Abstract
Our understanding of the molecular mechanisms underlying differential vulnerability of substantia nigra dopamine neurons in Parkinson’s disease (PD) remains limited, and previous therapeutic efforts targeting rodent nigral neurons have not been successfully translated to humans. However, recent emergence of induced pluripotent stem cell technology has highlighted some fundamental differences between human and rodent midbrain dopamine neurons that may at least in part explain relative resistance of rodent neurons to degeneration in genetic models of PD. Using GBA1-linked PD as an example, we discuss cellular pathways that may predispose human neurons to degeneration in PD, including mitochondrial oxidant stress, elevated intracellular calcium, altered synaptic vesicle endocytosis, accumulation of oxidized dopamine and neuromelanin. Recent studies have suggested that a combination of mitochondrial oxidant stress and accumulation of oxidized dopamine contribute to dysfunction of nigral neurons in various genetic and sporadic forms of PD. We also briefly summarize the development of targeted therapies for GBA1-associated synucleinopathies and highlight that modulation of wild-type GCase activity serves as an important target for the treatment of genetic and idiopathic forms of PD and dementia with Lewy bodies.
Introduction
Neurodegenerative disorders, such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and Alzheimer’s disease (AD), are debilitating conditions characterized by progressive degeneration of specific neurons within the brain of affected individuals. Animal models have offered insights into the understanding of the pathogenesis of disease processes and have been useful for testing new neuroprotective approaches, but the results of preclinical studies in animal models have not often translated to clinical trials in patients. Recent discovery of induced pluripotent stem cell (iPSC) technology has enabled the study of patient-derived cells (Takahashi et al., 2007) and offered an opportunity to perform detailed mechanistic studies of disease in human neurons (Unternaehrer and Daley, 2011), as well as created a source of neurons for transplantation studies and drug screens (Shi et al., 2017). In this review, we will focus on the potential of iPSC-derived neurons to uncover mechanisms of selective vulnerability of midbrain dopaminergic neurons in PD and discuss therapeutic strategies currently being developed using such disease models. We will discuss GBA1-linked PD as an example of genetic forms of PD.
The role of GBA1 in Parkinson’s disease and other synucleinopathies
Parkinson’s disease is estimated to affect approximately 1% of the population older than 65 years of age and 4% of the population above 85. Although most cases of PD are sporadic, about 5%–10% are attributed to inherited PD cases (de Lau and Breteler, 2006). Early prominent degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) results in dopamine deficiency within the basal ganglia that leads to classical parkinsonian motor symptoms such as bradykinesia, muscular rigidity, resting tremors and postural instability (Kalia and Lang, 2015; Schapira and Jenner, 2011). PD is also associated with a number of non-motor symptoms including cognitive impairments, anxiety, and depression, some of which precede the motor dysfunction by more than a decade (Gonera et al., 1997). The discovery of genes linked to rare monogenic forms of the disease has provided novel insight into the molecular mechanisms involved in the pathogenesis of familial and sporadic PD. For example, heterozygous mutations in GBA1, the gene encoding for glucocerebrosidase (GCase), are considered one of the most common genetic risk factors for PD (Wong and Krainc, 2016). GCase is a hydrolase that converts glycosphingolipid glucosylceramide into glucose and ceramide inside the lysosome (Blanz and Saftig, 2016). Compared to idiopathic PD cases, patients with GBA1 mutations have distinctive features including earlier age at onset, greater cognitive decline, and a greater likelihood to have atypical clinical manifestations (Sidransky et al., 2009). Biallelic GBA1 mutations lead to Gaucher’s disease (GD), a rare autosomal recessive lysosomal storage disorder characterized by the accumulation of glycolipids within lysosomes leading to their massive engorgement, the typical hallmark of GD (Aflaki et al., 2017; Hruska et al., 2008). Cognitive impairment is a common problem in the later stages of PD, and mild deficits can be identified even in early-stage disease (Hely et al., 2008). Carriers of GBA1 mutations have a 6-fold increased risk of developing dementia compared with non-carriers (Seto-Salvia et al., 2012). In fact mutations in GBA1 are also a risk factor for the development of dementia with Lewy bodies (DLB), thus pointing towards a link between GBA1, parkinsonism and dementia (Tsuang et al., 2012). But the relationship between GBA1 mutations and the pathogenesis of PD and other Lewy body disorders has not yet been resolved. The neuropathological findings in patients with DLB, PD or GD appear to be typical of other synucleinopathies, characterized by the deposition of Lewy bodies, intraneuronal inclusions composed predominantly of insoluble aggregates of α-synuclein in the brainstem or cortical regions (McKeith et al., 2017; McKeith et al., 2005; Spillantini et al., 1997). This suggests that GCase may contribute to aggregation of α-synuclein through an enhanced protein aggregation mechanism or as a consequence of GCase deficiency (Velayati et al., 2010). A positive feedback loop between diminished GCase function and accumulation of α-synuclein has been proposed (Mazzulli et al., 2011). Perturbed GCase activity leads to accumulation of α-synuclein, which in turn has been shown to interfere with endoplasmic reticulum (ER)-Golgi trafficking of GCase irrespective of the presence of GBA1 mutations (Mazzulli et al., 2011). The build-up of glucosylceramide, the toxic lipid substrate of GCase, interferes with lysosomal processing of α-synuclein by stabilizing soluble oligomeric intermediates leading to toxic α-synuclein aggregation (Mazzulli et al., 2011). These bidirectional effects between GCase and α-synuclein might form a self-propagating positive-feedback loop in PD and other synucleinopathies and also suggest that GCase plays a role in the pathogenesis of sporadic PD.
