Abstract
Obesity is a global pandemic associated with macro- and microvascular endothelial dysfunction. Microvascular endothelial dysfunction has recently emerged as a significant risk factor for the development of cognitive impairment. In this review, we present evidence from clinical and preclinical studies supporting a role for obesity in cognitive impairment. Next, we discuss how obesity-related hyperinsulinemia/insulin resistance, systemic inflammation, and gut dysbiosis lead to cognitive impairment through induction of endothelial dysfunction and disruption of the blood brain barrier. Finally, we outline the potential clinical utility of dietary interventions, exercise, and bariatric surgery in circumventing the impacts of obesity on cognitive function.
Keywords: hyperinsulinemia, insulin resistance, inflammation, gut dysbiosis, diet, memory
Introduction
Obesity, a pandemic impacting over 1 billion people in the world and 90 million Americans, is a risk factor for cardiovascular disease, cognitive impairment, and dementia (Toth et al., 2017). Age and cardiovascular risk factors adversely affect vascular health, which is critical to normal brain function including cognitive function (DeCarli et al., 2001; Hanon et al., 2005; Hoth et al., 2007; Panza et al., 2006; Solfrizzi et al., 2004). Disruptions in the vascular system, which involves the macro- and microvasculature and the endothelium, occurs prior to the onset of cognitive impairment (Corriveau et al., 2016). In fact, this is now widely recognized as a broad-spectrum disorder referred to as vascular contributions to cognitive impairment and dementia (VCID) (Gorelick et al., 2011).
Endothelial cells have distinct functions in vascular biology. Endothelial cells are responsible for maintaining vascular tone, regulating blood flow, modulating the inflammatory response, and the trafficking of molecules between the periphery and the brain. Endothelial dysfunction disrupts blood flow, reduces vascular tone, and impairs the blood brain barrier (BBB) (Wardlaw et al., 2013). This is mediated in part by disruption in specialized receptors on endothelial cells that transduce mechanical and chemical stimuli to facilitate the release of signaling molecules such as nitric oxide (NO), endothelin, and prostanoids.
Both clinical and preclinical studies demonstrate that obesity reduces the bioactivity of NO (Bender et al., 2007; Bourgoin et al., 2008; Damjanovic and Barton, 2008; Rask-Madsen and King, 2007). Severely obese children display evidence of endothelial dysfunction (Tounian et al., 2001). Furthermore, severely obese (body mass index, [BMI] ≥34 kg/m2) insulin resistant individuals demonstrate impairments in blood flow and vascular tone (Steinberg et al., 1996). This is likely due the downstream signaling consequences associated with hyperinsulinemia/insulin resistance. Insulin resistance is characterized by reductions in phosphoinositide 3-kinase (PI3K) signaling and increased mitogen-activated protein kinase signaling, which leads to decreased NO production, a characteristic of endothelial dysfunction (Williams et al., 2002). These processes work synergistically to promote continuous blood flow to the brain, a process known as neurovascular coupling. Obesity induced disruption of NO has been linked to impaired neurovascular coupling in preclinical models (Tarantini et al., 2018; Tucsek et al., 2014b). Furthermore, this impairment in neurovascular coupling has been associated with cognitive impairment and neurodegeneration (Riddle et al., 2003; Troen et al., 2008; Tucsek et al., 2014a).
Understanding additional mechanisms that contribute to the development of insulin resistance may have therapeutic potential for preventing or reversing obesity-associated endothelial dysfunction. Herein, we discuss the role of specific obesity-related mechanisms including inflammation, hyperinsulinemia/insulin resistance, and gut dysbiosis on endothelial function and cognitive impairment. Likely, obesity induces a feed-forward cycle among these various mechanisms, which ultimately culminates into endothelial cell dysfunction and consequently cognitive impairment (Figure 1). While other mechanisms such as oxidative stress, mitochondrial dysfunction, and neurotrophic factors are important, they have been extensively reviewed elsewhere (de Mello et al., 2018; Sripetchwandee et al., 2018), and thus will not be discussed here. We conclude our review with a summary of therapeutic strategies currently being investigated for alleviating the detrimental impacts of obesity-induced cognitive impairment. Understanding the impact of the relationship among these phenomena may help bring forward new therapeutic strategies to mitigate obesity-induced cognitive impairment.
Figure 1. Obesity induces cognitive decline.
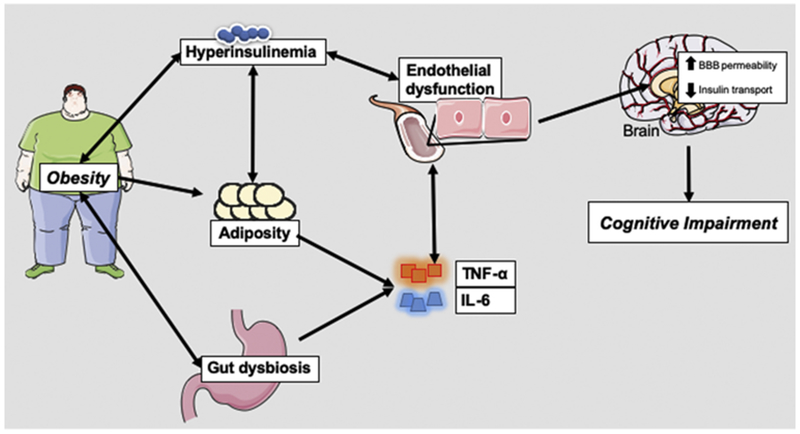
Schematic illustrating how the consequences of obesity (hyperinsulinemia, adiposity, and gut dysbiosis) results in the production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). This culminates into endothelial dysfunction, which leads to increased cytokine influx, increased blood brain barrier (BBB) permeability, and reduced insulin transport in the brain. This feed forward cycle of insults ultimately contribute to cognitive impairment.
The link between obesity and cognitive impairment
Clinical Studies
Clinical studies evaluating the link between obesity and cognitive impairment demonstrate inconsistent findings. Some support an association between obesity and cognitive impairment (Gunstad et al., 2010; Prickett et al., 2015; Wang et al., 2016a) while others fail to demonstrate a relationship(Reviewed in (Bischof and Park, 2015; Gunstad et al., 2010; Han et al., 2009). These inconsistent findings may be due to variations in study design. First, clinical studies may differ based on the population age, which consist of early life (4–18 years old), early to mid-life (19–65 years old), or mid-life to late-life (65+ years old) (Smith et al., 2011). Overall, studies suggest that early and mid-life obesity are associated with worse cognitive outcomes. Of these two population ages, mid-life obesity has the most adverse effects on cognition (Dye et al., 2017). In fact, lower scores on the Mini-Mental State Examination (MMSE) correlate with midlife obesity (Bischof and Park, 2015). Cognitive performance on tests involving visual memory, organization, executive function, attention and visuomotor speed was worse in individuals with central obesity in midlife (Wolf et al., 2007). A previous study reported that late-life obesity correlated with better performance on attention and executive function tasks (Gunstad et al., 2010); however, a recent study reported no association between late-life obesity and cognitive impairment (Deckers et al., 2017). Overall, these studies suggest that aging may play a role in obesity-related cognitive impairment; however, the mechanisms have not been elucidated.
In addition to cognitive deficits, recent data suggest that differences in brain structure in obese and non-obese populations are present. For example, volumes of brainstem and diencephalon reduction were noted in early adulthood obesity (Marques-Iturria et al., 2013). Likewise, lower cortical thickness was observed in the left superior frontal and right medial orbitofrontal cortex in a similar group of patients, which may provide some explanation about the association between obesity and cognitive dysfunction in obese individuals (Marques-Iturria et al., 2013). Others have demonstrated that populations with increased BMI display decreased global brain volume and gray-matter volume with decreased neural viability in both frontal and parietal cortices (Gazdzinski et al., 2008). Similarly, reductions in global white matter integrity (Verstynen et al., 2012) and atrophy of the temporal, frontal, occipital cortices, hippocampus, thalamus, and midbrain have also been noted in other populations with increased BMI (as reviewed in Shafer et al. (Shefer et al., 2013). Coinciding with these findings is the observation that a high BMI in midlife leads to declines in neuron and myelin viability (Gazdzinski et al., 2008) and may be associated with abnormalities in altered brain plasticity (Wang et al., 2016a). Collectively, these studies show that obesity may be a causal link for deleterious changes in brain structure. Additional studies are needed to confirm the aforementioned findings.
Second, clinical studies may use different indices for “obesity” designation. The majority of clinical studies use body mass index (BMI≥30 kg/m2) as the obesity metric. Unfortunately, BMI does not account for alterations in body composition; hence, it inadequately correlates with adiposity. Central adiposity metrics, such as waist circumference (≥ 40 inches for men and ≥ 35 inches for women) or waist-hip ratio (≥ 1 for men and ≥ 0.8 for women), correlate better with cognitive impairment (Han et al., 2009; Nilsson and Nilsson, 2009; Wang et al., 2016a).
Third, studies differ based on the cognitive test used to assess cognitive impairment. This stems from the fact that the term “impairment” is broad and involves multiple cognitive domains. Thus, it is difficult to assess every cognitive domain in a single study, which may lead to conflicting reports. Still, strong evidence from the extant literature suggest that obesity negatively impacts memory and is associated with poor performance on psychomotor, selective attention, decision making, and executive function tests (as reviewed in Dye et al. (Dye et al., 2017).
Finally, while many clinical studies report the presence or absence of comorbidities such as hypertension, hypercholesterolemia, diabetes, dyslipidemia, or hyperglycemia, some studies fail to adjust for comorbidities when assessing the link between obesity and cognitive deficiencies. A study by Singh-Manoux and colleagues demonstrated that obese individuals who were metabolically unhealthy had a faster rate of cognitive decline compared to obese individuals without metabolic abnormalities over a 10-year period (Singh-Manoux et al., 2012). While this study demonstrates obesity works synergistically with metabolic disorders to drive changes in cognition, the authors also showed that obesity in the absence of metabolic disorders increased the rate of global cognitive decline in comparison to the rate of decline in normal weight individuals (Singh-Manoux et al., 2012). These reports have since been confirmed by other groups as well (Ala Abu Saleh, 2015; Farah et al., 2016). Table 1 summarizes clinical studies from the past decade taking into consideration study population age, obesity metrics, cognitive test, and comorbidities for each of the specified studies. Given the great variability in clinical studies, it is difficult to evaluate potential mechanisms that drive obesity-related cognitive impairment. Preclinical animal models provide the opportunity to explore novel mechanisms underlying the pathogenesis of obesity-related cognitive impairment and to develop innovative therapies.
Table 1.
Impacts of obesity on cognition across the lifespan
| Authors | Population | Age range (mean, sd) | N | Study Design | Obesity Indices | Areas of cognitive functioning assessed (measures) | Findings (abbreviated) | Risk Factors Considered (adjusted for?) |
|---|---|---|---|---|---|---|---|---|
| (Davis and Cooper, 2011) | Early life, obese | 7-11 years (9.3±1.1) | 170 | Cross-sectional cohort study | BMI, WC, X-ray adiposity | executive function (cognitive assessment system) | [−]:executive function and obesity | none |
| (Jansen et al., 2011) | Early life, healthy weight and overweight | Overweight (10±0.9), healthy weight(9.9±0.7) | 32 | Cross-sectional study | BMI | perceptual reasoning (Colored Progressive Matrices test); perception and movement (Chronometric mental rotation test) | [−]: perception and movement, perceptual reasoning and obesity | none |
| (Schwartz et al., 2013) | Early life, obese | 12-18 years; males (14.9±1.8); females (15.1±1.9) | 911 | Cross-sectional retrospective cohort study | Visceral fat (MRI), total body fat | processing speed (automatic and controlled detection speed, symbol sear, coding); interference (Stroop Color-Word Test) Ruff 2 & 7 Selective Attention Test); working memory(Self-Ordered Pointing Task); visuospatial memory(Dot location Learning); verbal memory(Stories subtests of the Children’s Memory Scale); cognitive flexibility (Semantic and Phonemic fluency); all domains (WISC-III) | [−]: processing speed, working memory, resistance to interference, cognitive flexibility and visceral fat [+]: processing speed, verbal memory and total body fat |
none |
| (Verdejo-Garcia et al., 2010) | Early life, healthy and overweight | 13-16 years; overweight/obese (14.3± 1.2); healthy (15.3±0.9) | 61 | Cross-sectional | BMI | anxiety and impulsivity(Impulsive behavior scale, sensitivity to punishment and reward questionnaire); mental flexibility (Letter-number sequencing, TMTB); reasoning (Analogical reasoning similarities); planning (zoo map); interference(Stroop test); flexibility (Five Digit Test, TMTA); self-regulation (revised-strategy application test R-SAT); decision making (Iowa Gambling Test) | [-]:Inhibition, flexibility, decision making, flexibility and BMI [ø]: working memory, planning, memory and BMI |
none |
| (Lokken et al., 2009) | Early life, obese | 13-19 years (15.9± 1.7) | 25 | Cross-sectional retrospective cohort study | BMI | reading ability(Wde Range achievement test); intelligence (Wechsler Abbreviate Scale of Intelligence); global cognition (Computerized Cognitive Test Battery); verbal fluency and memory(Digitspan, Verbal Interference); attention (Continuous performance task, Switching of Attention); visuospatial (Mask task); Impulsivity(Go-No Go test) | [−]: attention, executive function and obesity | Hypertension, Type 2 diabetes (no) |
| (Fergenbaum et al., 2009) | Early- Mid-life, healthy, overweight, obese | 19-65 years | 207 | Cross-sectional | BMI, WC | executive function (Clock Drawing Test), visual attention and task switching (TMTA/B) | [−]: cognitive performance and obesity/increased waist circumference; [ø]: cognitive performance and overweight or healthy weight |
Metabolic syndrome, dyslipidemia, insulin resistance, hypertension diabetes (yes) |
| (Gunstad et al., 2010) | Mid- late life, overweight | 19-93 (55± 16.9) | 1,703 | Cross-sectional and longitudinal, prospective cohort study | BMI, WHR, weight | global cognition (MMSE);working memory (WAIS-R); visual attention/cognitive flexibility (TMTA/B); verbal learning, fluency and memory(California Verbal Learning Test, Letter Fluency, Boston Naming Test,); Memory (Prospective Memory Test Benton Visual Retention Test, Blessed Information-Memory-Concentration test); visuo-spatial (Card Rotations) | [−]: global cognitive performance, attention, memory, verbal fluency, and BMI, WC, or WHC [+]: visuospatial/TMTB and BMI, WC, or WHC |
Type 2 diabetes/glucose intolerance, hypertension (yes) |
| (Deckers et al., 2017) | Early-late life, obese | 24-81 years (58± 15) | 1,823 | Longitudinal cohort study | BMI, WC | verbal learning and memory (Visual Verbal Learning Test); executive function (Concept Shifting Test); processing speed (Letter Digit Substitution Test) | [−]: memory, executive function processing speed and obesity | Hypertension, type 2 diabetes, cardiovascular disease, depressive symptoms (no) |
| (Nilsson and Nilsson, 2009) | Early, Mid-late, Late life, normal weight, overweight | 35-55 years (47.0±6.0); 60-70 years | 3,526 | Cross-sectional, prospective cohort study | BMI, WHR | word comprehension task, global cognition (MMSE); memory(prospective memory test), free recall test, immediate free recall test, cued test, name-stem completion task, name recognition task, fluency test | [−]: semantic memory and oven/weight; [+]: spatial ability and middle-aged overweight [ø]: episodic memory and overweight after adjusting for CVD risk |
Hypertension, diabetes, stroke (no) |
