Abstract
Background:
Patients having bariatric surgery have lower mortality when compared to those with similar body mass index who do not undergo surgery. It is unclear whether mortality post-bariatric surgery is similar to the general population. The benefit of bariatric surgery would be highlighted should people previously at high risk for premature death have comparable, or better, mortality experience as the general population.
Objective:
To compare mortality following bariatric surgery to the general U.S. population of the same age, sex, and race.
Setting:
The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) prospective cohort of 2,458 adults who underwent bariatric surgery at ten U.S. hospitals between 2006 and 2009.
Methods:
Deaths were identified via LABS-2 follow-up and the National Death Index. Standardized mortality ratios (SMR) of post-bariatric surgery mortality observed in LABS-2 versus age, sex, race and year-adjusted expected mortality in the general U.S. population were calculated and compared to 1 which results when the number of observed and expected deaths are equal.
Results:
LABS-2 median follow-up was 6.6 (IQR: 5.9–7.0) years post-surgery. Seventy-six deaths were observed over 15616 person-years (PY) of observation (4.9 deaths/1,000 PY). The rate expected in the general U.S. population with the same age, sex, race, and year distribution was 4.8/1,000 PY (SMR=1.02, 95%CI=0.80–1.27). There were no significant differences between observed and expected mortality by surgical procedure. Compared to expected mortality in the general U.S. population, people 35–44 years old at time of surgery had significantly more deaths (SMR=2.06, 95%CI=1.22–3.25), while people at least 55 years of age had significantly fewer (SMR=0.63, 95%CI=0.42–0.92). Significantly more deaths than expected occurred in the perioperative period and 5–7 years following surgery.
Conclusions:
Mortality within 7 years of bariatric surgery is comparable to the general U.S. population which is likely to have better survival than people with severe obesity. However, more deaths than expected were identified 5–7 years after surgery.
Keywords: Mortality, bariatric surgery, long-term mortality, Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding
Introduction
Obesity is a leading cause of preventable death in the United States.(1,2) Bariatric surgery is the most effective long-term treatment for severe obesity.(3) In addition to sustained weight loss, bariatric surgery may result in remission of, or improvement in, comorbid conditions(3–5) associated with premature death.(6,7) Compared to control groups with severe obesity, lower short-(8–10) and long-term(9,11–18) (i.e., ≥ 5 years) mortality rates following bariatric surgery have been well documented. There is, however, conflicting evidence as to whether the benefits of bariatric surgery extend to long-term mortality when compared to the general population.(12)
Although there have been studies comparing the mortality experience of people undergoing bariatric surgery to mortality in a general population,(19–22) the distribution of types of bariatric surgeries performed has changed over time,(23) and those studies either compare to populations outside of the United States(19,20) or are restricted to just two states(21,22). This study was undertaken to examine whether mortality following a different distribution of bariatric surgeries than in those studies differs from the general population when using more recent and geographically diverse data from the United States.
Using data from the Longitudinal Assessment of Bariatric Surgery (LABS)-2, a 7-year, multi-center prospective cohort study of U.S. adults undergoing their first bariatric surgery, and from the National Death Index, the primary aim of this study was to compare observed mortality following bariatric surgery to the expected mortality of the general U.S. population with similar age, sex and race over the same calendar years. A secondary aim was to compare observed mortality to expected by sex, age group, surgical procedure and time since surgery. Because of the paucity of information about pre-surgery factors associated with long-term mortality following bariatric surgery,(13,22,24) a third aim was to identify pre-surgery factors associated with mortality following bariatric surgery.
Methods
Design and Participants
LABS-2 was a prospective cohort study of 2,458 adults who underwent bariatric surgery at one of 10 hospitals at 6 clinical centers in the United States between April, 2006 and April, 2009. Participants attended pre-surgery, 30 day, 6 month, and annual post-surgery follow-up assessments for up to 7 years or until January 31, 2015, whichever came first. Institutional Review Boards at each center approved protocols and all participants provided informed consent. Flow of participants through recruitment has been previously described.(3)
Vital status for LABS-2 participants was determined through study follow-up and a National Death Index(25) search for those who were not known to have died through December 31, 2014. Each clinical site provided to the National Death Index, to use in a search, participant identifying information (first and last name, date of birth, social security number, and a combination of other personal identifiers as available such as sex, race, marital status, state of residency, and date of last contact).
The Centers for Disease Control and Prevention (CDC) Wonder Underlying Cause of Death database compiles death certificate data from 50 states and the District of Columbia.(26) Age-, sex-, race-, and year-specific crude mortality rates for each calendar year from 2006–2014 were downloaded from CDC Wonder(27) and used to calculate expected mortality.
Measures
Underlying cause of death was obtained from death certificates. Cause of death categories were created using the World Health Organization Global Burden of Disease Study cause list(28) as reference (summarized in eTable 1).
Participants’ weights were measured to the nearest pound and height to the nearest inch. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.(29)
Participants were asked their date of birth and race by research staff. Race was considered missing for participants who did not report race as at least 1 of: white or Caucasian, Black or African American, Asian, American Indian or Alaska Native, or Native Hawaiian or Other Pacific Islander. Annual household income, education, and smoking were assessed using self-administered questionnaires. Smoking was categorized as any versus no cigarette smoking in the past year. Participants who underwent a revision or reversal of their bariatric procedure were included in analyses of their original bariatric procedure.
Regular alcohol use, defined as drinking at least twice per week, was assessed using the Alcohol Use Disorder Identification Test (AUDIT)(30,31) Symptoms of alcohol use disorder (AUD) was defined as an AUDIT of score at least 8, symptoms of alcohol dependence, or symptoms of alcohol-related harm at least once in the past 12 months.(31,32)
Sleep apnea, diabetes, hypertension, dyslipidemia and history of deep vein thrombosis/pulmonary embolism were determined using a combination of laboratory values, physical examination measures, participant-reported medication use, and comorbid diagnoses from healthcare providers and medical records review.(33,34)
Statistical Analysis
Observed mortality rates were calculated by dividing the number of deaths within 7 years of the initial bariatric surgery by the number of person-years (PY) of observation. Age-, sex-, race-, and calendar year-specific expected number of deaths were calculated by multiplying the crude mortality rate per 1,000 in the general population by the number of LABS-2 participants in that group divided by 1,000. Age-, sex-, and calendar year-specific mortality rates for races other than Black or White are suppressed in CDC Wonder to avoid revealing identities of individuals, so total age-, sex-, and calendar year-specific crude mortality rates from CDC Wonder were used for the 100 LABS-2 participants who were not Black or White. The total expected number of deaths in the general U.S. population is the sum of expected deaths across all LABS-2 participants. Standardized mortality ratios (SMRs) were calculated as the ratio of observed to expected deaths. The test statistics for whether the observed number of deaths differed significantly from expected (i.e., SMR=1) and 95% confidence intervals (CI) around SMRs were calculated assuming the Poisson distribution using methods proposed by Byar(35).