Determinants of degeneration of midbrain dopamine neurons in GBA1-linked PD
Although the bidirectional feedback loop uncovers a possible mechanism of how loss of GCase and accumulation of α-synuclein are linked in PD pathogenesis, it does not explain the preferential loss of midbrain dopamine neurons in PD. In fact, α-synuclein and GCase – and other disease-associated proteins – are widely expressed in the brain and selective neuronal vulnerability occurring in a distinct brain region cannot be based on their (wildtype or mutant) expression alone. Also, among synucleinopathies different cell types are affected, all of which accumulate α-synuclein and undergo degeneration at a certain stage, i.e. dopaminergic neurons in the SNpc in PD and cortical neurons in DLB (Wong and Krainc, 2017). In PD, degeneration of the neuromelanin-containing dopaminergic neurons in the SNpc is by far the most pronounced but degeneration is not limited to this region. Disease pathology also occurs in other brain stem nuclei among which are the dorsal motor nucleus of the vagus and the locus coeruleus (Surmeier et al., 2017). These neuronal populations appear to share high basal mitochondrial stress that predisposes them to other insults such as genetic mutations or toxin exposure (Surmeier and Sulzer, 2013). This basal stress appears to be due to high mitochondrial oxidant stress (Pacelli et al., 2015), broad action potentials and autonomous pacemaking that are accompanied by large oscillations in intracellular calcium concentration combined with low intrinsic calcium buffering (Surmeier et al., 2017). Affected neuronal populations counteract this bioenergetic burden by buffering extensive (mitochondrial) oxidant stress through accumulation of neuromelanin, apparently as a neuroprotective response. While neuromelanin has traditionally been considered an inert cellular byproduct of dopamine metabolism, recent evidence suggests a possible physiological role of neuromelanin (Fedorow et al., 2005). It has been proposed that the formation of neuromelanin protects the cell against an overload of harmful agents by interacting with transition metals, especially iron, and removing excess cytosolic dopamine, which otherwise would be oxidized by iron to form dopamine-o-quinones (DAQs), toxic dopamine derivates that can enter into pathways forming adducts with amino acid residues (mainly cysteine residues) of proteins (Zucca et al., 2017). However, it has also been demonstrated that neuromelanin released from damaged or dying dopaminergic neurons into the extracellular environment can activate surrounding microglia thereby promoting the degeneration of neighboring neurons inducing a neurodegenerative process via an inflammatory mechanism (Wilms et al., 2003; Zecca et al., 2008; Zecca et al., 2003; Zhang et al., 2011). In addition, murine and human stem cell-derived dopamine neurons have been shown to express the major histocompatibility complex class I (MHC-I) for the presentation of antigenic peptides on neuronal membranes in response to microglia activation suggesting another potential toxic process involving neuromelanin (Cebrian et al., 2014). Cytosolic dopamine has long been known to be potentially toxic because of its oxidation to reactive DAQs (Greenamyre and Hastings, 2004). Several studies have shown that the buildup of cytosolic dopamine is associated with oxidant stress and that dopamine is found to react, modify or inactivate lipids and proteins contributing to selective vulnerability and reduced survival of dopaminergic neurons in PD (Burbulla et al., 2017; Caudle et al., 2008; Chen et al., 2008; Conway et al., 2001; LaVoie et al., 2005; Norris et al., 2005; Sulzer and Zecca, 2000). Recently, we showed that dopamine oxidation is a common feature in multiple forms of PD using iPSC-derived dopamine neurons from familial and sporadic PD cases (Burbulla et al., 2017). We identified a time-dependent pathogenic cascade starting with mitochondrial oxidant stress that promotes the generation of DAQs. These species of oxidized dopamine led to disruption of GCase activity, diminished lysosomal function and accumulation of α-synuclein. Previous reports from our and other laboratories have shown that wild-type GCase activity is reduced in brain tissue and iPSC-derived neurons of idiopathic PD patients, as well as other genetic forms of PD without GBA1 mutation (Burbulla et al., 2017; Chiasserini et al., 2015; Gegg et al., 2012; Mazzulli et al., 2016; Murphy et al., 2014; Nguyen and Krainc, 2018), but the mechanism has been unclear. We found that dopamine modifies GCase on one its key cysteine residues in the catalytic site, and hypothesized that this modification causes the decline in wild-type enzymatic activity in dopamine neurons (Burbulla et al., 2017).