| (Singh-Manoux et al., 2012) | Early to Mid-life, metabolically normal, abnormal obese | 39-63 years (49.7±9.1) | 6,401 | Longitudinal cohort, prospective study | BMI | reasoning (Alice Heim 4-I); memory(20-word free recall test); verbal fluency (phonemic and semantic) | [−]: cognitive performance over time and metabolically abnormal obesity | Hypertension, cholesterol, diabetes, dyslipidemia, and hyperglycemia (yes) |
| (Wolf et al., 2007) | Early-Late life, obese | 40-69 years (52±7.9) | 1,814 | Longitudinal cohort, prospective study | BMI, WHR | Executive function (neuropsychological test battery), Visual attention (TMTB, HVOT, Visual Reproductions test immediate and delayed recall)verbal and working memory (Paired Associates test immediate and delayed recall, logical memory test immediate and delayed recall) | [−] executive function, visuomotor skills | Hypertension (yes) |
| (Rochette et al., 2016) | Mid-late life, obese | (43±11.2) | 171 | Longitudinal prospective study | BMI | global cognition (Neurocognitive battery); language (verbal list recognition); verbal memory (dig it span forward, digit span backwards); Attention (sustained attention) Verbal Fluency (switching of attention-digits); interference (verbal interference-word); verbal fluency (letter fluency, animal fluency); task switching (switching of attention); executive function (maze errors and maze overrun errors) | [−]: cognitive performance and severe obesity | Hypertension, diabetes (no) |
| (Debette et al., 2011) | Early - Mid-life, obese | (54±9) | 1,352 | Longitudinal cohort, prospective study | BMI, WC, WHR | global cognitive function/executive function (Neuropsychological test battery); working visual memory(delayed recall component of the Visual Reproductions test, Logical Memory subtest from the Wechsler Memory Scale); visual attention/cognitive flexibility; (Visual Reproductions test, TMTB and A) | [−]:executive function and mid-life obesity | Hypertension, diabetes, hypercholesterolemia (no) |
| (Sabia et al., 2009) | Mid-life, healthy underweight overweight, obese | (60.8±5.9) | 5,131 | Cross-sectional and longitudinal, prospective cohort study | BMI | global cognition (30-item MMSE); memory (20-word free recall test); reasoning (Alice Heim 4-I); verbal fluency test | [−]: cognitive performance over time and overweight or obesity [−]:MMSE, memory, executive function over time and obesity or underweight |
diabetes, hypertension, cholesterol, coronary heart disease, stroke, glucose tolerance test(yes) |
| (Han et al., 2009) | Mid- Late life, obese | 63-89 years (70±5.05) |
2,767 | Longitudinal, cross-sectional, prospective | BMI, WC, WHR, PBF | Global cognitive function (NA: CERAD-K) | [−] global cognitive function | Hypertension, diabetes, high cholesterol (yes) |
| (Benito-Leon et al., 2013) | Mid - Late life, obese | (74.9±5.5) | 1,949 | Cross sectional cohort, prospective study | BMI | global cognition (37-item version of MMSE), attention (easier form of TMTA), verbal learning and memory(Naming Test); immediate memory(immediate free recall); verbal and working memory(delayed recall, story recall, immediate logical memory, delayed logical memory); verbal fluency (Word Accentuation Test, Verbal fluency) | [−]: global cognition, attention, verbal fluency, memory and overweight/obesity | diabetes, hypertension, high cholesterol, dementia (yes) |
Studies in table come from previous reviews Smith et al., 2011; Benito-Leon et al 2013; Bischof et al., 2015; Wang et al., 2016; Dye et al., 2017. AH4-I, Alice Heim 4-I; IMC, Blessed Information-Memory-Concentration test; MMSE, Mini-Mental State Exam; neuropsychological assessment CERAD-K, Consortium to Establish a Registry for Alzheimer’s Disease, Korean; R-SAT, revised-strategy application test; TMT, Trail making test; WAIS-R, Wechsler Adult Intelligence Scale Revised; WISC, Wechsler Intelligence Scale for Children; HVOT, Hooper Visual Organization Test BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; Ø indicates null findings. (−) indicates inverse correlation between weight and speafic area of cognitive functioning. (+) indicates positive correlation between weight and area of cognitive functioning.
Preclinical Studies
The western diet has been regarded as playing a significant role in the obesity epidemic. In animal models, the diet is comprised of at least 40% of calories from fat and supplemented with a lesser amount of simple carbohydrates (Lesniewski et al., 2013). Diet-induced obesity tends to vary between laboratories, with some using both high-fat and high simple-carbohydrate concentrations, while others use one or the other. Western and high simple-carbohydrate diet models induce cognitive impairment and brain dysregulation in rodent models (Darling et al., 2013; Hsu et al., 2015; Jurdak and Kanarek, 2009; Kanoski and Davidson, 2011; Yeomans, 2017). This cognitive deficit is apparent despite either no change in weight reported or minimal changes compared to a high-fat diet (HFD) (Jurdak and Kanarek, 2009; Kanoski and Davidson, 2011). Standing alone, the HFD mouse model of obesity has clinical translatability (Aroor et al., 2018; Pulakat et al., 2011), and will be discussed here.
Similar to clinical studies, preclinical animal studies also suffer from the lack of consistency in study design. First, the age of HFD induction and the duration of feeding differs across preclinical studies. The impact of juvenile onset HFD feeding on cognitive performance has been rigorously reviewed, with the vast majority of studies demonstrating a causative relationship (Del Olmo and Ruiz-Gayo, 2018; Noble and Kanoski, 2016). This has been corroborated in both the adult and aged mouse phenotypes (reviewed (Cordner and Tamashiro, 2015). Studies demonstrate cognitive impairment after two weeks (McLean et al., 2018; Sims-Robinson et al., 2016a), 9 days (Murray et al., 2009), and even as early as 3 days on a HFD (Thaler et al., 2012), indicating that the length of feeding does not appear to be a major factor.
Second, the percentage of fat used for HFD treatment varies among the preclinical studies. Typically, obesity is induced by feeding animals a diet containing 32-60% of calories from fat. Studies with diets consisting of ≥40% of kilocalories from fat report diet-induced cognitive impairment in both mice and rats (Fu et al., 2017; Underwood and Thompson, 2016; Wang et al., 2016b). In contrast, some studies demonstrate that HFD does not correlate with cognitive impairment (Kosari et al., 2012; Li et al., 2013; Mielke et al., 2006). The impact of HFD in preclinical models on cognitive function has been extensively reviewed (Cordner and Tamashiro, 2015).
Third, similar to clinical studies, preclinical studies differ in the type of cognitive test utilized to assess cognitive impairment. Studies demonstrate that diet-induced obesity reduces performance in Morris water maze (Cordner and Tamashiro, 2015; Gladding et al., 2018; Kasper et al., 2018; Sims-Robinson et al., 2016a; Spinelli et al., 2017), novel object recognition (Cordner and Tamashiro, 2015; Kadish et al., 2016; Sims-Robinson et al., 2016a; Sona et al., 2018), and Y-maze (Almeida-Suhett et al., 2017; Cordner and Tamashiro, 2015; Gladding et al., 2018; Labouesse et al., 2018; Martins et al., 2017; Sona et al., 2018). The Morris water maze, Y-maze, and Novel Object Recognition task are designed to test spatial, working, and recognition memory, respectively. Finally, HFD mice display multiple comorbidities such as hyperinsulinemia, glucose intolerance, and hypertension (Buettner et al., 2007; Oakes et al., 1997; Tschop and Heiman, 2001; Vincent et al., 2009) depending on the percentage of fat used and the duration of HFD feeding. Table 2 summarizes preclinical studies published from 2016-2019 comparing differences in the timeline of HFD feeding, percentage of fat in the experimental diet, cognitive test, and the presence of comorbidities. Despite the variability among preclinical studies, animal models provide the opportunity to explore the molecular mechanisms underlying obesity-related cognitive impairment.
Table 2.
Animal models of obesity-induced cognitive impairment.
| Author | Animal Background | Age Of High-Fat Feeding Specific HFD | Behavioral Task And Phenotype | Risk Factors Considered | Findings (Abbreviated) |
|---|---|---|---|---|---|
| (MARTINS ET AL., 2017) | C57BL6×129SV | From 2–14 months of age CD - 58G7, Test Diets HFD-58G9, Test Diets; 60% calories from lard |
Novel Smell recognition test Y-Maze spontaneous alternation Significant impairment at 14 months in Y-maze performance in HFD group |
Insulin concentrations | Fewer hippocampal synapses at 8 months in HFD group |
| (GLADDING ET AL., 2018) | C57BL/6J | From 4-16 months of age CD - Specialty Feeds; 7% fat w/w HFD - Specialty Feeds; 21 % fat w/w from safflower oil and clarified butter/ghee |
Morris Water Maze Y-Maze Less time in the target quadrant in MWM and fewer novel arm entries in Y-maze in HFD group |
Insulin sensitivity, Glucose tolerance, Leptin, hippocampal inflammation | Intrahippocampal insulin infusion alleviated behavioral deficits in HFD group |
| (VINUESA ET AL., 2018) | C57BL/6J | From 3-9 weeks of age CD - Gepsa Feeds; 2.5 kcal/g energy/pellet, 3.6% fat HFD - Gepsa Feeds; 3.9 kcal/g energy / pellet, 21.6% fat: monounsaturated fatty acids (44.7%), saturated fatty acids (29.8%) and polyunsaturated fatty acids (20.9%) |
Elevated Plus Maze, Novel Object Location Recognition test Impaired novel object location recognition, no significant difference in EPM performance in HFD group |
Pancreatic insulin, inflammation, DG neurogenic capacity | Inflammation changes in the hippocampus, decreased DG capacity, dendritic spine morphology alterations in HFD group |
| (SONA ET AL., 2018) | C57BL/6J | From 4-20 weeks of age CD – D12450 Research Diets; 10 kcal % fat HFD – D12492 Research Diets; 60 kcal % fat from lard |
Two-way active avoidance, Y-maze test, passive avoidance, Object Recognition Impaired performance in novel recognition, Y-maze, and avoidance tests in HFD group |
Glucose tolerance | Decrease in expression of both BDNF and GRP40 in HFD group |
| (LABOUESSE ET AL., 2018) | C57BL/6N | From 4-12 weeks of age CD-SSNIFF Diets; 10 kcal % fat HFD - SSNIFF Diets; 60 kcal % fat from lard |
Y-maze Impaired Y-maze function in HFD group |
None | Reduced miRNA associated with axon guidance and cognition, down regulation of axon guidance genes in HFD group |
| (SPINELLI ET AL., 2017) | C57BL/6 | From 4-10 weeks of age CD – Mucedola, Italy; unreported HFD - Mucedola, Italy; unreported |
MWM Impaired MWM performance in HFD group |
Hippocampal insulin resistance | Impaired synaptic plasticity and insulin resistance in HFD group |
| (KASPER ET AL., 2018) | C57B1/6J | From 12-22 weeks of age CD - Teklad 7912; 17 kcal % fat HFD - D12492 Research Diets; 60 kcal % fat from lard |
MWM Impaired MWM performance in HFD group |
Peripheral insulin resistance | None |
| (ALMEIDA-SUHETT ET AL., 2017) | C57BL/6J | From 5 to 21 weeks of age CD – Harlan Teklad Global diet 2018 HFD-TD.06414, Harlan Laboratories; 60 kcal % fat from lard |
Open field, elevated zero maze, Y-maze, forced swim test Increased anxiety behavior in open field and elevated zero maze, impaired cognitive performance in y-maze, increased depressive behavior in forced swim in HFD group |
Glucose tolerance, IL-1B in hippocampus, amygdala, frontal cortex, and hypothalamus | Increased IL-1B in hippocampus and amygdala in HFD group |
| (KADISH ET AL., 2016) | C57BI/6 | From 2-8 months of age CD – NIH.31 diet, Harlan Teklan HFD - TD.120084, Harlan Teklan; 41 kcal % fat, source unlisted, High-protein diet-TD.120083 High-carbohydrate diet- TD.120082 |
Open field, zero maze, social recognition, water maze* not MWM Impaired social recognition and water maze performance in HFD group |
Body composition, hippocampal inflammation | Increased body fat percentage and total weight in HFD group. No change in hippocampal inflammation |
| (SIMS-ROBINSON ET AL., 2016) | C57BL/6J | From 4-12 weeks of age for dietary reversal and 4-20 weeks of age for HFD group CD – D12450B, Research Diets; 10 kcal % from fat HFD – D05090701, Research Diets; 54% kcal % fat from lard |
NOR, MWM Impaired NOR and MWM after 16 weeks HFD group, recovered in the dietary reversal group |
Glucose Tolerance, hippocampal insulin signaling | Impaired hippocampal insulin signaling in HFD group, recovered in the dietary reversal group |
| (MCLEAN ET AL., 2018) | C57BI/6J | From 12-14 weeks of age CD - D12450B, Research Diets; 10 kcal % from fat HFD – D12492 Research Diets; 60 kcal % fat from lard |
NOR, object context task Impaired NOR and object context task |
Glucose Tolerance | Impaired glucose tolerance in HFD mice, reversed in dietary reversal group |
| (SOONTORNNIYOMKIJ ET AL., 2016) | C57BL/6 | From 5-10 and 15-20 months of age CD - 8604 Teklad; Harlan Laboratories HFD – D12492 Research Diets; 60 kcal % fat from lard |
Novel place recognition task | Liver and hippocampal glutamine synthase immunoreactivity | Higher glutamine synthase activity in liver of HFD mice, higher glutamine synthase activity in the hippocampus of aged HFD mice. |
| (UNDERWOOD AND THOMPSON, 2016) | Long Evans | From 3-18 weeks of age CD – Open Source Diets; 14 kcal % fat HFD –Open Source Diets; 58 kcal % fat, augmented with coconut oil and casein protein |
Spatial Object Recognition Impaired spatial memory in both sexes in HFD groups |
Blood glucose, Glucose-tolerance testing, insulin tolerance testing, plasma corticosterone, leptin, and estradiol |
Males became obese (not females)on HFD. HFD augmented corticosterone. HFD induced changes to insulin and glucose tolerance in males. |
| (WANG ET AL., 2016) | Sprague-Dawley | From 4-20 weeks of age CD – 8062, Chengdu Dossy Biological Technology Co. Ltd. HFD –Chengdu Dossy Biological Technology Co. Ltd.; 40 kcal % fat (unlisted source) |
MWM, Object recognition task, open field Impaired object recognition, impaired reference and working memory(MWM) in the HFD group |
Lipid levels (TC, LDL, TG, HDL) | TC, LDL, TG were significantly increased in the HFD group |
| (FU ET AL., 2017) | Sprague Dawley | From 2-10 months of age CD - protein 28 kcal %, carbohydrate 60 kcal %, and fat 12 kcal %) HFD – D12492 Research Diets; 60 kcal % fat from lard |
Spontaneous alternation behavior, Novel Object Recognition Increased spontaneous alternation, impaired NOR for the HFD group. |
Glucose Tolerance, euglycemic-hyperinsulinemic clamping, Heart rate, blood pressure, hippocampal microvascular perfusion | Impaired microvascular perfusion in hippocampus, impaired insulin signaling in HFD group |
ABREVIATIONS: BRAIN DERIVED NEUROTROPHIC FACTOR (BDNF); CONTROL DIET (CD); FREE FATTY ACID RECEPTOR 1 (GPR40); HIGH DENSITY LIPOPROTEIN (HDL); HIGH-FAT DIET (HFD); INTERLEUKIN (IL); LOW DENSITY LIPOPROTEIN (LDL) MORIS WATER MAZE (MWM); NOVEL OBJECT RECOGNITION (NOR); TOTAL CHOLESTEROL (TC); TRIGLYCERIDES (TG)
Molecular mechanisms contributing to obesity-induced cognitive impairment
Several mechanisms play a role in obesity-related cognitive impairment (Dye et al., 2017; Kanoski and Davidson, 2011; Miller and Spencer, 2014; Noble and Kanoski, 2016). Vascular contributions to cognitive impairment has been increasingly recognized in both preclinical and clinical studies (Akiguchi and Yamamoto, 2010; Fulop et al., 2018; Khan et al., 2018; Toth et al., 2017). HFD is associated with decreased vascular integrity in the brain of rodent models (de Aquino et al., 2018; Fu et al., 2017; Kalyan-Masih et al., 2016). Furthermore, BBB integrity is compromised following HFD (Freeman and Granholm, 2012; Kanoski et al., 2010). The BBB plays a fundamental role in maintaining brain homeostasis. Given that endothelial cells are one of the major cell types that form the BBB, obesity driven endothelial dysfunction contributes to BBB impairment through several mechanisms (Wardlaw et al., 2013). Hence, understanding the potential impact of obesity on endothelial cell function may be a key factor reversing obesity-associated cognitive impairment.
