Abstract
From simultaneous air and water polychlorinated biphenyl (PCB) measurements collected in September 2010, we re-evaluated the direction and magnitude of net air-water exchange of PCBs in southwest Lake Michigan and compared them with estimations made using similar approaches 15 years prior. Air and water samples were collected during a research expedition on Lake Michigan at 5 km off the coast of Chicago, with prevailing winds from the southwest of our location. Gas-phase ΣPCB concentrations ranged from 190 to 1100 pg m−3 with a median of 770 pg m−3, which is similar to the concentrations measured in the City of Chicago at the same time and similar to concentrations measured in this part of the lake over the last 20 years. Water dissolved-phase ΣPCB concentrations ranged from 150 to 170 pg L−1 with a median of 160 pg L−1, which is one-tenth of that measured in the 1990s. ΣPCB net fluxes showed a slightly absorptive behavior, with a median of (−) 21 ng m−2 d−1 and an interquartile range of (−) 47 to (+) 5 ng m−2 d−1, where (−) and (+) fluxes indicate absorption and volatilization, respectively. Airborne PCB concentrations were higher when the winds were coming from Chicago and drive the deposition. Our fluxes are not significantly different from estimations from 1994 and 1995, and suggest that absorption of PCBs into the waters is slightly more prevalent than 15 years ago. It was confirmed that Chicago remains an important atmospheric source of PCBs to Lake Michigan.
Keywords: Lake Michigan, Chicago, polychlorinated biphenyls, air-water fluxes
INTRODUCTION
Determination of the fate and transport of polychlorinated biphenyls (PCBs) and other semivolatile organic compounds in the Great Lakes has been investigated since the 1970s. Indeed, there were at least 150 studies between 1981 and 2017 conducted to better understand the behavior of these chemicals in this region. Examples include those supported by the Integrated Atmospheric Deposition Network (IADN) since 1991 (Salamova et al., 2015) and the multi-institutional Lake Michigan Mass Balance (LMMB) study (Hornbuckle et al., 2006; USEPA, 2004). These major efforts have illustrated, among many findings, that PCB levels in most environmental compartments in the region are decreasing, but not enough to lift consumption advisories in the Great Lakes. Furthermore, with the reduction of important tributary riverine sources (e.g., Green Bay), atmospheric sources from urban-industrial areas surrounding the lakes are driving the fate and transport of PCBs in this region (Hornbuckle et al., 2006).
However, it is not clear that the concentrations of PCBs in all environmental matrices in Lake Michigan have followed the trend predicted by the LMMB study after twenty years. For example, there is some evidence of an increase in the water PCB levels of Lake Michigan (Venier et al., 2014), after a significant decrease until the 1990s. Furthermore, the last time atmospheric PCB concentrations above the water of southern Lake Michigan were measured was in 2000 (Miller and Hornbuckle, 2010) and PCB fluxes were estimated more than two decades ago (Miller et al., 2001; Zhang et al., 1999). Thus, there is an important lack of these type of PCB data during these last two decades.
Therefore, the main aim of this study was to re-estimate air-water PCB fluxes from the southwestern part of Lake Michigan, from samples collected September 2010, and compare them to estimations carried out over two decades earlier. Furthermore, congener specific analysis was conducted on the fluxes, including PCB11, which has never been reported for open waters of Lake Michigan.
MATERIALS AND METHODS
Sampling Methods.
Air and water PCB samples were simultaneously collected aboard the U.S. EPA R/V Lake Guardian, on September 20-23rd of 2010 (Fig S1); approximately 5 km off the coast of Chicago. Prior to sampling, XAD-2 resin was thoroughly cleaned utilizing multiple 24 hour Soxhlet extractions, placed in amber glass jars, and stored in a freezer at −20 °C (Martinez et al., 2010). Air samples (n = 6) were collected by employing a high volumetric air sampler (Hi-Vol) mounted to an aluminum boom connected to the bow of the vessel. The Hi-Vol sampled air through a glass fiber filter (GFF), collecting the particulate phase, followed by ~ 40 g of XAD-2 resin; collecting the gas phase at a volumetric rate of 0.4 m3 min−1. The GFF samples were archived and not used for this study. The Hi-Vol was operated continuously throughout the collection of the water samples for a 12 hour sampling period. Water samples (n = 4) were collected at the rate of ~500 mL min−1, utilizing a submersible centrifugal pump and pumped through a stainless steel pentaplate containing five 0.7 μm pore size GFFs (47 mm diameter × 0.42 mm thickness); collecting the particulate phase. The filtrate, stored in stainless steel tanks, was pumped at 510 ± 30 mL min−1 through 3 cm i.d. × 30 cm long glass column containing ~75 g of XAD-2 resin; collecting the dissolved phase. An average volume of 400 ± 0.6 L of water was passed through the XAD-2 resin. The GFF water filters were archived and not used for this study.
Analytical Methods.
PCBs in both air and water were measured utilizing a published method from our group (Awad et al., 2016; Hu et al., 2008; Martinez et al., 2010). Briefly, prior to sample extraction, the XAD-2 samples were transferred to 100 mL stainless steel cells and spiked with 50 ng and 30 ng for air and water samples, respectively, of the following PCB surrogate standards: PCB 14 (3,5-dichlorobiphenyl), PCB65-d5 (2,3,5,6-tetrachlorabiphenyl-d5, deuterated) and PCB166 (2,3,4,4′,5,6-hexachlorobiphenyl). Samples were extracted utilizing accelerated solvent extraction (Accelerated Solvent Extractor, Dionex ASE-300) with equal parts of acetone and hexane. The air sample extracts were cleaned by elution through a Pasteur pipette filled with 0.7 g of 3% (w/w) reagent grade water deactivated silica gel utilizing 1:1 (v/v) hexane:dichloromethane. The water sample extracts were mixed and centrifuged with sulfuric acid, and then liquid-liquid extracted with hexane, which was repeated three times. The clean extracts were eluted with hexane through a pipette column containing 1 g of acidified silica gel. The eluates were concentrated to ~ 0.5 mL, transferred to GC vials and spiked with 20 ng and 30 ng for air and water samples, respectively, of internal standard: PCB204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl). In addition to PCB204, the water sample extracts were spike with 30 ng of an additional internal standard: PCB30-d5 (2,4,6-trichlorobiphenyl-2′,3′,4′,5′,6′-d5, deuterated).
