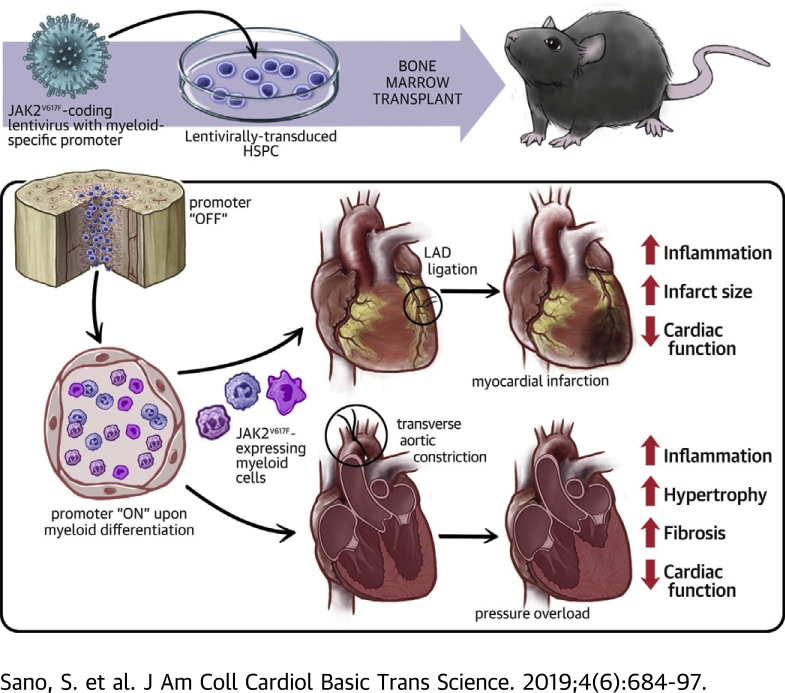
Visual Abstract
Key Words: clonal hematopoiesis, left ventricular hypertrophy, myocardial infarction
Abbreviations and Acronyms: AIM2, absence in melanoma 2; ANOVA, analysis of variance; ARCH, age-related clonal hematopoiesis; BMT, bone marrow transplant; CCL2, C-C motif chemokine ligand 2; CHIP, clonal hematopoiesis of indeterminate potential; GFP, green fluorescent protein; HSC, hematopoietic stem cell; HSPC, hematopoietic stem and progenitor cell; IFNGR1, interferon gamma receptor 1; IL, interleukin; JAK2, Janus kinase 2; JAK2V617F, mutant Janus kinase 2 (valine to phenylalanine at residue 617); JAK2WT, wild-type Janus kinase 2; LPS, lipopolysaccharide; LT-HSC, long-term hematopoietic stem cell; MI, myocardial infarction; MPN, myeloproliferative neoplasm; NET, neutrophil extracellular traps; STAT, signal transducer and activator of transcription; TAC, transverse aortic constriction surgery
Highlights
-
•
Clonal hematopoiesis can develop from JAK2V617F mutant cells, but mouse models harboring this mutation are confounded by myeloproliferative disease phenotypes.
-
•
To establish a model of JAK2V617F clonal hematopoiesis, a lentivirus vector was used to transduce hematopoietic stem and progenitor cells with a construct that expresses this mutation from a myeloid-specific promoter.
-
•
When transduced hematopoietic stem and progenitor cells were implanted into mice, JAK2V617F chimerism was achieved in monocytes and neutrophils in the absence of changes in blood cell counts, and these mice exhibited greater myocardial inflammation and accelerated heart failure when subjected to models of cardiac injury.
-
•
These data suggest that clonal hematopoiesis can arise from the acquisition of JAK2V617F mutations in a progenitor cell subpopulation that gives rise to circulating myeloid cells, and that this condition can promote cardiovascular disease through proinflammatory mechanisms.
Summary
Janus kinase 2 (valine to phenylalanine at residue 617) (JAK2V617F) mutations lead to myeloproliferative neoplasms associated with elevated myeloid, erythroid, and megakaryocytic cells. Alternatively these same mutations can lead to the condition of clonal hematopoiesis with no impact on blood cell counts. Here, a model of myeloid-restricted JAK2V617F expression from lineage-negative bone marrow cells was developed and evaluated. This model displayed greater cardiac inflammation and dysfunction following permanent left anterior descending artery ligation and transverse aortic constriction. These data suggest that JAK2V617Fmutations arising in myeloid progenitor cells may contribute to cardiovascular disease by promoting the proinflammatory properties of circulating myeloid cells.
Clonal hematopoiesis of indeterminate potential (CHIP) or age-related clonal hematopoiesis (ARCH) is a prevalent condition in elderly individuals in which a substantial portion of mature blood cells are derived from a single dominant hematopoietic stem cell (HSC) clone 1, 2, 3. In a portion of individuals, this clonal hematopoiesis event can be attributed to mutations in “driver” genes that are recurrently mutated in hematologic malignancies. These mutated genes include DNMT3A, TET2, ASXL1, and others. These mutations are thought to provide the HSC with a competitive advantage such that it undergoes clonal amplification and gives rise to differentiated blood cell progeny that also harbor pre-leukemic mutations. Notably, the mutations that give rise to clonal hematopoiesis do not overtly alter blood cell counts or give rise to other features of hematologic malignancy. The existence of clonal hematopoiesis has been known for decades 4, 5, but it had generally been viewed as a benign condition and that it might provide a counterbalance to HSC exhaustion that occurs in elderly individuals (6). However, recent epidemiological studies have shown that clonal hematopoiesis is associated with an appreciable increase in mortality in the general population as well as in patient cohorts 7, 8, 9, 10, 11, 12. In some instances, clonal hematopoiesis has been associated with an increased risk of cardiovascular disease, including coronary artery disease, ischemic stroke, and early onset myocardial infarction 10, 13. Studies in experimental models have provided evidence that inactivating mutations in TET2 can causally contribute to atherosclerosis and heart failure through an interleukin (IL)-1 beta–dependent mechanism 14, 15. Similarly, experimental studies have shown that mutations in DNMT3A can contribute to myocardial inflammation and heart failure (16). Recently, hematopoietic mutations in TET2 and DNMT3A have been associated with the progression and poor prognosis in patients with chronic ischemic heart failure (17).
Janus kinase 2 (JAK2) is a nonreceptor tyrosine kinase that transmits intracellular signals downstream of various cytokine receptors. While JAK2 is broadly expressed, the activating mutation JAK2 G1849T (V617F) (JAK2V617F) in hematopoietic cells is commonly associated with rare myeloproliferative neoplasms (MPNs) including polycythemia vera, essential thrombocytopenia, and myelofibrosis that are generally associated with the aberrant production of red blood cells, platelets, and leukocytes 18, 19. These diseases frequently lead to increased incidences of myocardial infarction, stroke, and deep vein thrombosis due to increased blood viscosity, clotting, and leukocytosis.
It is increasingly appreciated that there are many individuals who harbor the JAK2V617F allele in leukocytes yet do not exhibit overt changes in levels of erythrocytes, platelets or leukocytes. A number of studies have detected the presence of the JAK2V617F mutation in the leukocytes of individuals with no diagnosis of MPNs at frequencies ranging from 0.1% to 9.6% of the population depending on the method of detection and the cohort analyzed 20, 21, 22, 23. More recently, the JAK2V617F mutation in leukocytes has been appreciated to be associated with the condition of CHIP or ARCH (i.e., the detectable clonal amplification of the mutation with no associated changes in blood cell counts) 8, 10, 12, 13, 24, 25, 26. This JAK2V617F -mediated clonal hematopoiesis has been associated with an increased incidence of cardiovascular disease (13). In light of these considerations, experimental studies are warranted to elucidate whether the JAK2V617F mutation in the myeloid lineage can contribute to cardiovascular disease independently of high blood cell counts and the prothrombotic complications associated with MPNs. However, these experiments are confounded by the neoplasm phenotypes that are exhibited by murine models that harbor Jak2V617F mutations 27, 28, 29, 30, 31.
