Abstract
Alzheimer’s disease (AD) is a growing problem worldwide, and there are currently no effective treatments for this devastating disease. The neurotrophic growth factors insulin and insulin-like growth factor-I (IGF-I) are currently being investigated as potential therapeutic approaches for AD in preclinical and clinical studies. However, given that the metabolic syndrome (MetS) and diabetes are risk factors for AD, it is unknown how associated insulin resistance (IR) in the brain may impact the effectiveness of these therapies for AD. In this report, we therefore investigated the mechanisms underlying the effects of insulin and IGF-I on AD-associated pathology in the context of IR, with particular emphasis on phosphorylation of amyloid precursor protein (APP), a key step in promoting amyloid plaque formation in AD. Both insulin and IGF-I decreased APP phosphorylation in cultured primary cortical neurons, supporting their therapeutic use in AD. Induction of IR blocked the beneficial effect of insulin and reduced the effect of IGF-I on APP dephosphorylation. These effects were mediated by the phosphatidylinositol 3- kinase (PI3-K)/protein kinase B (Akt) pathway, as inhibition of this pathway during IR restored the effect of IGF-I on APP dephosphorylation. Finally, we explored the translational relevance of these results in vivo by demonstrating that high fat diet fed mice, a robust model of IR and MetS, exhibited the expected increased brain APP phosphorylation. Overall, these data suggest that the beneficial therapeutic effect of insulin and IGF-I on APP phosphorylation is negatively impacted by IR, and suggest that insulin and IGF-I alone may not be appropriate therapies for AD patients with IR, MetS, or diabetes.
Keywords: Alzheimer’s disease, IGF-I, insulin, amyloid precursor protein, insulin resistance
Introduction
Alzheimer’s disease (AD) affects millions of people worldwide (Brookmeyer et al., 2018; Fiest et al., 2016); however, there are currently no effective disease modifying treatments for patients. The two pathological hallmarks of AD are toxic protein aggregates of amyloid beta (Aβ) peptides, and neurofibrillary tangles composed of hyperphosphorylated tau (Masters CL, 2015; Selkoe et al., 2012). Aβ peptides are generated via amyloidogenic processing of amyloid precursor protein (APP) by β- and ү-secretases (Kim and Feldman, 2012; Masters CL, 2015). In addition to proteolytic cleavage, the phosphorylation state of APP contributes to amyloid pathology. APP phosphorylation at the threonine 668 residue increases Aβ generation (Lee et al., 2003) and produces neurotoxic cytoplasmic fragments that promote tau hyperphosphorylation (Shin et al., 2007). Pathological changes appear in the brains of patients long before the onset of clinical symptoms of cognitive decline (Ballard et al.; Villemagne et al., 2013), and many therapies aimed at combating cognitive symptoms and AD pathology have failed clinical trials (Anderson et al., 2017; Gauthier et al., 2016). Why these therapies have failed is a matter of debate; however, it is theorized that starting treatment following the onset of cognitive symptoms may be too late to alter disease progression (Aisen et al., 2013; Anderson et al., 2017; Mehta et al., 2017).
Insulin and insulin-like growth factor-I (IGF-I) are neurotrophic factors currently being investigated as therapies for AD and cognitive decline (Ashpole et al., 2015; Avgerinos et al., 2018; Benedict et al., 2004; Claxton et al., 2015; Rajasekaret al., 2017; Reger et al., 2008; Trejo et al., 2007). In the brain, insulin binds its cognate receptor to activate downstream pathways (Gubbi et al., 2018; Kim and Feldman, 2012; van der Heide et al., 2006), resulting in pleotropic neuroprotective effects (Kim and Feldman, 2015; van der Heide et al., 2006). Insulin signaling via activation of phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (Akt) and extracellular signal regulated kinase (ERK) pathways improves learning and memory (Kim and Feldman, 2015; van der Heide et al., 2006), promotes neuronal growth, and increases neuronal survival (Kim and Feldman, 2012; Renault et al., 2009). IGF-I primarily signals through the IGF-I receptor (IGF-IR) to activate PI3-K/Akt and ERK pathways, promoting neuronal differentiation, survival, and synapse formation (Hodge et al., 2007; McGinley et al., 2016; Zaka et al., 2005). IGF-I regulates neurogenesis (Mir et al., 2017; Nieto-Estevez et al., 2016; Ziegler et al., 2015), and like insulin, mediates neuroprotection (Fernandez and Torres-Aleman, 2012). IGF-I is produced locally within the central nervous system (CNS), and both insulin and IGF-I can enter from the systemic circulation by crossing the blood brain barrier (Carro et al., 2000; Csajbók and Tamás, 2016; Fernandez et al., 1999; Fernandez and Torres-Aleman, 2012). The receptors and downstream signaling molecules for both factors are expressed throughout the periphery and CNS (Ashpole et al., 2015; Avgerinos et al., 2018; Claxton et al., 2015; Kim and Feldman, 2012; Rajasekaret al., 2017; Trejo et al., 2007). Pre-clinical and clinical studies of ischemic injury (Lioutas et al., 2015) and amyotrophic lateral sclerosis (Dodge et al., 2008) further show that the neurotrophic effects of insulin and IGF-I contribute to neuroprotection and mitigate pathology in the CNS.
The role of insulin and IGF-I in the development and progression of cognitive aging and AD, however, is controversial (Deak and Sonntag, 2012; Sonntag et al., 2005; Van Fleemst, 2010). There is evidence for altered insulin receptor, IGF-IR, and IGF-I levels in AD patients (Gasparini and Xu, 2003; Gubbi et al., 2018; Moloney et al., 2010; Westwood et al., 2014; Zemva and Schubert, 2011; Zheng and Tong, 2017), suggesting that abnormalities in insulin/IGF-I signaling may be relevant to disease pathology, but implications of these abnormalities are yet to be elucidated. Preclinical evidence from murine models of AD has also revealed the complexity and inconsistency of the role of insulin and IGF-I signaling in AD pathology (Frater et al., 2018; Guo et al., 2017; Pardo et al., 2016). Insulin decreases intracellular Aβ and promotes Aβ delivery to the plasma membrane and extracellular space in vitro (Gasparini et al., 2001). Peripheral administration of IGF-I likewise increases Aβ clearance from the brains of aging rats in one study (Carro et al., 2002), but this finding is not replicated in other AD models (Lanz et al., 2008). Adding to the inconsistencies regarding the role of insulin and IGF-I on Aβ clearance, findings on IGF-IR activation in the context of neuronal toxicity also remain controversial. IGF-I protects hippocampal neurons against Aβ toxicity (Dore et al., 1997), yet several studies using neuronal knockout models demonstrate that reduced IGF-I signaling can mitigate disease pathology (George et al., 2017; Gontier et al., 2015; Gubbi et al., 2018). Specific to APP metabolism, insulin and IGF-I decrease APP phosphorylation in neuronal cell lines (Pandini et al., 2013) and promote non-amyloidogenic processing (Song et al., 2018). Collectively, these studies provide rationale for investigating the potential of insulin and IGF-I as treatments for AD (Avgerinos et al., 2018; Hu et al., 2016).