Individual PCB congeners for both set of samples were quantified using Tandem Mass Spectrometry (GC/MS/MS) in multiple reaction monitoring (MRM) mode to identify all 209 PCB congeners in 160 individual or coeluting congener peaks. Air samples were run in an Agilent 6890N Quattro Micro™ GC, Waters Micromass MS Technologies, whereas the water samples were run in an Agilent 7890B GC, Agilent 7000C MS System. Both instruments were equipped with a Supelco SPB-Octyl fused silica capillary column (5% phenyl methyl siloxane, 30 m × 250 μm i.d., 0.25 μm film thickness) using helium as the carrier gas at a constant flow rate of 0.8 mL min−1 and nitrogen/argon as the collision gas. The GCs operated in solvent vent injection mode at the following injection conditions: initial temperature 45 °C, initial time 0.06 min, ramp 600 °C min−1 to inlet temperature 325 °C at 4.4 psi (Ampleman et al., 2015; Hu et al., 2008; Martinez et al., 2010).
Air-Water Exchange Model.
We calculated the direction and magnitude of the air-water exchange for individual and total PCB congeners using a well-established and described method in numerous peer-reviewed papers (Martinez et al., 2019; Martinez et al., 2010; Schwarzenbach et al., 2003; Zhang et al., 1999). We utilized the gradient-flux law, i.e., mass transfer velocity multiplied by concentration gradient (eq. 1):
| (eq. 1) |
where FPCBi,a/w is the flux between the air and water for the ith PCB (ng m−2 d−1), VPCBi,a/w is the air-water exchange velocity for the ith PCB (m d−1), is the freely-dissolved concentration of the ith PCB in the water column (ng m−3), and is the water-phase concentration of the ith PCB at equilibrium with the air-phase concentration (ng m−3). We calculated the net, gross volatilization (i.e., ) and gross absorption (i.e., ) fluxes using eq. 1. Negative net fluxes indicate net absorption fluxes [FPCBi,a/w < 0, depict it with a (−) sign], zero net fluxes indicate net equilibrium fluxes (FPCBi,a/w = 0) and positive net fluxes indicate net volatilization fluxes [FPCBi,a/w > 0, depict it with a (+) sign].
Since the water sampling method does not separate PCB sorbed to dissolved organic carbon (DOC) from the freely-dissolved phase, we included the effect of DOC on the water samples. That is, a two-phase partitioning model was implemented for the water samples (eqs. 2 & 3) (Burkhard, 2000; Martinez et al., 2017; Schwarzenbach et al., 2003):
| (eq. 2) |
| (eq. 3) |
where is the total concentration of the ith PCB in the water collected in the XAD-2 resin (ng m−3), Xfd is the fraction of chemical freely dissolved (dimensionless), CDOC is the concentration of DOC present in the water (kg L−1) and KDOC is the DOC-water partition coefficient (L kg−1). Because our water sampling method separated the particulate phase via the pentaplate, it was not necessary to include a particulate third phase in this model. In order to account for the non-linear effect of wind speed on the water-side mass transfer coefficient, a two parameter Weibull distribution was also included (eq. 4) (Schwarzenbach et al., 2003; Zhang et al., 1999):
| (eq. 4) |
where is the average water-phase exchange velocity of CO2 (cm s−1), u10 is the wind speed at a 10 m reference height (m s−1), η and ξ are the scale and shape parameters, respectively; both parameters were determined by maximum likelihood estimation (Livingstone and Imboden, 1993; Nwobi and Ugomma, 2014). Fluxes were calculated for the six air samples, where two water samples were applied twice due to their overlap sampling period with the air samples (Fig S2). Wind speeds measured over Lake Michigan, for the sampling periods (wind rose plots, Fig S3 and Fig S4), followed a Weibull distribution; determined by Anderson-Darling goodness-of-fit test (Table S1). The meteorological data used for the calculation of the fluxes are presented in Table S2. The mathematical summary of this flux approach with nomenclature can be found in the Supplementary Material and Fig S5.
Monte Carlo Simulations.
We used a Monte Carlo approach to estimate the uncertainty of the calculated fluxes (Martinez et al., 2019; Martinez et al., 2010). Frequency distributions for each parameter were obtained from available data, including air and water temperatures, wind speed and atmospheric pressure, and generated from the standard deviations from reported physical-chemical property values (see Supplementary Material and Table S2). Normal distributions were obtained and assumed for all the parameters, with the exception of the wind speed, that as noticed before, it followed a Weibull distribution. The meteorological data were obtained from the deck log of the research vessel, and are summarized in Table S3. We assumed normal distribution for the chemical measurements, with a 20% error due to chemical analysis. We utilized RStudio (Version 1.1.442) to perform the simulations, which were repeated 1000 times and provided frequency distribution of the fluxes. The RStudio codes are provided in the Supplementary Material.
Quality Assurance & Quality Control (QA/QC).