In this study, we document that mice expressing JAK2V617F display a strong bias toward amplification into the myeloid lineage in competitive bone marrow transplantation (BMT) experiments. Thus, a myeloid-specific lentivirus and BMT strategy was employed to specifically express JAK2V617F exclusively in monocytes and neutrophils in blood following the transduction of lineage-negative bone marrow cells. These mice displayed normal levels of leukocytes, erythrocytes, and platelets. However, when challenged in 2 models of cardiac injury the Jak2V617F mice displayed greater myocardial inflammation and pathological remodeling. These results raise the possibility that the acquisition and expansion of mutations within hypothetical monocyte or neutrophil-restricted progenitor cells could account for JAK2V617F-mediated clonal hematopoiesis and subsequent cardiovascular disease.
Methods
Mice
Jak2V617F transgenic mice were provided by Zhizhuang Joe Zhao at the University of Oklahoma (31). Briefly, the human JAK2V617F transgene is driven under the control of the vav1 promoter that drives expression in hematopoietic and vascular endothelial cells (32). The JAK2V617F line was backcrossed with control C57BL6/J mice for several generations, and brought to homozygosity. All reported results were performed in animals homozygotes for the transgene. Genotyping was performed using quantitative reverse transcription polymerase chain reaction of the human JAK2 gene (TaqMan primers from Applied Biosystems, Waltham, Massachusetts). Littermate wild-type mice were used as control animals. In lentivirus-mediated lineage-negative cell transfer experiments, wild-type C57BL/6J mice for both donor and recipient were purchased from The Jackson Laboratory (Stock# 000664) (Bar Harbor, Maine). Male mice were used for all the experiments. Mice were maintained on a 12-h light-dark schedule in a specific pathogen-free animal facility and given food and water ad libitum. The number of mice included in each study is indicated in the figures or the associated legends.
Plasmids and lentivirus production
Myeloid-specific SP146-gp91 promoter-enhancer sequence was synthesized as described previously with some modifications (33). Full sequences are provided in Supplemental Figure 1. psPAX2 and pMD2.G were a gift from Didier Trono (Addgene, Watertown, Massachusetts, plasmids 12260 and 12259). Lentivirus particles were generated as described previously (34). Briefly, the plasmids (pLenti-SP146-gp91-JAK2, psPAX2, pMD2.G) were co-transfected to HEK293T cells with polyethylenimine (Cat# 24765-1, Polysciences, Warrington, Pennsylvania) and the supernatant was collected at 48 h after transfection. After filtration (40 μm), virus particles were concentrated by ultracentrifugation at a speed of 20,000 rpm for 3 h. The virus pellet was suspended with StemSpan medium (Cat# 09600, Stemcell Technologies, Cambridge, Massachusetts) without aeration and kept at –80°C. Lentiviral particle titer was determined using a Lenti-X qRT-PCR Titration Kit (Cat# 631235, Clontech, Mountain View, California).
Isolation of lineage-negative cells and lentivirus transduction
Lineage-negative cells were isolated from the bone marrow of C57BL/6J wild-type mice using a Lineage Cell Depletion Kit (Cat #130-090-858, Miltenyi Biotec, Somerville, Massachusetts) according to manufacturer’s instructions. Cells were pre-incubated with StemSpan medium for 1.5 h at 37°C. Lentivirus transduction was performed in the presence of 20 ng/ml of thrombopoietin, 50 ng/ml of stem cell factor 1, 4 μg/ml of polybrene, and 5 μg/ml of rapamycin for 16 to 20 h (35). Cells were washed and resuspended with RPMI medium before transplantation via the retro-orbital vein.
Statistics
Data are expressed as mean ± SEM, except for the boxplots which show minimum, 25th percentile, median, 75th percentile, and maximum. Shapiro-Wilk normality test was used to evaluate data distribution, and F test was used to evaluate homogeneity of variance. For normally distributed data with 1 experimental variable, unpaired (2-tailed) Student’s t-test was used for comparing the difference between wild-type JAK2 (JAK2WT) and JAK2V617F of transgenic mice strain in: CD41 expression of long-term hematopoietic stem cells (LT-HSCs), absolute numbers of white blood cells at 16 weeks after BMT, cardiac function parameters (posterior wall thickness at diastole, fractional shortening) at 2 months post-BMT; and also used for comparing the difference between JAK2WT and JAK2V617F of myeloid JAK2V617F mice strain in absolute numbers of white blood cells, hemoglobin, and platelets at 8 weeks after BMT; cardiac fibrosis at 14 days post-myocardial infarction (MI); absolute numbers of neutrophils and macrophages of enzymatically digested infarct area at 4 days post-MI; cardiac myocyte hypertrophy and cardiac fibrosis at 8 weeks post-transverse aortic constriction surgery (TAC); and transcript expression of Col3a1 of heart tissue at 8 weeks post-TAC; and unequal variance t test was used for comparing the difference between JAK2WT and JAK2V617F of transgenic mice strain in absolute numbers of Hb at 16 weeks after BMT; and used for comparing the difference between JAK2WT and JAK2V617F of myeloid JAK2V617F mice strain in heart mass and lung weight at 8 weeks post-TAC, transcript expression of IL-6 and Col1a1 of heart tissue at 8 weeks post-TAC; and used for comparing the difference of JAK2 transgene expression between CD11b+ cells and CD31+ cells from hearts 7 days after MI; and 1-way analysis of variance (ANOVA) with post hoc Tukey’s multiple comparison test was used for comparing the differences among green fluorescent protein (GFP), JAK2WT and JAK2V617F of THP-1 cells in gene expression (Isg15, Mx1, Cxcl10) at baseline. For non-normally distributed data with 1 experimental variable, Kruskal-Wallis test was used for comparing the difference between JAK2WT and JAK2V617F of transgenic mice strain in absolute numbers of platelets at 16 weeks after BMT; the difference between JAK2WT and JAK2V617F of myeloid JAK2V617F mice strain in absolute numbers of Ly6Chi monocytes of enzymatically digested infarct area at 4 days post-MI; macrophage accumulation in myocardium at 8 weeks post-TAC; transcript expression of Anp, Bnp, and β/αMhc of heart tissue at 8 weeks post-TAC; and the difference of JAK2 transgene expression between CD11b+ cells and CD31+ cells from hearts 7 days after TAC. Kruskal-Wallis test with post hoc Dunn’s multiple comparison test was used for comparing the differences among GFP, Jak2WT, and Jak2V617F of THP-1 cells in gene expression (Oas1, Oas2) at baseline. For data with more than 1 experimental variable, 2-way ANOVA with post hoc Tukey’s multiple comparison test was used for comparing the difference among GFP, JAK2WT, and JAK2V617F of THP-1 cells in gene expression (IL-6, IL-1Β, tumor necrosis factor alpha, C-C motif chemokine ligand 2[CCL2], absence in melanoma 2 [AIM2]) after lipopolysaccharide (LPS) stimulation; between JAK2WT and JAK2V617F of myeloid JAK2V617F mice strain in cytokine gene expression of heart tissue at both sham state and 7 days post-MI; and among GFP, JAK2WT and JAK2V617F of THP-1 cells in the gene expression (Isg15, Mx1, Oas1, Oas2) with or without treatment of ruxolitinib. Two-way repeated measures ANOVA with Sidak’s multiple comparison test was selected as post hoc comparison for analysis between 2 groups at each time point. It was used for sequentially comparing the difference between JAK2WT and JAK2V617F of transgenic mice strain in the blood chimerism after BMT; and for sequentially comparing the difference between JAK2WT and JAK2V617F of myeloid JAK2V617F mice strain in cardiac function parameters (left ventricular end-systolic volume, left ventricular end-diastolic volume, ejection fraction) pre- and post-MI, and cardiac function parameters (posterior wall thickness at diastole, fractional shortening) pre- and post-TAC. Two-way repeated measures ANOVA with post hoc Tukey’s multiple comparison test was selected as post hoc comparison for analysis among 3 groups at each time point. It was used for sequentially comparing the difference of blood chimerism among JAK2WT-sham, JAK2V617F-sham, and JAK2V617F-MI mice pre- and post-surgery within each time point. All results were considered statistically significant at 0.05. All the statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software, San Diego, California).
