Abstract
Introduction
Anhedonia is a transdiagnostic psychopathological dimension, consisting in the impaired ability to experience pleasure. In order to further our understanding of its neural correlates and to explore its potential relevance as a predictor of treatment response, in this article we systematically reviewed studies involving anhedonia and neuromodulation interventions, across different disorders.
Methods
We included seven studies fulfilling inclusion/exclusion criteria and involving different measures of anticipatory and consummatory anhedonia, as well as different noninvasive brain stimulation interventions (transcranial magnetic stimulation and transcranial direct current stimulation). Studies not exploring hedonic measures or not involving neuromodulation intervention were excluded.
Results
All the included studies entailed the use of rTMS protocols in one of the diverse prefrontal targets. The limited amount of studies and the heterogeneity of stimulation protocols did not allow to draw any conclusion with regard to the efficacy of rTMS in the treatment of transnosographic anhedonia. A potential for anhedonia in dissecting possible endophenotypes of different psychopathological conditions preliminarily emerged.
Conclusions
Anhedonia is an underexplored condition in neuromodulation trials. It may represent a valuable transdiagnostic dimension that requires further examination in order to discover new clinical predictors for treatment response.
Keywords: addiction, depression, hedonic tone dysfunction, neuromodulation, schizophrenia, SHAPS, transcranial direct current stimulation, transcranial magnetic stimulation
1. BACKGROUND
Transdiagnostic psychopathological dimensions have been increasingly recognized as relevant factors in the forecast of a more accurate classification and treatment of mental disorders.1 The Research Domain Criteria (RDoC) strategic plan defines these markers as continuous dimensions increasingly present from general population to, in higher extent, the pathophysiology of mental conditions.2 Moreover, transdiagnostic dimensions could be relevant in the clustering of mental disorders, in order to define different pathophysiologically based disease subtypes and possible predictors of treatment outcome.3 Growing research is investigating possible biotypes of mental disorders,4 with a predictive potential in terms of treatment response.
Anhedonia is a relevant, and often under‐considered, transdiagnostic psychopathological dimension.5 It is defined as the inability to experience pleasure or interest in almost all activities of daily life.6 There are two faces of anhedonia that could be analyzed: the consummatory and anticipatory anhedonia. The consummatory pleasure consists in the immediate satisfaction and pleasurable feelings that are linked to the realization of a desire. On the other hand, the anticipatory pleasure is the association to the expectation of a pleasurable reward and therefore is connected to motivation.7
Recently, neuromodulation interventions—also called noninvasive brain stimulation (NIBS) —have reached greater attention as tools for modulating local bran activity and as treatment options in several disorders.8 Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are noninvasive and safe techniques that could inhibit or promote local neural activity in the underlying cortical areas. Also, following the neuronal projections of the targeted areas, it has been hypothesized that there could be a wider‐range action,9 while is not clear if in its mechanism of action, a modulation of the synaptic plasticity could be involved.10 The application of NIBS in different conditions has been found to determine a clinical response in a portion, but not all, of the treated patients.11 Nowadays, the efforts in medical research to improve and promote the neuromodulation interventions are focused on detecting different endophenotypes of mental diseases that could be more predictive of a better response to treatment.
Neuromodulation interventions have been tested in several conditions involving anhedonia by using different stimulation protocols and targeted areas, most leading to mixed results.12, 13, 14 Interestingly, in pharmacological studies of MDD samples, the occurrence of anhedonia has been found to be predictive of poor treatment response, limited recovery and lower quality of life.15 Considered the potential relevance of anhedonia in differentially affecting the clinical course of involved conditions, in this paper we aimed at exploring the concept of anhedonia as a putative transdiagnostic dimension characterizing different disorders’ endophenotypes. In order to preliminarily test the hypothesis that reward dysfunctions could be proposed as a predictor of NIBS treatment response, we systematically reviewed original articles both involving the application of neuromodulation interventions (ie, rTMS and tDCS) and focusing on psychometric scales and cognitive tasks measuring anhedonia and reward dysfunctions.