BBB breakdown precedes and activates neuroinflammation and neurodegeneration. Evidence from studies in aged animal models of obesity suggest that chronic HFD leads to enhanced plasma-derived IgG leakage into the hippocampal perivascular space (Tucsek et al., 2014a). Similarly, these authors showed that aged-mice fed a HFD for 24 months had decreased hippocampal microvascular density accompanied by impairments in hippocampal-dependent cognitive function compared to mice on a normal chow diet (Tucsek et al., 2014b). These studies suggest that increased neuroinflammation may induce declines in microvascular integrity leading to cognitive decline and that these changes are compounded with increasing age. Several mechanisms contribute to obesity-induced BBB breakdown and its deleterious cerebrovascular effects.
One mechanism of obesity mediated BBB breakdown is through disruption of tight junctions at the level of the endothelium (Zlokovic, 2008). Obese type 2 diabetic mice display declines in tight junction proteins Zonula occluden-1 (ZO-1) and claudin-12 (Salameh et al., 2019). Interestingly, treatment with the mitochondrial carbonic anhydrase inhibitor, topiramate, improved tight junction protein expression and restored BBB integrity in these animals (Salameh et al., 2019). Likewise, increases in thrombin induced pericyte activation led to declines in ZO-1 and occludin but not claudin-5 in a HFD mouse model (Machida et al., 2017). Collectively, these studies suggest that obesity may induce tight junction disruption leading to BBB breakdown.
In addition, infiltration of serum-derived substances into the hippocampal space allows for microglial activation and subsequent reductions in endothelial cell tight junction protein expression in obesity (Sumi et al., 2010)(Shigemoto-Mogami et al., 2018). Long chain saturated fatty acids derived from chronic HFD intake facilitate activation of microglia to promote chronic neuroinflammation (Dalvi et al., 2017; Fritsche, 2015; Thaler et al., 2012), which contributes to cognitive impairment (Kahn and Flier, 2000; Thaler et al., 2012). HFD aged mice also display exacerbated activation of microglia associated with impaired hippocampal learning and memory deficits (Valcarcel-Ares et al., 2019). Accordingly, Bocarsly et al. reported that diet-induced obesity led to reductions in dendritic spines as well as altered microglial morphology within the prefrontal cortex, a phenomenon accompanied by deficits in prefrontal cortex-dependent cognitive tasks (Bocarsly et al., 2015). Interestingly, pharmacological inhibition of microglia activation in obese mice was shown to be protective against cognitive degradation as a result of improvements in BBB integrity (Cope et al., 2018). Additional mechanisms underlying obesity-induced initiation of BBB breakdown will be described below.
Systemic Inflammation
Chronic consumption of a “western diet” promotes systemic inflammation. Although there are various sources of inflammation with HFD consumption, the main source stems from white adipose tissue hypertrophy and dysfunction. Migrant macrophages homing to hypertrophic adipose tissue adopt an atypical pro-inflammatory phenotype (Ghanim et al., 2004). Notably, multiple adipose tissue macrophage (ATM) subsets exist in obese adipose tissue. These populations have distinct functions, express specific markers, and have specific tissue distributions not typical of M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes (Coats et al., 2017; Gordon, 2003; Kratz et al., 2014; Xu et al., 2013). Instead, obese ATMs reflect a distinct metabolically active group of surface expression markers induced by free fatty acids, high glucose, and hyperinsulinemia (Kratz et al., 2014), which subsequently drive adipocyte hypertrophy (Lumeng et al., 2007; Weisberg et al., 2003). In mice, both resident and recruited ATMs make up about 50% of adipose tissue cells in obese animals compared to 10% in lean animals (Guilherme et al., 2008; Weisberg et al., 2003). Upon activation, ATMs produce pro-inflammatory cytokines including Interleukin (IL)-6 and tumor necrosis factor (TNF)-α.
Interleukin-6 (IL-6)
IL-6 is produced by ATMs and has pleiotropic effects on inflammation, the immune response, and vascular function (Roytblat et al., 2000; Yasukawa et al., 1987). Accumulating evidence suggest that IL-6 is a major inflammatory cytokine that increases with adipocyte hypertrophy in obesity (Almuraikhy et al., 2016). Clinical studies in obese patients demonstrate that IL-6 and IL-6 receptor expression are upregulated in subcutaneous adipose tissue from patients with increased BMI and percentage body fat compared to lean subjects (Mohamed-Ali et al., 1997; Sindhu et al., 2015). IL-6 is purportedly a better correlate of obesity and insulin resistance compared to other cytokines, however there are conflicting reports about IL-6 that make its role in obesity-induced pathophysiology unclear. Administration of Tocilizumab, the anti-IL-6 drug, reduced systemic inflammation but led to metabolic syndrome and weight gain (Febbraio et al., 2010), and IL-6 knockout mice demonstrate overt obesity onset, insulin resistance, and M1 macrophage polarization (Matthews et al., 2010; Mauer et al., 2014; Wallenius et al., 2002). As such, it has become increasingly evident that IL-6 in the CNS plays a critical role in modulating body weight and metabolism through signaling in the CNS (Fernandez-Gayol et al., 2019; Timper et al., 2017). Although IL-6 appears beneficial for modulating weight via CNS signaling, its chronic systemic presence in obesity may be linked to detrimental effects on cognition.
Accumulating evidence suggest that obesity-induced systemic inflammation is associated with poorer cognitive outcomes. IL-6 is known to disrupt neural circuitry responsible for cognitive functioning and task completion (Vallieres et al., 1997), inhibit neurogenesis (Monje et al., 2003), decrease synaptic plasticity (Poluektova et al., 2005), and impede learning and memory performance (Braida et al., 2004) in mice. Reports on the impact of IL-6 on cognitive decline in obesity have been inconsistent with some supporting a role for IL-6 in cognitive decline (Donzis and Tronson, 2014; Lai et al., 2017; Lampe et al., 2019; Palta et al., 2015; Singh-Manoux et al., 2014; Weaver et al., 2002; Yaffe et al., 2003) and others failing to demonstrate a role for IL-6 in cognitive decline (as reviewed in Donzis and Tronson, 2014). A longitudinal study in the Whitehall II cohort found that mid-life IL-6 plasma concentrations were predictive of cognitive decline (Singh-Manoux et al., 2014). Despite these positive associations, a recent study suggest that IL-6 is not associated with cognition (Wennberg et al., 2018). It is well established that IL-6 negatively impacts vascular function, which can have long-term deleterious impacts on the BBB.
An overwhelming body of evidence suggest that IL-6 has deleterious impacts on endothelial cells (Hartman and Frishman, 2014; Schwingshackl and Hoffmann, 2014). Although the endothelium does not express the membrane IL-6 receptor, IL-6 is secreted from endothelial cells and has various direct and indirect effects on the endothelium (Hou et al., 2008). One study demonstrated that IL-6 significantly impaired endothelial colony forming cell outgrowth, which was restored after inhibition of the IL-6 receptor (Shahrivari et al., 2017). Experimental overexpression of IL-6 in the brains of mice leads to BBB permeability and neuroinflammation, while IL-6 knockout mice have preserved BBB function despite an enhanced inflammatory response (Paul et al., 2003). IL-6 BBB dysfunction are likely caused by signaling at the cellular level, as IL-6 suppresses endothelial NO expression and bioavailability (Saura et al., 2006). IL-6 also works in concert with TNF-α to disrupt adherens/tight junction expression and increased permeability through suppression of tight junctions in brain microvascular endothelial cells (Rochfort et al., 2016; Rochfort and Cummins, 2015).
TNF-α
TNF-α is constitutively produced by ATMs in dysfunctional adipose tissue of obese subjects and acts as a potential mediator of insulin resistance (Cawthorn and Sethi, 2008; Hotamisligil et al., 1993) through activation of phosphatases, which impair insulin signaling (Nieto-Vazquez et al., 2008). Impaired insulin signaling contributes to increased adiposity (Zhou and Rui, 2013). A recent study in obese hemodialysis patients showed that TNF-α was associated with abdominal obesity (Beberashvili et al., 2019). It is particularly noteworthy that genetic ablation of the TNF-α receptor 1 makes mice resistant to diet-induced obesity (Romanatto et al., 2009).
Under normal physiological conditions, constitutive production of hippocampal TNF-α modulates synaptic strength (Beattie et al., 2002), and elevated hippocampal levels in HFD mice correlate with cognitive impairment (Jeon et al., 2012; Ma et al., 2018). Evidence that cognitive impairment is mitigated through suppression of systemic TNF-α concentrations within the hippocampus points to a role for TNF-α in modulating cognitive decline (Grundy et al., 2014; Labrousse et al., 2012). Despite these findings, there are conflicting reports about the role of TNF-α on cognition. An independent study in FIFD mice failed to demonstrate a correlation between TNF-α and cognitive function (Boitard et al., 2014). Despite these findings, a recent study reported that administration of infliximab, a TNF-α inhibitor impermeable to the BBB, improves pathology in transgenic Alzheimer’s disease mice, a model commonly associated with cognitive impairment (Paouri et al., 2017).
TNF-α may modulate cognitive function through its effects on the micro vasculature and endothelium. Small vessels excised from obese patient perivascular tissue are less responsive to endothelium-dependent relaxation, a mechanism likely due to TNF-α-induced endogenous oxidative stress signaling (Virdis et al., 2011). This same group demonstrated that small arteries in perivascular adipose tissue produce TNF-α and oxidative stress in excess, leading to loss of vasorelaxation (Virdis et al., 2015). Infliximab administration leads to improvements in vascular reactivity to acetylcholine, a vasodilator, in obese subjects with metabolic syndrome (El Assar et al., 2013; Tesauro et al., 2008). Small arteries dissected from the visceral fat of obese patients display suppressed endothelial dependent relaxation in response to acetylcholine; however, aberrant response was mitigated by infliximab (Virdis et al., 2011). Similar findings have been observed in rodent models of obesity and/or models with an inflammatory phenotype. HFD consumption increases TNF-α in both the femoral artery and corpus cavernosum of rats (Sponton et al., 2017). Isolated mesenteric vascular beds from HFD mice displayed improvements in insulin-mediated vasodilation upon treatment with infliximab (da Costa et al., 2016). Collectively, this evidence points to role for TNF-α in mediating microvascular damage in obesity.
As previously stated, damage to the microvasculature in the periphery ultimately leads to changes in vascular structures of the CNS. While this evidence points to a role for TNF-α in obesity-related endothelial dysfunction in the periphery, TNF-α mediated endothelial dysfunction also occurs at the BBB (Deli et al., 1995). TNF-α increases permeability of human brain microvascular endothelial cells (Didier et al., 2003). Thus, it’s no surprise that TNF-α modulates disruption of BBB integrity. The administration of the TNF-α inhibitor, etanercept, restores BBB integrity and cognition in mice (Cheng et al., 2018). Overall, these data suggest a role for inflammation in obesity-related cognitive impairment. Inadvertently, chronic low-grade inflammation, often observed in obesity, have been implicated in the pathogenesis of hyperinsulinemia and insulin resistance (Esser et al., 2014).
Hyperinsulinemia
CNS insulin receptor signaling is important for synaptic plasticity, neuronal survival, learning and memory, etc. (see Chiu and Cline (2010) for a comprehensive review). Diet-induced obesity leads to hyperinsulinemia and is associated with impaired CNS insulin signaling (Hussain et al., 2019; Petrov et al., 2015; Sims-Robinson et al., 2016a); however, the mechanisms are not known. Impaired CNS insulin signaling is typically attributed to diet-induced CNS insulin receptor resistance (Hussain et al., 2019; Kim et al., 2011a; Kim et al., 2011b; Petrov et al., 2015; Sims-Robinson et al., 2016b). Alternatively, impaired CNS insulin signaling may be due to a deficiency of insulin in the CNS. Hyperinsulinemia and diet-induced obesity are associated with decreased CNS insulin levels (Begg et al., 2013; Israel et al., 1993; Kaiyala K. J., 2000). CNS insulin must be transported via receptor-mediated transport through the BBB from the periphery (Banks et al., 1997; Banks et al., 2012).
Although limited, evidence from the literature support the notion that CNS insulin transport is mediated by the insulin receptor (Banks, 2004; Banks et al., 1997; Banks et al., 2012; King and Johnson, 1985). Insulin uptake in bovine aortic endothelial cells requires normal insulin signaling (Wang et al., 2013; Wang et al., 2008). Furthermore, these studies demonstrate that ablating insulin receptor function reduces insulin uptake. High concentrations of insulin receptors are reported in brain capillaries (Blumling Iii and Silva, 2012; Pardridge, 2008; Pardridge and Boado, 2012). Although these studies suggests that the insulin receptor is involved in receptor-mediated uptake in endothelial cells, it is worth noting that a recent study demonstrated that insulin can enter the CNS through non-insulin receptor mediated processes (Rhea et al., 2018). Overall, impaired CNS insulin receptor signaling coupled with the reduced CNS insulin transport provides a potential mechanism underlying obesity-related cognitive impairment.