Study approval
Study protocols were approved by the Institutional Animal Care and Use Committees at Boston University and the University of Virginia.
Additional materials and methods are described in the Supplemental Appendix.
Results
Hematopoietic stem and progenitor cells harboring JAK2V617F preferentially expand into myeloid cell populations
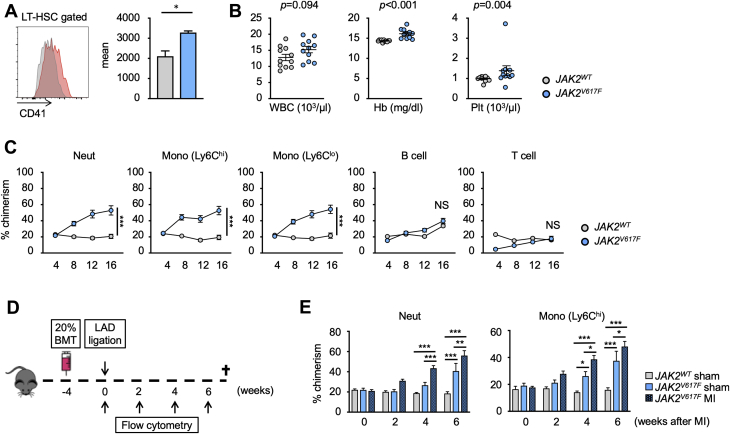
LT-HSCs, defined as CD48– CD150+ LSK cells, that harbor the JAK2V617F mutation, have been reported to display increased expression of CD41, a marker of a myeloid-biased HSC population, in the experimental setting of BMT (36). Using a transgenic mouse strain that expresses human JAK2V617F from the vav1 promoter (31), we find that the LT-HSC population also expresses an increased level of CD41 (p = 0.0125) (Figure 1A). To evaluate the functional characteristics of these LT-HSCs, we performed a competitive BMT assay in which lethally irradiated mice were transplanted with bone marrow cells containing 20% of test cells (vav1-JAK2V617F or nonmutant cells expressing the CD45.2 variant) and 80% of wild-type competitor cells that expressed the CD45.1 variant. As shown in Figure 1B, analysis of the peripheral blood at 16 weeks after transplantation revealed a significant increase in hemoglobin levels (p < 0.001) and platelet counts (p = 0.004) in mice that were transplanted with bone marrow cells from vav1-Jak2V617F mice, consistent with MPN-like phenotypes. CD45.2 cell chimerism was also examined to evaluate the competitive fitness of the vav1-Jak2V617F cells. The vav1-Jak2V617F cells displayed a distinct bias to expand into neutrophils (p < 0.001) and monocytes (p < 0.001), and little or no evidence of expansion into lymphoid populations could be detected (Figure 1C). Mice that underwent competitive BMT with bone marrow from the vav1-JAK2V617F mouse also displayed cardiac hypertrophy in the absence of surgical cardiac injury (posterior wall thickness at diastole, p = 0.003) (Supplemental Figure 2), consistent with a report showing that Jak2V617F transgenic mice develop cardiac hypertrophy in the absence of experimental cardiac injury (37).
Figure 1.
HSPCs Expressing JAK2V617F Display a Competitive Advantage in a Competitive BMT Assay That Is Highly Restricted to the Myeloid Lineage
(A) Representative flow cytometry data to show that mutant Janus kinase 2 (valine to phenylalanine at residue 617) (JAK2V617F)–harboring long-term hematopoietic stem cell (LT-HSCs) display higher expression of CD41 protein compared with wild-type cells. LT-HSC population was defined as lineage–, c-kit+, Sca-1+, CD48–, and CD150+ cells. n = 3 in each group. Data are presented as mean fluorescence intensity. Statistical analysis was performed using 2-tailed unpaired Student’s t-test. (B) Absolute numbers of white blood cells, hemoglobin, and platelets of mice that underwent competitive transplantation with 20% JAK2V617F bone marrow or 20% wild-type bone marrow at 16 weeks after bone marrow transplantation (BMT). Data are shown as mean ± SEM. Statistical analysis was performed using 2-tailed unpaired Student’s t test (white blood cells [WBCs]), unequal variance t test (hemoglobin [Hb]), and Kruskal-Wallis test (platelets [Plt]). n = 11 in each group. (C) Flow cytometry analysis of peripheral blood showing that JAK2V617F cells displayed a competitive advantage over wild-type Janus kinase 2 (Jak2WT) competitor cells in a myeloid-biased manner. Peripheral blood was obtained at 4, 8, 12, and 16 weeks after BMT. n = 6 in Jak2WT groups and n = 16 in JAK2V617F groups. Statistical analysis was performed using 2-way repeated measures analysis of variance with Sidak’s multiple comparison tests. Significance stars are from Sidak’s tests. (D) Schematic that describes the experimental protocol. Left anterior descending artery (LAD) ligation surgery was performed 4 weeks after 20% competitive BMT. The chimerism of test cells in peripheral blood was evaluated by sequential flow cytometry analysis. (E) Flow cytometry analysis showing that experimental myocardial infarction (MI) induced by LAD ligation accelerates the expansion of JAK2V617F myeloid cells in peripheral blood. n = 6 to 8 in each group. Statistical analysis was performed using 2-way repeated measures analysis of variance with Tukey’s multiple comparison tests. Significance stars are from Tukey’s tests. *p < 0.05, **p < 0.01, ***p < 0.001. NS = not significant.
Because it has been reported that inflammation favors the expansion of JAK2V617F cells relative to wild-type cells 38, 39, we tested whether the systemic sterile inflammation caused by LAD ligation, a model of myocardial infarction, could accelerate the expansion of vav1-Jak2V617F donor bone marrow–derived cells into myeloid cell populations (Figure 1D). LAD ligation or sham surgery was performed 1 month after competitive BMT with 20% vav1-Jak2V617F or 20% wild-type bone marrow cells. LAD ligation was found to accelerate the expansion of vav1-Jak2V617F cells into the myeloid lineage, suggesting that myocardial infarction confers an additional competitive advantage to the expansion of Jak2 mutant cells (neutrophil: p = 0.001 at 4 weeks and p = 0.003 at 6 weeks post-MI; Ly6Chi Monocyte: p = 0.014 at 4 weeks and p = 0.043 at 6 weeks post-MI) (Figure 1E). No differences were observed in the lymphoid populations (data not shown), and LAD ligation does not affect the frequencies of CD45.2-positive, wild-type cells in the different leukocyte populations (14).