Mortality rates and SMRs were calculated separately by sex and pre-specified age, and time since surgery groups. Stratified analyses were also conducted for surgical procedure. Results are presented for the RYGB and laparoscopic adjustable gastric banding (LAGB) surgery groups because there were only 78 “other” procedures (59 sleeve gastrectomy and 19 biliopancreatic diversion with duodenal switch).
Poisson mixed models with robust error variance(36) were used to identify pre-surgery factors associated with post-perioperative (i.e., 30 days to 7 years post-bariatric surgery) mortality. All models included clinical site as a random effect and fixed effects, based on published results,(13,22,24) were surgical procedure, age, sex, race, and pre-surgery BMI. Other pre-surgery variables considered for inclusion as independent variables, based on prior research,(13,22,24) were: annual household income, education, any cigarette smoking, any alcohol use, regular alcohol use, AUD symptoms, sleep apnea, diabetes, hypertension, dyslipidemia, and deep vein thrombosis/pulmonary embolism. Due to the large number of variables under consideration and relatively few deaths observed, base models that included clinical site, surgical procedure, age, sex, race, and pre-surgery BMI were fit with each factor under consideration, one at a time. From these models, factors with P<0.20 were entered into a multivariable model and retained if statistically significant (P<0.05). Results are presented as adjusted relative risks (ARR) with their 95% confidence intervals (CI).
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). All P values are two-sided. P values less than 0.05 are considered statistically significant.
Results
Pre-surgery characteristics of the LABS-2 cohort are described in Table 1. RYGB was the most common procedure (n=1770, 72.0% (n=1550) of which were performed laparoscopically and 220 open), followed by LAGB (n=610, 24.8%).
Table 1.
Pre-surgery characteristics of Longitudinal Assessment of Bariatric Surgery-2 cohort
| Total (n=2458) |
RYGB (n=1770) |
LAGB (n=610) |
Othera (n=78) |
|
|---|---|---|---|---|
| Age, years, median (Q1, Q3) | 46 (37, 54) | 45 (47–54) | 48 (37–56) | 45 (35–53) |
| Age group, No. (%) | ||||
| 18–34 | 470 (19.1) | 337 (19.0) | 115 (18.9) | 18 (23.1) |
| 35–44 | 674 (27.4) | 515 (29.1) | 141 (23.1) | 18 (23.1) |
| 45–54 | 714 (29.0) | 515 (29.1) | 174 (28.5) | 25 (32.1) |
| 55+ | 600 (24.4) | 403 (22.8) | 180 (29.5) | 17 (21.8) |
| Sex, No. (%) | ||||
| Female | 1931 (78.6) | 1413 (79.8) | 465 (76.2) | 53 (67.9) |
| Male | 527 (21.4) | 357 (20.2) | 145 (23.8) | 25 (32.1) |
| Race, No. (%) | No.=2433 | No.=1751 | No.=606 | No.=76 |
| White | 2102 (86.4) | 1494 (85.3) | 543 (89.6) | 65 (85.5) |
| Black | 256 (10.5) | 196 (11.2) | 51 (8.4) | 9 (11.8) |
| Other | 75 (3.1) | 61 (3.5) | 12 (2.0) | 2 (2.6) |
| Annual household income, No. (%) | No.=2204 | No.=1589 | No.=546 | No.=69 |
| <$25,000 | 402 (18.2) | 316 (19.9) | 70 (12.8) | 16 (23.2) |
| $25,000–$49,999 | 568 (25.8) | 447 (28.1) | 110 (20.1) | 11 (15.9) |
| $50,000–$74,999 | 522 (23.7) | 378 (23.8) | 135 (24.7) | 9 (13.0) |
| ≥$75,000 | 712 (32.3) | 448 (28.2) | 231 (42.3) | 33 (47.8) |
| Education, No. (%) | No.=2264 | No.=1635 | No.=559 | No.=70 |
| ≤ High school | 521 (23.0) | 385 (23.5) | 125 (22.4) | 11 (15.7) |
| Some college | 918 (40.5) | 700 (42.8) | 191 (34.2) | 27 (38.6) |
| ≥ College | 825 (36.4) | 550 (33.6) | 243 (43.5) | 32 (45.7) |
| Body mass index, median (Q1, Q3) | 45.9(41.8,51.4) | 46.6 (42.4, 52.0) | 43.9(40.4,48.1) | 53.4(45.4,61.8) |
| Any cigarette smoking, No. (%) | No.=2454 | No.=1766 | No.=610 | No.=78 |
| No | 2130 (86.8) | 1512 (85.6) | 554 (90.8) | 64 (82.1) |
| Yes | 324 (13.2) | 254 (14.4) | 56 (9.2) | 14 (17.9) |
| Any alcohol use, No. (%) | No.=2262 | No.=1632 | No.=559 | No.=71 |
| No | 926 (40.9) | 704 (43.1) | 196 (35.1) | 26 (36.6) |
| Yes | 1336 (59.1) | 928 (56.9) | 363 (64.9) | 45 (63.4) |
| Regular alcohol use, No. (%) | No.=2262 | No.=1632 | No.=559 | No.=71 |
| No | 2116 (93.5) | 1539 (94.3) | 516 (92.3) | 61 (85.9) |
| Yes | 146 (6.5) | 93 (5.7) | 43 (7.7) | 10 (14.1) |
| AUD symptoms, No. (%) | No.=2256 | No.=1627 | No.=564 | No.=71 |
| No | 2108 (93.4) | 1521 (93.5) | 521 (93.4) | 66 (93.0) |
| Yes | 148 (6.6) | 106 (6.5) | 37 (6.6) | 5 (7.0) |
| Sleep apnea, No. (%) | No.=2455 | No.=1768 | No.=610 | No.=77 |
| No | 1167 (47.5) | 854 (48.3) | 272 (44.6) | 41 (53.2) |
| Yes | 1288 (52.5) | 914 (51.7) | 338 (55.4) | 36 (46.8) |
| Diabetes, No. (%) | No.=2405 | No.=1737 | No.=595 | No.=73 |
| No | 1589 (66.1) | 1122 (64.6) | 418 (70.3) | 49 (67.1) |
| Yes | 816 (33.9) | 615 (35.4) | 177 (29.7) | 24 (32.9) |
| Hypertension, No. (%) | No.=2408 | No.=1740 | No.=593 | No.=75 |
| No | 771 (32.0) | 533 (30.6) | 218 (36.8) | 20 (26.7) |
| Yes | 1637 (68.0) | 1207 (69.4) | 375 (63.2) | 55 (73.3) |
| Dyslipidemia, No. (%) | No.=2219 | No.=1606 | No.=537 | No.=76 |
| No | 722 (32.5) | 509 (31.7) | 187 (34.8) | 26 (34.2) |
| Yes | 1497 (67.5) | 1097 (68.3) | 350 (65.2) | 50 (65.8) |
| Deep vein thrombosis/ pulmonary embolism, No. (%) | No.=2229 | No.=1606 | No.=555 | No.=68 |
| No | 2138 (95.9) | 1552 (96.6) | 522 (94.1) | 64 (94.1) |
| Yes | 91 (4.1) | 54 (3.4) | 33 (5.9) | 4 (5.9) |
AUD=Alcohol use disorder; LAGB=laparoscopic adjustable gastric band; RYGB=Roux-en-Y gastric bypass.