Obesity is associated with lower cerebrospinal fluid (CSF) insulin, a measure of CNS insulin levels (Kern et al., 2006; Kullmann et al., 2016). Interestingly, reduced CSF insulin concentrations are also observed in patients with mild cognitive impairment (Craft et al., 1998; Gil-Bea et al., 2010). This is associated with an decrease in underlying brain insulin sensitization (Sims-Robinson et al., 2010), observed through aberrant signaling of the insulin receptor (Kim et al., 2012) in both the hypothalamus (Ono, 2019) and hippocampus (Pratchayasakul et al., 2011; Sims-Robinson et al., 2016a). Insulin dysregulation in the hippocampus have been directly implicated in impaired memory processes such as synaptic plasticity (McNay et al., 2010; Spinelli et al., 2017; Spolcova et al., 2014; Suarez et al., 2019). Impaired insulin sensing in the hypothalamus is linked to decreased glucose sensing, which may eventually lead to a feed forward cycle in the obesity pathology (Chen et al., 2017; Ono, 2019; Weissmann et al., 2014).
Intravenous insulin has been shown to improve cognition in healthy subjects (Craft et al., 1999; Craft et al., 1996; Kern et al., 2001), however, this is not a viable treatment option for cognitive impairment due to secondary complications such as hypoglycemia (Morris and Burns, 2012). Intranasal insulin has been offered as a way of directly increasing CNS insulin concentrations, while avoiding altering systemic glucose and insulin levels (Claxton et al., 2015; Craft et al., 2012; Reger et al., 2006). CNS insulin receptors are highly expressed in areas of the brain important for memory consolidation and executive functioning such as the hippocampus (Baskin et al., 1983; Hill et al., 1986), and are specifically concentrated at the synapses (Laron, 2009). Hence, it is not surprising that intranasal insulin improves memory in cognitively normal humans (Benedict et al., 2004; Benedict et al., 2007). Additional studies are warranted to explore the potential role of CNS insulin signaling and transport on obesity-related cognitive impairment.
Hyperinsulinemia and insulin resistance are linked to decreased BBB integrity (Arnold et al., 2018) and endothelial cell dysfunction (Muniyappa and Sowers, 2013). Insulin is known to have a direct vasodilatory effect, mediated through the stimulation of NO production in endothelial cells (Kuboki et al., 2000) via protein kinase B (Akt) activation (Muniyappa and Sowers, 2013). Hence, hyperinsulinemia impairs vascular tone. HFD animals exhibit impaired neurovascular coupling (Tarantini et al., 2018) as well as diminished insulin-mediated BBB responses, microvascular perfusion, and cognitive decline (de Aquino et al., 2018; Fu et al., 2017). Interestingly, some studies suggest that insulin sensitizers such as metformin and pioglitazone may confer beneficial effects on endothelial function (Muniyappa and Sowers, 2013; Naka et al., 2011; Radenkovic, 2014). These findings taken together suggest that obesity coupled with hyperinsulinemia may have detrimental effects on endothelial function. While the inflammation and hyperinsulinemia connection is grounded in the literature, a mounting body of evidence suggest that the gut microbiome may play a role in modulating hyperinsulinemia and obesity’s impact on cognition.
Gut Microbiome
The gut microbiome is an aggregate of more than 100 trillion microorganisms, including bacteria, viruses, fungi and protozoa (Gill et al., 2006). The microbiome is essential for microbiota-gut-brain bidirectional communication (Rhee et al., 2009). The two most prominent bacterial divisions in the gut include gram positive Firmicutes and gram negative Bacteroidetes, which make up 90% of all phylogenetic types. Changes to food and environment have drastically altered the microbiome (Gomez, 2017). Disturbance in gut homeostasis is often due to loss of beneficial bacterial, overgrowth of harmful bacterial, or loss in microbial diversity also known as gut dysbiosis (DeGruttola et al., 2016). This is particularly true in obesity; the obese gut in mice and humans has an increased Firmicutes/Bacteroidetes (F/B) ratio compared to lean controls (Angelakis et al., 2012; Furet et al., 2010; Kong et al., 2013), leading to greater adiposity (Turnbaugh et al., 2006). Higher F/B ratios have been linked to numerous disease processes (Flint et al., 2007; Ley et al., 2006) likely through changes in body-weight, inflammation, insulin sensitivity, and behavior (Allen et al., 2017; Liang et al., 2018). Furthermore, there is evidence suggesting a decline in overall bacterial diversity in obese individuals (Yun et al., 2017). While some controversy exists whether gut dysbiosis impacts obesity, (Sze and Schloss, 2016; Sze and Schloss, 2017), animal models provide more direct approaches at studying the phenomenon. Fecal transplant from obese mice to germ-free mice leads to the development of obesity (Backhed et al., 2007; Turnbaugh et al., 2008). Interestingly, the opposite effect can occur with fecal transplants from lean to obese mice (Sun et al., 2018). Collectively, these studies suggest that gut dysbiosis may be both a result and potentiator of obesity. Mechanisms underlying the role of gut dysbiosis in obesity and obesity-induced cognitive impairment have been reviewed elsewhere (Cuevas-Sierra et al., 2019; Noble et al., 2017).
Gut microbiota homeostasis promotes optimal brain development and cognitive functioning (Diaz Heijtz et al., 2011) Clinical and preclinical evidence suggests that obesity-induced changes in the gut microbiome may play a role in the development of cognitive dysfunction. Patients with dementia have a higher F/B ratio compared to non-demented patients (Saji et al., 2019), and elderly adults with increased F/B ratios have poorer immediate and delayed recall scores (Manderino et al., 2017). This is also the case preclinically. Obese mice demonstrating an increase in F/B ratios and a decline in gut microbiota diversity had impaired recognition and spatial memory (Zhang et al., 2018). In line with this finding is the discovery that administration of antibiotics in DIO mice led to improved insulin signaling in the brain and improved anxiety and depression associated with cognitive functioning(Soto et al., 2018) Moreover, aged normal weight mice with obese-type gut microbiota displayed BBB dysfunction, reduced CBF and deteriorations in cognition (Hoffman et al., 2017). Similarly, a recent discovery by Bruce-Keller et al demonstrated that mice with obese-type gut microbiota displayed neurocognitive and behavioral disruptions in the absence of obesity (Bruce-Keller et al., 2015). Interestingly, rats subjected to a western diet had alterations in hippocampal genes important for neuroplasticity; however, these abnormalities were reversed with probiotic treatment (Beilharz et al., 2018). Notably, the authors reported that probiotic administration led to increases in Streptococcus and Lactobacillus in the gut (Beilharz et al., 2018).
Diet-induced obesity models that exhibit gut microbiome imbalance and cognitive impairment also demonstrate reduced tight junction proteins (Zhang et al., 2018). There appears to be a role for vascular dysfunction in mediating these effects (Braniste et al., 2014). Microbiome diversity is inversely correlated with arterial stiffness (Li et al., 2017), and obese-type gut microbiota induces BBB dysfunction and reduced CBF (Hoffman et al., 2017). Interestingly, intestinal microbiota modulates the expression of BBB tight junction proteins (Kelly et al., 2015).
One mechanism by which alterations in the microbiome promotes cognitive dysfunction is through increasing BBB permeability (Braniste et al., 2014). The BBB is comprised of a tightly sealed monolayer of brain endothelial cells connected at a junctional complex by tight junction and adherens junction proteins (Hawkins and Davis, 2005). Diet-induced obesity models that exhibit alterations within the microbiome and cognitive impairment demonstrated reduced tight junction proteins in the BBB (Kelly et al., 2015). One mechanism through which microbial gut imbalance may lead to reduced tight junction proteins at the BBB is through diminished short chain fatty acids (SCFAs) typically produced during dietary fiber fermentation (Pryde et al., 2002). SCFAs modulate tight junction formation through enhancing the expression of tight junction proteins within the prefrontal cortex and hippocampus of the brain of germ-free adult mice (Braniste et al., 2014). Macrovascular changes also impact cognitive decline. It is well established that declines in central artery elasticity negatively impacts cognitive function (Palta et al., 2019). Interestingly, a recent study showed that gut microbial diversity is inversely associated with central artery stiffness in women, even after adjusting for insulin resistance and other cardiovascular disease risk factors (Menni et al., 2018). Likewise, antibiotic treatment in aged mice reversed endothelial dysfunction and arterial stiffening through attenuation of inflammation and oxidative stress (Brunt et al., 2019). Taken together, these studies suggest that gut dysbiosis may lead to changes in vascular structure or BBB integrity that are detrimental for cognitive function. Additional studies are needed in obese preclinical models and patients to further confirm these findings.
Therapeutic strategies
Dietary Interventions
Diets are often characterized by the macronutrient, which includes fats, proteins, and carbohydrates, that is primarily providing the source of energy for the body. Given the role of fatty acids in the development of insulin resistance (Thomas and Pfeiffer, 2012), it is generally assumed that reducing the dietary intake of fat will be beneficial for improving insulin resistance; however, such diets are often difficult to maintain. The Mediterranean diet is not comprised of a fat-restriction, but rather consists of fruits, vegetables, legumes, cereals, and olive oil. The Mediterranean diet has beneficial effects on insulin resistance, diabetes risk and overall cardiovascular health (Kastorini et al., 2011; Riserus et al., 2009). Similarly, the ketogenic diet, a low carbohydrate diet with fat as the primary energy source, improves glycemic control and insulin sensitivity (Forsythe et al., 2008).
Several previous studies have highlighted the role of diet on inflammation associated with obesity (Bullo et al., 2007; Calder et al., 2011; Galland, 2010; Lee et al., 2013). The Mediterranean diet exerts anti-inflammatory effects by decreasing inflammatory cytokines including IL-6 and TNF-α (Calder et al., 2011; Casas et al., 2014; Casas et al., 2016; Hu et al., 1997; Mena et al., 2009; Urpi-Sarda et al., 2012). The ketogenic diet is associated with a reduction in inflammatory markers including but not limited to TNF-α (Forsythe et al., 2008; Ruskin et al., 2009). Cross sectional studies have demonstrated a link between carbohydrates and inflammatory cytokines (Du et al., 2008; Levitan et al., 2008; Pischon et al., 2005; Qi et al., 2006a; Qi et al., 2006b). It is clear that diet has impacts on neuroinflammation. However, conflicting reports exist regarding the role of diet modification on gliosis.
The composition of the gut microbiota is largely dependent upon diet (Finegold and Rolfe, 1983; Graf et al., 2015; Hayashi et al., 2002; Mueller et al., 2006). A change from a high-fat, low fiber diet to a low-fat high-fiber diet leads to marked changes in the microbiota within 24 hours (Wu et al., 2011). Western style diets alter the gut microbiota resulting in a decrease in bacterial diversity (Wu et al., 2011). Whereas, elevated levels of Bacteriodetes are observed in individuals adhering to the Mediterranean diet (Gutierrez-Diaz et al., 2016). Likewise, in obese patients with severe metabolic disease, consumption of a Mediterranean diet or a low fat diet reversed gut dysbiosis (Haro et al., 2017). Unfortunately, the validity and reproducibility of studies focused on the impact of diet on gut microbiota in humans is challenging since most investigators rely on self-reporting of dietary habits. Animal models, however, have provided some useful information. Diet modification from an ad libitum low fat diet to caloric restriction in young rats reduced the F/B ratio (Tanca et al., 2018). A recent study in monkeys compared the changes in gut microbiota following a western and Mediterranean-type diets (Nagpal et al., 2018). Similar to previous studies, alterations in the gut microbiota were observed (Carmody et al., 2015; David et al., 2014; De Filippo et al., 2010; Hale et al., 2018; Nagpal et al., 2018). Whether improved outcomes due to dietary intervention is facilitated through alterations in the microbiome is not well understood.
In the past decade, lifestyle modifications have emerged as an alternative strategy to reduce the risk of cognitive impairment (Daviglus et al., 2010). A recent review provides a comprehensive report of randomized controlled trials published from 2014–2016 exploring the efficacy of various nutritional interventions on preventing the onset of cognitive disorders and dementia (Agosti, 2018). The Mediterranean diet is one of the most studied dietary interventions to protect against cognitive decline. According to observational studies, the Mediterranean diet is associated with a reduced risk of cognitive impairment, mild cognitive impairment, and Alzheimer’s disease (Lourida et al., 2013; Psaltopoulou et al., 2013; Singh et al., 2014; Solfrizzi et al., 2017). A randomized controlled trial demonstrated that subjects randomly assigned to the Mediterranean diet supplemented with extra virgin olive oil performed better on episodic memory and attention tasks compared with the control group. Furthermore, the Mediterranean diet subjects demonstrated a significant improvement in frontal and global cognition (Valls-Pedret et al., 2015). Despite the positive effects from observational studies, more interventional studies are needed to validate these findings.
In 2011, the American Heart Association established the “Life’s Simple 7,” for achieving ideal cardiovascular health. The Life’s Simple 7 definition of an ideal cardiovascular diet includes a diet rich in fruits and vegetables, oily fish, fiber, and low in sodium which is similar to the Mediterranean diet (Sacco, 2011). In a large clinical trial known as the EVIDENT study, participants who had a high Mediterranean Diet adherence score had higher arterial elasticity (Garcia-Hermoso et al., 2018). Likewise, in a randomized controlled trial, adherence to the Mediterranean diet for 1.5 years improved endothelial function, as measured by flow mediated dilation, in diabetic and pre-diabetic patients compared to a low-fat diet alone (Torres-Pena et al., 2018). These changes in endothelial function may be due to increases in serum NO and declines in endothelin-1 (Storniolo et al., 2017) and reactive oxygen species production (Carnevale et al., 2014; Giordano et al., 2012). Subsequent reports suggest that the Mediterranean diet reduces endothelial cell damage and improves the regenerative capacity of endothelial progenitor and circulating progenitor cells (Cesari et al., 2018; Marin et al., 2011). Conversely, carotid atherosclerosis patients on a modified Mediterranean diet for 20 weeks did not demonstrate improvements in internal carotid or large basal cerebral artery blood flow or cognitive function (Droste et al., 2014). The benefits of the diet were likely masked by statin use in two-thirds of the study population as statins improve NO bioavailability and increase CBF (Droste et al., 2014). The impacts of the ketogenic diet on the vascular function are not as well understood and additional studies are needed to understand its impact in cardiovascular health.
Bariatric Surgery
Decades of obesity research indicates that lifestyle interventions including diet and exercise, are not effective for helping severely obese individuals. The National Institutes of Health (NIH) established guidelines, which specify that obese individuals with a BMI≥35 with comorbidities or BMI≥40 without comorbidities are ideal candidates for weight loss surgery, known bariatric surgery. Bariatric surgery has emerged as an effective therapy for these individuals yielding sustained reductions in weight (Adams et al., 2007; Buchwald et al., 2004; Gloy et al., 2013; Maggard et al., 2005; Maggard-Gibbons et al., 2013; O’Brien et al., 2013; Padwal et al., 2011; Picot et al., 2009; Sjostrom, 2013; Sjostrom et al., 2007). Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric banding (LAGB) and biliopancreatic diversion with duodenal switch represents the three most commonly types of bariatric surgeries(Buchwald and Oien, 2013). An analysis of various randomized controlled trials (Arterburn and Courcoulas, 2014) revealed that bariatric surgical procedures results in greater average weight loss of (~57 pounds) compared with non-surgical options (Dixon et al., 2008; Ikramuddin et al., 2013; Ikramuddin and Livingston, 2013; Mingrone et al., 2012; O’Brien et al., 2006; Schauer et al., 2012).