Insulin resistance (IR) is a common pathological characteristic of metabolic dysfunction (Frisardi et al., 2010; Grundy, 1999; Kim and Feldman, 2015; Tabák et al., 2009; Weyer et al., 1999) and there is growing literature to suggest the presence of IR in the CNS of AD patients (Arnold et al., 2018; Kim and Feldman, 2015). IR is a central feature of metabolic syndrome (MetS) and type 2 diabetes (Grundy, 1999; Tabák et al., 2009; Weyer et al., 1999), which are considered risk factors for AD (Frisardi et al., 2010; Kim et al., 2015; Li et al., 2015). IR develops primarily in response to lifestyle factors, particularly consumption of a high fat or western diet (Czech, 2017; Meikle and Summers, 2017), and has detrimental effects on many tissues and systems, including the CNS (Arnold et al., 2018; Kim and Feldman, 2015; Moloney et al., 2010; Talbot et al., 2012). Similar to pathological AD changes in the brain, IR can occur years prior to the onset of clinical symptoms (Talbot et al., 2012). Interestingly, some studies report that improving IR by metformin, the most widely prescribed anti-diabetic medicine, alleviates AD symptoms (Chen et al., 2016; Koenig et al., 2017; Li et al., 2012), although the efficacy and mechanisms of metformin in AD are still controversial (Barini et al., 2016; Chiang et al., 2016; Chung et al., 2017; Son et al., 2016). Hence, while clinical studies link IR and AD, currently little is known about how IR may influence the effectiveness of either insulin or IGF-I in the treatment of AD, and how the downstream signaling of insulin or IGF-I intersects with IR to affect APP processing in the brain. In the current study, we used our established in vitro (Kim et al., 2015; Kim et al., 2011b) model of AD pathology to investigate how insulin and IGF-I signaling impact APP phosphorylation alone and in the presence of IR, and we further utilized an in vivo model of IR and MetS (Kim et al., 2009; Kim et al., 2015) to examine the presence of APP phosphorylation in the brain under IR conditions.
Materials and methods
Antibodies and chemicals
All antibodies were purchased from Cell Signaling (Beverly, MA), except the polyclonal antibody against actin (Abcam, Cambridge, MA) and monoclonal antibody against total APP (Millipore, Billerica, MA). Inhibitors (LY294002 and U0126) were purchased from Calbiochem (La Jolla, CA). All other chemicals were purchased from either Sigma (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ).
Rat embryonic cortical neuron (eCN) preparation
Primary eCN were harvested from E15 embryos of Sprague Dawley rats according to published protocols (Kim et al., 2015; Kim et al., 2011b). Briefly, the cortex was dissected and dissociated using trypsin and plated in 12-well tissue culture plates coated with poly-L-lysine. Cells were maintained in feed media [(Neurobasal media (Invitrogen, Grand Island, NY), supplemented with 1X B27 (Invitrogen), antibiotics (penicillin, streptomycin, and neomycin; Sigma), 2.5 μg/ml albumin, 10 μg/ml apo-transferrin, 0.1 μg/ml biotin, 15 μg/ml D-galactose, 7 ng/ml progesterone, 16 μg/ml putrescine, 4 ng/ml selenium, 3 ng/ml μ-estradiol, 4 ng/ml hydrocortisone, 3 μg/ml catalase, and 2.5 μg/ml SOD]. Half of the media was replaced with fresh feed media every other day. On day 7, to exclude any effect of insulin contained in B27, culture media was changed to treatment media (feed media without B27) 2-4 h before treatment.
Western immunoblotting
eCN were lysed in RIPA buffer (Pierce, Rockford, IL) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Lysates were collected, briefly sonicated, and centrifuged at 13,000 rpm for 20 min at 4°C. Western immunoblotting was performed as described previously (Kim et al., 2015). TBS with 0.01% Tween-20 and 3% BSA were used for blocking and primary antibody dilutions. Primary antibodies were incubated at 4°C overnight. Secondary antibodies were diluted in 5% fat-free milk and incubated for 2 h at room temperature. Signal was visualized using enhanced chemiluminescence reagents (ECL, Amersham Bioscience, Piscataway, NJ) or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL). Images were captured using the Chemidoc XRS system and analyzed by Quantity One software (Bio-Rad Laboratory, Hercules, CA).
Animals
Animal protocols were approved by the University of Michigan Institutional Animal Care and Use Committee. Mice were housed in a pathogen-free environment and cared for in accordance with University of Michigan guidelines. C57BL6 mice, a strain commonly used to study diet-induced obesity (Hinder et al., 2017; O’Brien et al., 2018; O’Brien et al., 2014), were purchased from Jackson Laboratory (C57BL/6J #000664). Obesity was induced by placing mice on a high fat diet (HFD) consisting of 54% kcal from fat (Research Diets Inc., New Brunswick, NJ #D05090701) at 4 wk of age until 24 wk of age, while age-matched controls were fed a standard diet consisting of 10% kcal from fat (Research Diets Inc. #D12450B). Our laboratory has previously published a detailed phenotypical analysis of these HFD mice (Hinder et al., 2017; Kim et al., 2015; O’Brien et al., 2018), which exhibit a MetS phenotype, including obesity, impaired glucose tolerance, and hyperinsulinemia, as well as cognitive deficits.
Mice were euthanized per our published protocols (O’Brien et al., 2018; Oh et al., 2010) with an overdose of sodium pentobarbital (Fatal-plus, Vortech Pharmaceutical, Dearborn, MI). For Western immunoblotting analyses, brains were cut in half and the cortex halves were separated, snap frozen in liquid nitrogen, and stored at −80°C until use. At least 6 animals were analyzed for each treatment, and representative Western immunoblotting results are presented in the corresponding figure.
Statistical analysis
All experiments were repeated at least 4 times and results are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed by Student’s t-test using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Statistical significance was defined as p < 0.05.