Myeloid cells harboring the JAK2V617F mutation display enhanced inflammatory properties
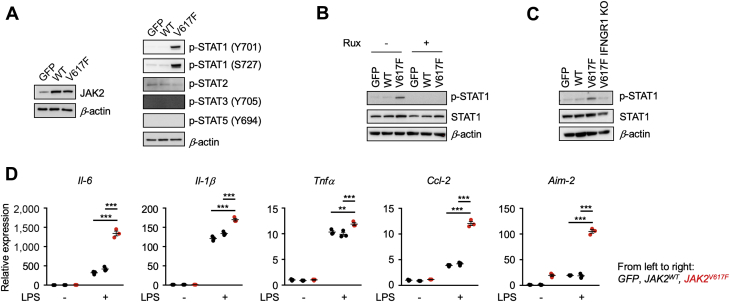
To address the effect of JAK2V617F mutation in myeloid populations, THP-1 cells were transduced with lentivirus expressing GFP, JAK2WT, or JAK2V617F from the SP146-gp47 myeloid-specific promoter/enhancer (Supplemental Figure 1A). Overexpression of exogenous wild-type JAK2 protein did not affect the activation status of signal transducer and activator of transcription (STAT) proteins by phosphorylation, but cells expressing JAK2V617F displayed activation of STAT1 signaling that was indicated by robust phosphorylation of STAT1 at the Y701 and S727 residues (Figure 2A). The activation of STAT1 under these conditions was dependent on JAK2V617F enzymatic activity, as it could be blocked by the JAK1/2 inhibitor ruxolitinib (Figure 2B). In the unstimulated state, THP-1 cells transduced with the mutated Jak2V617F allele displayed upregulation of several interferon-responsive genes, Isg15 (p < 0.001), Mx1 (p < 0.001), Oas1 (p < 0.05), Oas2 (p < 0.05), and Cxcl10 (p < 0.001) (Supplemental Figure 3), which is consistent with constitutive STAT1 activation. Ruxolitinib blocked the upregulation of these genes (p < 0.001) (Supplemental Figure 4).
Figure 2.
Myeloid Cells Transduced With the JAK2V617F Allele Exhibit Enhanced Proinflammatory Properties
(A) Immunoblot analysis reveals modest overexpression of exogenous human JAK2WT and JAK2V617F in THP-1 cells using the lentivirus system (left). Green fluorescent protein (GFP) was expressed in control cells. Signal transducer and activator of transcription (STAT) activities in each experimental group of cells were evaluated by immunoblot with antibodies that detect the level of activating phosphorylation. (B) THP-1 cells were treated with 1 μM of ruxolitinib or vehicle. STAT1 phosphorylation was evaluated by immunoblot analysis. (C) THP-1 cells harboring JAK2V617F were transduced with a lentivirus encoding clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, red fluorescent protein, and single guide RNA targeting human interferon gamma receptor 1. STAT1 phosphorylation was evaluated by immunoblot analysis that detected decreased levels of phosphorylation in IFNGR1 knockout Jak2V617F THP-1 cells. (D) Gene expression analysis of THP-1 cells transduced with lentivirus encoding GFP, JAK2WT, or JAK2V617F at 8 h after stimulation with 10 ng/ml lipopolysaccharide (LPS). n = 3 in each group. Data are shown as mean ± SEM. Statistical analysis was performed using 2-way analysis of variance with Tukey’s multiple comparison tests. Significance stars are from Tukey’s tests. *p < 0.05, **p < 0.01, ***p < 0.001. AIM2 = AIM2, interferon-inducible protein A2; CCL2 = C-C motif chemokine ligand 2; IL = interleukin; p-STAT = phosphorylated; signal transducer and activator of transcription; Rux = ruxolitinib; TNF = tumor necrosis factor; other abbreviations as in Figure 1.
Jak2V617F requires interactions with homodimer type 1 cytokine receptors for growth factor-independent activation of JAK-STAT signaling (40). Thus, to identify the receptor in the monocytic cell line that fulfills this role, THP-1 cells were transduced with lentivirus encoding Cas9 clustered regularly interspaced short palindromic repeat-associated 9 (CRISPR), red fluorescent protein and a guide RNA targeting human interferon gamma receptor 1 (IFNGR1). Gene editing was confirmed by sequencing of the IFNGR1 locus (Supplemental Figure 5). This manipulation led to reductions in STAT1 phosphorylation in the Jak2V617F-expressing cells (Figure 2C). These results indicate that Jak2V617F requires IFNGR1 for downstream signal transduction in THP-1 cells. In contrast, similar manipulations targeting other cytokine receptors, including the interferon lambda receptor, the erythropoietin receptor, or the granulocyte colony-stimulating factor receptor, did not affect JAK2V617F-STAT1 signaling (data not shown).
Upon stimulation with LPS, cells transduced with Jak2V617F displayed significant upregulation of transcripts of various cytokines and chemokines, including IL-6 (p < 0.001), IL-1β (p < 0.001), tumor necrosis factor alpha (p = 0.0001) and CCL2 (p < 0.001), in addition to upregulation of the AIM2 inflammasome component (p < 0.001) (Figure 2D). In contrast, THP-1 cells expressing Jak2WT did not exhibit enhanced inflammatory responses.
Myeloid JAK2V617F Expression accelerates heart failure in response to experimental MI
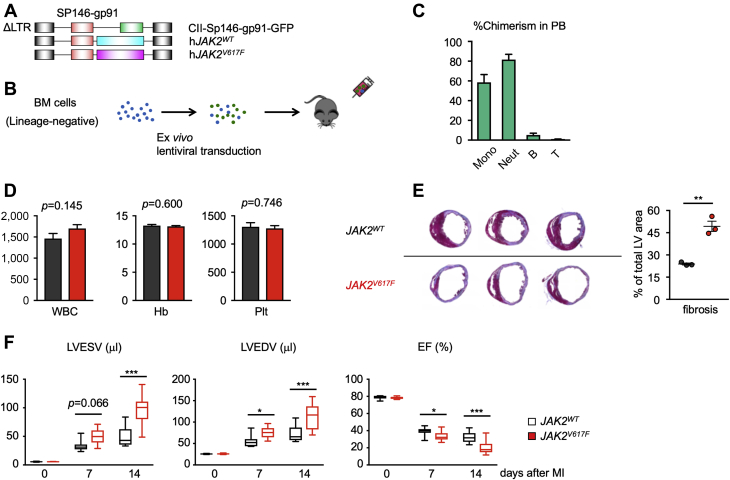
To address whether Jak2V617F-mediated clonal expansion of myeloid cells contributes to cardiac dysfunction, we developed a strategy in which the expression of the Jak2V617F mutation is restricted to myeloid populations. The goal was to avoid the expression of Jak2V617F in vascular endothelial cells and in the erythroid and megakaryocyte populations that would lead to changes in erythrocyte and platelet numbers and confound the analysis of Jak2V617F-mediated clonal hematopoiesis in the cardiovascular system. In this regard, the conditional Cre-mediated expression system that employs the Lyz2 promoter to drive Jak2V617F expression in myeloid cells will also give rise to confounding MPN-like phenotypes due to a low level of Cre protein expression in hematopoietic stem and progenitor cell (HSPC) populations (30). Thus, we generated a lentivirus vector in which exogenous Jak2 expression is under the control of the myeloid-specific SP146/gp91 promoter/enhancer, in which a minimal promoter sequence of human gp91phox gene is fused to the synthetic SP146 element 33, 41 (Figure 3A, Supplemental Figure 1B). To evaluate the fidelity of this system, we transduced lineage-negative bone marrow cells from wild-type mice with a lentivirus encoding GFP from the SP146/gp91 promoter/enhancer and transplanted these cells into lethally irradiated wild-type mice (Figure 3B). Flow cytometry analysis of peripheral blood at 8 weeks after transplantation revealed that GFP signal was predominantly observed in monocyte and neutrophil cell populations, with negligible GFP-positivity in lymphoid cells (Figure 3C). We also found little or no expression of exogenous JAK2 gene in endothelial cells after cardiac injury models, further highlighting the specificity of our myeloid-specific promoter (Supplemental Figure 6). We also analyzed immune cell populations isolated from hearts at 4 days after LAD ligation and found that the lentivirus vector expressed the GFP transgene in cardiac neutrophils, monocytes, and macrophages (Supplemental Figure 7). Encouraged by these data, we then transduced lineage-negative cells from wild-type mice with a lentivirus encoding JAK2WT or JAK2V617F under the control of the myeloid-specific promoter and enhancer and transplanted these cells into lethally irradiated wild-type mice. Notably, these mice did not display MPN-like phenotypes and exhibited normal levels of hemoglobin and platelet counts at 8 weeks after transplantation (Figure 3D).
