimeavibactam resistance by KPC-producing Enterobacteriaceae.
- 54.Melinta Therapeutics, Inc. VABOMERE® (meropenem and vaborbactam) for injection, for intravenous use. Lincolnshire: IL 60069 USA; 2019. February. [Google Scholar]
- 55.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7:439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jorgensen SCJ, Rybak MJ. Meropenem and vaborbactam: stepping up the battle against carbapenem-resistant Enterobacteriaceae. Pharmacotherapy. 2018;38:444–461. [DOI] [PubMed] [Google Scholar]
- 57.Wu G, Cheon E. Meropenem-vaborbactam for the treatment of complicated urinary tract infections including acute pyelonephritis. Expert Opin Pharmacother. 2018;19:1495–1502. [DOI] [PubMed] [Google Scholar]
- 58.Albin OR, Patel TS, Kaye KS. Meropenem-vaborbactam for adults with complicated urinary tract and other invasive infections. Expert Rev Anti Infect Ther. 2018;16:865–876. [DOI] [PubMed] [Google Scholar]
- 59.Cho JC, Zmarlicka MT, Shaeer KM, et al. Meropenem/vaborbactam, the first carbapenem/β-lactamase Inhibitor combination. Ann Pharmacother. 2018;52:769–779. [DOI] [PubMed] [Google Scholar]
- 60.Langley GW, Cain R, Tyrrell JM, et al. Profiling interactions of vaborbactam with metallo-β-lactamases. Bioorg Med Chem Lett. 2019;29:1981–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A manuscript revealing that the vaborbactam boronate is able to inhibit all subclasses of metallo-β-lactamases.
- 61.Castanheira M, Rhomberg PR, Flamm RK, et al. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016;60:5454–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hackel MA, Lomovskaya O, Dudley MN, et al. In vitro Activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62: e01904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castanheira M, Huband MD, Mendes RE, et al. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61: e00567–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? clinical and formulary considerations. Clin Infect Dis. 2019;68:519–524. [DOI] [PubMed] [Google Scholar]
- 65.Sabet M, Tarazi Z, Griffith DC. Activity of meropenem-vaborbactam against Pseudomonas aeruginosa and Acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother. 2019;63:e01665–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of β-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61:e01443–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun D, Rubio-Aparicio D, Nelson K, et al. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61:e01694–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson WR, Kline EG, Jones CE, et al. Effects of KPC variant and porin genotype on the in vitro activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63:e02048–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merck & Co., Inc. RECARBRIO™ (imipenem, cilastatin, and relebactam) for injection, for intravenous use. Whitehouse Station: NJ 08889 USA; 2019. [Google Scholar]
- 70.Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem-relebactam against clinical isolates of Gram-negative bacilli isolated in hospital laboratories in the United States as part of the SMART 2016 program. Antimicrob Agents Chemother. 2018;62:e00169–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lob SH, Hackel MA, Kazmierczak KM, et al. In vitro activity of imipenem-relebactam against Gram-negative bacilli isolated from patients with lower respiratory tract infections in the United States in 2015 - Results from the SMART global surveillance program. Diagn Microbiol Infect Dis. 2017;88:171–176. [DOI] [PubMed] [Google Scholar]
- 72.Lob SH, Hackel MA, Kazmierczak KM, et al. In vitro activity of imipenem-relebactam against Gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (results from the SMART global surveillance program). Antimicrob Agents Chemother. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A manuscript describing the in vitro activity of imipenem-relebactam against ESKAPE pathogens.