Results
Insulin and IGF-I reduce T668-APP phosphorylation via Akt
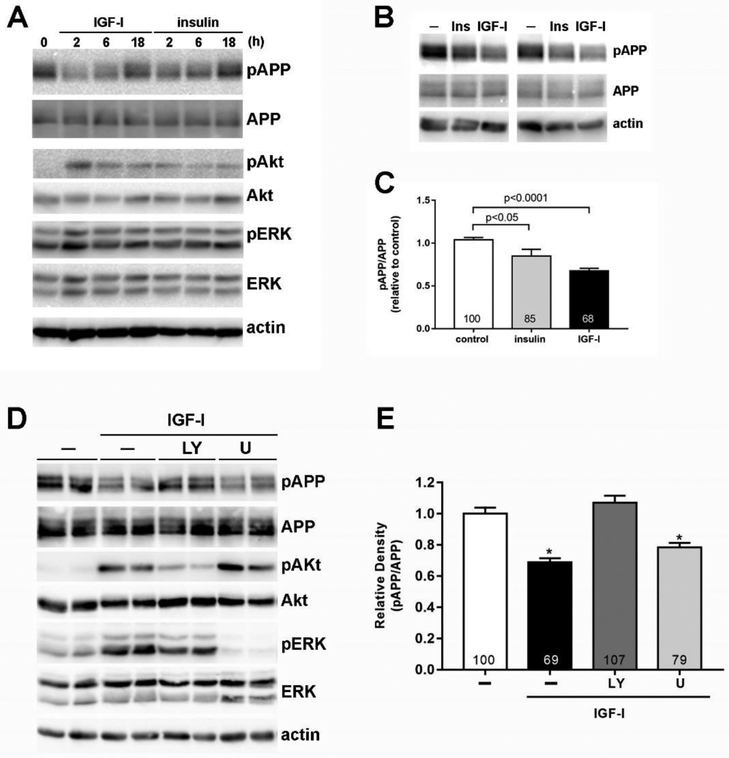
APP phosphorylation at threonine 668 (T668) is one of the key posttranslational modifications that yields the production of Aβ (Matsushima et al., 2012; Pandini et al., 2013; Ramelot and Nicholson, 2001). Therefore, we first examined the effect of insulin and IGF-I on the phosphorylation of T668-APP in rat eCN. Both insulin and IGF-I decreased APP phosphorylation for up to 6 h in a time-dependent manner, with phosphorylation levels returning to baseline after 24 h (Fig 1A). This effect was more significant with IGF-I (68% of the control, p<0.0001) compared to insulin (85% of the control, p<0.05; Fig 1C). We also confirmed that insulin and IGF-I increased Akt Ser473 and ERK Thr202/Thr204 phosphorylation (Fig. 1A). We found that the effect of IGF-I on APP phosphorylation was blocked by inhibiting Akt activation using the PI3-K/Akt inhibitor LY294002; however, the ERK inhibitor, U0126, had no effect on IGF-I-mediated APP dephosphorylation (Fig 1D & E). LY294002 and U016 had a similar effect on insulin-induced decreases in APP phosphorylation (data not shown). These results demonstrate that insulin and IGF-I decrease APP phosphorylation, and that this decrease in phosphorylation is mediated through the PI3-K/Akt pathway.
Figure 1. Insulin and IGF-I decrease APP phosphorylation through PI3-K/Akt-mediated pathways in embryonic cortical neurons (eCN).
(A) Rat primary eCN cells were treated with 20 nM insulin or IGF-I for the indicated times and cell lysates were analyzed by Western blotting with the indicated antibodies. (B) eCN were treated without or with 20 nM insulin or IGF-I for 2 h and (C) changes in APP phosphorylation were analyzed against total APP by densitometry. Fold changes compared to control are indicated in the bars. (D) eCN were treated with 20 μM LY294002 (LY) or U0126 (U) for 1 h and then treated with 20 nM IGF-I for 2 h. (E) The effect of LY and U on APP phosphorylation was analyzed by densitometry. Results are shown mean ± SEM by Student t-test. % changes compared to control are indicated in the bars. *p<0.05 compared to control or LY94002 treated cells. All experiments were repeated at least 4 times and representative images are shown.
IR reduces the effects of insulin and IGF-I on T668-APP phosphorylation
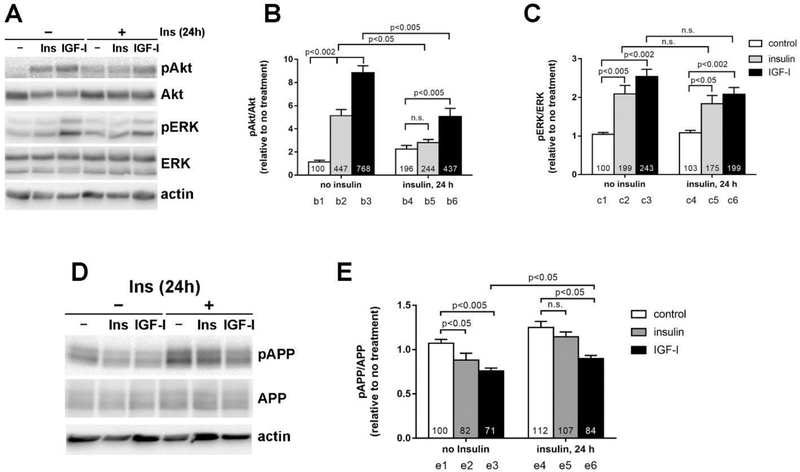
We next employed our previously established in vitro model of hyperinsulinemia induced IR in eCN (Kim et al., 2011a; Kim et al., 2011b), which consists of chronic 50 nM insulin pretreatment for 24 h. We then treated the cells acutely with 20 nM insulin or IGF-I. In agreement with our previous report (Kim et al., 2011b), acute insulin treatment of IR eCN failed to further increase Akt phosphorylation (p>0.05; Fig 2A). Flowever, IGF-I retained the ability to significantly increase Akt phosphorylation in IR eCN, although this increase was significantly lower (60% lower, Fig 2B bars b3 vs b6, p <0.005) than in non-IR cells. Insulin- and IGF-I-induced ERK phosphorylation was unaffected by chronic insulin pretreatment (p>0.05; Fig 2A). Induction of IR increased T668-APP phosphorylation (12% greater than the control, Fig 2E compare bars e1 to e4; p<0.05). In addition, both insulin (80% of the controls, Fig 2E bar e2; p<0.05) and IGF-I (70% of the controls, Fig 2E bar e3; p<0.005) reduced APP phosphorylation under normal non-IR conditions. However, in IR eCN, insulin failed to reduce APP phosphorylation (Fig 2E bar e5; p>0.05). Although IGF-I maintained the ability to reduce APP phosphorylation in IR cells (Fig 2E bar e6; p<0.05), its effects were diminished, with a significant difference in APP dephosphorylation response between IR and normal non-IR conditions (15% difference, Fig 2E bars e3 vs e6, p<0.05). These results suggest that IR induced by chronic insulin treatment prevents the effect of insulin and reduces the effect of IGF-I on T668-APP phosphorylation.
Figure 2. Chronic insulin treatment (IR) and reduced insulin- and IGF-I-mediated decrease in APP phosphorylation.
eCN were treated with 50 nM insulin for 24 h to model IR and then treated with 20 nM insulin or IGF-I for 2 h (A). Akt (B) and ERK (C) phosphorylation were analyzed against the total protein. (D) APP phosphorylation was analyzed by Western blotting and (E) measured against total APP level. The numbers inside the bars indicate the % changes compared to no treatment. Results are shown mean ± SEM by Student t-test.