59 sleeve gastrectomy and 19 biliopancreatic diversion – duodenal switch
Median follow-up for the 2,458 LABS-2 participants was 6.6 (25th-75th percentiles: 5.9–7.0) years. There were 76 deaths observed in 15616.15 PY of observation for a mortality rate of 4.9/1,000 PY compared to a rate of 4.8/1,000 PY expected in the general U.S. population with the same age, sex, and race distribution followed over the same calendar years as the LABS-2 cohort. This yielded an SMR of 1.02 (95%CI, 0.80–1.27, P=0.93), thus, the observed and expected mortality did not significantly differ.
Mortality rates and SMRs by sex, age, surgical procedure and time since surgery are reported in Table 2. Observed and expected deaths for men (SMR=0.95, 95%CI=0.62–1.40, P=0.90), women (SMR=1.05, 95%CI=0.78–1.38, P=0.77), post-RYGB (SMR=1.19, 95%CI=0.91–1.54, P=0.21 and post-LAGB (SMR=0.67, 95%CI=0.38–1.11, P=0.14) did not differ significantly. However, the 18 observed deaths was significantly more than the 8.8 expected in the general U.S. population among the 674 participants who were 35–44 years old at time of surgery (SMR=2.06, 95%CI=1.22–3.25, P=0.01), and there were significantly fewer deaths than expected among the 600 participants who were at least 55 years old at time of surgery (SMR=0.63, 95%CI=0.42–0.92, P=0.01). There were also significant differences between the observed and expected number of deaths in the perioperative (within 30 days) and latest (5–7 years after surgery) time frames. Although peri-operative mortality rates were low (3/1770=0.17% undergoing RYGB, 0/610=0.00% undergoing LAGB, and 1/78=1.3% undergoing another type of bariatric procedure), the 4 deaths within 30 days of surgery were significantly (P=0.01) more than the 0.7 expected in the general U.S. population (SMR=5.37, 95%CI=1.46–13.76, P=0.01). At least 5 years after surgery, there were significantly more deaths (n=33) than expected in the general U.S. population (n=21.4; SMR=1.55, 95%CI=1.06–2.17, P=0.02).
Table 2.
Post-bariatric surgery deaths
| Observed number | Observed rate /1,000PY | Expected number | Expected rate /1,000PY | SMR (95% Cl) | P value |
|
|---|---|---|---|---|---|---|
| Total (n=2458, PY=15616.15) | 76 | 4.9 | 74.9 | 4.8 | 1.02 (0.80–1.27) | 0.93 |
| Sex | ||||||
| Men (n=527, PY=3331.88) | 25 | 7.5 | 26.3 | 7.9 | 0.95 (0.62–1.40) | 0.90 |
| Women (n=1931, PY=12284.27) | 51 | 4.2 | 48.6 | 4.0 | 1.05 (0.78–1.38) | 0.77 |
| Age at time of surgery | ||||||
| 18–34 (n=470, PY=3035.57) | 6 | 2.0 | 2.8 | 0.9 | 2.14 (0.79–4.66) | 0.13 |
| 35–44 (n=674, PY=4300.12) | 18 | 4.2 | 8.8 | 2.0 | 2.06 (1.22–3.25) | 0.01 |
| 45–54 (n=714, PY=4491.43) | 25 | 5.6 | 20.6 | 4.6 | 1.21 (0.79–1.79) | 0.38 |
| ≥55 (n=600, PY=3789.04) | 27 | 7.1 | 42.7 | 11.3 | 0.63 (0.42–0.92) | 0.01 |
| Surgical procedure | ||||||
| RYGB (n=1770, PY=11289.88) | 59 | 5.2 | 49.6 | 4.4 | 1.19 (0.91–1.54) | 0.21 |
| LAGB (n=610, PY=3817.54) | 15 | 3.9 | 22.3 | 5.8 | 0.67 (0.38–1.11) | 0.14 |
| Other (n=78, PY=508.73)a | 2 | 3.9 | 2.4 | 4.6 | 0.85 (0.10–3.07) | 0.86 |
| Time since surgery | ||||||
| ≤30 days (n=2458, PY=201.67) | 4 | 19.8 | 0.7 | 3.7 | 5.37 (1.46–13.76) | 0.01 |
| >30 days and <1 year (n=2454, PY=2244.12) | 6 | 2.7 | 8.6 | 3.8 | 0.70 (0.26–1.52) | 0.49 |
| ≥1 year and <3 years (n=2432, PY=4831.23) | 12 | 2.5 | 20.7 | 4.3 | 0.58 (0.30–1.01) | 0.06 |
| ≥3 years and <5 years (n=2399, PY=4749.54) | 21 | 4.4 | 23.5 | 4.9 | 0.89 (0.55–1.37) | 0.70 |