- 73.Papp-Wallace KM, Barnes MD, Alsop J, et al. Relebactam is a potent inhibitor of the KPC-2 β-lactamase and restores imipenem susceptibility in KPC-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62:e00174–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnes MD, Bethel CR, Alsop J, et al. Inactivation of the Pseudomonas-derived cephalosporinase-3 (PDC-3) by relebactam. Antimicrob Agents Chemother. 2018;62:e02406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powles MA, Galgoci A, Misura A, et al. In vivo efficacy of relebactam (MK-7655) in combination with imipenem-cilastatin in murine infection models. Antimicrob Agents Chemother. 2018;62:e02577–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes MD, Rutter JD, Papp-Wallace KM, et al. O0284: Imipenemrelebactam efficiently inhibits D179 variants of the KPC-2 β-lactamase European Congress of clinical microbiology and infectious diseases Amsterdam, Netherlands; 2019. [Google Scholar]
- 77.Haidar G, Clancy CJ, Chen L, et al. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61:e00642–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balabanian G, Rose M, Manning N, et al. Effect of porins and blaKPC expression on activity of imipenem with relebactam in Klebsiella pneumoniae: can antibiotic combinations overcome resistance? Microb Drug Resist. 2018;24:877–881. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Simmonds A, Stump S, Giddins MJ, et al. Clonal background, resistance gene profile, and porin gene mutations modulate in vitro susceptibility to imipenem-relebactam in diverse Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62: e00573–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of imipenem with relebactam against Gram-negative pathogens from New York City. Antimicrob Agents Chemother. 2015;59:5029–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68:2286–2290. [DOI] [PubMed] [Google Scholar]
- 82.Livermore DM. Interplay of impermeability and chromosomal β-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:2046–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morinaka A, Tsutsumi Y, Yamada M, et al. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J Antimicrob Chemother. 2015;70:2779–2786. [DOI] [PubMed] [Google Scholar]
- 84.Moya B, Barcelo IM, Bhagwat S, et al. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother. 2017;61:e02529–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durand-Reville TF, Guler S, Comita-Prevoir J, et al. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol. 2017;2:17104. [DOI] [PubMed] [Google Scholar]; • A manuscript detailing the rationale design of a novel DBO subclass with activity against A. baumannii.
- 86.Dychter SS, Gold DA, Carson D, et al. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs. 2012;35:84–91. [DOI] [PubMed] [Google Scholar]
- 87.Hendrickson JR, North DS. Pharmacoeconomic benefit of antibiotic step-down therapy: converting patients from intravenous ceftriaxone to oral cefpodoxime proxetil. Ann Pharmacother. 1995;29:561–565. [DOI] [PubMed] [Google Scholar]
- 88.Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019;179:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veve MP, Wagner JL, Kenney RM, et al. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extendedspectrum β-lactamase urinary tract infections. Int J Antimicrob Agents. 2016;48:56–60. [DOI] [PubMed] [Google Scholar]
- 90.Beique L, Zvonar R. Addressing concerns about changing the route of antimicrobial administration from intravenous to oral in adult inpatients. Can J Hosp Pharm. 2015;68:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shrayteh ZM, Rahal MK, Malaeb DN. Practice of switch from intravenous to oral antibiotics. Springerplus. 2014;3:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brack E, Bodmer N, Simon A, et al. First-day step-down to oral outpatient treatment versus continued standard treatment in children with cancer and low-risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatr Blood Cancer. 2012;59:423–430. [DOI] [PubMed] [Google Scholar]
- 93.Mertz D, Koller M, Haller P, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother. 2009;64:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daver NG, Shelburne SA, Atmar RL, et al. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect. 2007;54:539–544. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Zhang F, Zhao C, et al. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother. 2014;58:1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biedenbach DJ, Kazmierczak K, Bouchillon SK, et al. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother. 2015;59:4239–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kazmierczak KM, Rabine S, Hackel M, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crandon JL, Nicolau DP. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother. 2013;57:3299–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alm RA, Johnstone MR, Lahiri SD. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother. 2015;70:1420–1428. [DOI] [PubMed] [Google Scholar]
- 100.Crandon JL, Nicolau DP. In vitro activity of cefepime/AAI101 and comparators against cefepime non-susceptible Enterobacteriaceae. Pathogens. 2015;4:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrissey I, Magnet S, Hawser S, et al. In vitro activity of cefepimeenmetazobactam against Gram-negative isolates collected from United States and European hospitals during 2014–2015. Antimicrob Agents Chemother. 2019;e00514–19. DOI: 10.1128/AAC.00514-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papp-Wallace KM, Bethel CR, Caillon J, et al. Beyond piperacillintazobactam: cefepime and AAI101 as a potent β-lactam-β-lactamase inhibitor combination. Antimicrob Agents Chemother. 2019;63:e00105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A paper describing the biochemical characterization of enmetazobactam and its unique mechanism of action.