Akt mediates IR effects on APP phosphorylation
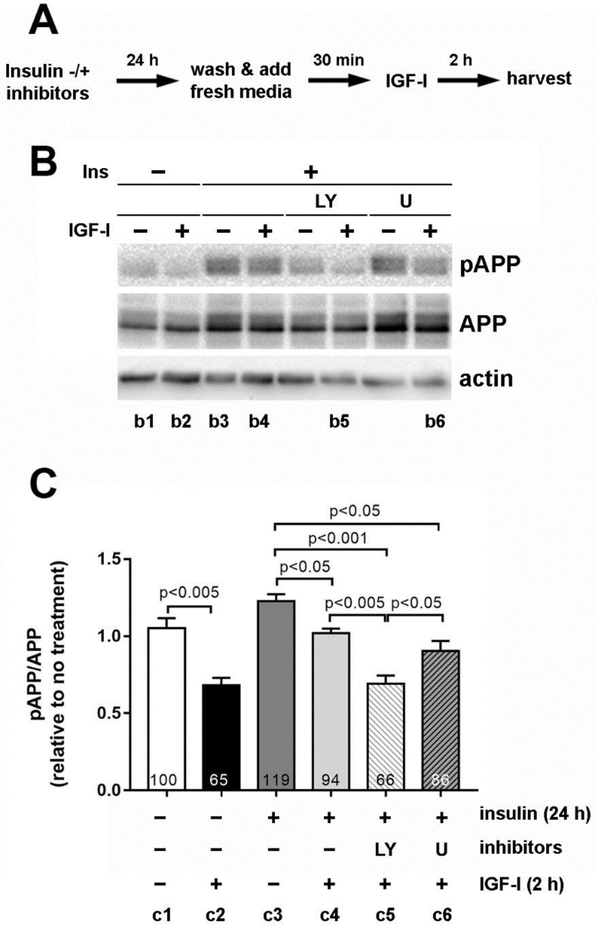
We and others previously hypothesized that over-activation of Akt signaling due to hyperinsulinemia contributes to decreased insulin sensitivity and signaling, resulting in IR (Kim et al., 2011a; Kim et al., 2011b; Liu et al., 2009). We therefore confirmed the role of PI3-K/Akt signaling on T668-APP phosphorylation. We blocked this pathway using specific inhibitors for PI3-K (LY294002) and ERK (U0126) in our in vitro model of IR. During chronic insulin pretreatment for 24 h (IR), eCN were incubated without or with the inhibitors LY294002 or U0126, followed by acute insulin or IGF-I treatment without inhibitors. In line with the above results (Fig 2B), the ability of IGF-I to decrease APP phosphorylation was again diminished in IR (Fig 3B). However, LY294002 restored the effect of IGF-I on APP phosphorylation in the presence of IR (Fig 3C compare bars c2, c4 & c5). The ERK inhibitor U0126 had no effect (bars 4 vs 6, p>0.05), indicating a biological regulation specific to the Akt pathway. Densitometric analysis (Fig 3C) confirmed that LY294002 treatment restored APP dephosphorylation (compare bars c2 and c5, p>0.05), which was significantly lower than in cells without inhibitors (bar c4, p<0.005) or in cells treated with U0126 (bar c6, p<0.05). Additionally, there was no significant difference between LY294002 (Fig 3C bar c5) and non-IR neurons stimulated acutely with insulin (bar c2, p>0.05). These results suggest that IR induces chronic hyperactivation of Akt and subsequent desensitization of PI3-K/Akt pathway, which prevents an insulin and IGF-I-mediated decrease in T668-APP phosphorylation.
Figure 3. Preventing over-activation of PI3-K/Akt pathway restored IGF-I-mediated decrease in APP phosphorylation under IR conditions.
Rat eCN were incubated with 50 nM insulin along with 20 μM LY294002 (LY) or U0126 (U) for 24 h. The cells were washed and incubated in fresh treatment media for 30 min before 20 nM IGF-I treatment for 2 h. (A) The experimental scheme. (B) Immunoblotting was performed using antibodies against pAPP and APP. Equal protein loading was confirmed with actin immunoblotting. (C) Densitometric analysis of the immunoblot. The relative changes compared to control (c1) are indicated in % inside each bar. All experiments were performed at least 3 times in duplicate and representative images are shown. Results are shown mean ± SEM and analyzed by Student t-test.
T668-APP phosphorylation is increased in the cortex of MetS mice
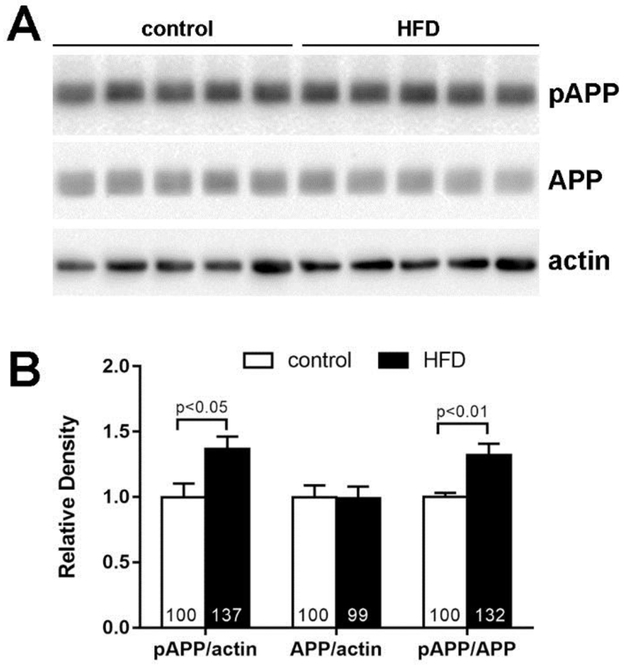
Our in vitro results suggest that neuronal IR negatively affects the therapeutic impact of insulin and IGF-I on AD pathology. Therefore, we confirmed the translational relevance of our in vitro results using a HFD mouse model of MetS that presents with obesity, IR, and cognitive decline (Kim et al., 2015; Sims-Robinson et al., 2016). We observed a significant increase in T668-APP phosphorylation in the cortex of HFD mice compared to age-matched controls fed a standard diet (20% higher than control, p<0.005; Fig 4B). Our results provide a promising animal model to study the effects of AD therapies, including insulin and IGF-I, on tau and APP pathology in the presence of IR.
Figure 4. APP phosphorylation is increased in HFD mouse cortex.
(A) Cortex homogenates from 24 wk control (n=5) and high fat diet (HFD; n=5) mice were analyzed by the Western immunoblotting. Each lane represents a cortex from one animal. (B) The relative density determined by densitometric analysis. The % change is indicated in the bars. Results are shown as mean ± SEM and analyzed by Student t-test.
Discussion
Although AD is the most common neurodegenerative disease worldwide (Fiest et al., 2016), there are no viable treatments for this morbid disorder (Anderson et al., 2017; Liao et al., 2015). In humans, metabolic dysfunction, specifically MetS and diabetes, increases the risk of AD, with IR playing a key role in this process (Ferreira et al., 2018; Kim and Feldman, 2012; Kim and Feldman, 2015). Therefore, the current study addressed the impact of IR on the therapeutic potential of insulin and IGF-I in AD. We show that in the absence of IR, acute insulin and IGF-I treatment reduce T668-APP phosphorylation in eCN. These results suggest that in addition to their neuroprotective effects (Hamabe et al., 2005; Hodge et al., 2007), insulin and IGF-I could improve AD pathology by reducing APP phosphorylation, which may then lead to a decrease in pathogenic Aβ plaques (Shin et al., 2007). In contrast, IR increased T668-APP phosphorylation in eCN and blocked the ability of insulin to reduce APP phosphorylation. IGF-I retained the ability to reduce APP phosphorylation, but to a lesser degree than under non-IR conditions, secondary to continued IGF-I activation of the PI3-K/Akt pathway. Further, we confirmed the translational relevance of our in vitro results when we observed an increase in T668-APP phosphorylation in the cortex of a mouse model of MetS, IR, and cognitive decline (Sims-Robinson et al., 2016), providing a murine model for testing the effects of evolving therapies on cognition and APP biology. Not only do the novel data presented here further support an important role for IR in AD pathology, but they also indicate that insulin and IGF-I alone may not be effective treatments for AD in patients with IR.