| ≥5 years and <7 years (n=2353, PY=3589.60) | 33 | 9.2 | 21.4 | 5.9 | 1.55 (1.06–2.17) | 0.02 |
| Among RYGB (n=1770, PY=11289.88) | ||||||
| Sex | ||||||
| Men (n=357, PY=2268.48) | 17 | 7.5 | 16.3 | 7.2 | 1.04 (0.62–1.71) | 0.93 |
| Women (n=1413, PY=9021.41) | 42 | 4.7 | 32.3 | 3.7 | 1.26 (0.93–1.74) | 0.11 |
| Age at time of surgery | ||||||
| 18–34 (n=337,PY=2196.71) | 4 | 1.8 | 2.0 | 0.9 | 1.98 (0.54–5.07) | 0.28 |
| 35–44 (n=515, PY=3299.54) | 16 | 4.8 | 6.8 | 2.0 | 2.37 (1.35–3.85) | 0.004 |
| 45–54 (n=515, PY=3240.03) | 19 | 5.9 | 14.8 | 4.6 | 1.28 (0.77–2.00) | 0.33 |
| ≥55 (n=403, PY=2553.59) | 20 | 7.8 | 25.8 | 10.1 | 0.78 (0.47–1.20) | 0.29 |
| Time since surgery | ||||||
| ≤30 days (n=1770, PY=145.22) | 3 | 20.7 | 0.5 | 3.4 | 6.06 (1.25–17.71) | 0.03 |
| >30 days and <1 year (n=1767, PY=1614.32) | 6 | 3.7 | 5.7 | 3.5 | 1.05 (0.39–2.29) | 0.99 |
| ≥1 year and <3 years (n=1747, PY=3478.85) | 7 | 2.0 | 13.7 | 3.9 | 0.51 (0.21–1.06) | 0.07 |
| ≥3 years and <5 years (n=1730, PY=3427.14) | 15 | 4.4 | 15.5 | 4.5 | 0.97 (0.54–1.59) | 0.97 |
| ≥5 years and <7 years (n=1700, PY=2624.36) | 28 | 10.7 | 14.2 | 5.5 | 1.94 (1.29–2.81) | 0.002 |
| Among LAGB (n=610, PY=3817.54) | ||||||
| Sex | ||||||
| Men (n=145, PY=903.17) | 7 | 7.8 | 8.8 | 9.8 | 0.79 (0.32–1.63) | 0.70 |
| Women (n=465, PY=2914.37) | 8 | 2.7 | 13.4 | 4.6 | 0.60 (0.26–1.17) | 0.17 |
| Age at time of surgery | ||||||
| 18–34 (n=115, PY=720.81) | 2 | 2.8 | 0.6 | 0.9 | 3.12 (0.38–11.26) | 0.24 |
| 35–44 (n=141, PY=887.96) | 0 | 0.0 | 1.7 | 1.9 | 0.00 (0.00–2.16) | 0.36 |
| 45–54 (n=174, PY=1086.50) | 6 | 5.5 | 4.9 | 4.5 | 1.22 (0.45–2.66) | 0.73 |
| ≥55(n=180, PY=1122.27) | 7 | 6.2 | 15.0 | 13.4 | 0.47 (0.19–0.96) | 0.04 |
| Time since surgery | ||||||
| ≤30 days (n=610, PY=50.10) | 0 | 0.0 | 0.2 | 4.5 | 0.00 (0.00–18.34) | 0.36 |
| >30 days and <1 year (n=610, PY=559.13) | 0 | 0.0 | 2.6 | 4.7 | 0.00 (0.00–1.41) | 0.14 |
| ≥1 year and <3 years (n=608, PY=1198.38) | 5 | 4.2 | 6.3 | 5.3 | 0.79 (0.26–1.84) | 0.80 |
| ≥3 years and <5 years (n=592, PY=1168.61) | 5 | 4.3 | 7.2 | 6.1 | 0.70 (0.23–1.62) | 0.55 |
| ≥5 years and ≤7 years (n=577, PY=841.33) | 5 | 5.9 | 5.9 | 7.0 | 0.85 (0.28–1.98) | 0.92 |
CI=confidence interval; LAGB=laparoscopic adjustable gastric band; PY=person years; RYGB=Roux-en-Y gastric bypass; SMR=standardized mortality ratio
59 sleeve gastrectomy and 19 biliopancreatic diversion – duodenal switch
Although there was not a significant difference between the observed and expected mortality for either RYGB or LAGB, there were differences in mortality by age and length of follow-up according to procedure. Specifically, the observed mortality was significantly higher than expected only among individuals aged 35–44 who underwent RYGB (SMR=2.37, 95%CI=1.35–3.85, P=0.004), while the observed mortality was significantly lower than expected only among individuals aged 55 and older who underwent LAGB (SMR=0.47, 95%CI=0.19–0.96, P=0.04). The observed mortality was significantly higher than expected within 30 days (SMR=6.06, 95%CI=1.25–17.71, P=0.03) and 5–7 years post-RYGB (SMR=1.94, 95%CI=1.29–2.81, P=0.002) but not post-LAGB (Table 2).
Across all time points, the most common causes of death were cardiovascular disease (n=18), neoplasm, all of which were malignant, (n=13), and diabetes mellitus, endocrine, blood, immune, and genitourinary disease (n=12). The number of deaths by cause of death are in Table 3.
Table 3.