Figure 3.
Mice With Myeloid-Specific JAK2V617F Mutation Have Inferior Outcome After MI
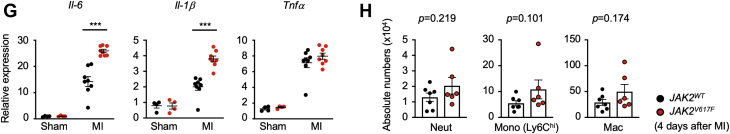
(A) Construction of the lentivirus vectors used in this study. SP146-gp91 is a myeloid-specific enhancer and promoter, respectively. (B) Schematic of the study. Bone marrow–derived lineage-negative cells were transduced with lentivirus ex vivo and transplanted to lethally irradiated mice. Mice were subjected to LAD ligation surgery 8 weeks after BMT and were observed for an additional 8 weeks. (C) The proportion of GFP-positive cells in the peripheral blood from mice reconstituted with lineage-negative cells transduced with GFP-encoding lentivirus vector. Blood cell populations were analyzed by flow cytometry. n = 3 in each group. Data are shown as mean ± SEM. (D) Analysis of peripheral blood parameters of mice from each group (JAK2WT in black and JAK2V617F in red, n = 6). Data are shown as mean ± SEM. Statistical analysis was performed using 2-tailed unpaired Student’s t tests. (E) Representative cross-sectional images and analysis of Masson’s trichrome staining for cardiac fibrosis from each group of mice (n = 3) after LAD ligation surgery at the end of the study. Data are shown as mean ± SEM. Statistical analysis was performed using 2-tailed unpaired Student’s t-tests. (F) Sequential echocardiographic analysis of mice from each group (n = 11) before and after LAD ligation surgery at the indicated time points. Data are shown as minimum to maximum. Statistical analysis was performed using 2-way repeated measures analysis of variance with Sidak’s multiple comparison tests. Significance stars are from Sidak’s tests. (G) Analysis of transcript expression of proinflammatory cytokines in the infarct area were obtained from each group of mice 4 days after LAD ligation surgery. 36b4 was used as a reference for normalization. n = 8 in surgical groups and n = 4 in sham groups. Data are shown as mean ± SEM. Statistical analysis was performed by 2-way analysis of variance with Tukey’s multiple comparison tests. Significance stars are from Tukey’s tests. (H) Flow cytometry analysis of myeloid cell populations in the infarct area obtained from each group of mice 4 days after LAD ligation surgery. Cell numbers were normalized per 100-mg tissue weight. n = 6–7 in each group. Data are shown as mean ± SEM. Statistical analysis was performed using 2-tailed unpaired Student’s t test (neutrophil [Neut], macrophage) or Kruskal-Wallis test (Ly6ChiMono). *p < 0.05, **p < 0.01, ***p < 0.001. B = B cell; EF = ejection fraction; LTR = long-terminal repeat; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; Mono = monocyte; PB = peripheral blood; T = T cell; other abbreviations as in Figures 1 and 2.
LAD ligation was then performed to establish a model of myocardial infarction, and the cardiac phenotypes of mice transduced with the myeloid-specific vectors expressing JAK2V617F or JAK2WT as control animals. At the 14 day termination of the experiment, mice transplanted with bone marrow lineage-negative cells transduced with the myeloid-specific lentiviral vector expressing JAK2V617F displayed enlarged infarct areas in histological analysis and an increase in fibrosis (p = 0.002) (Figure 3E). Before sacrifice, serial echocardiographic analysis revealed progressive dilatation of cardiac chamber size and deterioration of cardiac function in the JAK2V617F group (p < 0.001) (Figure 3F). To evaluate the inflammatory status of the heart, quantitative polymerase chain reaction analysis was performed on tissues from the infarct areas at 7 days after LAD ligation in a separate group of mice. Consistent with our observations in the transduced THP-1 cells (Figure 2), the infarcted myocardium of mice from the JAK2V617F expression group displayed significantly increased expression of IL-6 (p < 0.001) and IL-1β (p < 0.001) transcript compared with mice from the Jak2WT group (Figure 3G), indicating an enhanced inflammatory response in the infarct zone. Flow cytometry analysis of enzymatically digested infarct area 4 days after myocardial infarction showed trends toward increases in Ly6Chi monocytes (p = 0.101), neutrophils (p = 0.219), and macrophages (p = 0.174) in the JAK2V617F group (Figure 3H).
Myeloid JAK2V617F expression accelerates nonischemic cardiac remodeling
To corroborate and extend these findings in another model of heart failure, experiments were conducted in a model of pressure overload cardiac hypertrophy because there is a growing awareness that myeloid cell–mediated inflammatory responses contribute to pathological cardiac remodeling under these conditions 42, 43, 44, 45. Thus, experimental groups of mice were transplanted with bone marrow lineage-negative cells transduced with the myeloid-specific lentiviral vector expressing the JAK2WT and JAK2V617F from the SP146/gp91 promoter/enhancer before TAC to promote cardiac hypertrophy (Figure 4A). Notably, these mice did not display cardiac hypertrophy in the absence of surgical cardiac injury (Figure 4C). This finding is in contrast to competitive BMT experiments employing bone marrow from mice that express Jak2V617F under the vav1 promoter (Supplemental Figure 2), suggesting that cardiac hypertrophy in the absence of surgical cardiac injury is secondary to conditions associated with MPN phenotype and not a feature of myeloid restricted Jak2V617F expression.
Figure 4.