Insulin and IGF-I are currently being investigated as novel therapeutics for AD and cognitive decline; however, effects on cognitive deterioration and AD pathology have been mixed. Clinical studies demonstrate that intranasal insulin administration in humans (for >3 weeks) with mild cognitive impairment (MCI) or AD positively impacts cognitive performance (Claxton et al., 2015; Craft et al., 2017). It appears that IGF-I administration has not been investigated in humans with AD or MCI, but treatment of both healthy and MCI patients with growth hormone releasing hormone (GHRH), which increases serum IGF-I levels via the somatotrophic axis, results in improved executive function and a trend towards improved memory (Baker et al., 2012; Sevigny et al., 2008). In contrast, use of a GH secretagogue in patients with AD did not alter disease progression (Sevigny et al., 2008). While these studies evaluated the clinical outcome using cognitive function measures, the role of insulin and IGF-I in regulating amyloid and tau pathology in humans is unclear.
The published literature on the effects of insulin and IGF-I, including endogenous protein and exogenous treatment, on AD pathology to date suggest a complex and often contradictory role for these growth factors in regulation of APP processing, Aβ, or tau pathology. Insulin administration protects against Αβ induced synapse loss in neurons (De Felice et al., 2009; Lourenco et al., 2013), and IGF-I protects against Αβ induced toxicity in hippocampal neurons (Dore et al., 1997). Based on existing studies, it is unclear whether IGF-I promotes Αβ clearance from the brain (Dore et al., 1997; Lanz et al., 2008). In contrast to findings suggesting that IGF-I reduces Aβ levels and toxicity, IGF-IR knockout studies suggest that reducing IGF-IR activation may be beneficial (George et al., 2017; Gontier et al., 2015; Gubbi et al., 2018). Similar to the anti-amyloidogenic effects presented here, others have also observed decreased T668- APP phosphorylation (Pandini et al., 2013), decreased Aβ production (Song et al., 2018), and decreased APP processing (Wang et al., 2014) in transformed human neuroblastoma cell lines and/or primary rat eCN upon insulin or IGF-I treatment. Furthermore, we and others have shown in vitro effects of insulin and IGF-I treatment on tau pathology in primary eCN, including decreased tau phosphorylation (Flong and Lee, 1997; Kim et al., 2015). Collectively, our new data in a primary nontransformed cell, eCN, along with our previously published findings on tau biology (Kim et al., 2015), support the idea that insulin and IGF-I may mitigate AD-associated pathology.
Metabolic dysfunction is a known risk factor for the development of AD (Frisardi et al., 2010; Kim et al., 2015; Li et al., 2015). In particular, IR is a common pathological characteristic linking metabolic dysfunction and AD in both animal models and humans (Friedlander et al., 2001; Frisardi et al., 2010; Kim and Feldman, 2015; Li et al., 2015; Sperling et al., 2019; Talbot et al., 2012). Studies in rodents have demonstrated that brain IR results in AD pathology and cognitive decline (de la Monte et al., 2017; Lester-Coll et al., 2006), and pathology is amplified when Aβ is infused under systemic diabetic conditions (Ma et al., 2013; Su et al., 2019). IR has also been identified in the CNS of AD patients postmortem (Moloney et al., 2010; Talbot et al., 2012). In the current study, using our in vitro model of IR induced by chronic insulin treatment (Kim et al., 2015; Kim et al., 2011b), we show for the first time that IR results in increased T668-APP phosphorylation. Phosphorylation at T668 leads to intracellular sorting and trafficking of APP (Pandini et al., 2013; Ramelot and Nicholson, 2001), which in turn impacts proteolytic cleavage and increases A;β production (Matsushima et al., 2012). Phosphorylated T668-APP also promotes tau accumulation and Aβ plaques (Shin et al., 2007), promoting the spread of AD pathology (Aplin et al., 1996; Suzuki et al., 1994; Taru et al., 2002) and increasing cognitive impairment (Sperling et al., 2019). As data here and from others (Frisardi et al., 2010; Kim and Feldman, 2012; Moloney et al., 2010; Talbot et al., 2012) continue to emphasize that IR is not only present in AD, but contributes to AD pathology, it is essential to consider the clinical importance of IR when studying therapeutic approaches.
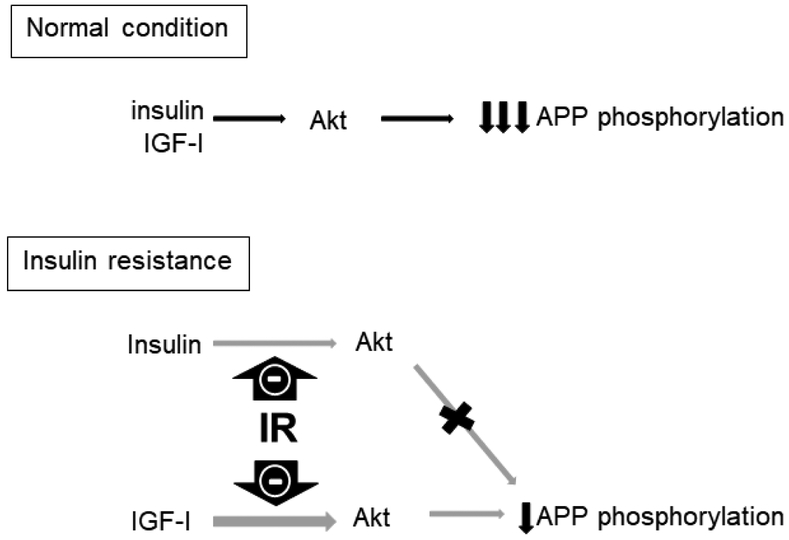
In the present study we also show that IR impacts the therapeutic effectiveness of insulin and IGF-I treatment. IR blocked the insulin-mediated decrease in T668-APP phosphorylation in eCN and partially prevented the ability of IGF-I to lower T668-APP phosphorylation in these same cells. A similar decrease in T668-APP phosphorylation has been reported in response to nerve growth factor treatment of mouse septal neurons that resulted in further anti-amyloidogenic effects (Triaca et al., 2016); however, studies assessing the treatment effects of insulin and IGF-I on AD pathology under IR conditions have not quantified functional changes in learning and memory in vivo, although they have demonstrated improvements in AD pathology (Pandini et al., 2013; Song et al., 2018; Wang et al., 2014). To our knowledge, only one other report demonstrates alterations in AD pathology in response to IR conditions, namely a change in Aβ generation and secretase activity in IR SH-SY5Y cells and in the brains of db/db mice (Son et al., 2012). However, in that study, positive results were only observed under IR conditions where supraphysiological insulin concentrations were used (Son et al., 2012). Insulin and IGF-I can both signal through the PI3-K/Akt pathway, which is typically protective in cells under metabolically normal, healthy conditions (Duarte et al., 2008; Sanderson et al., 2009). However, both insulin and IGF-I signaling are impaired and the PI3-K/Akt pathway is disrupted as a consequence of IR in dorsal root ganglion neurons and HK-532 human cortical stem cell lines (Kim et al., 2015; Kim et al., 2011b), so the current data in eCN may be generalizable to other neuronal cell types as well. Our data support a mechanism whereby under normal metabolic conditions, treatment with insulin and IGF-I result in an PI3-K/Akt-mediated decrease in APP phosphorylation; however, under conditions of metabolic stress and IR, the beneficial effects of insulin and IGF-I are either blocked or reduced (Figure 5).