1.1. Transdiagnostic relevance of anhedonia in psychopathology
Anhedonia is a core symptom of major depressive disorder (MDD; especially in the definition of melancholic subtype). It is commonly found in the mental health history of patients who attempted suicide and have succeeded; anhedonic patients have greater social impairment, higher levels of hopelessness and are usually younger than nonanhedonic patients. 16, 17 Interestingly, anhedonia has been found in bipolar disorder also during euthymic phases,18 and it has been suggested as vulnerability factor involved in comorbid bipolar conditions.19
Anhedonia is a relevant negative symptom in schizophrenia (SZ)20 and social anhedonia, defined as the lack of pleasure or reward from social situations, is a key aspect of schizotypal personality disorder.21
Moreover, anhedonia is a relevant symptom in patients with substance use disorders (SUDs), since it can represent a symptom of abstinence and therefore it may predict relapse,22, 23, 24 posttraumatic stress disorder (PTSD),25, 26 anxiety disorder,27 and obsessive‐compulsive disorder.7 Nonetheless, anhedonia is not only a symptom of various psychiatric disorders but is also often present in other conditions such as Parkinson's disease,28, 29 over‐eating or risky behaviors.30, 31, 32 According to a recent review,33 anhedonia appears to be a heritable trait with both a biological and clinical basis and an anhedonic endophenotype could possibly be identified. Anhedonia is a trait linked to many mental diseases, and it represents a potential marker of vulnerability, especially for depression. The biological hypothesis could be that anhedonia is a symptom connected to a dysfunctional mechanism between life stressors and the brain reward system.34
Recent evidence indicates the presence of possible overlapping in the neural substrates of behavioral and cognitive processing in MDD and SZ conditions sharing marked anhedonia.35, 36 Although the transnosographic nature of anhedonia is well‐established, the neurobiological underpinnings of hedonic dysfunctions are still unclear,37 as well as it is uncertain if the neurobiological mechanisms are the same in the different mental diseases.
Animal models of anhedonia are valuable tools allowing to explore the neurobiological underpinnings as well as to search for possible predictors of treatment outcome. Data from rodent models are starting to provide important insights into the pathophysiology of anhedonia,38 especially through models impairing the responses to rewarding stimuli (eg, sucrose preference) or through the use of dopamine‐related transgenic approaches.39
1.2. Anhedonia and pharmacological treatments: outcome measure or predictor of response?
Since anhedonia is a difficult‐to‐treat target, several therapeutic approaches have been proposed, including psychosocial interventions, antipsychotics (for SZ conditions40), antidepressants (in mood disorders41), and neuromodulation interventions (in addictive use disorders12, 42).
The hypothesis that anhedonia is due to a dopaminergic hypofunction of the reward system leads to the implementation of pharmacological therapeutic approaches that are based on drugs that modulate that neurotransmitter as well as psychostimulants, dopamine agonists and norepinephrine or dopamine reuptake inhibitor such as Bupropion.23, 43, 44 Since, as mentioned before, anhedonia is a well‐known symptom in drugs’ withdrawal, many efforts are made in finding a symptomatic treatment for patients with substance use disorder. A recent study proved the efficacy on the melancholic trait of acetyl‐l‐carnitine (ALC) on alcoholic patients with anhedonic features.45 Recent evidence on this field highlighted the efficacy of second‐generation antipsychotics on anhedonic traits, like quetiapine and aripiprazole.46, 47, 48 Hence, such therapies could be also useful in dual diagnosis patients with anhedonia.49
Only a few studies investigated the effect of antidepressants on anhedonia. A recent review50 analyzed the possible effect of agomelatine. Its action appears to be mediated mainly through neurotrophins elevation,51 but further studies are required to prove its efficacy in reducing anhedonic symptoms.50
Preliminary studies on rats showed that while fluoxetine did not show any antianhedonic properties, imipramine did, but only on a subgroup. In this trial, the researchers have also studied the effect of other drugs, understanding that while clozapine and lithium showed an antianhedonic effect, haloperidol did not equivalently. Such findings confirm that dopaminergic dysfunction has a relevant role in anhedonia; drugs that interact with the dopaminergic system could be utilized to treat anhedonia and therefore the underlying mental illness.52
Finally, a recent systematic review by Cao and colleagues53 analyzed 17 studies on MDD patients with anhedonic features treated with 14 different antidepressants. This review suggested that antidepressants improve anhedonic symptoms, yet no significant difference was found among subjects.
Another valid therapeutic approach could be psychotherapy. Behavioral activation (BA), which was initially developed as part of cognitive behavioral therapy (CBT), seems to be useful in cases with anticipatory anhedonia. Such results seem to be confirmed by fMRI studies.54
2. MATERIALS AND METHODS
A literature research of the databases PubMed/MEDLINE, ISI Web of Science, and Scopus was conducted in order to find appropriate published articles on the application of neuromodulation interventions (ie, TMS and tDCS) in any clinical and nonclinical sample in which anhedonic symptoms were assessed.