The impact of bariatric surgery on insulin resistance has been extensively reviewed (Rao et al., 2012). A reduction in fasting glucose and insulin levels as well as improvements in insulin sensitivity are reported within 3 months following bariatric surgery (Leichman et al., 2008). Some argue that weight loss is responsible for the improvements in glucose metabolism and insulin resistance following bariatric surgery (Adami et al., 2004; Castagneto et al., 1994; Pereira et al., 2003; Summers, 2002). Others suggest that the reversal of insulin resistance occurs prior to the manifestation of substantial weight loss (Leichman et al., 2008; Rubino et al., 2010; Schauer et al., 2003; Sugerman et al., 2003)..
Given that obesity is characterized as a state of chronic inflammation, the anti -inflammatory changes observed with weight loss play a significant role in overall health (Cottam et al., 2004). TNF-α is the most frequent cytokine assessed following bariatric surgery due to its strong associated with insulin resistance (Moller, 2000). Reports on the impact of RYGB surgery on TNF-α are contradictory demonstrating either no change (Catalan et al., 2007; Sams et al., 2016), an increase (Illan-Gomez et al., 2012), or a decrease (Miller et al., 2011). The latter clinical study is consistent with preclinical studies, which reveal a decrease in TNF-α levels in adipose tissue at 9 weeks post-surgery in rats (Rideout et al., 2010). In contrast to RYGB, the reports of TNF-α following LAGB are relatively consistent with multiple groups demonstrating no change in serum levels of TNF-α (Kopp et al., 2003; Laimer et al., 2002) and a decrease in subcutaneous adipose tissue (Moschen et al., 2010). The reports for IL-6 levels following RYGB surgery are also inconsistent demonstrating either an increase (Illan-Gomez et al., 2012), or a decrease (Lindegaard et al., 2015). Similar to RYGB, the serum levels of IL-6 also varied across different studies following LAGB with either no change (Laimer et al., 2002; Moschen et al., 2010) or a decrease (Samaras et al., 2013). Taken together, these studies suggest that the surgical procedure, post-surgical time point, and tissue evaluated contribute to the inconsistencies in the field regarding the potential role of inflammation.
Comprehensive data exploring the impact of bariatric surgery on the microbiome has been extensively reviewed (Ulker and Yildiran, 2019). Bariatric surgery increased microbial richness. Previous studies observed an increase in microbial diversity and altered microbial composition in both man (Furet et al., 2010; Graessler et al., 2013; Kong et al., 2013; Zhang et al., 2009) and rodents (Li et al., 2011; Liou et al., 2013). Studies suggest that these changes in the microbiota may be independent of weight loss or caloric restriction and are maintained up to 9 years post-surgery (Liou et al., 2013; Tremaroli et al., 2015). Furthermore, colonization of germ-free mice with fecal material from RYGB mice resulted in weight loss and reduced adiposity, suggesting that RYGB-associated microbiota can improve host metabolism (Liou et al., 2013; Tremaroli et al., 2015). Overall, these studies suggest that bariatric surgery leads to alterations in the microbiota.
Severely obese patients seeking bariatric surgery have poorer baseline cognition compared to healthy weight controls (Prickett et al., 2018). The Longitudinal Assessment of Bariatric Surgery (LABS) project is a multi-site, prospective longitudinal examination of the safety and efficacy of bariatric surgery and the impact on cognitive function. Improvements in multiple cognitive domains following surgery persisted for several years. Executive function and memory performance remained at this improved level however, attention scores declines in participants that regained a substantial amount of weight (Alosco et al., 2014a; Alosco et al., 2014b; Gunstad et al., 2011; Miller et al., 2013). Overall, these data suggest that bariatric surgery improves obesity-related cognitive impairment.
Severely obese patients experience various structural adaptations in the arteries over time that lead to increases in blood pressure and subsequent changes in arterial stiffening. While still limited, studies assessing the long-term impact of bariatric surgery on vascular disease outcomes are underway. A recent study revealed that morbidly obese patients who underwent LAGB experienced weight reduction but did not exhibit improvements in endothelial function and arterial stiffness after 4-years (Galkine et al., 2018). Conversely, a study in 16 morbidly obese subjects undergoing bariatric surgery demonstrated that study participants had improvements in retinal microvascular health after 4 years but not in large arterial stiffness (Streese et al., 2019). Still, others demonstrated that laparoscopic sleeve gastrectomy improved insulin mediated microvascular function in morbidly obese patients with insulin resistance or diabetes (Ministrini et al., 2018). Nevertheless, improvements in endothelial function and arterial stiffness post-bariatric surgery may be due to surgery-induced reversal of underlying chronic conditions including sleep apnea (de Assuncao Machado et al., 2018), high levels of subcutaneous adipose tissue (Backdahl et al., 2018), insulin resistance and inflammation (Vazquez et al., 2005) that are common amongst obese patients. Accordingly, studies revealed that markers of inflammation and endothelial function including ICAM-1, E-selectin, and P-selectin were improved following RYGB surgery (Stolberg et al., 2018; Vazquez et al., 2005; Yadav et al., 2017) but, these studies failed to assess changes at the blood vessel level. Moreover, studies examining the impacts of bariatric surgery on changes at the level of the blood brain barrier are missing. Additional studies are needed to fully understanding the role of bariatric surgery in improving vascular dysfunction in obesity.
Exercise
Structured exercise training improves cardiometabolic health indices (Campbell et al., 2015; Stefanov et al., 2013; Umpierre et al., 2011). Adolescent girls on a prescriptive resistance and aerobic exercise training 12 week intervention display 50% lower plasma insulin concentrations at the end of the intervention period (Bharath et al., 2018). Likewise, combined resistance and aerobic exercise significantly reduces homeostatic model assessment of insulin resistance (HOMA-IR) and blood pressure parallel to body fat in obese adolescent girls (Son et al., 2017). Obese adults with type 2 diabetes demonstrate improved glycemic control with supervised exercise training, but this effect is not sustained with unsupervised training (Gajanand et al., 2019). Likewise, running wheel exercise training for 6 weeks led to significant declines in fasting blood glucose levels in mice and HbA1c in HFD rats (Mehta et al., 2018). Voluntary exercise in HFD mice also led to improved insulin sensitivity (Fjaere et al., 2019). Collectively, these studies suggest that supervised, prescriptive exercise training is an effective intervention for obesity-induced cardiometabolic risk factors.
Adipose tissue hypertrophy leads to increased macrophage infiltration and activation yielding increases in inflammatory cytokine production including IL-6 and TNF-α (Bjorntorp et al., 1971a; Bjorntorp et al., 1971b; Drolet et al., 2008). Interestingly, completion of a 3 week high intensity interval training (HIIT) program increased IL-6 in obese adults while a 3 week moderate-intensity continuous training (MICT) exercise intervention lead to cytokine suppression (Vella et al., 2017a; Vella et al., 2017b). A conflicting study in obese elderly adults demonstrated elevated levels of IL-6 following a prescriptive MICT program (Pedrinolla et al., 2018). HFD rodents undergoing an 8 week endurance training displayed reductions in TNF-α, and IL-6 (Rocha-Rodrigues et al., 2017). Combined aerobic and resistance training for 8-weeks in obese subjects leads to reductions in TNF-α (Jin et al., 2018). Moreover, a 24 week HIIT yields suppression of TNF-α in obese adolescents (Tenorio et al., 2018). TNF-α levels are reduced in HFD mice following wheel running and treadmill exercise (Bradley et al., 2008; Kim and Yi, 2015). Overall, these studies support a role for exercise in modulating TNF-α in clinical and experimental models of obesity.
Exercise reportedly increases the diversity of the gut microbiome (Clarke et al., 2014). Interestingly, obese women exposed only to an endurance exercise intervention for 6 weeks display inert taxonomic modifications in the gut microbiome (Munukka et al., 2018). BMI and exercise frequency purportedly dictate gut microbiota diversity (Bai et al., 2018). For example, two independent studies report that HFD mice experience a shift in microbiota composition with wheel running exercise (Evans et al., 2014; Schipke et al., 2019). Notably, a 6-week HIIT treatment in HFD mice led to increased microbiome diversity and F/B ratio in the fecal microbiota and distal gut (Denou et al., 2016). Conversely, a recent report demonstrated that low-to-moderate exercise training program was not effective in reversing HFD induced changes in the microbiome of mice (Ribeiro et al., 2019). Taken together, these studies suggest that exercise may modulate changes in the microbiome; however, additional human studies are warranted to clarify the role of exercise in modulating the gut microbiome.
The effectiveness of exercise on improving cognitive function in obese patients is inconsistent (Espeland et al., 2017a; Espeland et al., 2017b; Smith et al., 2010) but there is substantially more evidence supporting a role for the benefits of exercise. For example, a 4-month high intensity training (HIT) program improves short-term and verbal memory along with attention and processing speed in middle-age obese patients (Drigny et al., 2014). Results from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial demonstrated that a 2-year diet and structural exercise intervention improves processing speed and executive function amongst overweight, elderly subjects compared controls (Ngandu et al., 2015). Interestingly, the benefits of exercise alone and exercise in combination with diet provided similar improvements in cognition amongst frail, obese elderly patients (Napoli et al., 2014). These findings are in line with studies in preclinical models. An early preclinical study demonstrated that HFD-induced cognitive deficits in hippocampal-dependent memory improved with both voluntary running wheel or forced treadmill exercise training in Sprague-Dawley (Noble et al., 2014). Moreover, Jeong et al. revealed that treadmill exercise training in HFD rats improves memory restoration (Jeong and Kang, 2018). While aerobic interval training in HFD mice demonstrated improvements in spatial learning and memory (Shi et al., 2018). Collectively, these studies suggest that exercise training has beneficial effects on obesity-associated cognitive function in both clinical and preclinical studies.
The majority of studies examining the therapeutic capacity of exercise on arterial de-stiffening and vascular function in obesity support a role for increased physical activity in limiting obesity-induced damage to the vasculature. A recent study examining the effectiveness of a low-volume, HIT training program in obese individuals showed that skeletal muscle capillarization increased while aortic pulse wave velocity (PWV), a measure of aortic stiffness downstream of endothelial dysfunction, decreased in obese individuals (Scott et al., 2019). Interestingly, acute maximal exercise increased carotid-femoral fPWV in obese individuals, which may reflect underlying preclinical vascular disease in obesity (Bunsawat et al., 2017). Still, others have demonstrated that in populations with metabolic syndrome, an 8-week supervised training program lead to significant improvements in vascular stiffness and subsequent improvements in metabolic disease and fitness (Slivovskaja et al., 2018). Consistent with this finding is the discovery that exercise training effectively de-stiffened the central arteries in obese men independent of weight loss and dietary modification (Maeda et al., 2015).
In animal models of diet-induced obesity, exercise prevented cerebrovascular damage despite increases in weight gain (Graham et al., 2019). Similarly, exercise prevented diastolic dysfunction likely due to declines in oxidative stress and improved mitochondrial architecture (Bostick et al., 2017). In addition, chronic exercise therapy in obese rats led to restoration of insulin-mediated vasodilation as well as improvements and skeletal muscle and cerebral microcirculation (Olver et al., 2017). Additional studies are needed to fully understand mechanisms responsible for exercise-induced changes at the vascular level in obese populations.
Conclusions and perspectives
Obesity is a global pandemic that is still on the rise in developing countries. The interconnected relationship between early- to mid-life obesity and cognitive impairment makes the public health and economic implications of this issue urgent. Thus, understanding mechanistic pathways at the intersection of these diseases is important for guiding the development of therapeutic strategies that prevent and/or reverse disease. The framework presented in this review is based on our current knowledge of how aberrant endothelial function in obesity drives changes in the brain that may culminate into cognitive impairment. Presently, factors associated with obesity including hyperinsulinemia/insulin resistance, inflammation, and disruption at the microbiota-gut-brain axis appear to orchestrate pathophysiologic insults at the level of the endothelium. Endothelial dysfunction is an early event in the manifestation of cognitive impairment and dementia. Establishing a clinical tool that will detect endothelial dysfunction may be useful for assessing cognitive impairment risk. Given that endothelial dysfunction promotes premature arterial stiffening, measuring arterial stiffness may be promising. This can be accomplished by measuring carotid-femoral pulse wave velocity (cfPWV), which inversely correlates with cognitive function (Triantafyllidi et al., 2009). Additional studies are needed to understand the clinical utility of cfPWV in predicting cognitive impairment risk associated with obesity.
Acknowledgements.
The authors wish to acknowledge Ms. Taylor Lowry and Mrs. Janet Boggs for editorial assistance.
Funding: The authors were supported by the following funding sources: American Heart Association (#15SFDRN25870000; #15SFDRN24480016) and NIH NINDS (1U24NS107232-01) to J.N.J.B.; the National Institute of Health (NIH) NHLBI (HL007260) to L.S.W.; NIH NIGMS (2R25GM072643-14A1) to C.J.S.; NIH NINDS (1R01NS099595) and Alzheimer’s Association (AARGD -16-440893) to C.S-R.
Abbreviations
- ATM
Adipose tissue macrophage
- BBB
blood brain barrier
- BMI
body mass index
- cfPWV
carotid-femoral pulse wave velocity
- CNS
central nervous system
- CSF
cerebral spinal fluid
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- F/B
Firmicutes/Bacteroidetes
- HFD
high-fat diet
- HIIT
high intensity interval training
- HOMA-IR
homeostatic model assessment of insulin resistance
- IL
interleukin
- LAGB
laparoscopic adjustable gastric banding
- LABS
Longitudinal Assessment of Bariatric Surgery
- MICT
moderate-intensity continuous training
- NIH
National Institutes of Health
- NO
nitric oxide
- PI3k
phosphoinositide 3-kinase
- Akt
protein kinase b
- RYGB
Roux-en-Y gastric bypass
- TNF
tumor necrosis factor
- VCID
vascular contributions to cognitive impairment and dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing interests.