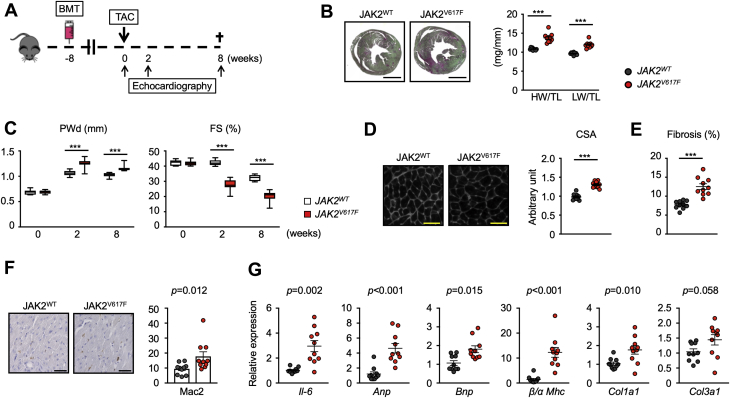
Mice With Myeloid-Specific JAK2V617F Mutation Display Greater Dysfunction in a Model of Pressure-Overload Hypertrophy
(A) Schematic of the study. Lethally irradiated wild-type mice were transplanted with lineage-negative cells that were transduced by myeloid-specific lentivirus expression vectors. These mice were subjected to transverse aortic constriction surgery (TAC) 8 weeks after BMT. Echocardiography was performed at the times indicated and mice were euthanized 8 weeks after TAC. (B) Representative images of Picrosirius red/Fast Green staining of the heart (left), and heart weight and lung weight adjusted by tibia length (right) from each group (n = 10) at the end of the study. Scale bar = 3 mm. Data are shown as mean ± SEM. Statistical analysis was evaluated by unequal variance t test. (C) Sequential echocardiographic analysis of mice from each group (n = 10) before and after TAC at the indicated time points. Data are shown as minimum to maximum. Statistical analysis was evaluated by 2-way repeated measures analysis of variance with Sidak’s multiple comparison tests. Significance stars are from Sidak’s tests. (D) Representative images and analysis of wheat germ agglutinin staining of the heart sections from each group (n = 10) at the end of study. Scale bar = 100 μm. Data are shown as mean number per field. ± SEM. Statistical analysis was evaluated by 2-tailed unpaired Student’s t-test. (E) Analysis of Picrosirius red/Fast Green staining of the heart sections from each group (n = 10) presented in B, at the end of study. Data are shown as mean ± SEM. Statistical analysis was evaluated by unequal variance t test. (F) Representative images and analysis of Mac2 staining of the sections of hearts from mice of each group (n = 10) at the end of study. Scale bar = 100 um. Data are shown as mean ± SEM, Mac2+ cells per field. Statistical analysis was evaluated by Kruskal-Wallis test. (G) Analysis of transcript expression in the myocardium obtained from each group of mice (n = 10) 8 weeks after TAC surgery. 36b4 was used as a reference for normalization. Data are shown as mean ± SEM. Statistical analysis was evaluated by 2-tailed unpaired Student’s t test (Col3a1), unequal variance t test (IL-6, Col1a1) or by Kruskal-Wallis test (Anp, Bnp, β/α MHC). *p < 0.05, **p < 0.01, ***p < 0.001. CSA = cross-sectional area of myocyte; FS = fractional shortening; HW = heart weight; LW = lung weight; PWd = posterior wall thickness at diastole; TL = tibia length; other abbreviations as in Figure 1.
In response to pressure overload hypertrophy, the JAK2V617F experimental group displayed significant increases in heart mass (p < 0.001) and lung weight (p < 0.001) indicative of congestion compared with mice from the JAK2WT experimental group at 8 weeks post-surgery (Figure 4B). Sequential analysis of echocardiography revealed that the JAK2V617F experimental group displayed significantly increased cardiac posterior wall thickness (Sidak’s 95% confidence interval: –0.19 to –0.07; p < 0.001) and a progressive reduction of fractional shortening (Sidak’s 95% confidence interval: 9.30 to 14.73; p < 0.001) (Figure 4C). Correspondingly, histological analyses revealed that the TAC-treated JAK2V617F group displayed more cardiac myocyte hypertrophy (p < 0.001) (Figure 4D) and cardiac fibrosis (p < 0.001) (Figure 4E) following TAC. Immunohistological staining with Mac2 antibody revealed greater macrophage accumulation in the myocardium of the TAC JAK2V617F group (p = 0.012) (Figure 4F), and these mice displayed greater IL-6 (p = 0.002), Anp (p < 0.001), Bnp (p = 0.015), Col1a1 (p = 0.010), and Col3a1 (p = 0.058) transcript expression and an increase in the ratio of β-to-α myosin heavy chain isoform (p < 0.001) (Figure 4G), indicative of greater inflammation, fibrosis, and cardiac dysfunction.
Discussion
Myeloproliferative neoplasms are rare blood disorders that are frequently associated with somatic JAK2V617F mutation in hematopoietic cells. These conditions lead to elevations in erythrocytes and platelets that have the potential to contribute to cardiovascular disease through increased blood viscosity and thrombotic complications 18, 19, 46. Additionally, these conditions are associated with leukocytosis that can also contribute to cardiovascular diseases 47, 48, 49. Recently, it has been recognized that asymptomatic adults display clonal events in their hematopoietic system that result from JAK2V617F mutations, yet they do not display overt changes in leukocytes, erythrocytes, or platelets. This condition, referred to as clonal hematopoiesis (or CHIP or ARCH), is prevalent in the elderly population and has been associated with increased mortality and cardiovascular disease incidence (13). Clonal hematopoiesis associated with candidate genes that are recurrently mutated in hematologic malignancies is estimated to occur in 10% of individuals who are older than 70 years of age. Of these, the activating JAK2V617F mutation can account for a portion of the reported cases of clonal hematopoiesis cases, yet these individuals do not display abnormalities in total blood counts 10, 13. Thus, the mechanisms leading to the increased cardiovascular disease incidence caused by JAK2V617F-mediated clonal hematopoiesis are enigmatic.
Here, we evaluated the fitness of HSCs expressing a JAK2V617F transgene to repopulate bone marrow in lethally irradiated mice using a competitive transplantation approach. Analysis of the blood of transplanted mice established that this BMT led to the preferential expansion of mutant JAK2 hematopoietic cells to an extent that was comparable to the allelic fractions that are observed in individuals with clonal hematopoiesis 8, 10, 13. The kinetics of this expansion was similar to that previously observed in competitive transplantation experiments using bone marrow harboring inactivating mutations in Tet2 but much more robust than what was observed with inactivating mutations in Dnmt3a 14, 15, 16, indicative of gene-specific effects of these mutations in the HSPC compartment. A particularly striking observation was that while the Tet2 and Dnmt3a mutations in HSPCs tended to be multipotent and represented in all progeny leukocytes, BMT experiments with the JAK2V617F mutation displayed a nearly exclusive bias toward expansion into neutrophils and monocytes versus the lymphoid lineage. Consistent with these findings, a model of Jak2V617F knock-in mice also display a myeloid bias of cell expansion 50, 51. We and others also find that the JAK2V617F mutation promotes the expression of CD41 in the LT-HSC population, a marker that is expressed on a subpopulation of myeloid-biased HSC that accumulate with age (36). Along these lines, lineage-restricted expansion is generally observed in patients with clonal hematopoiesis, typically with much higher mutant allele fractions in the myeloid population 52, 53.
Although mice transplanted with JAK2V617F bone marrow developed a strong expansion bias into myeloid cell populations, they also developed elevations in hemoglobin, platelets, and leukocytes that are associated with MPNs. These phenotypes are also observed in murine models of hematopoietic cell-specific Jak2V617F expression 28, 29, 30, 31. However, alterations in blood cell counts are generally not a feature of the clonal hematopoiesis that can arise from mutations in any 1 of multiple pre-leukemic genes including the Jak2V617F variant. To account for these discrepant phenotypes between JAK2V617F-mediated MPNs and clonal hematopoiesis, it has been proposed that heterogeneity among the HSC populations that acquire the JAK2V617F mutation may contribute to the phenotypic diversity observed in this patient population (54). It is becoming increasingly recognized that distinct HSC subpopulations differ in their functional properties and display restricted lineage biases 55, 56, 57, 58, 59. Thus, it has been proposed that essential thrombocytopenia can result from a JAK2V617F mutation that is acquired in megakaryocyte-restricted HSCs, whereas polycythemia vera can result when the mutation is acquired in HSCs that are destined for myeloid- or erythroid-restricted progeny (54). Support for these more complex lineage schemes comes from evidence of bypass pathways involving lineage-restricted progenitors that are self-renewing 56, 57, and long-lived, lineage-biased HSCs that predominate in native hematopoiesis 58, 60. Alternatively, it remains possible that clonal hematopoiesis and the diverse MPN disease phenotypes could result from the length of time that a patient harbors the mutation, the size of the clone, or the acquisition of additional driver gene mutations 61, 62.