- 103.Crandon JL, Nicolau DP. In vivo activities of simulated human doses of cefepime and cefepime-AAI101 against multidrug-resistant Gram-negative Enterobacteriaceae. Antimicrob Agents Chemother. 2015;59:2688–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barnes MD, Kumar V, Bethel CR, et al. Targeting multidrug-resistant Acinetobacter spp.: sulbactam and the diazabicyclooctenone β-lactamase inhibitor ETX2514 as a novel therapeutic agent. MBio. 2019;10:e00159–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis. 2017;65:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McLeod SM, Shapiro AB, Moussa SH, et al. Frequency and mechanism of spontaneous resistance to sulbactam combined with the novel β-lactamase inhibitor ETX2514 in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2018;62:e01576–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sader HS, Castanheira M, Mendes RE, et al. Antimicrobial activity of high-proportion cefepime-tazobactam (WCK 4282) against a large number of Gram-negative isolates collected worldwide in 2014. Antimicrob Agents Chemother. 2017;61:e02409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castanheira M, Duncan LR, Rhomberg PR, et al. Enhanced activity of cefepime-tazobactam (WCK 4282) against KPC-producing Enterobacteriaceae when tested in media supplemented with human serum or sodium chloride. Diagn Microbiol Infect Dis. 2017;89:305–309. [DOI] [PubMed] [Google Scholar]
- 109.Livermore DM, Mushtaq S, Warner M, et al. Potential of high-dose cefepime/tazobactam against multiresistant Gram-negative pathogens. J Antimicrob Chemother. 2018;73:126–133. [DOI] [PubMed] [Google Scholar]; • A manuscript describing that higher dosing of cefepime-tazobactam may achieve a carbapenem-like spectrum.
- 110.Mushtaq S, Vickers A, Woodford N, et al. P1536: Potentiation of cefepime by the boronate VNRX-5133 versus Gram-negative bacteria with known β-lactamases. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 111.Kazmierczak K, Hackel M, Sahm D. P1544: In vitro activity of cefepime in combination VNRX-5133 when tested against cephalosporin and carbapenem resistant β-lactamase producing Gramnegative isolates. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 112.Estabrook M, Hackel M, Sahm D. P1542: In vitro activity of cefepime in combination with VNRX-5133 against meropenem and/or cefepime resistant clinical isolates of Pseudomonas aeruginosa. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid Spain. [Google Scholar]
- 113.Daigle D, Burns C, Pevear D. O0606: Kinetic mechanism and parameters of inhibition of serine KPC-2, CTX-M-15, P99 AmpC and metallo VIM-2 by the broad-spectrum β-lactamase inhibitor VNRX-5133. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 114.Daigle D, Hamrick J, Chatwin C, et al. 1370: Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug resistant K. pneumoniae and P. aeruginosa. ID Week; San Francisco, California; 2018. [Google Scholar]
- 115.Weiss W, Pulse M, Nguyen P, et al. O0600: Efficacy of cefepime/VNRX-5133, a novel β-lactamase inhibitor, against cephalosporinresistant, ESBL-producing K. pneumoniae in a murine lung-infection model. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 116.Weiss W, Pulse M, Nguyen P, et al. P1538: Efficacy of cefepime/VNRX-5133, a novel broad-spectrum β-lactamase inhibitor, in a murine bacteraemia infection model with carbapenem-resistant Enterobacteriaceae (CREs). European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 117.Georgiou PC, Siopi M, Tsala M, et al. P1540: VNRX-5133, a novel broad-spectrum β-lactamase inhibitor, enhances the activity of cefepime against resistant Enterobacteriaceae and P. aeruginosa isolates in a neutropenic mouse-thigh infection model. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 118.Tyrrell JM, Wali M, Daigle D, et al. P1541: Susceptibility to cefepime/VNRX-5133 in 298 carbapenem-resistant Enterobacteriaceae producing serine and metallo-β-lactamases. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 119.Sader HS, Castanheira M, Huband M, et al. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother. 2017;61:e00072–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Livermore DM, Mushtaq S, Warner M, et al. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother. 2017;72:1373–1385. [DOI] [PubMed] [Google Scholar]
- 121.Thomson KS, AbdelGhani S, Snyder JW, et al. Activity of cefepime-zidebactam against multidrug-resistant (MDR) Gram-negative pathogens. Antibiotics (Basel). 2019;8:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sader HS, Rhomberg PR, Flamm RK, et al. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother. 2017;72:1696–1703. [DOI] [PubMed] [Google Scholar]
- 123.Papp-Wallace KM, Nguyen NQ, Jacobs MR, et al. Strategic approaches to overcome resistance against Gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem. 2018;61:4067–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A paper describing the microbiological potency and biochemical characterization of WCK 4234, WCK 5153, and zidebactam.