Mechanistic pathways driving accumulation of oxidized dopamine in human neurons
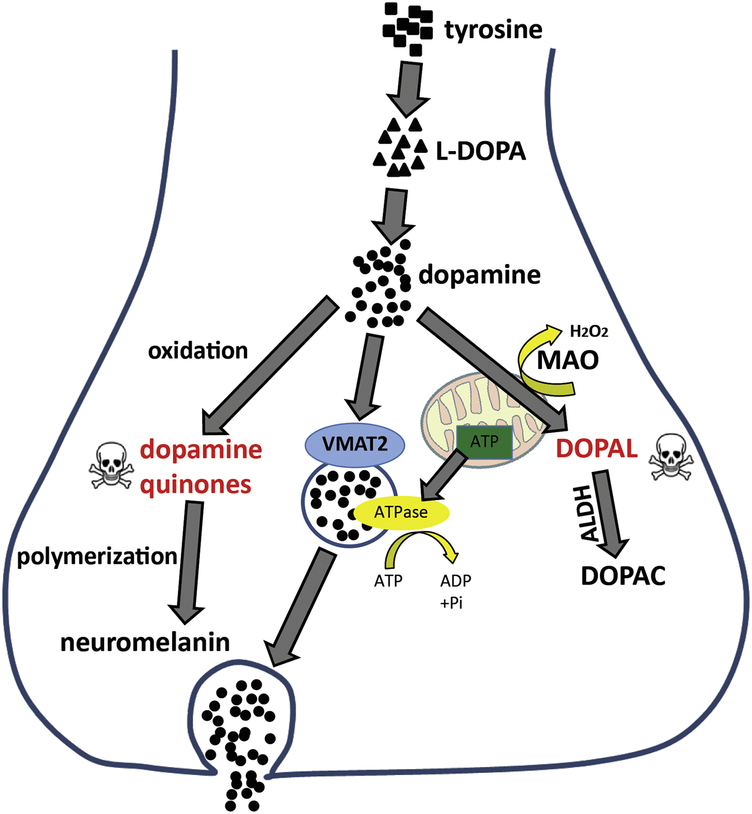
Several mechanistic pathways have been suggested to be involved in the alteration of dopamine metabolism resulting in the oxidation of dopamine. To prevent the generation of DAQs, dopamine must either be efficiently sequestered into synaptic vesicles through active, ATP dependent transport by vesicular monoamine transporter 2 (VMAT2) or catabolized through mitochondrial monoamine oxidase (MAO) and aldehyde dehydrogenase enzymes (ALDH) to non-toxic metabolites (Fig. 1). Failure of one of these pathways or mitochondrial dysfunction – and consequently reduced ATP supply – will lead to disturbances in presynaptic dopamine handling, accumulation of the toxic intermediate 3,4-dihydroxyphenylacetaldehyde (DOPAL) and may result in neuron death. Monoamine oxidases (MAO) A and B, mitochondrial enzymes responsible for the oxidative deamination of dopamine, are essential for the catabolism of cytosolic dopamine (Shih et al., 1999). Keeping MAO at balanced levels seems to be critical as lack of enzymatic activity would subsequently increase the availability of cytosolic dopamine while lowering MAO activities would prevent the production of large quantities of toxic dopamine metabolites such as DOPAL and H2O2, two potent oxidizing agents (Burke et al., 2004; Eisenhofer et al., 2004; Goldstein et al., 2013; Meiser et al., 2013). Data from studies investigating the role of MAO in neurodegeneration in PD support the use of MAO inhibitors as treatment for PD to prevent the buildup of catabolic dopamine products to modulate susceptibility of neurons to stress (Jiang et al., 2012; Woodard et al., 2014). Jiang and colleagues found that levels of MAO-A and MAO-B transcripts as well as enzymatic activities were elevated in iPSC-derived midbrain dopamine neurons from PD patients carrying Parkin mutations (Jiang et al., 2012), consistent with previous findings of the same group that Parkin suppresses the transcription of MAO-A and -B in other cell systems (Jiang et al., 2006). Further, these results underscore the role of Parkin in balancing oxidative stress and dopamine metabolism in PD neurons.
Figure 1:
The hypothesized mechanisms linking mitochondrial dysfunction, enzymatic dopamine degradation and VMAT2-mediated dopamine packaging into synaptic vesicles in human dopamine neurons.
The enzyme ALDH turns DOPAL into the less reactive 3,4-dihydroxyphenylacetic acid (DOPAC) (Meiser et al., 2013). Reports of reduced ALDH levels and enzymatic activity in PD patients suggest its possible involvement in defective dopamine metabolism (Galter et al., 2003; Molochnikov et al., 2012). Further, ALDH enzymes are sensitive to oxidative stress and interfere with lipid peroxidation (Esterbauer et al., 1991; Jinsmaa et al., 2009). Therefore, ALDH inhibition may be another mechanistic link between altered dopamine metabolism and pathologic events underlying neurodegeneration, but has not yet been explored in human iPSC-derived neuronal culture.