Specific causes of death following bariatric surgery
| Cause of death category | N | Specific cause of death | N |
|---|---|---|---|
| Communicable, maternal, perinatal, nutritional | 3 | Sepsis, unspecified | 3 |
| Malignant and non-malignant neoplasms | 13 | Malignant neoplasm: Colon, unspecified | 3 |
| Malignant neoplasm of rectosigmoid junction | 1 | ||
| Malignant neoplasm: Intrahepatic bile duct carcinoma | 1 | ||
| Malignant neoplasm: Pancreas, unspecified | 2 | ||
| Malignant neoplasm: Bronchus or lung, unspecified | 1 | ||
| Malignant neoplasm: Breast, unspecified | 2 | ||
| Malignant neoplasm of prostate | 1 | ||
| Malignant neoplasm of kidney, except renal pelvis | 1 | ||
| Secondary malignant neoplasm of liver and intrahepatic bile duct | 1 | ||
| Diabetes mellitus, endocrine, blood, immune, genitourinary | 12 | Thyrotoxicosis with diffuse goiter | 1 |
| Unspecified diabetes mellitus: Without complications | 3 | ||
| Obesity | 6 | ||
| Chronic kidney disease, stage 5 | 1 | ||
| Urinary tract infection, site not specified | 1 | ||
| Mental and substance use disorders | 7 | Accidental poisoning by and exposure to noxious substances | 6 |
| Poisoning by and exposure to other and unspecified drugs, medicaments and biological substances, undetermined intent | 1 | ||
| Cardiovascular | 18 | Mitral valve disease, unspecified | 1 |
| Hypertensive heart disease without (congestive) heart failure | 1 | ||
| Acute myocardial infarction, unspecified | 3 | ||
| Chronic ischemic heart disease | 6 | ||
| Pulmonary embolism without mention of acute cor pulmonale | 1 | ||
| Endocarditis, valve unspecified | 2 | ||
| Other hypertrophic cardiomyopathy | 1 | ||
| Cardiac arrest, unspecified | 1 | ||
| Cardiomegaly | 1 | ||
| Phlebitis and thrombophlebitis of other deep vessels of lower extremities | 1 | ||
| Cerebrovascular | 5 | Intracerebral hemorrhage, unspecified | 1 |
| Intracranial hemorrhage (nontraumatic), unspecified | 1 | ||
| Cerebral infarction, unspecified | 1 | ||
| Stroke, not specified as hemorrhage or infarction | 1 | ||
| Cerebral arteritis, not elsewhere classified | 1 | ||
| Respiratory | 3 | Chronic obstructive pulmonary disease with acute lower respiratory infection | 1 |
| Other specified pleural conditions | 1 | ||
| Other disorders of lung | 1 | ||
| Digestive | 7 | Unspecified abdominal hernia without obstruction or gangrene | 1 |
| Alcoholic liver disease | 2 | ||
| Fatty (change of) liver, not elsewhere classified | 3 | ||
| Gastrointestinal hemorrhage, unspecified | 1 | ||
| Unintentional injuries | 3 | Pedestrian injured in traffic accident involving other and unspecified motor vehicles | 1 |
| Other fall on same level | 2 | ||
| Intentional injuries | 2 | Intentional self-poisoning by and exposure to other and unspecified drugs, medicaments and biological substances | 1 |
| Intentional self-harm by other and unspecified firearm discharge | 1 | ||
| Other | 3 | Rheumatoid arthritis, unspecified | 1 |
| Other and unspecified abdominal pain | 1 | ||
| Cardiogenic shock | 1 |
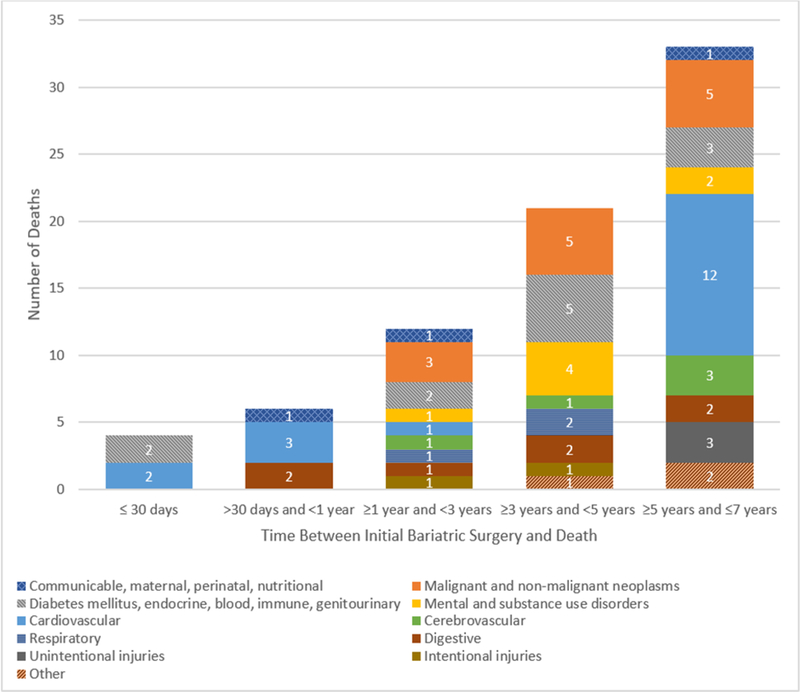
The number of deaths by cause of death and time since surgery are shown in Figure 1. There were 10 deaths observed within 1 year of surgery, half of which were due to a cardiovascular cause. After that, the number of deaths in each 2-year time-period increased such that there were the same number of deaths occurring in the last 2 years of follow-up (n=33 5–7 years post-surgery) as in the 4 years before that (n=33 ≥1-<5 years post-surgery). Only one death due to cardiovascular disease occurred among the 33 reported 1 to 5 years post bariatric surgery (3% of deaths). However, cardiovascular disease deaths accounted for 12/33 (36%) of all deaths 5–7 years following bariatric surgery.
Figure 1.
Causes of death by time since bariatric surgery
In the base models of pre-surgery factors predicting mortality 30 days to 7 years post-surgery, higher BMI (ARR=1.34 per 10 kg/m2, 95%CI=1.01–1.80), sleep apnea (ARR=1.77, 95%CI=1.04–3.00), and diabetes (ARR=1.92, 95%CI=1.16–3.19) were significantly related to higher risk of death, while any alcohol use was significantly related to lower risk of death (ARR=0.61, 95%CI=0.38–0.99). However, none of these factors was independently associated with post-perioperative mortality in the final model. Pre-operative older age (ARR=1.66 per 10 years, 95%CI=1.31–2.11), lower household income (ARR=4.40 for <$25,000 versus ≥75,000, 95%CI=1.89–9.76), smoking (ARR=2.75, 95%CI=1.53–4.95), and dyslipidemia (ARR=2.19, 95%CI=1.07–4.48) were independently associated with higher mortality in the full model (Table 4).
Table 4.