Previous studies have implicated JAK2V617F-mediated clonal hematopoiesis without an MPN disease phenotype in cardiovascular disease (13). To model the effect of myeloid-restricted JAK2V617F expression on the cardiovascular system, BMT experiments were conducted using lineage-negative cells that were transduced with a lentivirus vector expressing JAK2V617F from the SP146/gp91 promoter/enhancer. This synthetic promoter/enhancer is active in myeloid cells of the blood and tissues 33, 41, and it is more tissue restricted in this context than the LyzM promoter that is active in HSPCs in this context (30). Irradiated mice implanted with lineage negative cells transduced with the SP146/gp91–directed expression vector displayed high levels of transgene chimerism in the myeloid cells of the blood, but JAK2V617F expression from this vector did not alter leukocyte, platelet, or hemoglobin levels. Mice treated in this manner were then subjected to the permanent LAD ligation model of myocardial infarction. In this model, myeloid-directed JAK2V617F expression led to greater infarct size and a reduction in cardiac function that was associated with greater expression of IL-6 and IL-1β. To extend these studies, BMT using lineage negative cells transduced with the lentivirus vector expressing the JAK2V617F allele from the myeloid-specific promoter/enhancer were also subjected to a model of pressure overload hypertrophy that is achieved by TAC. In this second model, myeloid-directed JAK2V617F expression led to greater cardiac hypertrophy and fibrosis, which was accompanied by diminished cardiac function and increased lung congestion. Hearts from these mice also display greater macrophage infiltration and IL-6 expression. Based on these results, we hypothesize that clonal hematopoiesis that results in the expression of the JAK2V617F mutation in circulating myeloid cells can contribute to myocardial disease independent of thrombocytosis, erythrocytosis or leukocytosis.
It is increasingly appreciated that inflammation plays a causal role in cardiovascular diseases 63, 64, 65, 66. Here, we find that myeloid-directed JAK2V617F expression can increase myocardial inflammation in murine models of heart failure and increase inflammatory responses in the THP-1 human monocytic cell line. Specifically, JAK2V617F promotes the activating phosphorylation of STAT1 and increases the production of IL-6, IL-1β, tumor necrosis factor alpha, CCL2, and AIM2 in response to stimulation with LPS. Wild-type JAK2 is normally associated with a cytokine receptor, and cytokine binding to its cognate receptor leads to the activation of JAK2 via the transphosphorylation of a tyrosine residue in its activation loop (67). In contrast, the JAK2V617F allele activates downstream targets without the requirement for cytokine stimulation, and it is therefore widely recognized as a constitutively active form. However, binding to a cytokine receptor scaffold is still required for JAK2V617F to transmit a signal (40). While the receptors involved in JAK2V617F activation have been reported in several cell types, the receptors that confer this function in myeloid cells have not been elucidated. In the current study, we find that IFNGR1 is necessary for JAK2V617F to activate phosphorylated STAT1 signaling in THP-1 myeloid cells. Finally, because it has been reported that inflammation favors the expression of JAK2V617F hematopoietic cells to undergo clonal expansion relative to wild-type cells 38, 39, we investigated whether the sterile inflammation brought about by infarction could accelerate the expansion of JAK2V617F mutant LT-HSCs. In a competitive BMT experiment, LAD ligation accelerated the expansion of vav1-JAK2V617F cells into the myeloid lineage. These data provide experimental evidence for a positive feedback loop where JAK2V617F-mediated clonal hematopoiesis promotes cardiovascular disease, and vice versa, via modulation of inflammatory pathways.
A recent publication showed that JAK2V617F mutant neutrophils are prone to form neutrophil extracellular traps (NETs) and contribute to the thrombotic events that accompany myeloproliferative disease (68). NETs have been reported to promote cardiac dysfunction in the context of myocardial ischemia (69) and pressure overload (70). Thus, the formation of NETs could be another mechanism that can contribute to the cardiovascular consequences of the JAK2V617F mutation. However, JAK2 has cell type–specific functions, as it functions downstream of multiple receptors in different cell types to differentially activate specific downstream signaling pathways and produce different outcomes. Thus, in the current study, we focused on analyzing JAK2V617F mutations in the monocyte or macrophage population because they are widely recognized to be critical cells in cardiovascular disease models (64).
Study limitations
In this study, we employed lentivirus-mediated expression of human JAK2V617F protein under synthetic promoter/enhancer to achieve myeloid-restricted expression of the protein to avoid confounding effects of polycythemia vera or essential thrombocythemia phenotypes. However, this is an overexpression and may not reflect the phenotype obtained from physiological levels of the driver gene mutation. Furthermore, these studies expressed the human JAK2 mutant in mouse hematopoietic cells, and this species mismatch could produce an additional confounding factor. Because of these limitations, further evaluation of JAK2V617F mutation in myeloid populations is warranted using more physiologically relevant models.
In addition, niche signals can shape tissue-resident immune cell function, For example, the transcriptomic landscapes of resident macrophage are dependent upon the tissue where they reside. Thus a deeper analysis of JAK2 mutant immune cells recruited to the heart could provide additional information about the pathogenic impact of JAK2-mediated clonal hematopoiesis in the setting of cardiac disease, which was not addressed in this study.
Conclusions
We show that JAK2V617F expression in HSPCs leads to the expansion of the mutant clones in a manner that is highly restricted to myeloid cells. This expression pattern differs markedly from HSPC that harbor mutations in Tet2 or Dnmt3a, which display the ability to expand into all leukocyte populations in the competitive BMT model 14, 15, 16. Further, we developed a system to restrict JAK2V617F expression to differentiated blood myeloid cells following transduction of lineage-negative bone marrow cells that were implanted into lethally irradiated mice. Mice treated in this manner did not display alterations in blood cell or platelet levels, but they were more susceptible to myocardial inflammation and cardiac dysfunction in models of heart failure. We propose that JAK2V617F mutations can occur in a clonal subpopulation of HSC that exclusively gives rise to circulating myeloid cells that, in turn, contribute to cardiovascular disease risk through the overactivation of cytokine pathways. Thus, patients with JAK2V617F-mediated clonal hematopoiesis may benefit form therapies that target pathways activated by this mutant kinase.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: It is not clear why JAK2V617F mutations in hematopoietic cells will lead to an MPN in some individuals and the condition of clonal hematopoiesis with no changes in blood cell counts in others. Furthermore, it is unknown how JAK2V617F-mediated clonal hematopoiesis can contribute to cardiovascular disease risk independent of alterations in blood cell counts and pro-thrombotic complications associated with MPNs. Our competitive BMT studies in mice show that myeloid-restricted expression of the Jak2V617F mutation will promote cardiac inflammation and dysfunction in models of heart failure in the absence of erythrocytosis, thrombosis, or leukocytosis.