Dopamine is normally thought to be protected from auto-oxidation in the cytosol, as the majority is normally sequestered in secretory monoaminergic vesicles through clathrin-mediated synaptic vesicle endocytosis, the process by which a new synaptic vesicle invaginates from the plasma membrane (Saheki and De Camilli, 2012). However, if this pathway is defective and packaging of dopamine into vesicles is inefficient, cytosolic dopamine could ultimately become oxidized, accumulate and lead to toxic effects and nigrostriatal neurodegeneration further downstream (Caudle et al., 2007; Hastings et al., 1996; Vergo et al., 2007). A recent study by Nguyen and Krainc uses patient-derived dopaminergic neurons from patients carrying a mutation in leucine-rich repeat kinase 2 (LRRK2), to show that impaired synaptic vesicle endocytosis leads to altered dopamine metabolism and dopamine-mediated toxic effects (Nguyen and Krainc, 2018). Since LRRK2 phosphorylates several proteins involved in synaptic vesicle endocytosis (Biskup et al., 2006; Islam et al., 2016; Matta et al., 2012), we hypothesized that LRRK2 might have important kinase function at the synapse and that PD-linked mutations, which alter kinase activity, would affect synaptic integrity. Indeed, the altered phosphorylation of the PD-associated clathrinuncoating co-chaperone auxilin (DNAJC6 gene) (Edvardson et al., 2012; Koroglu et al., 2013; Olgiati et al., 2016) at amino acid position Ser627, located within its clathrin-binding domain, leads to differential association of auxilin with clathrin and disrupts synaptic vesicle endocytosis, resulting in decreased synaptic vesicle density in LRRK2 mutant dopaminergic neuron synapses (Nguyen and Krainc, 2018). This ultimately manifested in inefficient packaging of dopamine into synaptic vesicles, and consequently the accumulation of oxidized dopamine and its downstream effects, including decreased GCase activity and accumulation of α-synuclein, which were all attenuated by restoring auxilin function.
High mitochondrial oxidant stress is thought to have several deleterious effects on vulnerable dopamine neurons and is a major source of dopamine oxidation (Guzman et al., 2010). Several studies using iPSC-derived dopamine neurons have demonstrated the impact of oxidant stress on these vulnerable neurons and discussed its contribution to neurodegeneration (Burbulla et al., 2017; Chung et al., 2016; Devine et al., 2011; Little et al., 2018; Nguyen et al., 2011; Seibler et al., 2011). It has been shown in patient-derived neurons that reactive oxygen species can interact with cytosolic dopamine and boost its conversion to DAQs (Burbulla et al., 2017). Further, when mitochondrial function is compromised, it is proposed that the resultant decrease in ATP formation leads to inefficient uptake of dopamine into synaptic vesicles by VMAT2 since the energy for amine transport is derived from a proton gradient generated by ATP hydrolysis (Rudnick, 1998; Schuldiner et al., 1998). In turn, cytosolic dopamine levels increase and accelerate the oxidation of dopamine.
Mitochondrial oxidant stress is in part created by the intrinsic pacemaker activity in SNpc dopamine neurons. In fact, oxidation of mitochondrial thiol proteins increases when dopamine neurons are simply pacemaking (Guzman et al., 2010). Moreover, calcium influx through autonomous pacemaking results in an elevation of mitochondrial oxidant stress as well as synthesis of dopamine in substantia nigra neurons through activation of tyrosine hydroxylase, the rate-limiting enzyme of catecholamine synthesis (Guzman et al., 2010; Menezes et al., 1996; Mosharov et al., 2009; Rittenhouse and Zigmond, 1999). Patient-derived GBA1 mutant dopamine neurons have been shown to exhibit impaired cellular calcium homeostasis (Schondorf et al., 2014). Several in vivo and in vitro animal studies have discussed the interaction between calcium, dopamine and α-synuclein underlying the susceptibility of SNpc neurons in PD (Chan et al., 2007; Guzman et al., 2010; Lieberman et al., 2017; Mosharov et al., 2009). The relatively high cytosolic calcium concentrations in combination with low intrinsic calcium buffering capacity contributes to greater mitochondrial oxidant stress and adds to the bioenergetics burden in SNpc dopamine neurons (Foehring et al., 2009). Studies in PD patient-derived neurons showed that lowering levels of the calcium-dependent serine/threonine phosphatase calcineurin was sufficient to partly reduce the accumulation of oxidized dopamine suggesting that calcium homeostasis plays a role in dopamine metabolism and oxidation (Burbulla et al., 2017). Interestingly, when comparing iPSC-derived mouse dopaminergic wild-type neurons with iPSC-derived human dopaminergic wild-type neurons species-specific differences in the levels and activity of calcineurin were detected (Burbulla et al., 2017). Moreover, we showed that dopamine turnover was decreased in mouse compared to human dopaminergic neurons. This might also explain the fact that oxidized dopamine was only detected in mouse neurons upon addition of the dopamine precursor L-DOPA. Artificially increased dopamine levels in mouse brain neurons recapitulated other pathogenic findings that were observed in human dopamine neurons, including accumulation of α-synuclein and diminished GCase activity (Burbulla et al., 2017). This observation may at least partially explain why human pathology is not fully recapitulated in existing mouse models of PD. Phenotypes seen in PD mouse models have been subtle and generally do not exhibit degeneration of SNc neurons (Dawson et al., 2018), representing a major caveat in modeling of PD. Furthermore, important age-dependent phenotypes arising in a normal human lifespan, often accelerated in disease, are not present in mice including the formation of neuromelanin and age-dependent telomere shortening (Fedorow et al., 2005; Zhang et al., 2016). Together, these studies highlight the importance of human iPSC-derived neurons for studies for PD disease mechanisms in SNc neurons.