Predictors of mortality 30 days-7 years post-bariatric surgery
| Base Modelsa | Full Multivariable Modelb | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Deathsc | No. of Participantsc | ARR (95%CI) | P | No. of Deaths | No. of Participants | ARR (95%CI) | P | |
| Age, per 10 y older | 71 | 2430 | 1.62 (1.31–1.99) | <.001 | 59 | 1985 | 1.66 (1.31–2.11) | <.001 |
| Sex | 0.08 | 0.27 | ||||||
| Female | 48 | 1910 | 1.0[Reference] | 41 | 1538 | 1.0[Reference] | ||
| Male | 23 | 520 | 1.56 (0.95–2.56) | 18 | 447 | 1.34 (0.79–2.29) | ||
| Race | 0.72 | 0.73 | ||||||
| White | 61 | 2099 | 1.0[Reference] | 54 | 1752 | 1.0[Reference] | ||
| Black | 8 | 256 | 1.34 (0.65–2.78) | 4 | 168 | 0.94 (0.34–2.62) | ||
| Other | 2 | 75 | 0.92 (0.23–3.61) | 1 | 65 | 0.46 (0.07–3.18) | ||
| Annual household income | <.001 | 0.005 | ||||||
| <$25,000 | 24 | 393 | 5.44 (2.40–12.32) | 22 | 365 | 4.30 (1.89–9.76) | ||
| $25,000-$49,999 | 16 | 563 | 2.71 (1.13–6.47) | 16 | 511 | 2.68 (1.13–6.35) | ||
| $50,000-$74,999 | 13 | 519 | 2.26 (0.94–5.43) | 13 | 468 | 2.18 (0.92–5.20) | ||
| ≥$75,000 | 8 | 705 | 1.0[Reference] | 8 | 641 | 1.0[Reference] | ||
| Education | 0.50 | |||||||
| ≤ High school | 18 | 510 | 1.16 (0.64–2.09) | |||||
| Some college | 25 | 908 | 0.80 (0.44–1.44) | |||||
| ≥ College | 18 | 819 | 1.0[Reference] | |||||
| Body mass index, per 10 more | 71 | 2430 | 1.34 (1.01–1.80) | 0.046 | 59 | 1985 | 1.15 (0.82–1.60) | 0.42 |
| Any cigarette smoking | <.001 | <.001 | ||||||
| No | 56 | 2108 | 1.0[Reference] | 45 | 1735 | 1.0[Reference] | ||
| Yes | 15 | 318 | 2.59 (1.48–4.53) | 14 | 250 | 2.75 (1.53–4.95) | ||
| Any alcohol use | 0.04 | |||||||
| No | 36 | 914 | 1.0[Reference] | |||||
| Yes | 27 | 1321 | 0.61 (0.38–0.99) | |||||
| Regular alcohol use | 0.24 | |||||||
| No | 61 | 2090 | 1.0[Reference] | |||||
| Yes | 2 | 145 | 0.44 (0.11–1.73) | |||||
| AUD symptoms | 0.69 | |||||||
| No | 59 | 2081 | 1.0[Reference] | |||||
| Yes | 4 | 148 | 1.22 (0.45–3.26) | |||||
| Sleep apnea | 0.03 | |||||||
| No | 19 | 1156 | 1.0[Reference] | |||||
| Yes | 51 | 1271 | 1.77 (1.04–3.00) | |||||
| Diabetes | 0.01 | |||||||
| No | 29 | 1573 | 1.0[Reference] | |||||
| Yes | 40 | 804 | 1.92 (1.16–3.19) | |||||
| Hypertension | 0.36 | |||||||
| No | 12 | 760 | 1.0[Reference] | |||||
| Yes | 57 | 1620 | 1.35 (0.71–2.57) | |||||
| Dyslipidemia | 0.01 | 0.03 | ||||||
| No | 9 | 712 | 1.0[Reference] | 8 | 654 | 1.0[Reference] | ||
| Yes | 59 | 1482 | 2.47 (1.25–4.89) | 51 | 1331 | 2.19 (1.07–4.48) | ||
| Deep vein thrombosis/ | 0.35 | |||||||
| pulmonary embolism | ||||||||
| No | 56 | 2112 | 1.0[Reference] | |||||
| Yes | 5 | 90 | 1.51 (0.64–3.56) | |||||
| Surgical procedure | 0.33 | 0.73 | ||||||
| RYGB | 55 | 1717 | 1.36 (0.77–2.39) | 45 | 1433 | 1.08 (0.59–1.98) | ||
| LAGB | 15 | 606 | 1.0[Reference] | 13 | 486 | 1.0[Reference] | ||
| Other | 1 | 107 | 0.46 (0.06–3.47) | 1 | 66 | 0.53 (0.08–3.37) | ||
AUD=Alcohol use disorder; ARR=adjusted relative risk; CI=confidence interval; LAGB=laparoscopic adjustable gastric band; RYGB=Roux-en-Y gastric bypass.
Poisson mixed models with robust error variance adjusted for site, surgical procedure, age, sex, race, and BMI. 4 participants who died in perioperative period (≤30 days post-bariatric surgery) were excluded. 24 participants were missing information on race and not included in models. One participant missing data on race had died 30 days to 7 years post-surgery.
Poisson mixed model with robust error variance adjusted for site, surgical procedure, age, sex, race, BMI, and other variables as indicated in this table. Any alcohol use, sleep apnea, and diabetes were considered in the model but not retained. 4 participants who died in perioperative period (≤30 days post-bariatric surgery) were excluded. 469 participants were missing information on race, annual household income, cigarette smoking, or dyslipidemia and not included in model. 13 participants with missing data had died 30 days to 7 years post-surgery.
Sums may not add to total due to missing data.
Discussion
People who are eligible to undergo bariatric surgery have increased mortality risk compared to the general population due to excess weight and often have comorbidities that could reduce longevity, e.g., cardiovascular, diabetes. This study was undertaken to determine whether that excess risk persists following bariatric surgery since, despite substantial weight loss and comorbidity resolution occurring in many cases, the increased risk may not entirely disappear and weight regain and comorbidity reoccurrence or new onset is not uncommon.(3)
Despite the suspected increased risk for mortality among adults who have undergone bariatric surgery compared to the general population due to residual excess adiposity and related health conditions, LABS-2 participants did not have significantly higher mortality up to 7 years after bariatric surgery than age-, sex-, and race- similar adults from the general U.S. population. This was true for both surgical procedures, RYGB and LAGB, studied in this multi-center cohort.