A search algorithm was employed based on appropriate combinations of the terms: “anhedonia”, “pleasure”, “SHAPS”, “hedonic”, “neuromodulation”, “noninvasive brain stimulation”, “transcranial magnetic stimulation”, “repetitive “transcranial magnetic stimulation”, “direct transcranial current stimulation”, “tDCS”, “TMS”, “rTMS”.
The search was conducted on December 14, 2018. It included all publications prior to December 2018; no earliest date limit was applied. To expand the search, reference lists of the retrieved articles were also screened for additional studies.
The search strategy yielded 22 records. All original articles [open‐label trials (OLTs) or double‐blind trials, prospective or retrospective observational studies, case reports, and letter to editor], written in English language, were eligible for inclusion. We excluded studies focusing on animal models, reviews, commentaries, meta‐analysis, studies not involving any neuromodulation intervention, and studies not analyzing data referred to anhedonic measures.
Two researchers (MCS and ML) independently reviewed the titles and abstracts of the retrieved articles, applying the above‐mentioned inclusion and exclusion criteria. By reading titles and abstracts, 10 records were excluded. The researchers then independently reviewed the full‐text version of the articles to confirm their eligibility for inclusion, or subsequent exclusion. Seven out of 12 studies fulfilled the above‐mentioned criteria and were therefore included in the qualitative synthesis (Figure 1).
Figure 1.
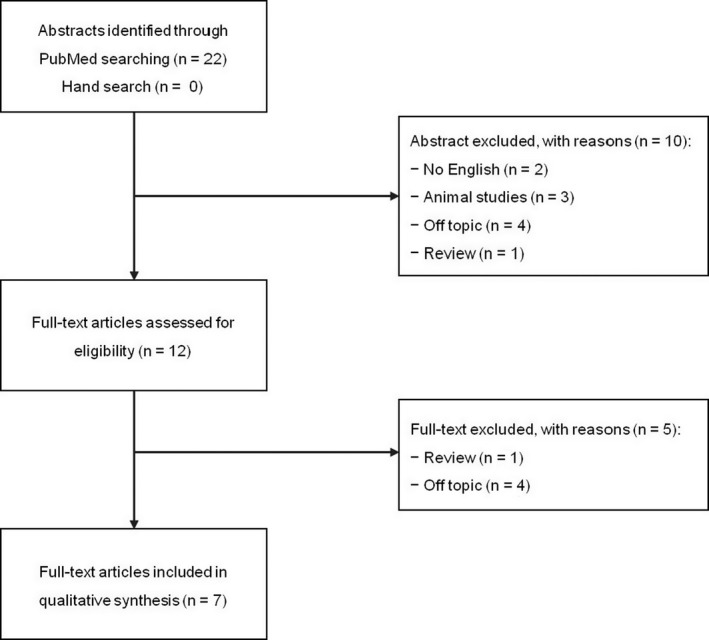
PRISMA flow diagram of systematic review
For each study selected for inclusion, details regarding the publication were gathered (ie, author names, year of publication, and country of origin); data were collected on the diagnosis, on neuromodulation interventions and protocols applied (type, targeted area, and number of sessions), as well as on methods used for the assessment of anhedonia on the main findings concerning hedonic functions reported in the study. All of this information was extracted by one author and subsequently verified, independently, by the other.
3. RESULTS
The qualitative synthesis included seven papers (Table 1), for a total number of 201 subjects (161 males and 40 females) with 83 healthy controls and 118 patients affected by: schizophrenia (SZ; N = 45), treatment‐resistant depression (TRD; N = 58), cocaine use disorder (CUD; N = 15). Concerning the design, the studies included were as follows: one randomized controlled trial (RCT) or sham‐controlled intervention; five open‐label studies(OL); and one study with a crossover design.
Table 1.