References
- Adami GF, et al. , 2004. Long-term normalization of insulin sensitivity following biliopancreatic diversion for obesity. Int J Obes Relat Metab Disord. 28, 671–3. [DOI] [PubMed] [Google Scholar]
- Adams TD, et al. , 2007. Long-term mortality after gastric bypass surgery. N Engl J Med. 357, 753–61. [DOI] [PubMed] [Google Scholar]
- Agosti PC,C; Schilardi A;, D’lntrono A; Valiani V; Lozupone M; Panza F; Dibello V; Piccininni C; Solfrizzi V; Sabbà C, 2018. Dietary intervention and prevention of cognitive-related outcomes in healthy older adults without cognitive dysfunction. Journal of Gerontology and Geriatrics. 87–100. [Google Scholar]
- Akiguchi I, Yamamoto Y, 2010. Vascular mechanisms of cognitive impairment: roles of hypertension and subsequent small vessel disease under sympathetic influences. Hypertens Res 33,29–31. [DOI] [PubMed] [Google Scholar]
- Ala Abu Saleh RS, Nimer Assy, 2015. Cognitive Dysfunction in Obese Individuals With or Without Metabolic Risk Factors (I12-5A). Neurology. 84,I12–5A. [Google Scholar]
- Allen AP, et al. , 2017. A psychology of the human brain-gut-microbiome axis. Soc Personal Psychol Compass. 11, e12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Suhett CP, et al. , 2017. Behavioral changes in male mice fed a high-fat diet are associated with IL-1beta expression in specific brain regions. Physiol Behav 169, 130–140. [DOI] [PubMed] [Google Scholar]
- Almuraikhy S,et al. , 2016. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 59, 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, et al. ,2014a. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg 207, 870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, et al. , 2014b. Improved memory function two years after bariatric surgery. Obesity (Silver Spring). 22,32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E, et al. , 2012. The relationship between gut microbiota and weight gain in humans. Future Microbiol 7,91–109. [DOI] [PubMed] [Google Scholar]
- Arnold SE, et al. , 2018. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 14,168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, et al. , 2018. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol 314, R387–R398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterburn DE, Courcoulas AP, 2014. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 349, g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backdahl J,et al. , 2018. Long-Term Improvement in Aortic Pulse Wave Velocity After Weight Loss Can Be Predicted by White Adipose Tissue Factors. Am J Hypertens 31, 450–457. [DOI] [PubMed] [Google Scholar]
- Backhed F, et al. , 2007. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 104, 979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, et al. , 2018. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7-18 years old children from the American Gut Project. Pediatr Obes. [DOI] [PubMed] [Google Scholar]
- Banks WA, 2004. The source of cerebral insulin. Eur J Pharmacol 490, 5–12. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. , 1997. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 18,1423–9. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. , 2012. Insulin in the brain: there and back again. Pharmacol Ther 136, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, et al. , 1983. Regional concentrations of insulin in the rat brain. Endocrinology. 112, 898–903. [DOI] [PubMed] [Google Scholar]
- Beattie EC, et al. , 2002. Control of synaptic strength by glial TNF alpha. Science. 295, 2282–5. [DOI] [PubMed] [Google Scholar]
- Beberashvili I, et al. , 2019. Abdominal obesity in normal weight versus overweight and obese hemodialysis patients: Associations with nutrition, inflammation, muscle strength, and quality of life. Nutrition. 59,7–13. [DOI] [PubMed] [Google Scholar]
- Begg DP, et al. , 2013. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology. 154,1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, et al. , 2018. Cafeteria diet and probiotic therapy: cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Mol Psychiatry. 23, 351–361. [DOI] [PubMed] [Google Scholar]
- Bender SB, et al. , 2007. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab. 9, 688–96. [DOI] [PubMed] [Google Scholar]
- Benedict C, et al. , 2004. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 29,1326–34. [DOI] [PubMed] [Google Scholar]
- Benedict C, et al. , 2007. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 86,136–42. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, et al. , 2013. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES). Eur J Neurol 20,899–906, e76–7. [DOI] [PubMed] [Google Scholar]
- Bharath LP,et al. , 2018. Combined resistance and aerobic exercise training reduces insulin resistance and central adiposity in adolescent girls who are obese: randomized clinical trial. Eur J Appl Physiol 118,1653–1660. [DOI] [PubMed] [Google Scholar]
- Bischof GN, Park DC, 2015. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom Med 77, 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, et al. , 1971a. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 20, 927–35. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, et al. , 1971b. Adipose tissue fat cell size and number in relation to metabolism in endogenous hypertriglyceridemia. Acta Med Scand. 190, 363–7. [DOI] [PubMed] [Google Scholar]
- Blumling Iii JP, Silva GA, 2012. Targeting the brain: advances in drug delivery. Curr Pharm Biotechnol 13, 2417–26. [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, et al. , 2015. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci U S A. 112, 15731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, et al. , 2014. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun 40, 9–17. [DOI] [PubMed] [Google Scholar]
- Bostick B, et al. , 2017. Daily exercise prevents diastolic dysfunction and oxidative stress in a female mouse model of western diet induced obesity by maintaining cardiac heme oxygenase-1 levels. Metabolism. 66, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin F, et al. , 2008. Endothelial and vascular dysfunctions and insulin resistance in rats fed a high-fat, high-sucrose diet. Am J Physiol Heart Circ Physiol 295, H1044–H1055. [DOI] [PubMed] [Google Scholar]
- Bradley RL, et al. , 2008. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295, E586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, et al. , 2004. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res 153, 423–9. [DOI] [PubMed] [Google Scholar]
- Braniste V, et al. , 2014. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, et al. , 2015. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 77, 607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt VE, et al. , 2019. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 597, 2361–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H, et al. , 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA. 292, 1724–37. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Oien DM, 2013. Metabolic/bariatric surgery worldwide 2011. Obes Surg 23, 427–36. [DOI] [PubMed] [Google Scholar]
- Buettner R, et al. , 2007. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring). 15, 798–808. [DOI] [PubMed] [Google Scholar]
- Bullo M, et al. , 2007. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr 10, 1164–72. [DOI] [PubMed] [Google Scholar]
- Bunsawat K, et al. , 2017. The effect of acute maximal exercise on postexercise hemodynamics and central arterial stiffness in obese and normal-weight individuals. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, et al. , 2011. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 106 Suppl 3, S5–78. [DOI] [PubMed] [Google Scholar]
- Campbell MD, et al. , 2015. Simulated games activity vs continuous running exercise: a novel comparison of the glycemic and metabolic responses in T1DM patients. Scand J Med Sci Sports. 25, 216–22. [DOI] [PubMed] [Google Scholar]
- Carmody RN, et al. , 2015. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 17, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale R, et al. , 2014. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis. 235, 649–58. [DOI] [PubMed] [Google Scholar]
- Casas R, et al. , 2014. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One. 9, e100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas R, et al. , 2016. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvencion con DIeta MEDiterranea (PREDIMED) Randomized Controlled Trial. J Nutr 146, 1684–93. [DOI] [PubMed] [Google Scholar]
- Castagneto M, et al. , 1994. Normalization of Insulin Sensitivity in the Obese Patient after Stable Weight Reduction with Biliopancreatic Diversion. Obes Surg 4, 161–168. [DOI] [PubMed] [Google Scholar]
- Catalan V, et al. , 2007. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg 17, 1464–74. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Sethi JK, 2008. TNF-alpha and adipocyte biology. FEBS Lett 582, 117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari F, et al. , 2018. Aging process, adherence to Mediterranean diet and nutritional status in a large cohort of nonagenarians: Effects on endothelial progenitor cells. Nutr Metab Cardiovasc Dis 28, 84–90. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. , 2017. Hypothalamic Insulin Resistance in Obesity: Effects on Glucose Homeostasis. Neuroendocrinology. 104, 364–381. [DOI] [PubMed] [Google Scholar]
- Cheng Y, et al. , 2018. TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun 69, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Cline HT, 2010. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, et al. , 2014. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 63, 1913–20. [DOI] [PubMed] [Google Scholar]
- Claxton A, et al. , 2015. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44, 897–906. [DOI] [PubMed] [Google Scholar]
- Coats BR, et al. , 2017. Metabolically Activated Adipose Tissue Macrophages Perform Detrimental and Beneficial Functions during Diet-Induced Obesity. Cell Rep 20, 3149–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope EC, et al. , 2018. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J Neurosci 38, 8889–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordner ZA, Tamashiro KL, 2015. Effects of high-fat diet exposure on learning & memory. Physiol Behav 152, 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, et al. , 2016. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol 36, 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam DR, et al. , 2004. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 14, 589–600. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 1999. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 70, 146–52. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 2012. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, et al. , 1996. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 17, 123–30. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 1998. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 50, 164–8. [DOI] [PubMed] [Google Scholar]
- Cuevas-Sierra A, et al. , 2019. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv Nutr 10, S17–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa RM, et al. , 2016. TNF-alpha induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc Diabetol 15, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi PS, et al. , 2017. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-alpha on appetite regulating NPY neurons. Int J Obes (Lond). 41, 149–158. [DOI] [PubMed] [Google Scholar]
- Damjanovic M, Barton M, 2008. Fat intake and cardiovascular response. Curr Hypertens Rep 10, 25–31. [DOI] [PubMed] [Google Scholar]
- Darling JN, et al. , 2013. Predicting the effects of a high-energy diet on fatty liver and hippocampal-dependent memory in male rats. Obesity (Silver Spring). 21, 910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, et al. , 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505, 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, et al. , 2010. NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 27, 1–30. [PubMed] [Google Scholar]
- Davis CL, Cooper S, 2011. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: do cross-sectional associations correspond to exercise trial outcomes? PrevMed 52Suppl 1, S65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino CC, et al. , 2018. Effect of Hypoproteic and High-Fat Diets on Hippocampal Blood-Brain Barrier Permeability and Oxidative Stress. Front Nutr 5, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assuncao Machado AC, et al. , 2018. Endothelial Function of Patients with Morbid Obesity Submitted to Roux-en-Y Gastric Bypass With and Without Obstructive Sleep Apnea-Hypopnea Syndrome. Obes Surg 28, 3595–3603. [DOI] [PubMed] [Google Scholar]
- De Filippo C, et al. , 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 107, 14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello AH, et al. , 2018. Mitochondrial dysfunction in obesity. Life Sci 192, 26–32. [DOI] [PubMed] [Google Scholar]
- Debette S, et al. , 2011. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 77,461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, et al. , 2001. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 58, 643–7. [DOI] [PubMed] [Google Scholar]
- Deckers K, et al. , 2017. Obesity and Cognitive Decline in Adults: Effect of Methodological Choices and Confounding by Age in a Longitudinal Study. J Nutr Health Aging. 21, 546–553. [DOI] [PubMed] [Google Scholar]
- DeGruttola AK, et al. , 2016. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis 22, 1137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Olmo N, Ruiz-Gayo M, 2018. Influence of High-Fat Diets Consumed During the Juvenile Period on Hippocampal Morphology and Function. Front Cell Neurosci 12, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, et al. , 1995. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res 41, 717–26. [DOI] [PubMed] [Google Scholar]
- Denou E, et al. , 2016. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab 310, E982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, et al. , 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 108, 3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier N, et al. , 2003. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86, 246–54. [DOI] [PubMed] [Google Scholar]
- Dixon JB, et al. , 2008. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 299, 316–23. [DOI] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC, 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol Learn Mem 115, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigny J, et al. , 2014. Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: a pilot study. J Rehabil Med 46, 1050–4. [DOI] [PubMed] [Google Scholar]
- Drolet R, et al. , 2008. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond). 32, 283–91. [DOI] [PubMed] [Google Scholar]
- Droste DW, et al. , 2014. Advice on lifestyle changes (diet, red wine and physical activity) does not affect internal carotid and middle cerebral artery blood flow velocity in patients with carotid arteriosclerosis in a randomized controlled trial. Cerebrovasc Dis 37, 368–75. [DOI] [PubMed] [Google Scholar]
- Du H, et al. , 2008. Glycemic index and glycemic load in relation to food and nutrient intake and metabolic risk factors in a Dutch population. Am J Clin Nutr 87, 655–61. [DOI] [PubMed] [Google Scholar]
- Dye L, et al. , 2017. The relationship between obesity and cognitive health and decline. Proc Nutr Soc 76, 443–454. [DOI] [PubMed] [Google Scholar]
- El Assar M, et al. , 2013. Preserved endothelial function in human obesity in the absence of insulin resistance. J Transl Med 11, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, et al. , 2017a. Effects of Physical Activity Intervention on Physical and Cognitive Function in Sedentary Adults With and Without Diabetes. J Gerontol A Biol Sci Med Sci 72, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, et al. , 2017b. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 88, 2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N, et al. , 2014. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105, 141–50. [DOI] [PubMed] [Google Scholar]
- Evans CC, et al. , 2014. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 9, e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah R, et al. , 2016. Antioxidant Enzyme Activity and Cognition in Obese Individuals with or without Metabolic Risk Factors. Exp Clin Endocrinol Diabetes. 124, e3. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, et al. , 2010. Is interleukin-6 receptor blockade the Holy Grail for inflammatory diseases? Clin Pharmacol Ther 87, 396–8. [DOI] [PubMed] [Google Scholar]
- Fergenbaum JH, et al. , 2009. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity (Silver Spring). 17,1957–63. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gayol O, et al. , 2019. Different responses to a high-fat diet in IL-6 conditional knock-out mice driven by constitutive GFAP-Cre and Synapsin 1-Cre expression. Neuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Rolfe RD, 1983. Susceptibility testing of anaerobic bacteria. Diagn Microbiol Infect Dis 1, 33–40. [DOI] [PubMed] [Google Scholar]
- Fjaere E, et al. , 2019. Effects of exercise and dietary protein sources on adiposity and insulin sensitivity in obese mice. J Nutr Biochem 66, 98–109. [DOI] [PubMed] [Google Scholar]
- Flint HJ, et al. , 2007. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol 9, 1101–11. [DOI] [PubMed] [Google Scholar]
- Forsythe CE, et al. , 2008. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 43, 65–77. [DOI] [PubMed] [Google Scholar]
- Freeman LR, Granholm AC, 2012. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J Cereb Blood Flow Metab 32, 643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche KL, 2015. The science of fatty acids and inflammation. Adv Nutr 6, 293S–301S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, et al. , 2017. Long-term high-fat diet induces hippocampal microvascular insulin resistance and cognitive dysfunction. Am J Physiol Endocrinol Metab 312, E89–E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, et al. , 2018. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 40, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furet JP, et al. , 2010. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 59, 3049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajanand T, et al. , 2019. Comparing the Efficacy of Supervised and Unsupervised Exercise Training on Glycaemic Control in Type 2 Diabetes: A Systematic Review. Curr Diabetes Rev [DOI] [PubMed] [Google Scholar]
- Galkine A, et al. , 2018. Effects of Body Weight Reduction on Arterial Stiffness and Endothelial Function after Bariatric Surgery in Morbidly Obese Patients: A 4-Year Clinical Study. Acta Endocrinol (Buchar). 14, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L, 2010. Diet and inflammation. Nutr Clin Pract 25, 634–40. [DOI] [PubMed] [Google Scholar]
- Garcia-Hermoso A, et al. , 2018. Ideal Cardiovascular Health and Arterial Stiffness in Spanish Adults-The EVIDENT Study. J Stroke Cerebrovasc Dis 27, 1386–1394. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, et al. , 2008. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol 63, 652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim H, et al. , 2004. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 110, 1564–71. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, et al. , 2010. Insulin levels are decreased in the cerebrospinal fluid of women with prodomal Alzheimer’s disease. J Alzheimers Dis 22, 405–13. [DOI] [PubMed] [Google Scholar]
- Gill SR, et al. , 2006. Metagenomic analysis of the human distal gut microbiome. Science. 312, 1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano P, et al. , 2012. Carotenoids and cardiovascular risk. Curr Pharm Des 18, 5577–89. [DOI] [PubMed] [Google Scholar]
- Gladding JM, et al. , 2018. The Effect of Intrahippocampal Insulin Infusion on Spatial Cognitive Function and Markers of Neuroinflammation in Diet-induced Obesity. Front Endocrinol (Lausanne). 9, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloy VL, et al. , 2013. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 347, f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, 2017. Loss of gut microbiome diversity in industrialized societies: Alternative views (comment on DOI 10.1002/bies.201600145). Bioessays. 39. [DOI] [PubMed] [Google Scholar]