Surrogate standards, laboratory, field blanks and signal to noise ratio (> 5 times) were used to evaluate our QA/QC. The percentage recoveries of surrogate standards for the air samples were 88 ± 35, 62 ± 26 and 72 ± 34 for PCB14, PCB65-d5, and PCB166, respectively. For the water samples, 71 ± 2.5, 79 ± 6.3, and 86 ± 6.3 percentage recoveries for PCB14, PCB65-d5, and PCB166, respectively, were obtained. Recovery correction was performed to all samples to account for any losses during laboratory processes; PCB1 to 39, PCB40 to 127, and PCB128 to 209 corrected with recoveries from PCB14, PCB65-d5, and PCB166, respectively. The average ΣPCB mass measured in field air blanks was 41 ± 23 ng sample−1 (n = 5) and 8.5 ± 3.0 ng sample−1 for water samples (n = 4). Subsequently, a congener specific limit of quantification (LOQ) was applied to all samples; calculated as the upper limit of the 95% confidence interval of the blanks. Note that congener masses below the LOQ were substituted with a mass of zero. The congener specific LOQ for the air samples ranged from 13 pg sample−1 to 14.0 ng sample−1 with an average of 0.42 ng sample−1. For the water samples, the congener specific LOQ ranged from 0.0 to 2.2 ng sample−1 with an average of 0.11 ng sample−1.
All the data generated in this investigation, i.e., individual PCB congener concentrations in air and water, LOQ for both air and water, Henry’s law constants and octanol-water partition coefficients for individual PCB congeners, PCB fluxes (net, volatilization and absorption), and the meteorological data used to calculated the fluxes, are available at https://doi.org/10.1594/PANGAEA.897545 (Boesen et al., 2019).
RESULTS AND DISCUSSION
Airborne PCB Concentrations.
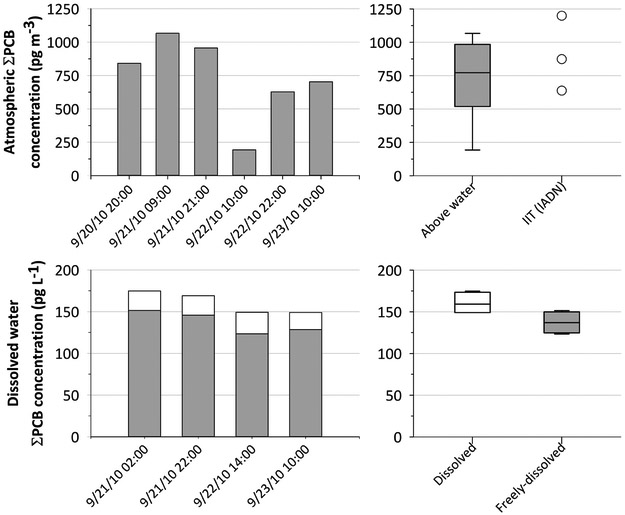
Gaseous concentrations of airborne ΣPCB ranged from 190 to 1100 pg m−3 with a median of 770 pg m−3 (Figure 1). Interestingly, our over water measurements were in the same range of measurements carried out at the ITT Chicago site for the same month using a similar Hi-Vol sampler (n = 3, 640 – 1200 pg m−3) (Indiana University, 2017). Indeed, no significant difference was found between both set of data (p-value = 0.2). Individual PCB congeners 52, 8, and coeluting congeners 20+28 yielded the highest average concentrations of 45 ± 17, 36 ± 20, and 33 ± 14 pg m−3, respectively (Table S4). Non-Aroclor PCB11, present in organic pigments (Anezaki and Nakano, 2014; Hu and Hornbuckle, 2010; Rastogi, 1992), but ubiquitous in ambient air in Chicago (Hu et al., 2008), yielded a concentration of 22 ± 6 pg m3. All these congeners were detected 100% of the time.
Figure 1.
Airborne (top plots), dissolved and calculated freely-dissolved (grey bars) water (bottom plots) concentrations of ΣPCBs over Lake Michigan September 20th-23rd of 2010. Freely-dissolved water concentrations were calculated using eqs. 2 and 3. The x-axes for plots indicate the start sampling time. The airborne PCB concentrations are compared to those reported by IADN for samples collected in September 2010 at the IIT site in Chicago (top right). The box plots include the maximum, 75th percentile, median, 25th percentile and the minimum value.
Although the samples were collected at the same location above the water over three days, a factor of 5.5 between the highest and the lowest samples shows an interesting concentration temporal variability. The highest concentrations were collected when the winds were coming from south west, whereas the lowest concentration was collected when the winds were coming from the north (see wind roses, Fig S4). Although the wind direction measurements clustered into two groups for the six airborne measurements, with only one measurement from the north and all the rest from the west/south west, we observed a significant correlation between the logarithm concentration of airborne PCBs and the cosine of the wind direction for ΣPCB and 57% of the individual congeners (R2 = 0.69 - 0.99, p-value < 0.05). All these individual congeners are present in Aroclor mixtures (Koh et al., 2015). Conversely and because PCB11 is ubiquitous in air and not associated to a specific airshed (Hu et al., 2008), PCB11 did not exhibit a statistically significant correlation with wind direction (R2 = 0.51, p-value = 0.1). This correlation between ΣPCB and 57% of individual congeners, and the wind direction also indicates that winds mostly coming from the Chicago region result in higher concentrations of airborne PCBs at our sampling location. This outcome is consistent with findings by others (Green et al., 2000; Offenberg and Baker, 2000; Simcik et al., 1997; Zhang et al., 1999), but almost 20 years ago, confirming that Chicago and the south west shore of Lake Michigan continue to be a relevant source of airborne PCBs to Lake Michigan.