Figure 5.
Proposed model summarizing the findings of this study. Under normal conditions, both insulin and IGF-I reduce APP phosphorylation through an Akt-mediated pathway. In IR, the PI3-K/Akt pathway is compromised, leading to an almost complete inhibition of the insulin-mediated reduction in APP phosphorylation. IGF-I still is able to reduce APP phosphorylation under IR conditions, but its effect is greatly reduced.
Another consideration that may also complicate the potential therapeutic effects of insulin and IGF-I is the presence of insulin/IGF-I hybrid receptors in the brain which have a higher affinity for IGF-I versus insulin and may therefore alter insulin signaling (Martinez-Rachadell et al., 2019; Slaaby, 2015). This may partially explain our results demonstrating that eCN are more sensitive to IGF-I treatment compared to insulin treatment. Further studies are thus warranted to better understand the potential contribution of the hybrid receptor to insulin- or IGF-I-mediated treatment effects in the presence of IR. Taken together, our data suggest that IR may be a confounding factor in the efficacy of therapeutic approaches for AD and emphasize the importance of efficacy testing in preclinical models that exhibit metabolic dysfunction as well as AD pathology.
We lastly established the translational relevance of our in vitro findings by demonstrating that a robust mouse model of MetS exhibited increased APP phosphorylation in brain cortex. Our laboratory has published a phenotypical analysis of this HFD model which presents with a MetS phenotype, including obesity and IR, as well as cognitive decline (Hinder et al., 2017; Kim et al., 2015; O’Brien et al., 2018; Sims-Robinson et al., 2016). Brain IR is present as early as 2 weeks after initiation of HFD (Sims-Robinson et al., 2016), and we have also previously shown that dysregulated IR and chronic overactivation of PI3-K/Akt signaling increases tau phosphorylation in the cortex of these mice (Kim et al., 2015). Here, we show that the other hallmark of AD pathology, APP phosphorylation (specifically T668-APP phosphorylation), is also increased in response to IR in this HFD mouse model of MetS. As it has been previously proposed that the development of brain IR is an early event in the progression of cognitive decline (Talbot et al., 2012), we propose a series of events where HFD feeding induces early IR, which then promotes T668-APP phosphorylation that drives subsequent generation of Aβ (Shin et al., 2007) and tau pathology (Kim et al., 2015). This supports our hypothesis that IR is an initial event contributing to pathological AD processing; however, more studies are needed to confirm our hypothesis and fully establish a time course of the disease.
IR-dependent hyperphosphorylation of T668-APP and the refractory response to insulin we observed in vitro are in line with other reports that brain IR in mouse models of diabetes results in increased AD pathology and cognitive decline (de la Monte et al., 2017; Lester-Coll et al., 2006; Ma et al., 2013; Su et al., 2019). Similarly, HFD or high sucrose diets in combination with altered insulin or IGF-I signaling promote AD pathology, memory deficits, and decreased Aβ uptake (Logan et al., 2018) (Busquets et al., 2017; Carvalho et al., 2012; Hiltunen et al., 2012; Ramos-Rodriguez et al., 2017). Most murine models of IR and AD are developed using genetically modified animals, and studies have shown mixed effects of insulin and IGF-I treatment on AD pathology and cognition (Lanz et al., 2008; Yang et al., 2013). Our model provides a unique opportunity to investigate treatment effects on APP and tau pathology in a non-genetically modified animal under IR conditions. Additional preclinical studies using this model will allow us to elucidate these mechanisms and fully understand the therapeutic potential of insulin, IGF-I, and other AD therapies under IR conditions.
Conclusion
In conclusion, our findings in eCN suggest that the therapeutic effectiveness of insulin and IGF-I on AD pathology is reduced under conditions of IR. Additional in vitro studies in other neuronal cell types as well as additional in vivo studies are needed to fully understand the use of insulin and IGF-I alone, or in combination, as therapeutic agents in preclinical models and in AD patients with IR. Further investigation of insulin and IGF-I in the context of IR will also help to clarify their complex role in AD. Overall, these data argue for a critical clinical impact of IR on AD therapies, and have important implications for the development and testing of future therapeutics.
Acknowledgements
Authors would like to acknowledge Faye Mendelson for technical assistance and Stacey Sakowski Jacoby for editorial assistance.
Funding: Funding for this study was provided by the Program for Neurology Research & Discovery, the A. Alfred Taubman Medical Research Institute, the Handelman Emerging Scholar Fund, the Robert E. Nederlander Sr. Program for Alzheimer’s Research, and the National Institutes of Health (T32 DK101357).