Using patient-specific dopamine neurons for development of targeted therapies in GBA1-PD
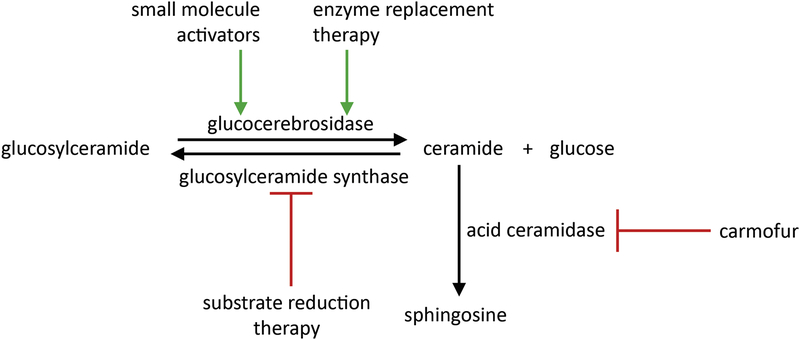
Targeting lysosomal GCase in patients with GBA1-linked PD has focused on direct activation (Mazzulli et al., 2016) or stabilization of misfolded mutant GCase to promote translocation of the mutant protein to the lysosome (Aflaki et al., 2016; Aflaki et al., 2014; Khanna et al., 2010). However, recent data suggests that wild-type GCase enzyme activity is also reduced in iPSC-derived dopaminergic neurons from genetic and idiopathic PD patients not harboring GBA1 mutations (Burbulla et al., 2017; Mazzulli et al., 2011; Nguyen and Krainc, 2018). Hence, modulation of GCase activity may be beneficial in both genetic and idiopathic forms of PD making GCase a valuable target for the treatment of multiple forms of PD (Fig. 2). Future studies will be needed to fully define the potential of novel GCase modulators in normal and affected tissues and to develop the most effective analogs for human clinical trials.
Figure 2:
Strategies of activation of GCase, inhibition of GCase lipid substrates and modulation of ceramide pathways.
Another approach to the treatment of GBA1-associated synucleinopathies is substrate reduction therapy. Under normal conditions, GCase converts glucosylceramide into ceramide (Kitatani et al., 2009), which is further converted into sphingosine by acid ceramidase. The loss of function of GCase leads to the accumulation of glucosylceramide, a toxic lipid substrate (Grabowski, 2008). Increases in glucosylceramide have been shown in iPSC-derived dopamine neurons from heterozygous GBA1 mutation carriers promoting the formation of α-synuclein oligomers (Mazzulli et al., 2011; Schondorf et al., 2014). This suggests that reduction of glucosylceramide may prove beneficial in PD therapies. Potent and selective inhibitors of glucosylceramide synthase such as miglustat (N-butyldeoxynojirimycin) have been reported to be a promising therapeutics as they also partially cross the blood-brain barrier (Aerts et al., 2006; Treiber et al., 2007). Miglustat is used in the treatment of progressive neurological manifestations in patients with the neurodegenerative condition Niemann-Pick disease type C (Pineda et al., 2018) in which cholesterol and glycosphingolipids such as glucosylceramide accumulate in the late endosomes/lysosomes of cells (Mukherjee and Maxfield, 2004). The previously established glucosylceramide synthase inhibitor eliglustat was proven to be beneficial in reversing disease-relevant phenotypes such as the reduction of aggregated insoluble α-synuclein in iPSC-derived PD patient neurons (Zunke et al., 2018). Furthermore, glucosylceramide inhibition by the brain-penetrant synthase inhibitor GZ667161 has been shown to reduce levels of glucosylceramide and glucosylsphingosine in the central nervous system of two murine models of synucleinopathy, slowing the accumulation of α-synuclein and ameliorating cognitive deficits (Sardi et al., 2017). In addition, recent studies have shown that targeting the downstream activity of acid ceramidase might represent another novel therapeutic angle for GBA1-PD. Chemical inhibition of acid ceramidase is sufficient to restore lysosomal ceramide and to promote the clearance of α-synuclein in a number of cellular models including GBA1-deficient iPSC-derived dopaminergic neurons from PD patients (Kim et al., 2018).