Water PCB Concentrations.
Dissolved water concentrations of ΣPCB ranged from 150 to 180 pg L−1 with a median of 160 pg L−1 (Figure 1). A very low concentration variability was found, with a factor of 1.2 between the highest and lowest sample. These results suggest, at least in comparison to the air samples, that our water measurements can be considered constant for the entire sampling period. Furthermore, and conversely to the air samples, no indication of wind direction effect on the dissolved water concentration was found. The latter is contrary to what Offenberg and Baker reported for 1994 - 1995 water samples located in the southern basin of Lake Michigan, where elevated dissolved PCBs were observed when winds were from the south and south west (Offenberg and Baker, 2000). Individual PCB congeners 52, coeluting congeners 61+70+74+76 and 110+115 yielded the highest average concentrations of 8.2 ± 1.4, 7.7 ± 0.4 and 7.5 ± 0.8 pg L−1, respectively (Table S5). Non-Aroclor PCB11 yielded a concentration of 7.1 ± 0.4 pg L−1. To our knowledge, this is the first report of PCB11 in open waters of Lake Michigan. PCB52 was the higher individual congener detected in both air and water samples. Individual PCB congeners for both air and water samples are presented in Table S4 and Table S5, respectively.
Calculated freely-dissolved water concentrations from eq. 2 resulted in a reduction of around 15% of the measure water concentrations, ranging from 120 to 150 ng L−1 (Figure 1). Generally, mono- to pentachlorobiphenyls were not affected by the DOC correction, with a small reduction (~15%). However, hepta- to decachlorobiphenyl congeners showed a larger reduction, from 50 to 80%.
Temporal Trends.
The airborne and dissolved water ΣPCB concentrations measured in this study are in the range to those measured by other studies but positioned in different places for their respective ranges. For example, between the 1980s and 2010, the magnitude of the measured airborne ΣPCB concentrations at southwest Lake Michigan ranged from 60 to 1500 pg m−3 with the average ranging anywhere from 300 to 870 pg m−3 (Doskey and Andren, 1981; Hornbuckle et al., 1995; Miller and Hornbuckle, 2010; Zhang et al., 1999), similar to our measurement average from 2010. Indeed, no significant correlation was found between the natural logarithm of the airborne concentrations against time (R2 = 0.02, p-value = 0.3), suggesting that there has been no significant change in the airborne PCB concentration above the southwest waters of Lake Michigan from 1977 to 2010. It is possible that this outcome is biased due to lack of data, especially where only one or two samples were available per year, seasonal effects, spatial location and sampling strategy (e.g., wind direction, temperature and stationary against transect sampling), but these are the only available values of airborne PCBs above the waters of southwest Lake Michigan. This small variation on airborne PCB concentration above the southwest water of Lake Michigan over ~30 years is contrary to what others have shown for Chicago, where a half-life of 12 years for total PCBs and a few individual congeners have been reported (Salamova et al., 2015). It is possible that over-water air samples are not only capturing airborne PCBs from the Chicago’s airshed, but the entire northwest and southwest shores of Lake Michigan, as well as volatilization from the waters. Further, we have reported that airborne PCB concentrations in Chicago spatially varies by a factor of ten (Hu et al., 2010). Thus, Salamova et al., (2015) half-life calculations from ITT could be biased and not represent the entire Chicago’s airshed.
Conversely, the dissolved water ΣPCB concentrations in the southern Lake Michigan has declined from 1980 by approximately 10-fold, from an average of 1800 pg L−1 to 200 pg L−1 (Anderson et al., 1999; Bicksler, 1996; Hornbuckle et al., 1995; Lefkovitz, 1987; Miller et al., 2001; Miller and Hornbuckle, 2010; Offenberg and Baker, 2000; Streets et al., 2006; Swackhamer and Armstrong, 1987; USEPA, 2004; Venier et al., 2014). The measured dissolved water concentrations, from 1980-2012, have ranged from 48 to 7900 pg L−1 with the average ranging anywhere from 150 to 1800 pg L−1. Surprisingly, the levels of PCBs from samples collected from 2000 to 2012 seems to be increasing. It is possible that once the main riverine PCB sources were reduced/controlled, e.g., Green Bay in WI, low contaminated PCB sediment from Lake Michigan could be driving the PCB water column concentration, as suggested by Li et al. (2009) or atmospheric absorption/deposition is playing a more important role than 10 years ago.
PCB Congener Profiles.
Both air and water samples, independent of their concentration levels and even averaging them by environmental compartment, exhibited very similar PCB congener profiles, especially the water samples (Fig S6). Indeed, little variability was found for the individual PCB congener fractions in the water samples, shown by the small standard deviations in relation to the means (Fig S6b). As expected, all top 10 PCB congeners in both air and water samples are present in Aroclor mixtures, except for PCB11. Lower chlorinated congeners (e.g., PCB8, PCBs18+30 and PCBs20+28) were greater contributors in the air sample profiles, while congeners with four to six chlorines (e.g. PCBs61+70+74+76, PCBs90+101+113, and PCBs110+115) were greater contributors to the water samples, with the surprising exception of PCB11, which was higher in the water samples (Fig S6). PCB52 was the highest contributor for both air and water samples (average of 6% and 5%, respectively).