A large study (N=7,862) examining mortality following laparoscopic RYGB (57.2%), LAGB (26.8%), and other laparoscopic bariatric procedures in New York State, reported a small but significant reduction in mortality across 8–14 years of follow-up when compared to age-, sex-, race-, and year- similar projections for the New York State population, (2.5% versus 3.1%, respectively; P=0.01).(22) The SMR point estimate calculated from the New York State study is 0.81, which is contained within the 95% confidence interval from our study, indicating that our results are consistent. The larger sample size, longer follow-up, and exclusion of open bariatric procedures and deaths that occurred immediately after surgery from the New York State study may explain why they found significantly lower mortality and we did not. It is important to note that LABS-2, which includes sites from across the country, including New York City, is more geographically representative of the United States. Additionally, mortality in LABS-2 was verified using data from the National Death Index which has better coverage than the Social Security Death Index(37,38) used to ascertain mortality in the New York State study. In contrast, studies in Sweden and Pennsylvania that compared post-bariatric surgery mortality to that of a general population found higher mortality following bariatric surgery than in the general population.(19–22) However, this is unsurprising since most patients in those studies(19,20) underwent gastric banding or vertical banded gastroplasty which are associated with less weight loss and comorbidity resolution than other procedures(3,39). Finally, several previous studies reported significantly lower mortality among adults who underwent bariatric surgery versus matched controls undergoing non-surgical usual care for obesity(9,11,12). However, the difference in reference groups from such studies prevents comparison with the current study.
We did not find significant differences between observed and expected mortality among men or women. That surgery imparts a small perioperative risk is not surprising.(8) However, that mortality, compared to the general population, was higher 5–7 years post-surgery, particularly with respect to cardiovascular causes, is a novel finding that warrants further investigation. It may be that the mortality-related benefits of surgery are limited to 5 years post-surgery, but it may also be that weight regain and comorbidity recurrence(3) impart greater mortality risk as time since surgery increases. We previously reported significant weight regain and increases in the prevalence of high triglycerides and hypertension 3–7 years following RYGB.3 Because weight regain, high triglycerides, and hypertension are cardiovascular disease risk factors, these findings may at least partially explain findings from the current study that twice as many cardiovascular disease deaths occurred 5–7 years post-surgery than in the first 5 years following surgery.
The two leading causes of death in the LABS-2 cohort, i.e., cardiovascular disease and neoplasms, are consistent with the leading causes of death in the general U.S. population(40) and in prior reports of bariatric surgery patients.(9,13,21,24) The small number of deaths in LABS-2 resulted in limited statistical power to perform tests of statistical significance for cause-specific mortality.
Many of the factors significantly associated with mortality in this cohort (i.e., older age, lower income, and cigarette smoking) are consistent with associations with mortality in the general population.(1,41–44) For example, we found that the presence of lower income, cigarette smoking, and dyslipidemia at time of surgery are associated with over twice the risk of death 30 days to 7 years following surgery. Our findings that the specific surgical procedure performed was not significantly associated with mortality is also consistent with published literature.(14,22) With adjustment for potential confounders, we did not find that pre-surgery diabetes status was independently associated with mortality 30 days to 7 years following bariatric surgery which is consistent with one prior report(24) but in contrast to two others(13,22). This may be due to differences among the studies in other risk factors that were considered since diabetes was significantly associated with mortality in the base model presented here. However, after adjusting for dyslipidemia, diabetes was no longer significant due to the association between those two risk factors. Of the two studies that identified pre-surgery diabetes status as an independent risk factor for post-bariatric surgery mortality, one controlled for total cholesterol but not dyslipidemia(13); it was not stated for what comorbidities the other study controlled for(22). Although dyslipidemia is an established risk factor for mortality in the general population,(45) to our knowledge, this is the first study to demonstrate that it is also a risk factor for mortality in the bariatric surgery population, which tends to have a high prevalence of the disorder. Dyslipidemia is thought to influence mortality through cardiovascular disease, the leading cause of death in this cohort.
Several limitations should be considered. First, due to the small number of deaths for many specific causes, only frequencies are presented. Second, to preserve statistical power, no adjustment for multiple comparisons was performed which may have led to increased type I errors with respect to subgroup analyses. Thus, findings in subgroups would benefit from validation using an independent sample. While many variables were considered in the models assessing pre-surgery factors related to post-perioperative mortality, it is possible that some were missed. Furthermore, though the LABS-2 participants were from several geographic regions of the United States, not all regions were represented, somewhat limiting the validity of the comparison with the general U.S. population. However, the results may still be more generalizable to the U.S. population than previous reports that are limited to particular states within the U.S. or were performed outside of the U.S. Additionally, this study takes advantage of the careful data collection in LABS-2, enabling us to examine risk factors for mortality not always available in administrative databases.
Conclusions
Mortality during the first 7 years following bariatric surgery is not statistically significantly different from the mortality expected in the general U.S. population of similar age, sex and race. However, the number of deaths was greater than expected in the first 30 days immediately following surgery and 5–7 years after surgery. This is driven by the surge in cardiovascular disease deaths 5–7 years after surgery which may be partially explained by post-surgery weight regain and recurrence of comorbidities(3). In addition, in the first 7 years following surgery, mortality was greater than expected in the general U.S. population among those 35–44 years old and less than expected among those over the age of 55 at time of surgery. Risk factors for mortality beyond the 30-day perioperative period to up to 7 years after bariatric surgery (i.e., older age, lower income, cigarette smoking, and dyslipidemia) are also risk factors for mortality in the general adult population.
Supplementary Material
Highlights.
Mortality ≤ 7 years after bariatric surgery is similar to the general population
Mortality is 60% higher 5–7 years post-bariatric surgery than in general population
The most common cause of death ≤ 7 years after bariatric surgery is cardiovascular
Post-surgery mortality risk factors are older age, lower income, smoking, dyslipidemia
Funding:
LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center -U01 DK066557; Columbia-Presbyterian - U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1- RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors will complete and submit the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs. Belle, Flum, King, Pories, White, Wolfe, and Yanovski have nothing to disclose. Dr. Courcoulas has received a research grant from Allurion, Inc. unrelated to this work. Dr. Pomp has received honorarium as a speaker from Medtronic and WL Gore and Associates. Dr. Spaniolas has received research support from Merck.