Conclusion and perspectives
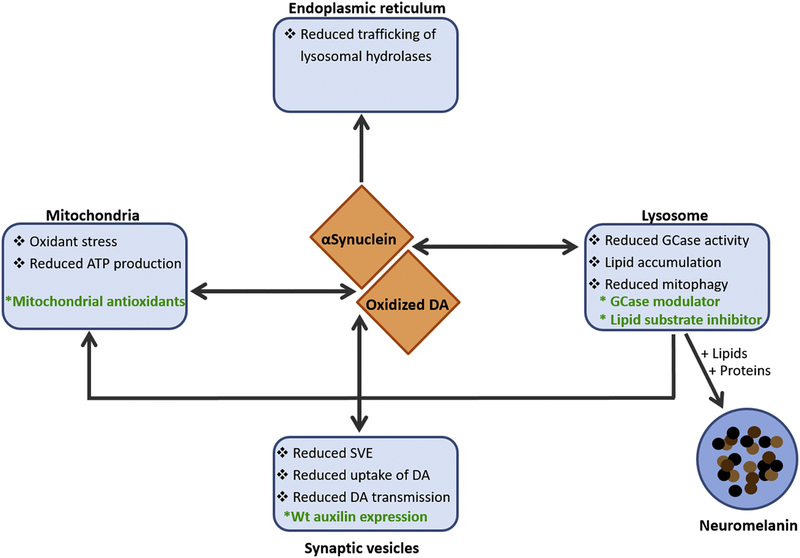
IPSC technology has made it possible to study molecular mechanisms of disease pathology using patient-derived cells, which has been especially valuable for neurodegenerative disorders such as PD which lack accurate animal models of disease (Fig. 3). Our recent work highlighted the key differences in dopamine metabolism between human and mouse SNc neurons that at least partially explains the relative vulnerability of human over mouse SNc neurons in modelling PD. Therefore, we hypothesize that human neuronal cultures may serve as more appropriate models for testing neuroprotective compounds and development of therapeutic interventions. However, more work is required to further develop iPSC models and recapitulate the interactions of neurons-glia in both cultured neurons and organoids. The obvious goal remains that, once this is achieved, patient-specific iPSCs will serve as resource for in vitro and in vivo disease modeling and drug screening as well as precursors for transplantation and tissue regeneration therapy.
Figure 3:
Hypothesized players of PD pathology and their involvement in the potential disease mechanisms discussed in the text. DA = dopamine. SVE = synaptic vesicle endocytosis.
Acknowledgments
This work was supported by NIH Grants R01 NS076054 and R37 NS096241 to D.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
D.K is Founder, Chair of SAB, Lysosomal Therapeutics, Inc, Cambridge, MA; SAB Member, The Silverstein Foundation; SAB Member, Prevail Therapeutics; Venture Partner, OrbiMed Advisors. L.F.B. declares that she has no competing interests.
References
- Aerts JM, Hollak CE, Boot RG, Groener JE and Maas M (2006) Substrate reduction therapy of glycosphingolipid storage disorders. Journal of inherited metabolic disease 29:449–456. [DOI] [PubMed] [Google Scholar]
- Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J and Sidransky E (2016) A New Glucocerebrosidase Chaperone Reduces alpha-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:7441–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, Marugan J, Patnaik S, Dutra A, Southall N, Zheng W, Tayebi N and Sidransky E (2014) Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Science translational medicine 6:240ra273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflaki E, Westbroek W and Sidransky E (2017) The Complicated Relationship between Gaucher Disease and Parkinsonism: Insights from a Rare Disease. Neuron 93:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM and Dawson VL (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Annals of neurology 60:557–569. [DOI] [PubMed] [Google Scholar]
- Blanz J and Saftig P (2016) Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. Journal of neurochemistry 139 Suppl 1:198–215. [DOI] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Kruger R, Surmeier DJ and Krainc D (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357:1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA and Zahm DS (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25:101–115. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Colebrooke RE, Emson PC and Miller GW (2008) Altered vesicular dopamine storage in Parkinson’s disease: a premature demise. Trends in neurosciences 31:303–308. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC and Miller GW (2007) Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:8138–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD and Sulzer D (2014) MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nature communications 5:3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE and Surmeier DJ (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447:1081–1086. [DOI] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ and Zhuang X (2008) Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasserini D, Paciotti S, Eusebi P, Persichetti E, Tasegian A, Kurzawa-Akanbi M, Chinnery PF, Morris CM, Calabresi P, Parnetti L and Beccari T (2015) Selective loss of glucocerebrosidase activity in sporadic Parkinson’s disease and dementia with Lewy bodies. Molecular neurodegeneration 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SY, Kishinevsky S, Mazzulli JR, Graziotto J, Mrejeru A, Mosharov EV, Puspita L, Valiulahi P, Sulzer D, Milner TA, Taldone T, Krainc D, Studer L and Shim JW (2016) Parkin and PINK1 Patient iPSC-Derived Midbrain Dopamine Neurons Exhibit Mitochondrial Dysfunction and alpha-Synuclein Accumulation. Stem cell reports 7:664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM and Lansbury PT Jr., (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294:1346–1349. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Golde TE and Lagier-Tourenne C (2018) Animal models of neurodegenerative diseases. Nature neuroscience 21:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM and Breteler MM (2006) Epidemiology of Parkinson’s disease. The Lancet Neurology 5:525–535. [DOI] [PubMed] [Google Scholar]
- Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA and Kunath T (2011) Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nature communications 2:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, Kaestner KH, Greene LE and Elpeleg O (2012) A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PloS one 7:e36458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ and Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacological reviews 56:331–349. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ and Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine 11:81–128. [DOI] [PubMed] [Google Scholar]
- Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P and Double KL (2005) Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson’s disease. Progress in neurobiology 75:109–124. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Zhang XF, Lee JC and Callaway JC (2009) Endogenous calcium buffering capacity of substantia nigral dopamine neurons. Journal of neurophysiology 102:2326–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Buervenich S, Carmine A, Anvret M and Olson L (2003) ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson’s disease and in the ventral tegmental area in schizophrenia. Neurobiology of disease 14:637–647. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW and Schapira AH (2012) Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Annals of neurology 72:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ and Sharabi Y (2013) Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. Journal of neurochemistry 126:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonera EG, van’t Hof M, Berger HJ, van Weel C and Horstink MW (1997) Symptoms and duration of the prodromal phase in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 12:871–876. [DOI] [PubMed] [Google Scholar]