To quantitatively evaluate PCB profile similarity, including Aroclor mixtures, we used the cosine theta metric (cos θ). This metric uses the cosine of the angle between two multivariable vectors (the profiles) where a value of 0.0 describes two completely different vectors and 1.0 describes two identical vectors (DeCaprio et al., 2005; Magar et al., 2005; Martinez and Hornbuckle, 2011). As expected, air and water sample profiles were similar in nature, where cos θ ranged from 0.80 to 0.99, with an average of 0.94 ± 0.05 for all the samples. Water PCB profiles were extremely similar, as shown in Fig S6b, with an average cos θ of 0.98 ± 0.01. Air samples also showed similar profiles between them, with an average cos θ of 0.92 ± 0.05 (Fig S6a). However, the air sample collected the 9/22/10 from 10:00 (Figure 1) with the lowest concentration, yielded the lowest similarity against the other air samples (0.86 ± 0.04). This subtle but interesting difference is due to a few individual congeners that were outliers (lower or higher) in relation to the other five air samples. For example, PCBs 11, 40+41+71, 44+47+65, 52, 56, 64 and 66 have a much higher proportion in this low concentration sample than the rest of the samples. Conversely, PCBs21+33 in this low concentration sample had a much lower proportion that the rest of the samples (Fig S7). The differences in these selected PCB congeners suggest dissimilar airborne sources, which is correlated with the wind direction, previously described.
Aroclor 1248 yielded the best match for both air and water, followed by Aroclor 1242 (i.e., cos θ = 0.80 ± 0.03 and 0.78 ± 0.05, respectively). Interestingly, Aroclor 1248 has not been reported as a major contributor to Chicago air (Hu et al., 2010; Rodenburg and Meng, 2013). However, biotic and abiotic processes can change the original Aroclor signal which could lead to misinterpretation of the original Aroclor mixtures that are present in the environment. For example, a mixture of Aroclors 1254 and 1242 weathered in lacustrine sediments also yielded a similar Aroclor 1248 profile (Magar et al., 2005). We note that if PCB bound to DOC were removed from the water samples, i.e., freely-dissolved water concentration, and the cos θs were recalculated, very similar agreement between the air, water and Aroclors are also obtained.
Air-Water PCB Fluxes.
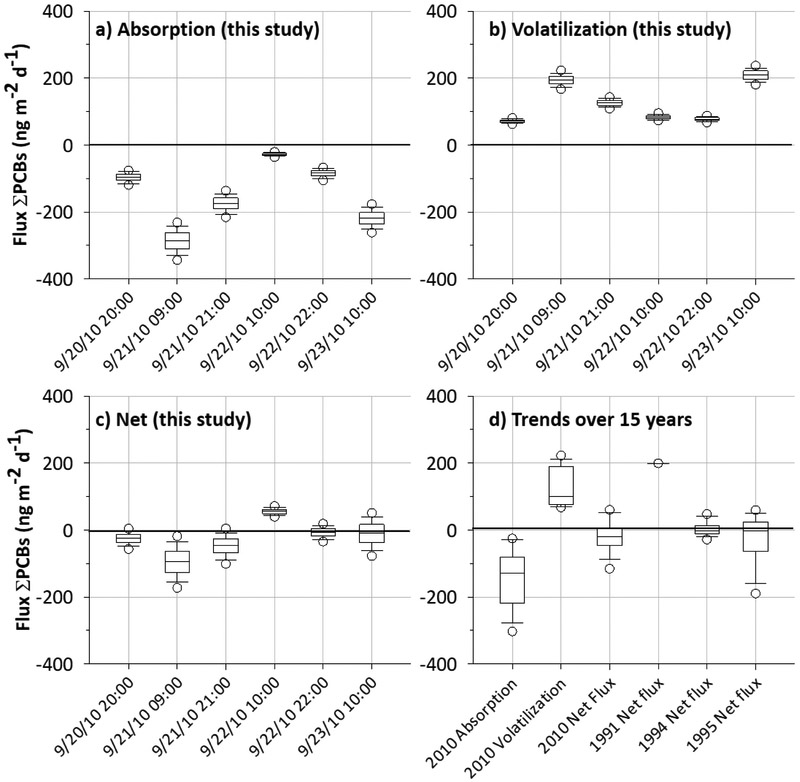
In general, net fluxes of ΣPCBs showed a slight tendency towards absorption, with a median of (−) 21 ng m−2 d−1 [interquartile range (IQR) of (−) 47 to (+) 5] (Figure 2d). Net flux medians from the individual pair samples ranged from (−) 90 to (+) 55 ng m−2 d−1 (Figure 2c). Two fluxes attract attention; the flux estimated with air sample collected 9/21/10 at 9:00 resulted in a net absorption flux, with a median of (−) 93 ng m−2 d−1 [IQR of (−) 130 to (−) 63]; conversely, the flux estimated with air sample collected 9/22/10 at 10:00 showed a volatilization trend, with a median of (+) 56 ng m−2 d−1 [IQR of (+) 50 to (+) 62] (Figure 2c). That is, fluxes estimated with air samples collected 9/21/10 at 9:00 and 9/22/10 at 10:00 yielded absorption and volatilization net fluxes, respectively, within a 95% CI. Overall, these results indicate how dynamic the air-water exchange of PCBs in Lake Michigan can be, even during only a 24 hour period.
Figure 2.
Absorption (a), volatilization (b) and net fluxes (c) of ΣPCBs for the six sampling periods (this study). Values were computed from the Monte Carlo simulations (n = 1000). Box plots describe from top to bottom the 95th percentile, 75th percentile, median, 25th percentile and 5th percentile values. The x-axes indicate the air starting sampling time. Plot (d) summarizes the absorption, volatilization and net fluxes for the six sampling periods (this study), including other net fluxes from 1991 to 1995. Net fluxes from 1991 (n = 1) are from Hornbuckle et al. (1995); 1994 (n = 22) and 1995 (n = 12) from Miller and Hornbuckle (2010) and Zhang et al. (1999), respectively.