Summary of included studies
| Authors | Study design | Sample, Diagnosis | Stimulation intervention | Targeted area | No. of Sessions | Assessment | Selected results |
|---|---|---|---|---|---|---|---|
| Downar et al (2016) | OL | 47 MDE: (38 MDD, 9 BD) | rTMS | DMPFC | 20 | HAM‐D‐17, BDI‐II, Beck Anxiety Inventory, 16‐item self‐rated Quick Inventory of Depressive Symptomatology (QIDS), Sheehan Disability Scale, Quality of Life Enjoyment and Satisfaction Questionnaire, Warwick‐Edinburgh Mental Well‐Being Scale | Nonresponders had a higher baseline anhedonia symptomatology. |
| Duprat et al (2016) | CO | 22 HC | iTBS | Left DLPFC | 1‐1 | Probabilistic Learnig Task, TEPS | The more hedonic the subjects (regarding consummatory aspects) is, the more iTBS could modulate reward system (increasing dopamine release). |
| Hurlermann et al (2015) | OL | 41 HC | iTBS | DMPFC DLPFC | 2 | fMRI localizer task, Cognitive emotion judgment task, emotion‐modulated startle paradigm. | NS |
| Ulrich et al (2018) | OL | 20 HC | iTBS + cTBS | Right‐medial VLPFC | 1‐1 | FCQ‐S | Modulation of processes of revaluation of hedonic food stimuli driven by TMS on rVLPFC. “Reshape” of hedonic food regulation. |
| Prikryl et al (2013) | RCT | 45 SZ | rTMS | Left PFC | 15 | SANS, SAPS, MADRS, CDSS | Significant improvement in anhedonia subscore (P < .001). |
| Russo et al (2018) | OL | 11 TRD | TMS | Left DLPFC | 36 | PES, IDS‐SR, PHQ‐9, SHAPS | Not significant improvement in SHAPS score. |
| Pettorruso et al (2018) | OL | 15 CUD | rTMS | Left DLPFC | 10 | TEPS, VAS, CSSA, UDS. | Improvements in anhedonia symptoms, more pronounced in high‐craving CUD subjects. |
Abbreviations: BDI‐II, Beck Depression Inventory; CDSS, Calgary Depression Scale for Schizophrenia; CO, crossover study; CSSA, Cocaine Selective Severity Assessment; cTBS, continuous theta‐burst stimulation; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; FCQ‐S, Food Craving Questionnaire‐Short; fMRI, functional magnetic resonance; HAM‐D‐17, Hamilton Depression Scale 17 items; IDS‐SR, Inventory of Depressive Symptomatology (Self‐Report); iTBS, intermittent theta‐burst stimulation; MADRS, The Montgomery‐Åsberg Depression Rating Scale; OL, open‐label study; PES, Pleasant Event Schedule; PHQ‐9, Patient Health Questionnaire‐9; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; SHAPS, Snaith‐Hamilton Pleasure Scale; TEPS, Temporal Experience of Pleasure Scale; UDS, urine drug screen; VAS, Visual Analogue Scale; VLPFC, ventromedial prefrontal cortex.
3.1. Depression
Downar and colleagues,55 in an open‐label study, recruited 47 patients (20 males and 27 females) with unipolar (n = 38) and bipolar (n = 9) TRD. These patients were treated with an add‐on high‐frequency rTMS over the dorsomedial prefrontal cortex (DMPFC), for a total of 20 daily sessions. Psychometric instruments showed a response rate in almost one‐half of the sample (50% reduction in symptoms' severity). They reported that nonrespondent patients were more likely to be anhedonic at the baseline. Furthermore, nonresponders showed significant lower connectivity through the reward pathway on baseline functional magnetic resonance.
Russo and colleagues14 explored the effects of a standard course of rTMS (5 daily sessions per week, up to 36 sessions) in an open‐design involving 11 female outpatients with a diagnosis of treatment‐resistant depression (TRD). From the second week of treatment, patients also received the behavioral activity therapy before the rTMS session. Six patients (55%) improved after rTMS treatment, along with a nonsignificant reduction in SHAPS scores.
3.2. Schizophrenia
Prikryl and colleagues56 conducted a 3‐week double‐blind RCT involving 45 schizophrenic male patients. The study protocol established five rTMS sessions per week for three weeks, added on the current pharmacological regimen and targeting the left PFC (tangential to the midline). Twenty‐five patients received the active stimulation, 20 the sham. A significant reduction of negative and affective symptoms severity was observed, particularly in the anhedonia subscale of SANS, both in the active and sham groups.
3.3. Substance use disorders
Our research group, in an open‐label pilot study,12 explored the effects of high‐frequency rTMS (10 sessions, two daily for five consecutive days) over the left DLPFC on anhedonic symptoms, in a sample of 15 treatment‐seeking subjects with cocaine use disorder (CUD). After the neuromodulation intervention, significant changes in Temporal Experience of Pleasure Scale (TEPS) scores were observed, indicative of reduction in both anticipatory and consummatory anhedonic symptoms. Furthermore, the results indicate an inverse correlation between craving reduction and improvements in anhedonia, suggesting a potential pathophysiological link between these clinical phenomena.