- Grabowski GA (2008) Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 372:1263–1271. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT and Hastings TG (2004) Biomedicine. Parkinson’s--divergent causes, convergent mechanisms. Science 304:1120–1122. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT and Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA and Zigmond MJ (1996) Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proceedings of the National Academy of Sciences of the United States of America 93:1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM and Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement disorders : official journal of the Movement Disorder Society 23:837–844. [DOI] [PubMed] [Google Scholar]
- Hruska KS, LaMarca ME, Scott CR and Sidransky E (2008) Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Human mutation 29:567–583. [DOI] [PubMed] [Google Scholar]
- Islam MS, Nolte H, Jacob W, Ziegler AB, Putz S, Grosjean Y, Szczepanowska K, Trifunovic A, Braun T, Heumann H, Heumann R, Hovemann B, Moore DJ and Kruger M (2016) Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Human molecular genetics 25:5365–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Jiang Q, Liu W and Feng J (2006) Parkin suppresses the expression of monoamine oxidases. The Journal of biological chemistry 281:8591–8599. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z and Feng J (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nature communications 3:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S and Doorn JA (2009) Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chemical research in toxicology 22:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV and Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. [DOI] [PubMed] [Google Scholar]
- Khanna R, Benjamin ER, Pellegrino L, Schilling A, Rigat BA, Soska R, Nafar H, Ranes BE, Feng J, Lun Y, Powe AC, Palling DJ, Wustman BA, Schiffmann R, Mahuran DJ, Lockhart DJ and Valenzano KJ (2010) The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. The FEBS journal 277:1618–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Jeon S, Burbulla LF and Krainc D (2018) Acid ceramidase inhibition ameliorates alpha-synuclein accumulation upon loss of GBA1 function. Human molecular genetics 27:1972–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM and Hannun YA (2009) Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. The Journal of biological chemistry 284:12972–12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroglu C, Baysal L, Cetinkaya M, Karasoy H and Tolun A (2013) DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism & related disorders 19:320–324. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG and Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nature medicine 11:1214–1221. [DOI] [PubMed] [Google Scholar]
- Lieberman OJ, Choi SJ, Kanter E, Saverchenko A, Frier MD, Fiore GM, Wu M, Kondapalli J, Zampese E, Surmeier DJ, Sulzer D and Mosharov EV (2017) alpha-Synuclein-Dependent Calcium Entry Underlies Differential Sensitivity of Cultured SN and VTA Dopaminergic Neurons to a Parkinsonian Neurotoxin. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D, Luft C, Mosaku O, Lorvellec M, Yao Z, Paillusson S, Kriston-Vizi J, Gandhi S, Abramov AY, Ketteler R, Devine MJ and Gissen P (2018) A single cell high content assay detects mitochondrial dysfunction in iPSC-derived neurons with mutations in SNCA. Scientific reports 8:9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, De Bock PJ, Morais VA, Vilain S, Haddad D, Delbroek L, Swerts J, Chavez-Gutierrez L, Esposito G, Daneels G, Karran E, Holt M, Gevaert K, Moechars DW, De Strooper B and Verstreken P (2012) LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75:1008–1021. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA and Krainc D (2011) Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF, Patnaik S, Sidransky E, Marugan JJ, Sue CM and Krainc D (2016) Activation of beta-Glucocerebrosidase Reduces Pathological alpha-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:7693–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M and Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M and Consortium on DLB (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. [DOI] [PubMed] [Google Scholar]
- Meiser J, Weindl D and Hiller K (2013) Complexity of dopamine metabolism. Cell communication and signaling : CCS 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes A, Zeman R and Sabban E (1996) Involvement of intracellular or extracellular calcium in activation of tyrosine hydroxylase gene expression in PC12 cells. Journal of neurochemistry 67:2316–2324. [DOI] [PubMed] [Google Scholar]
- Molochnikov L, Rabey JM, Dobronevsky E, Bonucelli U, Ceravolo R, Frosini D, Grunblatt E, Riederer P, Jacob C, Aharon-Peretz J, Bashenko Y, Youdim MB and Mandel SA (2012) A molecular signature in blood identifies early Parkinson’s disease. Molecular neurodegeneration 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH and Sulzer D (2009) Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S and Maxfield FR (2004) Lipid and cholesterol trafficking in NPC. Biochimica et biophysica acta 1685:28–37. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B and Halliday GM (2014) Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain : a journal of neurology 137:834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W, Palmer TD and Pera RR (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell stem cell 8:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M and Krainc D (2018) LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America 115:5576–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H and Lee VM (2005) Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. The Journal of biological chemistry 280:21212–21219. [DOI] [PubMed] [Google Scholar]