- 124.Moya B, Barcelo IM, Bhagwat S, et al. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother. 2017;61:e01238–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moya B, Barcelo IM, Cabot G, et al. In vitro and in vivo activities of β-lactams in combination with the novel β-lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2019;63:e00128–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Avery LM, Abdelraouf K, Nicolau DP. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother. 2018;62:e00948–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Almarzoky Abuhussain SS, Avery LM, Abdelraouf K, et al. In vivo efficacy of humanized WCK 5222 (cefepime-zidebactam) exposures against carbapenem-resistant Acinetobacter baumannii in the neutropenic thigh model. Antimicrob Agents Chemother. 2019;63: e01931–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bhagwat SS, Periasamy H, Takalkar SS, et al. The novel β-lactam enhancer zidebactam augments the in vivo pharmacodynamic activity of cefepime in a neutropenic mouse lung Acinetobacter baumannii infection model. Antimicrob Agents Chemother. 2019;63:e02146–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monogue ML, Tabor-Rennie J, Abdelraouf K, et al. In vivo efficacy of WCK 5222 (cefepime-zidebactam) against multidrug-resistant Pseudomonas aeruginosa in the neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2019;63:e00233–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bhagwat S, Kute A, Matuschek E, et al. P2770: Selection of EUCAST disk potency for WCK 5222 (cefepime-zidebactam, FEP-ZID) susceptibility testing against Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 131.Kline E, Jones C, Mettus R, et al. P1168: In vitro activity of meropenem-nacubactam against carbapenem-resistant and ceftazidime-avibactam-resistant Enterobacteriaceae. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 132.Morrissey I, Magnet S, Hawser S, et al. In vitro activity of nacubactam, a novel dual action diazabicyclooctane, alone and with meropenem, against β-lactamase-positive Enterobacteriaceae. European Congress of Clinical Microbiology and Infectious Diseases; 2018; Madrid, Spain. [Google Scholar]
- 133.Okujava R, Garcia F, Haldimann A, et al. Activity of meropenem/nacubactam combination against Gram-negative clinical isolates: ROSCO global surveillance 2017. ID Week; 2018; San Francisco, California. [Google Scholar]
- 134.Barnes MD, Taracila MA, Good CE, et al. Nacubactam enhances meropenem activity against carbapenem-resistant Klebsiella pneumoniae producing Klebsiella pneumoniae carbapenemases (KPC). Antimicrob Agents Chemother. 2019;e00432–19. DOI: 10.1128/AAC.00432-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mushtaq S, Vickers A, Woodford N, et al. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2019;74:953–960. [DOI] [PubMed] [Google Scholar]
- 136.Barnes MD, Good CE, Bajaksouzian S et al. : 698: Nacubactam inhibits class A β-lactamases. ID Week; 2018; San Francisco, California. [Google Scholar]
- 137.Asempa TE, Motos A, Abdelraouf K, et al. Efficacy of human-simulated epithelial lining fluid exposure of meropenem-nacubactam combination against class A serine β-lactamase-producing Enterobacteriaceae in the neutropenic murine lung infection model. Antimicrob Agents Chemother. 2019;63:e02383–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Monogue ML, Giovagnoli S, Bissantz C, et al. In vivo efficacy of meropenem with a novel non-β-lactam-β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother. 2018;62: e02596–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doumith M, Mushtaq S, Livermore DM, et al. New insights into the regulatory pathways associated with the activation of the stringent response in bacterial resistance to the PBP2-targeted antibiotics, mecillinam and OP0595/RG6080. J Antimicrob Chemother. 2016;71:2810–2814. [DOI] [PubMed] [Google Scholar]; • A manuscript discovering the role of the bacterial stringent response in resistance to nacubactam.