3.4. Healthy subjects
Duprat and colleagues57 conducted a crossover study by using an intermittent theta‐burst stimulation (iTBS) protocol in 22 male healthy subjects. All subjects received a single active and sham stimulation over the left DLPFC, with one‐week washout period. Active stimulation was able to influence the performance in a reward‐related task, with a faster action of iTBS in subjects with higher hedonic capacity to elicit the development of a response bias. Consistently with these results, the authors argued a more pronounced sensitivity to rewarding stimuli after the neuromodulation intervention, putatively related to increased dopamine release.
In a randomized controlled trial, Hurlermann and colleagues58 applied two sessions (targeting DMPFC and DLPFC areas) of continuous TBS (an inhibitory NIBS protocol) in a sample of 41 male healthy subjects. Both the inhibition of left DLPFC and DMPFC resulted in the attenuation of emotional responses to positive stimuli.
Recently, in an open‐label study,59 20 healthy males were recruited to perform a fMRI food/nonfood discrimination task before and after TBS. In particular, 10 patients started with iTBS followed by continuous TBS (cTBS) and vice‐versa. Both stimulation protocols targeted the right mid‐VLPFC at a 70% of RMT intensity. The two neuromodulation interventions were able to increase fMRI neural responses for low‐calorie food images. In addition, cTBS determined a significant decrease of the ventral tegmental area fMRI activation for high‐calorie foods.
4. DISCUSSION
Our revision of the literature highlighted only seven studies that have analyzed neuromodulation in correlation to the hedonic trait. The number of these studies is very limited, considering the constant spread of the NIBS worldwide and specifically in psychiatry. Moreover, the protocols used in the clinical trials were found to be very heterogeneous in terms of number of applications, targeted area, and study design. Anhedonia, as transnosographic symptom, has a good potential in dissecting the population involved in neuromodulation trials, in particular it could be used as an outcome predictor. As of today, only one study suggests that connectivity patterns in reward circuits and anhedonic symptoms could predict a negative answer to HF rTMS on DMPFC. On the other hand, HF rTMS seems to be efficacious in improving anhedonic function in nondepressed subjects (ie, in healthy and SUDs subjects) when applied on the left DLPFC, along with a modulation of the connected subcortical areas.12, 60, 61
All the included studies targeted a prefrontal area (mainly, the DLPFC and the DMPFC). Recently, different neurobiological correlates have been proposed for anticipatory and consummatory anhedonia.20 While consummatory anhedonia has been suggested as being mainly related to impaired ventral basal ganglia activation, the anticipatory component seems to be underpinned by frontal‐striatal networks involving dorsal anterior cingulate cortex and the middle/medial frontal gyrus. Interestingly, in a 1188 MDD patients study,4 four relevant biotypes based on fMRI dysfunctional connectivity patterns were identified. Two of these were found involving alterations in frontostriatal and orbitofrontal area, with significant correlations with anhedonia and psychomotor retardation. In addition, evidence highlighted an important contribution of the DMPFC to the reward value in the intertemporal choice. Furthermore, the stimulation of DMPFC was demonstrated to modulate striatal dopamine levels,62, 63 also involved in reward processing.64
The vast majority of clinical trials testing the efficacy of high‐frequency rTMS in patients with depressive disorders targeted the left dorsolateral prefrontal cortex, but some preliminary evidence demonstrated the efficacy of targeting different areas (ie, DMPFC).8 Possibly, dissecting endophenotypes based on hedonic functioning may allow the individuation of patients’ profiles differentially responding to diverse interventions. As our review shows, only a little part of clinical studies analyzed the anhedonic dimension. It would be important to better characterize clinical samples in the manner of pharmacological studies.65, 66 In fact, the occurrence of anhedonic symptoms would detect possible response predictors to neuromodulation treatment.51
As discussed, anhedonia has a relevant impact on patients’ quality of life. It worsens the rate of suicidal ideation in different samples67, 68, 69, 70, 71 and determines poor social functioning72 and higher and prolonged hospitalization.73
Lastly, anhedonia could be considered a pivotal psychopathological symptom, also noted in the DSM‐5 as a core symptom of MDD. Since it also correlates to more severe clinical frames and worse outcomes,74 it could be an important target for treatment. Treating anhedonia with specific tools could also improve the clinical course of these patients.