- Olgiati S, Quadri M, Fang M, Rood JP, Saute JA, Chien HF, Bouwkamp CG, Graafland J, Minneboo M, Breedveld GJ, Zhang J, International Parkinsonism Genetics N, Verheijen FW, Boon AJ, Kievit AJ, Jardim LB, Mandemakers W, Barbosa ER, Rieder CR, Leenders KL, Wang J and Bonifati V (2016) DNAJC6 Mutations Associated With Early-Onset Parkinson’s Disease. Annals of neurology 79:244–256. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS and Trudeau LE (2015) Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Current biology : CB 25:2349–2360. [DOI] [PubMed] [Google Scholar]
- Pineda M, Walterfang M and Patterson MC (2018) Miglustat in Niemann-Pick disease type C patients: a review. Orphanet journal of rare diseases 13:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse AR and Zigmond RE (1999) Role of N- and L-type calcium channels in depolarization-induced activation of tyrosine hydroxylase and release of norepinephrine by sympathetic cell bodies and nerve terminals. Journal of neurobiology 40:137–148. [DOI] [PubMed] [Google Scholar]
- Rudnick G (1998) Bioenergetics of neurotransmitter transport. Journal of bioenergetics and biomembranes 30:173–185. [DOI] [PubMed] [Google Scholar]
- Saheki Y and De Camilli P (2012) Synaptic vesicle endocytosis. Cold Spring Harbor perspectives in biology 4:a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, Viel C, Clarke J, Treleaven CM, Richards AM, Park H, Olszewski MA, Dodge JC, Marshall J, Makino E, Wang B, Sidman RL, Cheng SH and Shihabuddin LS (2017) Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proceedings of the National Academy of Sciences of the United States of America 114:2699–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH and Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 26:1049–1055. [DOI] [PubMed] [Google Scholar]
- Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T and Deleidi M (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nature communications 5:4028. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Steiner-Mordoch S and Yelin R (1998) Molecular and biochemical studies of rat vesicular monoamine transporter. Advances in pharmacology 42:223–227. [DOI] [PubMed] [Google Scholar]
- Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C and Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:5970–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, Paisan-Ruiz C, Campolongo A, Anton-Aguirre S, Martin I, Munoz L, Bufill E, Vilageliu L, Grinberg D, Cozar M, Blesa R, Lleo A, Hardy J, Kulisevsky J and Clarimon J (2012) Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Movement disorders : official journal of the Movement Disorder Society 27:393–399. [DOI] [PubMed] [Google Scholar]
- Shi Y, Inoue H, Wu JC and Yamanaka S (2017) Induced pluripotent stem cell technology: a decade of progress. Nature reviews Drug discovery 16:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Chen K and Ridd MJ (1999) Monoamine oxidase: from genes to behavior. Annual review of neuroscience 22:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y and Ziegler SG (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. The New England journal of medicine 361:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R and Goedert M (1997) Alpha synuclein in Lewy bodies. Nature 388:839–840. [DOI] [PubMed] [Google Scholar]
- Sulzer D and Zecca L (2000) Intraneuronal dopamine-quinone synthesis: a review. Neurotoxicity research 1:181–195. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA and Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nature reviews Neuroscience 18:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ and Sulzer D (2013) The pathology roadmap in Parkinson disease. Prion 7:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. [DOI] [PubMed] [Google Scholar]
- Treiber A, Morand O and Clozel M (2007) The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica; the fate of foreign compounds in biological systems 37:298–314. [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, Kramer P, Woltjer R, Kukull W, Nelson PT, Jicha GA, Neltner JH, Galasko D, Masliah E, Trojanowski JQ, Schellenberg GD, Yearout D, Huston H, Fritts-Penniman A, Mata IF, Wan JY, Edwards KL, Montine TJ and Zabetian CP (2012) GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79:1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer JJ and Daley GQ (2011) Induced pluripotent stem cells for modelling human diseases. Philosophical transactions of the Royal Society of London Series B, Biological sciences 366:2274–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati A, Yu WH and Sidransky E (2010) The role of glucocerebrosidase mutations in Parkinson disease and Lewy body disorders. Current neurology and neuroscience reports 10:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M and Lotharius J (2007) Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain research 1185:18–32. [DOI] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L and Lucius R (2003) Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 17:500–502. [DOI] [PubMed] [Google Scholar]
- Wong YC and Krainc D (2016) Lysosomal trafficking defects link Parkinson’s disease with Gaucher’s disease. Movement disorders : official journal of the Movement Disorder Society 31:1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC and Krainc D (2017) alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nature medicine 23:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard CM, Campos BA, Kuo SH, Nirenberg MJ, Nestor MW, Zimmer M, Mosharov EV, Sulzer D, Zhou H, Paull D, Clark L, Schadt EE, Sardi SP, Rubin L, Eggan K, Brock M, Lipnick S, Rao M, Chang S, Li A and Noggle SA (2014) iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell reports 9:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J and Lucius R (2008) Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta neuropathologica 116:47–55. [DOI] [PubMed] [Google Scholar]
- Zecca L, Zucca FA, Wilms H and Sulzer D (2003) Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends in neurosciences 26:578–580. [DOI] [PubMed] [Google Scholar]
- Zhang F, Cheng D, Wang S and Zhu J (2016) Human Specific Regulation of the Telomerase Reverse Transcriptase Gene. Genes 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D and Zecca L (2011) Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotoxicity research 19:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L and Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Progress in neurobiology 155:96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, Toker NJ, Jeon S, Fredriksen K and Mazzulli JR (2018) Reversible Conformational Conversion of alpha-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron 97:92–107 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]