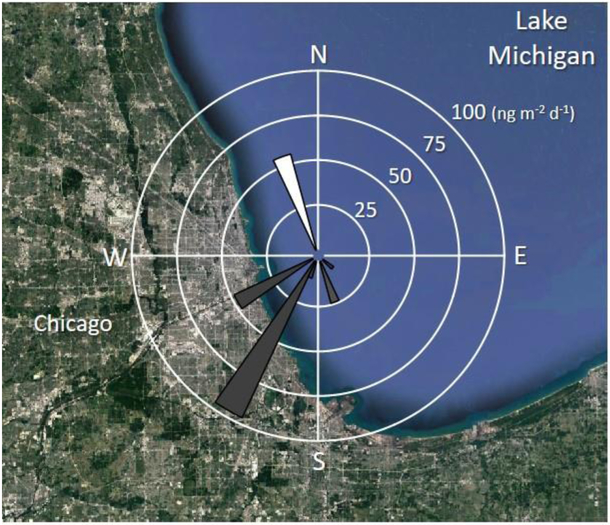
Airborne concentrations, which were correlated with wind direction, control the magnitude and direction of the net air-water exchange (Figure 3). However, wind direction was not significantly correlated with net fluxes (R2 = 0.5, p-value = 0.09), which could be due to the small number of samples collected when the wind was not coming from the west/south west.
Figure 3.
Wind flux rose plot for the six net fluxes estimated in ng m−2 d−1. Grey triangles correspond to net absorption, while white triangle corresponds to net volatilization. Flux values are the median of each estimated net flux (Figure 2c). The average of wind direction during the air sampling period was used as the wind direction (Table S3).
Gross absorption and gross volatilization yielded medians of (−) 130 ng m−2 d−1 [IQR of (−) 220 to (−) 81], and (+) 100 m−2 d−1 [IQR of (+) 77 to (+) 190], respectively (Figure 2d). Gross absorption flux medians from the individual samples ranged from (−) 290 to (−) 28 ng m−2 d−1 (Figure 2a), whereas gross volatilization medians from the individual samples ranged from (+) 71 to (+) 209 ng m−2 d−1 (Figure 2b).
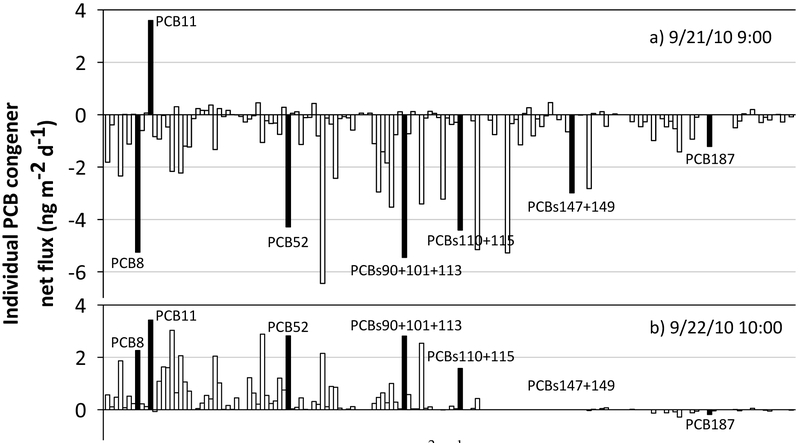
Individual PCB net fluxes from the six sample periods ranged from (−) 7 to (+) 5 ng m−2 d−1 for PCBs107+124 and PCB11, respectively. Only eleven PCB congeners showed within a 95% CI the same air-water exchange behavior during the six periods, i.e., net absorption or net volatilization. For example, PCBs12+13 always yielded net absorption, whereas PCBs43+73, 55 and 89 yielded net volatilization. In summary, within a 95% CI, individual PCB congener fluxes yielded net absorption 17% of the time, net volatilization 23% of the time, and 61% of the time it was not possible to determine the direction of the flux with certainty, i.e., the 2.5th and the 97.5th percentiles had a different sign for the same congener.
A clear difference in the PCB flux profile was found when net absorption or net volatilization occurred. For example, Figure 4 displays two different individual PCB flux calculations. When net absorption was estimated (Figure 4a), the PCB flux profile showed more mid to higher chlorinated PCB congeners, whereas when net volatilization was estimated (Figure 4b), the PCB flux profile showed more lower to mid chlorinated PCB congeners. PCB11 always volatilized from the water, but it was not within a 95% CI, i.e., not statistically significant under our criterion.
Figure 4.
Individual PCB congeners net flux (ng m−2 d−1) for air samples collected at a) 9/21/10 9:00 and b) 9/22/10 10:00. Negatives and positive flux values correspond to (−) net absorption and (+) net volatilization fluxes, respectively. Black bars indentify a few PCB congeners discussed in the text. X-axes are PCB congeners ordered by IUPAC nomenclature, from left to right, PCB1 to PCB209, respectively.
PCB air-water fluxes comparison.
Although comparing our flux estimations with other flux values is not straightforward due to differences in environmental conditions and sampling methods, we only found three studies, between 19 and 15 years ago, that reported ΣPCB net fluxes in the southwest part of Lake Michigan, (Hornbuckle et al., 1995; Miller et al., 2001; Zhang et al., 1999). Interestingly, our values are in the same range, but slightly lower than net fluxes calculated in 1991 (n = 1), 1994 (n = 22) and 1995 (n = 12) (Figure 2d). Indeed, the medians of our six samples were lower than the medians obtained in 1994 and 1995, but not significantly different (Kruskal-Wallis One Way ANOVA, p-value = 0.3). The lack of PCB flux estimations, both spatially and temporally, make it difficult to draw conclusions, but it seems that PCB absorption into the water from atmospheric input is increasing. This hypothesis could be supported by the reduction in water concentrations from samples 1994/95 to 2010, but also due to small variation/reduction of airborne concentrations, which it seems to be constant when the winds are coming from Chicago.