In conclusion, current literature does not support a specific role of neuromodulation interventions for the treatment of anhedonia. Nevertheless, it could be promising to dissect the involved endophenotypes to predict neuromodulation treatment response, but this proposal needs more research and verification in experimental conditions. Further studies are necessary to clarify the role of these approaches to reverse anhedonia and its disabling consequences.
ACKNOWLEDGMENTS
This work was supported by the "Departments of Excellence 2018‐2022" initiative of the Italian Ministry of Education, University and Research for the Department of Neuroscience, Imaging and Clinical Sciences (DNISC) of the University of Chieti‐Pescara. The authors would like to thank Carolina Lombardi for assistance with manuscript preparation and editing.
Spano MC, Lorusso M, Pettorruso M, et al. Anhedonia across borders: Transdiagnostic relevance of reward dysfunction for noninvasive brain stimulation endophenotypes. CNS Neurosci Ther. 2019;25:1229–1236. 10.1111/cns.13230
REFERENCES
- 1. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry Online. 2010;167(7):748‐751. [DOI] [PubMed] [Google Scholar]
- 3. Fusar‐Poli P, Solmi M, Brondino N, et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry. 2019;18(2):192‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drysdale AT, Grosenick L, Downar J, et al. Resting‐state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nusslock R, Alloy LB. Reward processing and mood‐related symptoms: an RDoC and translational neuroscience perspective. J Affect Disord. 2017;216:3‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5). Fifth; 2013. doi:10.1176/appi.books.9780890425596.744053.
- 7. Li S, Zhang Y, Fan J, et al. Patients with obsessive‐compulsive disorder exhibit deficits in consummatory but not anticipatory pleasure. Front Psychol. 2019;10:1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lefaucheur JP, André‐Obadia N, Antal A, et al. Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150‐2206. [DOI] [PubMed] [Google Scholar]
- 9. Valero‐Cabré A, Amengual JL, Stengel C, Pascual‐Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381‐404. [DOI] [PubMed] [Google Scholar]
- 10. Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vallence AM, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter‐ and intra‐subject variability of motor cortex plasticity following continuous theta‐burst stimulation. Neuroscience. 2015;304:266‐278. [DOI] [PubMed] [Google Scholar]
- 12. Pettorruso M, Spagnolo PA, Leggio L, et al. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: a pilot study. Brain Stimul. 2018;11(5):1195‐1197. [DOI] [PubMed] [Google Scholar]
- 13. Prikryl R, Kucerova H. Repetitive transcranial magnetic stimulation as new technique for treatment of negative symptoms of schizophrenia. Acta Psychiatr Scand. 2007;117(1):78‐79. [DOI] [PubMed] [Google Scholar]
- 14. Russo GB, Tirrell E, Busch A, Carpenter LL. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101‐104. [DOI] [PubMed] [Google Scholar]
- 15. Gao K, Sweet J, Su M, Calabrese JR. Depression severity and quality of life of qualified and unqualified patients with a mood disorder for a research study targeting anhedonia in a clinical sample. Asian J Psychiatr. 2017;27:40‐47. [DOI] [PubMed] [Google Scholar]
- 16. Fawcett J. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40(1):79. [DOI] [PubMed] [Google Scholar]
- 17. Buckner JD, Joiner TE, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM’s emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159(1‐2):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Nicola M, De Risio L, Battaglia C, et al. Reduced hedonic capacity in euthymic bipolar subjects: a trait‐like feature? J Affect Disord. 2013;147(1–3):446‐450. [DOI] [PubMed] [Google Scholar]
- 19. Pettorruso M, De Risio L, Di Nicola M, Martinotti G, Conte G, Janiri L. Allostasis as a conceptual framework linking bipolar disorder and addiction. Front Psychiatry. 2014;5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang B, Lin P, Shi H, et al. Mapping anhedonia‐specific dysfunction in a transdiagnostic approach: an ALE meta‐analysis. Brain Imaging Behav. 2016;10(3):920‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barkus E, Badcock JC. A transdiagnostic perspective on social anhedonia. Front Psychiatry. 2019;10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garfield J, Lubman DI, Yücel M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust N Z J Psychiatry. 2014;48(1):36‐51. [DOI] [PubMed] [Google Scholar]