- Gordon S, 2003. Alternative activation of macrophages. Nat Rev Immunol 3, 23–35. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, et al. , 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 42, 2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessler J, et al. , 2013. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 13, 514–22. [DOI] [PubMed] [Google Scholar]
- Graf D, et al. , 2015. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 26, 26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LC, et al. , 2019. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol Aging. 80, 154–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy T, et al. , 2014. Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-alpha expression in 7 month old unchallenged mice. Front Cell Neurosci 8, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, et al. , 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9, 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, et al. , 2010. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 34, 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, et al. , 2011. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis 7, 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Diaz I, et al. , 2016. Mediterranean diet and faecal microbiota: a transversal study. Food Funct 7, 2347–56. [DOI] [PubMed] [Google Scholar]
- Hale VL, et al. , 2018. Diet Versus Phylogeny: a Comparison of Gut Microbiota in Captive Colobine Monkey Species. Microb Ecol 75, 515–527. [DOI] [PubMed] [Google Scholar]
- Han C, et al. , 2009. Adiposity parameters and cognitive function in the elderly: application of “Jolly Fat” hypothesis to cognition. Arch Gerontol Geriatr 49, e133–8. [DOI] [PubMed] [Google Scholar]
- Hanon O, et al. , 2005. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 36, 2193–7. [DOI] [PubMed] [Google Scholar]
- Haro C, et al. , 2017. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol Nutr Food Res 61. [DOI] [PubMed] [Google Scholar]
- Hartman J, Frishman WH, 2014. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 22, 147–51. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP, 2005. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57, 173–85. [DOI] [PubMed] [Google Scholar]
- Hayashi H, et al. , 2002. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol Immunol 46, 819–31. [DOI] [PubMed] [Google Scholar]
- Hill JM, et al. , 1986. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 17, 1127–38. [DOI] [PubMed] [Google Scholar]
- Hoffman JD, et al. , 2017. Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome. Front Aging Neurosci 9, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, et al. , 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259, 87–91. [DOI] [PubMed] [Google Scholar]
- Hoth KF, et al. , 2007. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 38, 308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, et al. , 2008. Roles of IL-6-gp130 Signaling in Vascular Inflammation. Curr Cardiol Rev 4, 179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, et al. , 2015. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 25, 227–39. [DOI] [PubMed] [Google Scholar]
- Hu FB, et al. , 1997. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 337, 1491–9. [DOI] [PubMed] [Google Scholar]
- Hussain Y, et al. , 2019. Short-term westernized (HFFD) diet fed in adolescent rats: Effect on glucose homeostasis, hippocampal insulin signaling, apoptosis and related cognitive and recognition memory function. Behav Brain Res 361, 113–121. [DOI] [PubMed] [Google Scholar]
- Ikramuddin S, et al. , 2013. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 309, 2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikramuddin S, Livingston EH, 2013. New insights on bariatric surgery outcomes. JAMA. 310, 2401–2. [DOI] [PubMed] [Google Scholar]
- Illan-Gomez F, et al. , 2012. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg 22, 950–5. [DOI] [PubMed] [Google Scholar]
- Israel PA, et al. , 1993. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull 30, 571–5. [DOI] [PubMed] [Google Scholar]
- Jansen P, et al. , 2011. Impaired mental rotation performance in overweight children. Appetite. 56,766–9. [DOI] [PubMed] [Google Scholar]
- Jeon BT, et al. , 2012. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 61, 1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Kang EB, 2018. Effects of treadmill exercise on PI3K/AKT/GSK-3beta pathway and tau protein in high-fat diet-fed rats. J Exerc Nutrition Biochem 22, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CH, et al. , 2018. The effects of combined aerobic and resistance training on inflammatory markers in obese men. J Exerc Rehabil 14, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurdak N, Kanarek RB, 2009. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav 96, 1–5. [DOI] [PubMed] [Google Scholar]
- Kadish I, et al. , 2016. Dietary composition affects the development of cognitive deficits in WT and Tg AD model mice. Exp Gerontol 86, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Flier JS, 2000. Obesity and insulin resistance. J Clin Invest 106, 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, P. RL, Kahn SE, Woods SC, Schwartz MW, 2000. Obesity Induced by a High-Fat Diet Is Associated With Reduced Brain Insulin Transport in Dogs. Diabetes. 49, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Kalyan-Masih P, et al. , 2016. Western High-Fat Diet Consumption during Adolescence Increases Susceptibility to Traumatic Stress while Selectively Disrupting Hippocampal and Ventricular Volumes. eNeuro. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL, 2011. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 103, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, et al. , 2010. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis 21, 207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper JM, et al. , 2018. Cognitive deficits associated with a high-fat diet and insulin resistance are potentiated by overexpression of ecto-nucleotide pyrophosphatase phosphodiesterase-1. Int J Dev Neurosci 64, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastorini CM, et al. ,2011. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 57, 1299–313. [DOI] [PubMed] [Google Scholar]
- Kelly JR, et al. , 2015. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, et al. , 2006. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 49, 2790–2. [DOI] [PubMed] [Google Scholar]
- Kern W, et al. , 2001. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 74, 270–80. [DOI] [PubMed] [Google Scholar]
- Khan MB, et al. , 2018. Chronic Remote Ischemic Conditioning Is Cerebroprotective and Induces Vascular Remodeling in a VCID Model. Transl Stroke Res 9, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, et al. , 2011a. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 152, 3638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, et al. , 2011b. Cortical neurons develop insulin resistance and blunted Akt signaling: a potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid Redox Signal. 14, 1829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Yi HK, 2015. Intermittent bout exercise training down-regulates age-associated inflammation in skeletal muscles. Exp Gerontol 72, 261–8. [DOI] [PubMed] [Google Scholar]
- Kim OY, et al. , 2012. Arginase I and the very low-density lipoprotein receptor are associated with phenotypic biomarkers for obesity. Nutrition. 28, 635–9. [DOI] [PubMed] [Google Scholar]
- King GL, Johnson SM, 1985. Receptor-mediated transport of insulin across endothelial cells. Science. 227, 1583–6. [DOI] [PubMed] [Google Scholar]
- Kong LC, et al. , 2013. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 98, 16–24. [DOI] [PubMed] [Google Scholar]
- Kopp HP, et al. , 2003. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol 23, 1042–7. [DOI] [PubMed] [Google Scholar]
- Kosari S, et al. , 2012. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res 235, 98–103. [DOI] [PubMed] [Google Scholar]
- Kratz M, et al. , 2014. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20, 614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki K, et al. , 2000. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 101, 676–81. [DOI] [PubMed] [Google Scholar]
- Kullmann S, et al. , 2016. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev 96, 1169–209. [DOI] [PubMed] [Google Scholar]
- Labouesse MA, et al. , 2018. MicroRNA Expression Profiling in the Prefrontal Cortex: Putative Mechanisms for the Cognitive Effects of Adolescent High Fat Feeding. Sci Rep 8, 8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, et al. , 2012. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One. 7, e36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KSP, et al. , 2017. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 88, 876–882. [DOI] [PubMed] [Google Scholar]
- Laimer M, et al. , 2002. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes Relat Metab Disord 26, 659–62. [DOI] [PubMed] [Google Scholar]
- Lampe L, et al. , 2019. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol 85, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z, 2009. Insulin and the brain. Arch Physiol Biochem 115, 112–6. [DOI] [PubMed] [Google Scholar]
- Lee H, et al. , 2013. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr 16, 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichman JG, et al. , 2008. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med 121, 966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, et al. , 2013. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48, 1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan EB, et al. , 2008. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 57, 437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, et al. , 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 124, 837–48. [DOI] [PubMed] [Google Scholar]
- Li J, et al. , 2017. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JV, et al. , 2011. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 60, 1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. , 2013. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 8, e66069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, et al. , 2018. Gut-Brain Psychology: Rethinking Psychology From the Microbiota-Gut-Brain Axis. Front Integr Neurosci 12, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard KK, et al. , 2015. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AP, et al. , 2013. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5, 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken KL, et al. , 2009. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis 5,547–52. [DOI] [PubMed] [Google Scholar]
- Lourida I, et al. , 2013. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 24, 479–89. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, et al. , 2007. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 56, 16–23. [DOI] [PubMed] [Google Scholar]
- Ma YL, et al. , 2018. Suppressing Irf2bp2 expressions accelerates metabolic syndrome-associated brain injury and hepatic dyslipidemia. Biochem Biophys Res Commun 503, 1651–1658. [DOI] [PubMed] [Google Scholar]
- Machida T, et al. , 2017. Contribution of thrombin-reactive brain pericytes to blood-brain barrier dysfunction in an in vivo mouse model of obesity-associated diabetes and an in vitro rat model. PLoS One. 12, e0177447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, et al. , 2015. Lifestyle modification decreases arterial stiffness in overweight and obese men: dietary modification vs. exercise training. Int J Sport Nutr Exerc Metab 25, 69–77. [DOI] [PubMed] [Google Scholar]
- Maggard MA, et al. , 2005. Meta-analysis: surgical treatment of obesity. Ann Intern Med 142, 547–59. [DOI] [PubMed] [Google Scholar]
- Maggard-Gibbons M, et al. , 2013. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 309, 2250–61. [DOI] [PubMed] [Google Scholar]
- Manderino L, et al. , 2017. Preliminary Evidence for an Association Between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J Int Neuropsychol Soc 23, 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin C, et al. , 2011. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr 93, 267–74. [DOI] [PubMed] [Google Scholar]
- Marques-Iturria I, et al. , 2013. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res 214, 109–15. [DOI] [PubMed] [Google Scholar]
- Martins IV, et al. , 2017. Mitochondrial Abnormalities and Synaptic Loss Underlie Memory Deficits Seen in Mouse Models of Obesity and Alzheimer’s Disease. J Alzheimers Dis 55, 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews VB, et al. , 2010. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 53, 2431–41. [DOI] [PubMed] [Google Scholar]
- Mauer J, et al. , 2014. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15, 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean FH, et al. , 2018. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci Rep 8, 11976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, et al. , 2010. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem 93, 546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta BK, et al. , 2018. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int J Neurosci 1–12. [DOI] [PubMed] [Google Scholar]
- Mena MP, et al. , 2009. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr 89, 248–56. [DOI] [PubMed] [Google Scholar]
- Menni C, et al. , 2018. Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J 39, 2390–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, et al. , 2006. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res 175, 374–82. [DOI] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ, 2014. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun 42, 10–21. [DOI] [PubMed] [Google Scholar]
- Miller GD, et al. , 2011. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 7, 618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, et al. , 2013. Bariatric surgery patients exhibit improved memory function 12 months postoperatively. Obes Surg 23, 1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingrone G, et al. , 2012. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366, 1577–85. [DOI] [PubMed] [Google Scholar]
- Ministrini S, et al. , 2018. Microcirculatory Improvement Induced by Laparoscopic Sleeve Gastrectomy Is Related to Insulin Sensitivity Retrieval. Obes Surg 28, 3151–3158. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, et al. , 1997. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82, 4196–200. [DOI] [PubMed] [Google Scholar]
- Moller DE, 2000. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 11, 212–7. [DOI] [PubMed] [Google Scholar]
- Monje ML, et al. , 2003. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 302, 1760–5. [DOI] [PubMed] [Google Scholar]
- Morris JK, Burns JM, 2012. Insulin: an emerging treatment for Alzheimer’s disease dementia? Curr Neurol Neurosci Rep 12, 520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschen AR, et al. , 2010. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 59, 1259–64. [DOI] [PubMed] [Google Scholar]
- Mueller S, et al. , 2006. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 72, 1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Sowers JR, 2013. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 14, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munukka E, et al. , 2018. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol 9, 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, et al. , 2009. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J 23, 4353–60. [DOI] [PubMed] [Google Scholar]
- Nagpal R, et al. , 2018. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front Nutr 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KK, et al. , 2011. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril 95, 203–9. [DOI] [PubMed] [Google Scholar]
- Napoli N, et al. , 2014. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am J Clin Nutr 100, 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, et al. , 2015. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 385, 2255–63. [DOI] [PubMed] [Google Scholar]
- Nieto-Vazquez I, et al. , 2008. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 114, 183–94. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Nilsson E, 2009. Overweight and cognition. Scand J Psychol 50, 660–7. [DOI] [PubMed] [Google Scholar]
- Noble EE, et al. , 2017. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front Behav Neurosci 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Kanoski SE, 2016. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr Opin Behav Sci 9, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, et al. , 2014. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem 114, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PE, et al. , 2006. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 144, 625–33. [DOI] [PubMed] [Google Scholar]
- O’Brien PE, et al. , 2013. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg 257, 87–94. [DOI] [PubMed] [Google Scholar]
- Oakes ND, et al. , 1997. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 46, 1768–74. [DOI] [PubMed] [Google Scholar]
- Olver TD, et al. , 2017. A chronic physical activity treatment in obese rats normalizes the contributions of ET-1 and NO to insulin-mediated posterior cerebral artery vasodilation. J Appl Physiol (1985). 122, 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, 2019. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padwal R, et al. , 2011. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med 26, 1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta P, et al. , 2019. Central Arterial Stiffness Is Associated With Structural Brain Damage and Poorer Cognitive Performance: The ARIC Study. J Am Heart Assoc 8, e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta P, et al. , 2015. Interleukin-6 and C-Reactive Protein Levels and 9-Year Cognitive Decline in Community-Dwelling Older Women: The Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci 70, 873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, et al. , 2006. Cerebrovascular disease in the elderly: lipoprotein metabolism and cognitive decline. Aging Clin Exp Res 18, 144–8. [DOI] [PubMed] [Google Scholar]
- Paouri E, et al. , 2017. Peripheral Tumor Necrosis Factor-Alpha (TNF-alpha) Modulates Amyloid Pathology by Regulating Blood-Derived Immune Cells and Glial Response in the Brain of AD/TNF Transgenic Mice. J Neurosci 37, 5155–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, 2008. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug Chem 19, 1327–38. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, 2012. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol 503, 269–92. [DOI] [PubMed] [Google Scholar]
- Paul R, et al. , 2003. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain. 126, 1873–82. [DOI] [PubMed] [Google Scholar]
- Pedrinolla A, et al. , 2018. Role of Exercise in Vascular Function and Inflammatory Profile in Age-Related Obesity. J Immunol Res 2018, 7134235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, et al. , 2003. Insulin resistance in nondiabetic morbidly obese patients: effect of bariatric surgery. Obes Res 11, 1495–501. [DOI] [PubMed] [Google Scholar]
- Petrov D, et al. , 2015. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta. 1852, 1687–99. [DOI] [PubMed] [Google Scholar]
- Picot J, et al. , 2009. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13, 1–190, 215,–357, iii–iv. [DOI] [PubMed] [Google Scholar]
- Pischon T, et al. , 2005. Association between dietary factors and plasma adiponectin concentrations in men. Am J Clin Nutr 81, 780–6. [DOI] [PubMed] [Google Scholar]
- Poluektova L, et al. , 2005. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 52, 344–53. [DOI] [PubMed] [Google Scholar]
- Pratchayasakul W, et al. , 2011. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci 88, 619–27. [DOI] [PubMed] [Google Scholar]