CONCLUSIONS
Through field samples and specific PCB congener analysis, we were able to determine that more than 30 years after PCBs were banned, PCBs are still present in measurable quantities in air and water in the southern Lake Michigan region. As it was speculated 20 years ago, Chicago and the southwest shore of Lake Michigan, continues to be an important source of airborne PCBs to Lake Michigan. Due to the large decrease in the levels of PCBs in the water until 2000 and minor changes in airborne concentrations, we find that gas absorption into the water near Chicago has slightly increased compared to 20 years ago.
Supplementary Material
Acknowledgements
We thank the Superfund Research Program of the National Institute of Environmental Health Sciences (Grant No. NIH P42ES013661) and the U.S. Environmental Protection Agency’s Great Lakes National Program Office (Grant No. GL-00E00515-0) for funding; N. Herkert for assistance in laboratory work; Z. Rodenburg for the collection of the air and water samples including the extraction of the air samples; E. Jetter for managing the analytical laboratory; the Captain and crew of the EPA R/V Lake Guardian. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Environmental Protection Agency.
Footnotes
Supplementary Material
Supplementary material related to this article can be found at https://doi.org/10.1007/s11356-019-05159-1
The authors declare no competing financial interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Ampleman MD, Martinez A, DeWall J, Rawn DFK, Hornbuckle KC, Thorne PS, 2015. Inhalation and Dietary Exposure to PCBs in Urban and Rural Cohorts via Congener-Specific Measurements. Environ Sci Technol 49, 1156–1164. http://10.1021/es5048039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Bloem TB, Blankenbaker RK, Stanko TA, 1999. Concentrations of polychlorinated biphenyls in the water column of the Laurentian Great Lakes: Spring 1993. J Great Lakes Res 25, 160–170. 10.1016/S0380-1330(99)70724-0 [DOI] [Google Scholar]
- Anezaki K, Nakano T, 2014. Concentration levels and congener profiles of polychlorinated biphenyls, pentachlorobenzene, and hexachlorobenzene in commercial pigments. Environmental Science and Pollution Research 21, 998–1009. http://10.1007/s11356-013-1977-2 [DOI] [PubMed] [Google Scholar]
- Awad AM, Martinez A, Marek RF, Hornbuckle KC, 2016. Occurrence and Distribution of Two Hydroxylated Polychlorinated Biphenyl Congeners in Chicago Air. Environ Sci Technol Let 3, 47–51. http://10.1021/acs.estlett.5b00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicksler J, 1996. PCBs in spring-time water column of the Great Lakes. MS thesis, University of Minnesota, Minneapolis, MN. [Google Scholar]
- Boesen AC, Martinez A, Hornbuckle KC, 2019. PCB congener data of gas-phase, dissolved water, meteorological conditions, and air-water PCB fluxes in southwestern Lake Michigan, 2010. PANGAEA. 10.1594/PANGAEA.897545. [DOI] [Google Scholar]
- Burkhard LP, 2000. Estimating Dissolved Organic Carbon Partition Coefficients for Nonionic Organic Chemicals. Environ Sci Technol 34, 4663–4668. http://10.1021/es001269l [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ, 2005. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res 98, 284–302. http://10.1016/j.envres.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Doskey PV, Andren AW, 1981. Concentrations of Airborne PCBs Over Lake Michigan. J Great Lakes Res 7, 15–20. 10.1016/S0380-1330(81)72018-5 [DOI] [Google Scholar]
- Green ML, Depinto JV, Sweet C, Hornbuckle KC, 2000. Regional spatial and temporal interpolation of atmospheric PCBs: Interpretation of Lake Michigan mass balance data. Environ Sci Technol 34, 1833–1841. http://10.1021/es990374w [Google Scholar]
- Hornbuckle KC, Carlson DL, Swackhamer DL, Baker JE, Eisenreich SJ, 2006. Polychlorinated Biphenyls in the Great Lakes, in: Hites RA (Ed.), Persistent Organic Pollutants in the Great Lakes. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 13–70. [Google Scholar]
- Hornbuckle KC, Sweet CW, Pearson RF, Swackhamer DL, Eisenreich SJ, 1995. Assessing annual water-air fluxes of polychloirnated-biphenyls in Lake Michigan. Environ Sci Technol 29, 869–877. http://10.1021/es00004a006 [DOI] [PubMed] [Google Scholar]
- Hu DF, Hornbuckle KC, 2010. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ Sci Technol 44, 2822–2827. http://10.1021/es902413k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DF, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC, 2010. Atmospheric PCB congeners across Chicago. Atmos Environ 44, 1550–1557. http://10.1016/j.atmosenv.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DF, Martinez A, Hornbuckle KC, 2008. Discovery of Non-Aroclor PCB (3,3 '-Dichlorobiphenyl) in Chicago Air. Environ Sci Technol 42, 7873–7877. http://10.1021/es801823r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiana University, 2017. Integrated Atomospheric Deposition Network (IADN) Data Visualization Tool, IADN Data Viz. Indiana University, Bloomington, Indiana, USA. [Google Scholar]