- 23. Hatzigiakoumis DS, Martinotti G, Di GM, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front psychiatry. 2011;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janiri L, Martinotti G, Dario T, et al. Anhedonia and substance‐related symptoms in detoxified substance‐dependent subjects: a correlation study. Neuropsychobiology. 2005;52(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 25. Nawijn L, van Zuiden M, Frijling JL, Koch S, Veltman DJ, Olff M. Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev. 2015;51:189‐204. [DOI] [PubMed] [Google Scholar]
- 26. Martinotti G, Sepede G, Brunetti M, et al. BDNF concentration and impulsiveness level in post‐traumatic stress disorder. Psychiatry Res. 2015;229(3):814‐818. [DOI] [PubMed] [Google Scholar]
- 27. Silverstone PH. Is anhedonia a good measure of depression? Acta Psychiatr Scand. 1991;83(4):249‐250. [DOI] [PubMed] [Google Scholar]
- 28. Loas G, Krystkowiak P, Godefroy O. Anhedonia in parkinson’s disease: an overview. J Neuropsychiatry Clin Neurosci. 2012;24(4):444‐451. [DOI] [PubMed] [Google Scholar]
- 29. Pettorruso M, Martinotti G, Fasano A, et al. Anhedonia in Parkinson’s disease patients with and without pathological gambling: a case‐control study. Psychiatry Res. 2014;215(2):448‐452. [DOI] [PubMed] [Google Scholar]
- 30. Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry. 2002;43(3):189‐194. [DOI] [PubMed] [Google Scholar]
- 31. Franken I, Zijlstra C, Muris P. Are nonpharmacological induced rewards related to anhedonia? a study among skydivers. Prog Neuro‐Psychopharmacology Biol Psychiatry. 2006;30(2):297‐300. [DOI] [PubMed] [Google Scholar]
- 32. Franken I, Rassin E, Muris P. The assessment of anhedonia in clinical and non‐clinical populations: further validation of the Snaith‐Hamilton pleasure scale (SHAPS). J Affect Disord. 2007;99(1‐3):83‐89. [DOI] [PubMed] [Google Scholar]
- 33. Berghorst L, Pizzagalli DA. Defining depression endophenotypes In Beyer C, Stahl S. (eds.), Next Generation Antidepressants: Moving Beyond Monoamines to Discover Novel Treatment Strategies for Mood Disorders. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 34. Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10(1):393‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(6):1751‐1764. [DOI] [PubMed] [Google Scholar]
- 36. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH. Anhedonia in depression and schizophrenia: a transdiagnostic challenge. CNS Neurosci Ther. 2018;24(7):615‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheggi S, De Montis MG, Gambarana C. Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol. 2018;21(11):1049‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cinque S, Zoratto F, Poleggi A, et al. Behavioral phenotyping of dopamine transporter knockout rats: compulsive traits, motor stereotypies, and anhedonia. Front psychiatry. 2018;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. 2017;186:55‐62. [DOI] [PubMed] [Google Scholar]
- 41. Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ. Treatment for anhedonia: a neuroscience driven approach. Depress Anxiety. 2016;33(10):927‐938. [DOI] [PubMed] [Google Scholar]
- 42. Pettorruso M, di Giannantonio M, De Risio L, Martinotti G, Koob GF. A light in the darkness: repetitive transcranial magnetic stimulation (rTMS) to treat the hedonic dysregulation of addiction. J Addict Med. 2019. 10.1097/ADM.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101‐154. [DOI] [PubMed] [Google Scholar]
- 44. Stahl S. Stahl’s Essential Psychopharmacology : Neuroscientific Basis and Practical Application, 4th edn New York, NY: Cambridge University Press; 2013. [Google Scholar]
- 45. Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance‐dependent subjects. Am J Drug Alcohol Abuse. 2008;34(2):177‐183. [DOI] [PubMed] [Google Scholar]
- 46. Martinotti G, Andreoli S, di Nicola M, di Giannantonio M, Sarchiapone M, Janiri L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol. 2008;23(5):417‐424. [DOI] [PubMed] [Google Scholar]
- 47. Janiri L, Martinotti G, Di Nicola M. Aripiprazole for relapse prevention and craving in alcohol‐dependent subjects: Results from a pilot study [5]. J Clin Psychopharmacol. 2007;27(5):519‐520. [DOI] [PubMed] [Google Scholar]
- 48. Orsetti M, Di BF, Rinaldi M, Dallorto D, Ghi P. Some molecular effectors of antidepressant action of quetiapine revealed by DNA microarray in the frontal cortex of anhedonic rats. Pharmacogenet Genomics. 2009;19(8):600‐612. [DOI] [PubMed] [Google Scholar]
- 49. Sepede G, Lorusso M, Spano C, et al. Substance use in schizophrenia: Efficacy of atypical antipsychotics. J Schizophr Res. 2014;1(1):1‐12. [Google Scholar]