- 140.McLeod S, Carter N, Moussa S, et al. SUNDAY-AAR-714: The novel β-lactamase inhibitor ETX1317 restores the activity of cefpodoxime against drug-resistant Enterobacteriaceae ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 141.McLeod S, Moussa S, Huband M, et al. P1184: The novel β-lactamase inhibitor ETX1317 effectively restores the activity of cefpodoxime against recent global Enterobacteriaceae isolates from urinary tract infections. ECCMID; 2019; Amsterdam, Netherlands. [Google Scholar]
- 142.O’Donnell J, Chen A, Tanudra A, et al. Saturday-AAR287: Cefpodoxime proxetil/ETX0282: A novel oral β-lactam/lactam/β-lactamase inhibitor combination to treat the emerging threat of lactamase inhibitor combination to treat the emerging threat of multi-drug resistant Enterobacteriaceae. ASM Microbe; 2017; New Orleans, Louisiana. [Google Scholar]
- 143.Shapiro A, Moussa S, Carter N, et al. SUNDAY-AAR-715: Cefpodoxime-ETX1317 susceptibility is unaffected by ceftazidime-avibactam resistance mutations V240G, D179Y and D179Y/T243M in KPC-3 β-lactamase ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 144.Weiss W, Carter K, Pulse M, et al. P1991: Efficacy of cefpodoxime proxetil and ETX0282 in a murine UTI model with Escherichia coli and Klebsiella pneumoniae. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 145.Mushtaq S, Vickers A, Woodford N, et al. WCK 4234, a novel diazabicyclooctane potentiating carbapenems against Enterobacteriaceae, Pseudomonas and Acinetobacter with class A, C and D β-lactamases. J Antimicrob Chemother. 2017;72:1688–1695. [DOI] [PubMed] [Google Scholar]
- 146.Sader HS, Streit JM, Arends SJR, et al. SUNDAY-AAR-718 - Antimicrobial activity of ceftibuten-clavulanate when tested against clinical Enterobacteriaceae isolates collected worldwide in 2017 ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 147.Sader HS, Deshpande LM, Doyle TB, et al. SATURDAY-AAR-780: Ceftibuten-clavulanate activity against extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 148.Grupper M, Stainton SM, Nicolau DP, et al. In vitro pharmacodynamics of a novel ceftibuten-clavulanate combination antibiotic against Enterobacteriaceae. Antimicrob Agents Chemother. 2019;e00144–19. DOI: 10.1128/AAC.00144-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abdelraouf K, Stainton SM, Nicolau DP. In vivo pharmacodynamic profile of ceftibuten/clavulanate combination against extended spectrum β-lactamase-producing Enterobacteriaceae in the murine thigh infection model. Antimicrob Agents Chemother. 2019;e00145–19. DOI: 10.1128/AAC.00145-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burns CJ, Trout R, Zulli A, et al. MEDI 259: Discovery of VNRX-7145: A broad-spectrum orally bioavailable β-lactamase inhibitor (BLI) for highly resistant bacterial infections (“superbugs”). Florida: American Chemical Society National Meeting Orlando; 2019. [Google Scholar]
- 151.Hamrick JC, Chatwin CL, John KJ, et al. SUNDAY-AAR-720: The orally bioavailable β-lactamase inhibitor VNRX-7145 restores bactericidal activity of ceftibuten against Enterobacteriaceae expressing Ambler class A, C, and/or D enzymes ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 152.John KJ, Chatwin CL, Hamrick JC, et al. SUNDAY-AAR-719: Rescue of ceftibuten activity by the oral β-lactamase inhibitor VNRX-7145 against Enterobacteriaceae expressing class A, C and/or D β-lactamases ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 153.Hackel M, Sahm D. SUNDAY-AAR-721: In vitro activity of ceftibuten in combination with VNRX-7145 and comparators against 1,066 UTI isolates non-susceptible to amoxicillin-clavulanate and levofloxacin ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 154.Mendes RE, Rhomberg PR, Watters AA, et al. P1180: In vitro activity of the orally available ceftibuten/VNRX-7145 combination against a challenge set of Enterobacteriaceae pathogens carrying molecularly characterised β-lactamase genes. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 155.Myers CL, Daigle D, Burns CR, et al. P1182: Ceftibuten/VNRX-7145, an orally bioavailable β-lactam/β-lactamase inhibitor combination active against serine-β-lactamase producing Enterobacteriaceae. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 156.Pulse ME, Weiss WJ, Nguyen P, et al. SUNDAY-AAR-726: Efficacy of ceftibuten + VNRX-7145 a novel β-lactamase inhibitor against ESBL E. coli strains in a murine UTI model ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 157.Avery LM, Abdelraouf K, Nicolau DP. SUNDAY-AAR-727:Assessmentof the in vivo pharmacodynamic profile of ceftibuten (CTB)/VNRX-7145 combination against serine β-lactamase-producing Enterobacteriaceae (SBL-EB) in the neutropenic murine thigh infection model ASM Microbe; San Francisco, CA; 2019. [Google Scholar]
- 158.Lee J, Cho YL, Shin S, et al. P1187: Penicillin-binding protein activity of β-lactamase inhibitor GT-055. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 159.Sader HS, Duncan LR, Thompson J, et al. SUNDAY-AAR-571: Antimicrobial activity of the novel siderophore cephalosporin GT-1 tested alone and combined with the β-lactamase inhibitor GT-055 against molecularly characterized Enterobacteriaceae clinical isolates. ASM Microbe; 2018; Atlanta, Georgia. [Google Scholar]
- 160.Hackel M, Biek D, Cho YL, et al. SUNDAY-AAR-575: In vitro activity of GT-1 and GT-1/GT-055 against recent Gram-negative clinical isolates. ASM Microbe; 2018; Atlanta, Georgia. [Google Scholar]
- 161.Oh S, Lee J, Han H, et al. Sunday-AAR574: Activity of novel siderophore cephalosporin GT-1 and β-lactamase inhibitor GT-055 against resistant K. pneumoniae in time-kill and murine thigh infection studies. ASM Microbe; 2018; Atlanta, Georgia. [Google Scholar]
- 162.Ambrose PG, VanScoy BD, Trang M, et al. Pharmacokinetics-pharmacodynamics of CB-618 in combination with cefepime, ceftazidime, ceftolozane, or meropenem: the pharmacological basis for a stand-alone β-lactamase inhibitor. Antimicrob Agents Chemother. 2017;61:e00630–17. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A critical study that reveals PK/PD basis for a “standalone” BLI.
- 163.VanScoy BD, Trang M, McCauley J, et al. Pharmacokinetics-pharmacodynamics of a novel β-lactamase inhibitor, CB-618, in combination with meropenem in an in vitro infection model. Antimicrob Agents Chemother. 2016;60:3891–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reddy RK, Glinka T, Totrov M, et al. US 10,206,937 B2: Boronic acid derivatives and therapeutic uses thereof. In: Patent US, editor. United States; 2019;1–130. [Google Scholar]
- 165.Lomovskaya O, Hecker SJ, Reddy KR, et al. SUNDAY-AAR-706, AAR-707, AAR-708, AAR-709, AAR-710, AAR-711, AAR-712, AAR-713. ASM Microbe; 2019; San Francisco, California. [Google Scholar]
- 166.Gordon EM, Duncton MAJ, Gallop MA. Orally absorbed derivatives of the β-lactamase inhibitor avibactam. design of novel prodrugs of sulfate containing drugs. J Med Chem. 2018;61:10340–10344. [DOI] [PubMed] [Google Scholar]; • A key paper describing the synthesis and testing of the first orally-available DBO BLIs.
- 167.Gordon E, Duncton M, Trias J. SUNDAY-AAR-716: Oral prodrugs of avibactam. ASM Microbe; 2019; San Francisco, California. [Google Scholar]
- 168.Gordon E, Duncton M, Lal R, et al. P1159: Oral prodrugs of avibactam, medicinal chemistry, and synthesis of ARX-1796. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 169.Trias J, Sable C, Hackel M, et al. P1151: Potentiation of oral cephalosporins and carbapenems by the addition of avibactam. European Congress of Clinical Microbiology and Infectious Diseases; 2019; Amsterdam, Netherlands. [Google Scholar]
- 170.Duncan LR, Rhomberg PR, Mendes RE, et al. SUNDAY-AAR-717: Ceftibuten-avibactam activity against β-lactam-resistant Enterobacteriaceae clinical isolates. ASM Microbe; San Francisco, California. [Google Scholar]
- 171.Reck F, Bermingham A, Blais J, et al. IID572: A new potentially best-in-class β-lactamase inhibitor. ACS Infect Dis. 2019;5:1045–1051. [DOI] [PubMed] [Google Scholar]
- 172.Christensen C, Grossman JH, Hwang J. The innovator’s prescriptions: a disruptive solution for healthcare. New York: McGraw Hill; 2009. [Google Scholar]