Abbreviations
- (AD)
Alzheimer’s disease
- (Aβ)
Amyloid beta
- (APP)
Amyloid Precursor Protein
- (CNS)
Central nervous system
- (eCN)
Embryonic cortical neurons
- (ERK)
Extracellular signal regulated kinase
- (HFD)
High fat diet
- (IGF-I)
Insulin-like growth factor-I
- (IGF-IR)
Insulin-like growth factor-I receptor
- (IGFBP)
Insulin-like growth factor binding proteins
- (IR)
Insulin resistance
- (MCI)
Mild cognitive impairment
- (PI3-K)
Phosphatidylinositol 3-kinase
- (Akt)
Protein kinase B
- (MetS)
The Metabolic Syndrome
- (T668)
Threonine 668
- (T2D)
Type 2 diabetes
Footnotes
Declaration of interest: none (financial or other bias that may influence results)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisen PS, et al. , 2013. Moving towards early clinical trials for amyloid-targeted therapy in Alzheimer’s disease. Nature reviews Drug discovery. 12, 324. [DOI] [PubMed] [Google Scholar]
- Anderson RM, et al. , 2017. Why do so many clinical trials of therapies for Alzheimer’s disease fail? The Lancet. 390, 2327–2329. [DOI] [PubMed] [Google Scholar]
- Aplin AE, et al. , 1996. In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3β. Journal of neurochemistry. 67, 699–707. [DOI] [PubMed] [Google Scholar]
- Arnold SE, et al. , 2018. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nature Reviews Neurology. 14, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, et al. , 2015. Growth hormone, insulin-like growth factor-1 and the aging brain. Experimental gerontology. 68, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgerinos KI, et al. , 2018. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: a systematic review. Journal of neurology. 265, 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, et al. , 2012. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Archives of neurology. 69, 1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, et al. , 2011. 907 Jones E (2011) Alzheimer’s disease. Lancet. 377, 1019–908. [DOI] [PubMed] [Google Scholar]
- Barini E, et al. , 2016. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Molecular neurodegeneration. 11, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, et al. , 2004. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 29, 1326–1334. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, et al. , 2018. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s & Dementia. 14, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets O, et al. , 2017. Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mechanisms of ageing and development. 162, 38–45. [DOI] [PubMed] [Google Scholar]
- Carro E, et al. , 2000. Circulating insulin-like growth factor I mediates effects of exercise on the brain. Journal of Neuroscience. 20, 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, et al. , 2002. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nature medicine. 8, 1390–1397. [DOI] [PubMed] [Google Scholar]
- Carvalho C, et al. , 2012. Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes. 61, 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, et al. , 2016. Metformin alleviated Aβ-induced apoptosis via the suppression of JNK MAPK signaling pathway in cultured hippocampal neurons. BioMed research international. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M-C, et al. , 2016. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against Amyloid-beta-induced mitochondrial dysfunction. Experimental cell research. 347, 322–331. [DOI] [PubMed] [Google Scholar]
- Chung M-M, et al. , 2017. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Experimental cell research. 352, 75–83. [DOI] [PubMed] [Google Scholar]
- Claxton A, et al. , 2015. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. Journal of Alzheimer’s Disease. 44, 897–906. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 2017. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. Journal of Alzheimer’s Disease. 57, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csajbók EA, Tamás G, 2016. Cerebral cortex: a target and source of insulin? Diabetologia. 59, 1609–1615. [DOI] [PubMed] [Google Scholar]
- Czech MP, 2017. Insulin action and resistance in obesity and type 2 diabetes. Nature medicine. 23, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, et al. , 2009. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proceedings of the National Academy of Sciences. 106, 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, et al. , 2017. Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D-959 in an experimental model of sporadic Alzheimer’s disease. Journal of Alzheimer’s Disease. 55, 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Sonntag WE, 2012. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 67, 611–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, et al. , 2008. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Molecular Therapy. 16, 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore S, et al. , 1997. Insulin-like growth factor I protects and rescues hippocampal neurons against β-amyloid-and human amylin-induced toxicity. Proceedings of the National Academy of Sciences. 94, 4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AI, et al. , 2008. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3β signaling pathways and changes in protein expression. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1783, 994–1002. [DOI] [PubMed] [Google Scholar]
- Fernandez A, et al. , 1999. Neuroprotective actions of peripherally administered insulin like growth factor I in the injured olivo-cerebellar pathway. European Journal of Neuroscience. 11,2019–2030. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Alemán I, 2012. The many faces of insulin-like peptide signalling in the brain. Nature Reviews Neuroscience. 13, 225. [DOI] [PubMed] [Google Scholar]
- Ferreira LS, et al. , 2018. Insulin resistance in Alzheimer’s Disease. Frontiers in neuroscience. 12, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiest KM, et al. , 2016. The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Canadian Journal of Neurological Sciences. 43, S51–S82. [DOI] [PubMed] [Google Scholar]
- Frater J, et al. , 2018. Insulin-like growth factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing research reviews. 42, 14–27. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, et al. , 2001. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 86, 1496–1503. [DOI] [PubMed] [Google Scholar]
- Frisardi V, et al. , 2010. Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? Journal of Alzheimer’s Disease. 21, 57–63. [DOI] [PubMed] [Google Scholar]
- Gasparini L, et al. , 2001. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. Journal of Neuroscience. 21,2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Xu H, 2003. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends in neurosciences. 26, 404–406. [DOI] [PubMed] [Google Scholar]
- Gauthier S, et al. , 2016. Why has therapy development for dementia failed in the last two decades? Alzheimer’s & Dementia. 12, 60–64. [DOI] [PubMed] [Google Scholar]
- George C, et al. , 2017. The Alzheimer’s disease transcriptome mimics the neuroprotective signature of IGF-1 receptor-deficient neurons. Brain. 140, 2012–2027. [DOI] [PubMed] [Google Scholar]
- Gontier G, et al. , 2015. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. Journal of Neuroscience. 35, 11500–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, 1999. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. The American journal of cardiology. 83, 25–29. [DOI] [PubMed] [Google Scholar]
- Gubbi S, et al. , 2018. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. Journal of molecular endocrinology. 61, T171–T185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, et al. , 2017. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Scientific reports. 7, 45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamabe W, et al. , 2005. Insulin receptor-protein kinase C-γ signaling mediates inhibition of hypoxia-induced necrosis of cortical neurons. Journal of Pharmacology and Experimental Therapeutics. 313, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Hiltunen M, et al. , 2012. Contribution of genetic and dietary insulin resistance to Alzheimer phenotype in APP/PS1 transgenic mice. Journal of cellular and molecular medicine. 16, 1206–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder LM, et al. , 2017. Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis Model Mech. 10, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, et al. , 2007. Insulin-like growth factor-I (IGF-I) inhibits neuronal apoptosis in the developing cerebral cortex in vivo. International journal of developmental neuroscience. 25, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Lee VM-Y, 1997. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. Journal of Biological Chemistry. 272, 19547–19553. [DOI] [PubMed] [Google Scholar]
- Hu X, et al. , 2016. Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in Alzheimer’s disease: a meta-analysis. Neurological Sciences. 37, 1671–1677. [DOI] [PubMed] [Google Scholar]
- Kim B, et al. , 2009. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 150, 5294–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Feldman EL, 2012. Insulin resistance in the nervous system. Trends in Endocrinology & Metabolism. 23, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Feldman EL, 2015. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Experimental & molecular medicine. 47, e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, et al. , 2015. Insulin resistance prevents AMPK-induced tau dephosphorylation through Akt-mediated increase in AMPKSer485 phosphorylation. Journal of Biological Chemistry. 290, 19146–19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, et al. , 2011a. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 152, 3638–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, et al. , 2011b. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxidants & redox signaling. 14, 1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig AM, et al. , 2017. Effects of the insulin sensitizer metformin in Alzheimer’s disease: Pilot data from a randomized placebo-controlled crossover study. Alzheimer disease and associated disorders. 31, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, et al. , 2008. Peripheral elevation of IGF-1 fails to alter Aβ clearance in multiple in vivo models. Biochemical pharmacology. 75, 1093–1103. [DOI] [PubMed] [Google Scholar]