References
- 1.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courcoulas AP, King WC, Belle SH, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes−−3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015;150(10):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokes A, Preston SH. Deaths Attributable to Diabetes in the United States: Comparison of Data Sources and Estimation Approaches. PLoS One. 2017;12(1):e0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung HC, Xu J. Hypertension-related Mortality in the United States, 2000–2013. NCHS data brief. 2015(193):1–8. [PubMed] [Google Scholar]
- 8.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: A systematic review and meta-analysis. Diabetes, obesity & metabolism. 2017;19(9):1223–1232. [DOI] [PubMed] [Google Scholar]
- 10.Smith MD, Patterson E, Wahed AS, et al. Thirty-day mortality after bariatric surgery: independently adjudicated causes of death in the longitudinal assessment of bariatric surgery. Obes Surg. 2011;21(11):1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Yu J, Li L, et al. Effects of Bariatric Surgery on Mortality, Cardiovascular Events, and Cancer Outcomes in Obese Patients: Systematic Review and Meta-analysis. Obes Surg. 2016;26(11):2590–2601. [DOI] [PubMed] [Google Scholar]
- 12.Adams TD, Mehta TS, Davidson LE, Hunt SC. All-Cause and Cause-Specific Mortality Associated with Bariatric Surgery: A Review. Current atherosclerosis reports. 2015;17(12):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 14.Reges O, Greenland P, Dicker D, et al. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-en-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management With All-Cause Mortality. JAMA. 2018;319(3):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson LE, Adams TD, Kim J, et al. Association of Patient Age at Gastric Bypass Surgery With Long-term All-Cause and Cause-Specific Mortality. JAMA Surg. 2016;151(7):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. [DOI] [PubMed] [Google Scholar]
- 17.Christou NV, Sampalis JS, Liberman M, et al. Surgery Decreases Long-term Mortality, Morbidity, and Health Care Use in Morbidly Obese Patients. Annals of Surgery. 2004;240(3):416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 19.Plecka Ostlund M, Marsk R, Rasmussen F, Lagergren J, Naslund E. Morbidity and mortality before and after bariatric surgery for morbid obesity compared with the general population. Br J Surg. 2011;98(6):811–816. [DOI] [PubMed] [Google Scholar]
- 20.Marsk R, Naslund E, Freedman J, Tynelius P, Rasmussen F. Bariatric surgery reduces mortality in Swedish men. Br J Surg. 2010;97(6):877–883. [DOI] [PubMed] [Google Scholar]
- 21.Omalu BI, Ives DG, Buhari AM, et al. Death rates and causes of death after bariatric surgery for Pennsylvania residents, 1995 to 2004. Arch Surg. 2007;142(10):923–928; discussion 929. [DOI] [PubMed] [Google Scholar]
- 22.Telem DA, Talamini M, Shroyer AL, et al. Long-term mortality rates (>8-year) improve as compared to the general and obese population following bariatric surgery. Surgical endoscopy. 2015;29(3):529–536. [DOI] [PubMed] [Google Scholar]
- 23.American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers 2016; http://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
- 24.Sakran N, Sherf-Dagan S, Blumenfeld O, et al. Incidence and Risk Factors for Mortality Following Bariatric Surgery: a Nationwide Registry Study. Obes Surg. 2018;28(9):2661–2669. [DOI] [PubMed] [Google Scholar]
- 25.U.S.Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics. National Death Index. 2017; https://www.cdc.gov/nchs/ndi/index.htm. Accessed October 8, 2018.
- 26.Centers for Disease Control and Prevention. CDC Wonder, Underlying Causes of Death. 2017; https://wonder.cdc.gov/wonder/help/ucd.html. Accessed September 10, 2018.
- 27.Centers for Disease Control and Prevention NCfHS. Multiple Cause of Death 1999–2014 on CDC WONDER Online Database. In: Centers for Disease Control and Prevention NCfHS, 2015. [Google Scholar]
- 28.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. US County-Level Trends in Mortality Rates for Major Causes of Death, 1980–2014. JAMA. 2016;316(22):2385–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian NJ, King WC, Yanovski SZ, Courcoulas AP, Belle SH. Validity of self-reported weights following bariatric surgery. JAMA. 2013;310(22):2454–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcoholism, clinical and experimental research. 2002;26(2):272–279. [PubMed] [Google Scholar]
- 31.King WC, Chen JY, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. Jama. 2012;307(23):2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courcoulas AP, Christian NJ, O’Rourke RW, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015;11(5):1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslow NE, Day NE. Rates and Rate Standardization In: Heseltine E, ed. Statistical Methods in Cancer Research Volume II - The Design and Analysis of Cohort Studies. Vol IARC Scientific Publications No. 82. New York: Oxford University Press; 1987:69. [PubMed] [Google Scholar]
- 36.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 37.Sesso HD, Paffenbarger RS, Lee IM. Comparison of National Death Index and World Wide Web death searches. Am J Epidemiol. 2000;152(2):107–111. [DOI] [PubMed] [Google Scholar]
- 38.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468. [DOI] [PubMed] [Google Scholar]
- 39.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. [DOI] [PubMed] [Google Scholar]
- 40.Heron M Deaths: leading causes for 2010. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;62(6):1–96. [PubMed] [Google Scholar]
- 41.Woolhandler S, Himmelstein DU. The Relationship of Health Insurance and Mortality: Is Lack of Insurance Deadly? Ann Intern Med. 2017;167(6):424–431. [DOI] [PubMed] [Google Scholar]
- 42.Sommers BD, Gawande AA, Baicker K. Health Insurance Coverage and Health - What the Recent Evidence Tells Us. N Engl J Med. 2017;377(6):586–593. [DOI] [PubMed] [Google Scholar]
- 43.Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality--beyond established causes. N Engl J Med. 2015;372(7):631–640. [DOI] [PubMed] [Google Scholar]
- 44.Brodish PH, Hakes JK. Quantifying the individual-level association between income and mortality risk in the United States using the National Longitudinal Mortality Study. Social science & medicine (1982). 2016;170:180–187. [DOI] [PubMed] [Google Scholar]
- 45.Smith DG. Epidemiology of dyslipidemia and economic burden on the healthcare system. The American journal of managed care. 2007;13 Suppl 3:S68–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.