- Prickett C, et al. , 2015. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract 9, 93–113. [DOI] [PubMed] [Google Scholar]
- Prickett C, et al. , 2018. Neuropsychological Functioning in Mid-life Treatment-Seeking Adults with Obesity: a Cross-sectional Study. Obes Surg 28, 532–540. [DOI] [PubMed] [Google Scholar]
- Pryde SE, et al. , 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217, 133–9. [DOI] [PubMed] [Google Scholar]
- Psaltopoulou T, et al. , 2013. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol 74, 580–91. [DOI] [PubMed] [Google Scholar]
- Pulakat L, et al. , 2011. The Impact of Overnutrition on Insulin Metabolic Signaling in the Heart and the Kidney. Cardiorenal Med 1, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, et al. , 2006a. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care. 29, 1501–5. [DOI] [PubMed] [Google Scholar]
- Qi L, et al. , 2006b. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 29, 207–11. [DOI] [PubMed] [Google Scholar]
- Radenkovic M, 2014. Pioglitazone and Endothelial Dysfunction: Pleiotropic Effects and Possible Therapeutic Implications. Sci Pharm 82, 709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RS, et al. , 2012. Insulin resistance and bariatric surgery. Obes Rev 13, 316–28. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL, 2007. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3, 46–56. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. , 2006. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 27, 451–8. [DOI] [PubMed] [Google Scholar]
- Rhea EM, et al. , 2018. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol 596, 4753–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, et al. , 2009. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6, 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, et al. , 2019. Limited Effects of Low-to-Moderate Aerobic Exercise on the Gut Microbiota of Mice Subjected to a High-Fat Diet. Nutrients. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, et al. , 2003. Microvascular plasticity in aging. Ageing Res Rev 2, 149–68. [DOI] [PubMed] [Google Scholar]
- Rideout DA, et al. , 2010. Roux-en-Y gastric bypass alters tumor necrosis factor-alpha but not adiponectin signaling in immediate postoperative period in obese rats. Surg Obes Relat Dis 6, 676–80. [DOI] [PubMed] [Google Scholar]
- Riserus U, et al. , 2009. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 48, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Rodrigues S, et al. , 2017. Impact of physical exercise on visceral adipose tissue fatty acid profile and inflammation in response to a high-fat diet regimen. Int J Biochem Cell Biol 87, 114–124. [DOI] [PubMed] [Google Scholar]
- Rochette AD, et al. , 2016. Mild cognitive impairment is prevalent in persons with severe obesity. Obesity (Silver Spring). 24,1427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort KD, et al. , 2016. Tumour necrosis factor-alpha-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J Neurochem 136, 564–72. [DOI] [PubMed] [Google Scholar]
- Rochfort KD, Cummins PM, 2015. Cytokine-mediated dysregulation of zonula occludens-1 properties in human brain microvascular endothelium. Microvasc Res 100, 48–53. [DOI] [PubMed] [Google Scholar]
- Romanatto T, et al. , 2009. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 284, 36213–22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roytblat L, et al. , 2000. Raised interleukin-6 levels in obese patients. Obes Res 8, 673–5. [DOI] [PubMed] [Google Scholar]
- Rubino F, et al. , 2010. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 61, 393–411. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, et al. , 2009. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 4, e8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, et al. , 2009. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr 89, 601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, 2011. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). 12, 255–7. [DOI] [PubMed] [Google Scholar]
- Saji N, et al. , 2019. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep 9, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh TS, et al. , 2019. Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS. 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, et al. , 2013. Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia. 56, 2564–72. [DOI] [PubMed] [Google Scholar]
- Sams VG, et al. , 2016. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc 30, 3499–504. [DOI] [PubMed] [Google Scholar]
- Saura M, et al. , 2006. Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem 281, 30057–62. [DOI] [PubMed] [Google Scholar]
- Schauer PR, et al. , 2003. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238, 467–84; discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, et al. , 2012. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366, 1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke J, et al. , 2019. Spermidine and Voluntary Activity Exert Differential Effects on Sucrose-Compared with Fat-Induced Systemic Changes in Male Mice. J Nutr 149, 451–462. [DOI] [PubMed] [Google Scholar]
- Schwartz DH, et al. , 2013. Visceral fat is associated with lower executive functioning in adolescents. Int J Obes (Lond). 37,1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Hoffmann G, 2014. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 24, 929–39. [DOI] [PubMed] [Google Scholar]
- Scott SN, et al. , 2019. Home-HIT improves muscle capillarisation and eNOS/NAD(P)Hoxidase protein ratio in obese individuals with elevated cardiovascular disease risk. J Physiol [DOI] [PubMed] [Google Scholar]
- Shahrivari M, et al. , 2017. Peripheral Blood Cytokine Levels After Acute Myocardial Infarction: IL-1beta- and IL-6-Related Impairment of Bone Marrow Function. Circ Res 120, 1947–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, et al. , 2013. Is obesity a brain disease? Neurosci Biobehav Rev 37, 2489–503. [DOI] [PubMed] [Google Scholar]
- Shi Z, et al. , 2018. Aerobic Interval Training Regulated SIRT3 Attenuates High-Fat-Diet-Associated Cognitive Dysfunction. Biomed Res Int 2018, 2708491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, et al. , 2018. Activated Microglia Disrupt the Blood-Brain Barrier and Induce Chemokines and Cytokines in a Rat in vitro Model. Front Cell Neurosci 12, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, et al. , 2016a. Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life. PLoS One. 11, e0163883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, et al. , 2016b. The role of endoplasmic reticulum stress in hippocampal insulin resistance. Exp Neurol. 277, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, et al. , 2010. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 6, 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, et al. , 2016. Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life. PLoS One. 11, e0163883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu S, et al. , 2015. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS One. 10, e0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, et al. , 2014. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 39, 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, et al. , 2012. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology. 79, 755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, et al. , 2014. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 83, 486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L, 2013. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 273, 219–34. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, et al. , 2007. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357, 741–52. [DOI] [PubMed] [Google Scholar]
- Slivovskaja I, et al. , 2018. Aerobic Training Effect on Arterial Stiffness in Metabolic Syndrome. Am J Med 131, 148–155. [DOI] [PubMed] [Google Scholar]
- Smith E, et al. , 2011. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev 12, 740–55. [DOI] [PubMed] [Google Scholar]
- Smith PJ, et al. , 2010. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 72, 239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, et al. , 2017. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J Alzheimers Dis 59, 815–849. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, et al. , 2004. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 63, 1882–91. [DOI] [PubMed] [Google Scholar]
- Son WM, et al. , 2017. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens 39, 546–552. [DOI] [PubMed] [Google Scholar]
- Sona C, et al. , 2018. Docosahexaenoic acid modulates brain-derived neurotrophic factor via GPR40 in the brain and alleviates diabesity-associated learning and memory deficits in mice. Neurobiol Dis 118, 94–107. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, et al. , 2016. Age and High-Fat Diet Effects on Glutamine Synthetase Immunoreactivity in Liver and Hippocampus and Recognition Memory in Mice. Curr Aging Sci 9, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, et al. , 2018. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 23, 2287–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli M, et al. , 2017. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun 8, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolcova A, et al. , 2014. Deficient hippocampal insulin signaling and augmented Tau phosphorylation is related to obesity- and age-induced peripheral insulin resistance: a study in Zucker rats. BMC Neurosci 15, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponton AC, et al. , 2017. Circulating Concentrations of Adipocytokines and Their Receptors in the Isolated Corpus Cavernosum and Femoral Artery from Trained Rats on a High-Fat Diet. J Vasc Res 54, 33–50. [DOI] [PubMed] [Google Scholar]
- Sripetchwandee J, et al. , 2018. Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Front Endocrinol (Lausanne). 9, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanov T, et al. , 2013. Effects of supervised vs non-supervised combined aerobic and resistance exercise programme on cardiometabolic risk factors. Cent Eur J Public Health. 21, 8–16. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, et al. , 1996. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97, 2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolberg CR, et al. , 2018. Effects of gastric bypass surgery followed by supervised physical training on inflammation and endothelial function: A randomized controlled trial. Atherosclerosis. 273, 37–44. [DOI] [PubMed] [Google Scholar]
- Storniolo CE, et al. , 2017. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur J Nutr 56, 89–97. [DOI] [PubMed] [Google Scholar]
- Streese L, et al. , 2019. Short- and Long-Term Effects of Bariatric Surgery on Vascular Phenotype. Obes Surg 29, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Suarez AN, et al. , 2019. Regulation of Memory Function by Feeding-Relevant Biological Systems: Following the Breadcrumbs to the Hippocampus. Front Mol Neurosci 12, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugerman HJ, et al. , 2003. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237, 751–6; discussion 757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi N, et al. , 2010. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol 30, 247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers LK, 2002. Is surgery the best treatment for Type 2 diabetes in the obese? Diabet Med 19 Suppl 3, 14–7. [DOI] [PubMed] [Google Scholar]
- Sun W, et al. , 2018. Fecal Microbiota Transplantation Can Alleviate Gastrointestinal Transit in Rats with High-Fat Diet-Induced Obesity via Regulation of Serotonin Biosynthesis. Biomed Res Int 2018, 8308671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze MA, Schloss PD, 2016. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. MBio. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze MA, Schloss PD, 2017. Erratum for Sze and Schloss, “‘Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome”. MBio. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanca A, et al. , 2018. Caloric restriction promotes functional changes involving short-chain fatty acid biosynthesis in the rat gut microbiota. Sci Rep 8, 14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, et al. , 2018. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J Gerontol A Biol Sci Med Sci 73, 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio TRS, et al. , 2018. Effect of Low- Versus High-Intensity Exercise Training on Biomarkers of Inflammation and Endothelial Dysfunction in Adolescents With Obesity: A 6-Month Randomized Exercise Intervention Study. Pediatr Exerc Sci 30, 96–105. [DOI] [PubMed] [Google Scholar]
- Tesauro M, et al. , 2008. Tumor necrosis factor-alpha antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care. 31, 1439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, et al. , 2012. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122, 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Pfeiffer AF, 2012. Foods for the prevention of diabetes: how do they work? Diabetes Metab Res Rev 28, 25–49. [DOI] [PubMed] [Google Scholar]
- Timper K, et al. , 2017. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. Cell Rep 19, 267–280. [DOI] [PubMed] [Google Scholar]
- Torres-Pena JD, et al. , 2018. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis. 269, 50–56. [DOI] [PubMed] [Google Scholar]
- Toth P, et al. , 2017. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312, H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tounian P, et al. , 2001. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 358, 1400–4. [DOI] [PubMed] [Google Scholar]
- Tremaroli V, et al. , 2015. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab 22, 228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllidi H, et al. , 2009. Cognitive impairment is related to increased arterial stiffness and microvascular damage i n patients with never-treated essential hypertension. Am J Hypertens 22, 525–30. [DOI] [PubMed] [Google Scholar]
- Troen AM, et al. , 2008. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 105, 12474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Heiman ML, 2001. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes. 109, 307–19. [DOI] [PubMed] [Google Scholar]
- Tucsek Z, et al. , 2014a. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 69, 1212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, et al. , 2014b. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69, 1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, et al. , 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 3, 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, et al. , 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 444, 1027–31. [DOI] [PubMed] [Google Scholar]
- Ulker I, Yildiran H, 2019. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Biosci Microbiota Food Health. 38, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierre D, et al. , 2011. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 305, 1790–9. [DOI] [PubMed] [Google Scholar]
- Underwood EL, Thompson LT, 2016. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast 2016, 7385314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urpi-Sarda M, et al. , 2012. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr 142, 1019–25. [DOI] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, et al. , 2019. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J Gerontol A Biol Sci Med Sci 74, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, et al. , 1997. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: an effect depending upon the route of administration. Eur J Neurosci 9, 1461–72. [DOI] [PubMed] [Google Scholar]
- Valls-Pedret C, et al. , 2015. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med 175, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Vazquez LA, et al. , 2005. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab 90, 316–22. [DOI] [PubMed] [Google Scholar]
- Vella CA, et al. , 2017a. Physical Activity and Adiposity-related Inflammation: The MESA. Med Sci Sports Exerc 49, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella CA, et al. , 2017b. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur J Sport Sci 17, 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, et al. , 2010. Selective alterations within executive functions in adolescents with excess weight. Obesity (Silver Spring). 18,1572–8. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, et al. , 2012. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med 74, 682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, et al. , 2009. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 58, 2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa A, et al. , 2018. Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Mol Neurobiol [DOI] [PubMed] [Google Scholar]
- Virdis A, et al. , 2015. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J. 36, 784–94. [DOI] [PubMed] [Google Scholar]
- Virdis A, et al. , 2011. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol 58, 238–47. [DOI] [PubMed] [Google Scholar]
- Wallenius V, et al. , 2002. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8, 75–9. [DOI] [PubMed] [Google Scholar]
- Wang C, et al. , 2016a. Obesity Reduces Cognitive and Motor Functions across the Lifespan. Neural Plast. 2016, 2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. , 2013. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 62, 4030–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. , 2008. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 57, 540–7. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. , 2016b. Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci Lett 627, 185–91. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. , 2016. Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci Lett 627, 185–91. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, et al. , 2013. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, et al. , 2002. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 59, 371–8. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, et al. , 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann L, et al. , 2014. IKKepsilon is key to induction of insulin resistance in the hypothalamus, and its inhibition reverses obesity. Diabetes. 63, 3334–45. [DOI] [PubMed] [Google Scholar]
- Wennberg AMV, et al. , 2018. The Cross-Sectional and Longitudinal Associations Between IL-6, IL-10, and TNFalpha and Cognitive Outcomes in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams IL, et al. , 2002. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord 26, 754–64. [DOI] [PubMed] [Google Scholar]
- Wolf PA, et al. , 2007. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res 4, 111–6. [DOI] [PubMed] [Google Scholar]
- Wu GD, et al. , 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science. 334, 105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. , 2013. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab 18, 816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, et al. , 2017. Effect of Roux-en-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Front Immunol 8,1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, et al. , 2003. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 61,76–80. [DOI] [PubMed] [Google Scholar]
- Yasukawa K,et al. , 1987. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBOJ. 6, 2939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, 2017. Adverse effects of consuming high fat-sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc 76, 455–465. [DOI] [PubMed] [Google Scholar]
- Yun Y, et al. , 2017. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol 17,151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2009. Human gut microbiota in obesity and aftergastric bypass. Proc Natl Acad Sci USA. 106, 2365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P,et al. , 2018. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem 65, 54–65. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Rui L, 2013. Leptin signaling and leptin resistance. Front Med 7, 207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 57,178–201. [DOI] [PubMed] [Google Scholar]