- Lee M-S, et al. , 2003. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 163, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester-Coll N, et al. , 2006. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. Journal of Alzheimer’s Disease. 9, 13–33. [DOI] [PubMed] [Google Scholar]
- Li J, et al. , 2012. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacology biochemistry and behavior. 101,564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. , 2015. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clinical interventions in aging. 10, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, et al. , 2015. Repetitive transcranial magnetic stimulation as an alternative therapy for cognitive impairment in Alzheimer’s disease: a meta-analysis. Journal of Alzheimer’s Disease. 48, 463–472. [DOI] [PubMed] [Google Scholar]
- Lioutas V-A, et al. , 2015. Intranasal insulin and insulin-like growth factor 1 as neuroprotectants in acute ischemic stroke. Translational stroke research. 6, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. , 2009. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. American Journal of Physiology-Endocrinology and Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, et al. , 2018. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Molecular metabolism. 9, 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco MV, et al. , 2013. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell metabolism. 18, 831–843. [DOI] [PubMed] [Google Scholar]
- Ma Y-Q, et al. , 2013. mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Molecular medicine reports. 7, 623–627. [DOI] [PubMed] [Google Scholar]
- Martinez-Rachadell L, et al. , 2019. Cell-specific expression of insulin/insulin-like growth factor-I receptor hybrids in the mouse brain. Growth Hormone & IGF Research. 45, 25–30. [DOI] [PubMed] [Google Scholar]
- Masters CL, B. R., Blennow K, Rowe CC, Sperling RA, Cummings JL, 2015. Alzheimer’s disease. Nature Reviews Disease Primers. 1. [DOI] [PubMed] [Google Scholar]
- Matsushima T, et al. , 2012. Membrane-microdomain localization of amyloid β- precursor protein (APP) C-terminal fragments is regulated by phosphorylation of the cytoplasmic Thr668 residue. Journal of Biological Chemistry. 287, 19715–19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley LM, et al. , 2016. Human cortical neural stem cells expressing insulin-like growth factor-I: a novel cellular therapy for Alzheimer’s Disease. Stem cells translational medicine. 5, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, et al. , 2017. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert opinion on investigational drugs. 26, 735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle PJ, Summers SA, 2017. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nature Reviews Endocrinology. 13, 79. [DOI] [PubMed] [Google Scholar]
- Mir S, et al. , 2017. IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Scientific reports. 7, 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney AM, et al. , 2010. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 31,224–243. [DOI] [PubMed] [Google Scholar]
- Nieto-Estévez V, et al. , 2016. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Frontiers in neuroscience. 10, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, et al. , 2018. Juvenile murine models of prediabetes and type 2 diabetes develop neuropathy. Disease models & mechanisms. 11, dmm037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, et al. , 2014. Mouse models of diabetic neuropathy. ILAR journal. 54, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SS, et al. , 2010. The effects of anesthesia on measures of nerve conduction velocity in male C57BI6/J mice. Neuroscience letters. 483, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, et al. , 2013. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology. 154, 375–387. [DOI] [PubMed] [Google Scholar]
- Pardo J, et al. , 2016. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. European Journal of Neuroscience. 44, 2120–2128. [DOI] [PubMed] [Google Scholar]
- Rajasekar N, et al. , 2017. Intranasal insulin administration ameliorates streptozotocin (ICV)-induced insulin receptor dysfunction, neuroinflammation, amyloidogenesis, and memory impairment in rats. Molecular neurobiology. 54, 6507–6522. [DOI] [PubMed] [Google Scholar]
- Ramelot TA, Nicholson LK, 2001. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. Journal of molecular biology. 307, 871–884. [DOI] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, et al. , 2017. Progressive neuronal pathology and synaptic loss induced by prediabetes and type 2 diabetes in a mouse model of Alzheimer’s disease. Molecular neurobiology. 54, 3428–3438. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. , 2008. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-β in memory-impaired older adults. Journal of Alzheimer’s Disease. 13, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault VM, et al. , 2009. FoxO3 regulates neural stem cell homeostasis. Cell stem cell. 5, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TH, et al. , 2009. Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurological research. 31, 947–958. [DOI] [PubMed] [Google Scholar]
- Selkoe D, et al. , 2012. Deciphering alzheimer disease. Cold Spring Harbor perspectives in medicine. 2, a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, et al. , 2008. Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 71, 1702–1708. [DOI] [PubMed] [Google Scholar]
- Shin R-W, et al. , 2007. Amyloid precursor protein cytoplasmic domain with phospho-Thr668 accumulates in Alzheimer’s disease and its transgenic models: a role to mediate interaction of Aβ and tau. Acta neuropathologica. 113, 627–636. [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C, et al. , 2016. Dietary reversal ameliorates short-and long-term memory deficits induced by high-fat diet early in life. PloS one. 11, e0163883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaaby R, 2015. Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Scientific reports. 5, 7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SM, et al. , 2016. Metformin facilitates amyloid-β generation by β-and y-secretases via autophagy activation. Journal of Alzheimer’s Disease. 51, 1197–1208. [DOI] [PubMed] [Google Scholar]
- Son SM, et al. , 2012. Altered APP processing in insulin-resistant conditions is mediated by autophagosome accumulation via the inhibition of mammalian target of rapamycin pathway. Diabetes. 61,3126–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, et al. , 2018. Insulin-Like Growth Factor-1 Alleviates Expression of Aβ 1–40 and α-, β-, and γ-Secretases in the Cortex and Hippocampus of APP/PS1 Double Transgenic Mice. Journal of Molecular Neuroscience. 66, 595–603. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, et al. , 2005. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing research reviews. 4, 195–212. [DOI] [PubMed] [Google Scholar]
- Sperling RA, et al. , 2019. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Annals of Neurology. 85, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, et al. , 2019. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Molecular neurobiology. 1–20. [DOI] [PubMed] [Google Scholar]
- Suzuki T, et al. , 1994. Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. The EMBO journal. 13, 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tábak AG, et al. , 2009. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. The Lancet. 373, 2215–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, et al. , 2012. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taru H, et al. , 2002. Interaction of Alzheimer’s β-amyloid precursor family proteins with scaffold proteins of the JNK signaling cascade. Journal of Biological Chemistry. 277, 20070–20078. [DOI] [PubMed] [Google Scholar]
- Trejo J, et al. , 2007. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Molecular psychiatry. 12, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Triaca V, et al. , 2016. NGF controls APP cleavage by downregulating APP phosphorylation at Thr668: relevance for Alzheimer’s disease. Aging Cell. 15, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, et al. , 2006. Insulin signaling in the central nervous system: learning to survive. Progress in neurobiology. 79, 205–221. [DOI] [PubMed] [Google Scholar]
- Van Heemst D, 2010. Insulin, IGF-1 and longevity. Aging and disease. 1, 147. [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, et al. , 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. The Lancet Neurology. 12, 357–367. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. , 2014. Insulin inhibits Abeta production through modulation of APP processing in a cellular model of Alzheimer’s disease. Neuroendocrinology Letters. 35, 224–229. [PubMed] [Google Scholar]
- Westwood AJ, et al. , 2014. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 82, 1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, et al. , 1999. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. The Journal of clinical investigation. 104, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. , 2013. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. Journal of Alzheimer’s Disease. 33, 329–338. [DOI] [PubMed] [Google Scholar]
- Zaka M, et al. , 2005. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Molecular and Cellular Neuroscience. 30, 398–407. [DOI] [PubMed] [Google Scholar]
- Zemva J, Schubert M, 2011. Central insulin and insulin-like growth factor-1 signaling-implications for diabetes associated dementia. Current diabetes reviews. 7, 356–366. [DOI] [PubMed] [Google Scholar]
- Zheng P, Tong W, 2017. IGF-1: an endogenous link between traumatic brain injury and Alzheimer disease? Journal of neurosurgical sciences. 61,416–421. [DOI] [PubMed] [Google Scholar]
- Ziegler AN, et al. , 2015. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nature Reviews Endocrinology. 11, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]