- Koh WX, Hornbuckle KC, Thorne PS, 2015. Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures. Environ Sci Technol 49, 8105–8112. http://10.1021/acs.est.5b01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovitz LF, 1987. The Particle Mediated Fractionation of PCBs in Lake Michigan. MS thesis, University of Wisconsin, Madison, WI. [Google Scholar]
- Li A, Rockne KJ, Sturchio N, Song WL, Ford JC, Wei H, 2009. PCBs in sediments of the Great Lakes - Distribution and trends, homolog and chlorine patterns, and in situ degradation. Environ Pollut 157, 141–147. http://10.1016/j.envpol.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Livingstone DM, Imboden DM, 1993. The nonlinear influence of wind-speed variability on gas transfer in lakes. Tellus B 45, 275–295. http://10.1034/j.1600-0889.1993.t01-2-00005.x [Google Scholar]
- Magar VS, Brenner RC, Johnson GW, Quensen JF, 2005. Long-Term Recovery of PCB-Contaminated Sediments at the Lake Hartwell Superfund Site: PCB Dechlorination. 2. Rates and Extent. Environ Sci Technol 39, 3548–3554. http://10.1021/es0486216 [DOI] [PubMed] [Google Scholar]
- Martinez A, Awad AM, Herkert NJ, Hornbuckle KC, 2019. Determination of PCB fluxes from Indiana Harbor and Ship Canal using dual-deployed air and water passive samplers. Environ Pollut 244, 469–476. 10.1016/j.envpol.2018.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hadnott BN, Awad AM, Herkert NJ, Tomsho K, Basra K, Scammell MK, Heiger-Bernays W, Hornbuckle KC, 2017. Release of Airborne Polychlorinated Biphenyls from New Bedford Harbor Results in Elevated Concentrations in the Surrounding Air. Environ Sci Technol Let 4, 127–131. http://10.1021/acs.estlett.7b00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hornbuckle KC, 2011. Record of PCB congeners, sorbents and potential toxicity in core samples in Indiana Harbor and Ship Canal. Chemosphere 85, 542–547. http://10.1016/j.chemosphere.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Wang K, Hornbuckle KC, 2010. Fate of PCB Congeners in an Industrial Harbor of Lake Michigan. Environ Sci Technol 44, 2803–2808. http://10.1021/es902911a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Green ML, Depinto JV, Hornbuckle KC, 2001. Results from the Lake Michigan mass balance study: Concentrations and fluxes of atmospheric polychlorinated biphenyls and trans-nonachlor. Environ Sci Technol 35, 278–285. http://10.1021/es991463b [DOI] [PubMed] [Google Scholar]
- Miller SM, Hornbuckle KC, 2010. Spatial and temporal variations of persistent organic pollutants impacted by episodic sediment resuspension in southern Lake Michigan. J Great Lakes Res 36, 256–266. http://10.1016/j.jglr.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwobi FN, Ugomma CA, 2014. Comparison of Methods for the Estimation of Weibull Distribution Parameters. Metodol zv 1, 65–78. [Google Scholar]
- Offenberg JH, Baker JE, 2000. PCBs and PAHs in southern Lake Michigan in 1994 and 1995: Urban atmospheric influences and long-term declines. J Great Lakes Res 26, 196–208. 10.1016/S0380-1330(00)70686-1 [DOI] [Google Scholar]
- Rastogi SC, 1992. Investigaction of Isomer Specific Polychlorinated-Biphenyls in printing inks. Bull Environ Contam Toxicol 48, 567–571. [DOI] [PubMed] [Google Scholar]
- Rodenburg LA, Meng QY, 2013. Source Apportionment of Polychlorinated Biphenyls in Chicago Air from 1996 to 2007. Environ Sci Technol 47, 3774–3780. http://10.1021/es305024p [DOI] [PubMed] [Google Scholar]
- Salamova A, Venier M, Hites RA, 2015. Revised Temporal Trends of Persistent Organic Pollutant Concentrations in Air around the Great Lakes. Environ Sci Technol Let 2, 20–25. http://10.1021/acs.estlett.5b00003 [DOI] [PubMed] [Google Scholar]
- Schwarzenbach RP, Gschwend PM, Imboden DM, 2003. Environmental Organic Chemistry, 2nd ed. John Wiley & Sons Inc., New York. [Google Scholar]
- Simcik MF, Zhang HX, Eisenreich SJ, Franz TP, 1997. Urban contamination of the Chicago coastal Lake Michigan atmosphere by PCBs and PAHs during AEOLOS. Environ Sci Technol 31, 2141–2147. http://10.1021/es9609765 [Google Scholar]
- Streets SS, Henderson SA, Stoner AD, Carlson DL, Simcik MF, Swackhamer DL, 2006. Partitioning and bioaccumulation of PBDEs and PCBs in Lake Michigan. Environ Sci Technol 40, 7263–7269. http://10.1021/es061337p [DOI] [PubMed] [Google Scholar]
- Swackhamer DL, Armstrong DE, 1987. Distribution and characterization of PCBs in Lake Michigan water. J Great Lakes Res 13, 24–36. 10.1016/S0380-1330(87)71624-4 [DOI] [Google Scholar]
- USEPA, 2004. Results of the Lake Michigan Mass Balance Project; Polychlorinated Biphenyls and trans-Nonchlor Data Report. EPA 905 R-01-011.
- Venier M, Dove A, Romanak K, Backus S, Hites R, 2014. Flame Retardants and Legacy Chemicals in Great Lakes’ Water. Environ Sci Technol 48, 9563–9572. http://10.1021/es501509r [DOI] [PubMed] [Google Scholar]
- Zhang H, Eisenreich SJ, Franz TR, Baker JE, Offenberg JH, 1999. Evidence for Increased Gaseous PCB Fluxes to Lake Michigan from Chicago. Environ Sci Technol 33, 2129–2137. http://doi:10.1021/es981073+ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.