- 50. Thome J, Foley P. Agomelatine: an agent against anhedonia and abulia? J Neural Transm. 2015;122(S1):3‐7. [DOI] [PubMed] [Google Scholar]
- 51. Martinotti G, Pettorruso M, De Berardis D, et al. Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int J Neuropsychopharmacol. 2016;19(5):pyw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scheggi S, Pelliccia T, Ferrari A, De Montis MG, Gambarana C. Impramine, fluoxetine and clozapine differently affected reactivity to positive and negative stimuli in a model of motivational anhedonia in rats. Neuroscience. 2015;291:189‐202. [DOI] [PubMed] [Google Scholar]
- 53. Cao B, Zhu J, Zuckerman H, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109‐117. [DOI] [PubMed] [Google Scholar]
- 54. Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735‐738. [DOI] [PubMed] [Google Scholar]
- 55. Downar J, Geraci J, Salomons TV, et al. Anhedonia and reward‐circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76(3):176‐185. [DOI] [PubMed] [Google Scholar]
- 56. Prikryl R, Ustohal L, Prikrylova Kucerova H, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double‐blind trial. Schizophr Res. 2013;149(1‐3):167‐173. [DOI] [PubMed] [Google Scholar]
- 57. Duprat R, De Raedt R, Wu G‐R, Baeken C. Intermittent theta burst stimulation increases reward responsiveness in individuals with higher hedonic capacity. Front Hum Neurosci. 2016;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hurlemann R, Arndt S, Schlaepfer TE, Reul J, Maier W, Scheele D. Diminished appetitive startle modulation following targeted inhibition of prefrontal cortex. Sci Rep. 2015;5(1):8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ulrich M, Lorenz S, Spitzer MW, Steigleder L, Kammer T, Grön G. Theta‐burst modulation of mid‐ventrolateral prefrontal cortex affects salience coding in the human ventral tegmental area. Appetite. 2018;123:91‐100. [DOI] [PubMed] [Google Scholar]
- 60. Addolorato G, Vassallo GA, Antonelli G, et al. Author Correction: Binge Drinking among adolescents is related to the development of alcohol use disorders: results from a Cross‐Sectional Study. Sci Rep. 2018;8(1):15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pettorruso M, Di Giuda D, Martinotti G, et al. Dopaminergic and clinical correlates of high‐frequency repetitive transcranial magnetic stimulation in gambling addiction: a SPECT case study. Addict Behav. 2019;93:246‐249. [DOI] [PubMed] [Google Scholar]
- 62. Cho SS, Koshimori Y, Aminian K, et al. Investing in the future: Stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40(3):546‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4(8):e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lu H, Zou Q, Chefer S, et al. Abstinence from cocaine and sucrose self‐administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect. 2014;4(7):499‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao P, Xing J, Cao Y, et al. Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention‐deficit hyperactivity disorder. Neuropsychiatr Dis Treat. 2018;14:3231‐3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Auerbach RP, Millner AJ, Stewart JG, Esposito EC. Identifying differences between depressed adolescent suicide ideators and attempters. J Affect Disord. 2015;186:127‐133 031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ballard ED, Vande Voort JL, Luckenbaugh DA, Machado‐Vieira R, Tohen M, Zarate CA. Acute risk factors for suicide attempts and death: prospective findings from the STEP‐BD study. Bipolar Disord. 2016;18(4):363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ducasse D, Loas G, Dassa D, et al. Anhedonia is associated with suicidal ideation independently of depression: a meta‐analysis. Depress Anxiety. 2018;35(5):382‐392. [DOI] [PubMed] [Google Scholar]
- 70. Fawcett J, Scheftner WA, Fogg L, et al. Time‐related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189‐1194. [DOI] [PubMed] [Google Scholar]
- 71. Loas G, Lefebvre G, Rotsaert M, Englert Y. Relationships between anhedonia, suicidal ideation and suicide attempts in a large sample of physicians. PLoS ONE. 2018;13(3):e0193619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Llerena K, Park SG, Couture SM, Blanchard JJ. Social anhedonia and affiliation: examining behavior and subjective reactions within a social interaction. Psychiatry Res. 2012;200(2‐3):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patel R, Jayatilleke N, Broadbent M, et al. Negative symptoms in schizophrenia: a study in a large clinical sample of patients using a novel automated method. BMJ Open. 2015;5(9):e007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry 2017;44:1‐8. [DOI] [PubMed] [Google Scholar]