TRANSLATIONAL OUTLOOK: These studies suggest that JAK2V617F-mediated clonal hematopoiesis, in the absence of an MPN phenotype, can arise from the acquisition of these mutations in a hypothetical clonal population of progenitor cells that predominantly give rise to circulating myeloid cells. These JAK2V617F-postive myeloid cells can contribute to cardiovascular disease risk through the overactivation of cytokine signaling. Individuals with JAK2V617F-mediated clonal hematopoiesis may be protected from cardiovascular risk by JAK2 pathway inhibitors.
Acknowledgments
The authors thank Marieke Jones, PhD, and Data Services at the Health Sciences Library at the University of Virginia for advice on statistical analyses.
Footnotes
This work was supported by National Institutes of Health grant nos. HL138014, HL132564 (to Dr. Walsh), HL139819 (to Dr. Walsh), HL141256 (to Dr. Walsh), HL095685 (to Dr. Mohi), and HL136363 (to Dr. Ravid); American Heart Association Post-Doctoral Fellowship 17POST33670076 and Kanae Foundation for the Promotion of Medical Science (to Dr. Sano), and a China Scholarship Council grant (to Dr. Wang). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Fuster J.J., Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018;122:523–532. doi: 10.1161/CIRCRESAHA.117.312115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sano S., Wang Y., Walsh K. Clonal hematopoiesis and its impact on cardiovascular disease. Circ J. 2018;83:2–11. doi: 10.1253/circj.CJ-18-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jan M., Ebert B.L., Jaiswal S. Clonal hematopoiesis. Semin Hematol. 2017;54:43–50. doi: 10.1053/j.seminhematol.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busque L., Mio R., Mattioli J. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood. 1996;88:59–65. [PubMed] [Google Scholar]
- 5.Busque L., Patel J.P., Figueroa M.E. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodell M.A., Rando T.A. Stem cells and healthy aging. Science. 2015;350:1199–1204. doi: 10.1126/science.aab3388. [DOI] [PubMed] [Google Scholar]
- 7.Coombs C.C., Zehir A., Devlin S.M. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382 e4. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G., Kahler A.K., Handsaker R.E. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson C.J., Lindsley R.C., Tchekmedyian V. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiswal S., Fontanillas P., Flannick J. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh P.R., Genovese G., Handsaker R.E. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature. 2018;559:350–355. doi: 10.1038/s41586-018-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zink F., Stacey S.N., Norddahl G.L. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S., Natarajan P., Silver A.J. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sano S., Oshima K., Wang Y. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster J.J., MacLauchlan S., Zuriaga M.A. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano S., Oshima K., Wang Y. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123:335–341. doi: 10.1161/CIRCRESAHA.118.313225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorsheimer L., Assmus B., Rasper T. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4:25–33. doi: 10.1001/jamacardio.2018.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spivak J.L. Myeloproliferative Neoplasms. N Engl J Med. 2017;376:2168–2181. doi: 10.1056/NEJMra1406186. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A., Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1:97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen C., Birgens H.S., Nordestgaard B.G., Bojesen S.E. Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49 488 individuals from the general population. Br J Haematol. 2013;160:70–79. doi: 10.1111/bjh.12099. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen C., Birgens H.S., Nordestgaard B.G., Kjaer L., Bojesen S.E. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96:450–453. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidon P., El Housni H., Dessars B., Heimann P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia. 2006;20:1622. doi: 10.1038/sj.leu.2404292. [DOI] [PubMed] [Google Scholar]
- 23.Xu X., Zhang Q., Luo J. JAK2(V617F): Prevalence in a large Chinese hospital population. Blood. 2007;109:339–342. doi: 10.1182/blood-2006-03-009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abelson S., Collord G., Ng S.W.K. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinds D.A., Barnholt K.E., Mesa R.A. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood. 2016;128:1121–1128. doi: 10.1182/blood-2015-06-652941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie M., Lu C., Wang J. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Liu W., Fidler T. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123:e35–e47. doi: 10.1161/CIRCRESAHA.118.313283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akada H., Yan D., Zou H. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullally A., Lane S.W., Ball B. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Hayashi Y., Yokota A. Expansion of EPOR-negative macrophages besides erythroblasts by elevated EPOR signaling in erythrocytosis mouse models. Haematologica. 2018;103:40–50. doi: 10.3324/haematol.2017.172775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing S., Wanting T.H., Zhao W. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgiades P., Ogilvy S., Duval H. VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis. 2002;34:251–256. doi: 10.1002/gene.10161. [DOI] [PubMed] [Google Scholar]
- 33.Barde I., Laurenti E., Verp S. Lineage- and stage-restricted lentiviral vectors for the gene therapy of chronic granulomatous disease. Gene Ther. 2011;18:1087–1097. doi: 10.1038/gt.2011.65. [DOI] [PubMed] [Google Scholar]
- 34.Sano S., Wang Y., Evans M.A. Lentiviral CRISPR/Cas9-mediated genome editing for the study of hematopoietic cells in disease models. J Vis Exp. 2019;152:e59977. doi: 10.3791/59977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C.X., Sather B.D., Wang X. Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood. 2014;124:913–923. doi: 10.1182/blood-2013-12-546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gekas C., Graf T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood. 2013;121:4463–4472. doi: 10.1182/blood-2012-09-457929. [DOI] [PubMed] [Google Scholar]
- 37.Shi K., Zhao W., Chen Y. Cardiac hypertrophy associated with myeloproliferative neoplasms in JAK2V617F transgenic mice. J Hematol Oncol. 2014;7:25. doi: 10.1186/1756-8722-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arranz L., Sanchez-Aguilera A., Martin-Perez D. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 39.Fleischman A.G., Aichberger K.J., Luty S.B. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118:6392–6398. doi: 10.1182/blood-2011-04-348144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X., Levine R., Tong W. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He W., Qiang M., Ma W. Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther. 2006;17:949–959. doi: 10.1089/hum.2006.17.949. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Sano S., Oshima K. Wnt5a-mediated neutrophil recruitment has an obligatory role in pressure overload-induced cardiac dysfunction. Circulation. 2019;140:487–499. doi: 10.1161/CIRCULATIONAHA.118.038820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Zhang Y.L., Lin Q.Y. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39:1818–1831. doi: 10.1093/eurheartj/ehy085. [DOI] [PubMed] [Google Scholar]
- 44.Liao X., Shen Y., Zhang R. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A. 2018;115:E4661–E4669. doi: 10.1073/pnas.1720065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel B., Bansal S.S., Ismahil M.A. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci. 2018;3:230–244. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbui T., Finazzi G., Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176–2184. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 47.Carobbio A., Finazzi G., Guerini V. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109:2310–2313. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 48.Landolfi R., Di Gennaro L., Barbui T. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–2452. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 49.Campbell P.J., MacLean C., Beer P.A. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120:1409–1411. doi: 10.1182/blood-2012-04-424911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundberg P., Takizawa H., Kubovcakova L. Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F. J Exp Med. 2014;211:2213–2230. doi: 10.1084/jem.20131371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y., Akada H., Nath D., Hutchison R.E., Mohi G. Loss of Ezh2 cooperates with Jak2V617F in the development of myelofibrosis in a mouse model of myeloproliferative neoplasm. Blood. 2016;127:3410–3423. doi: 10.1182/blood-2015-11-679431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arends C.M., Galan-Sousa J., Hoyer K. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia. 2018;32:1908–1919. doi: 10.1038/s41375-018-0047-7. [DOI] [PubMed] [Google Scholar]
- 53.Buscarlet M., Provost S., Zada Y.F. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood. 2018;132:277–280. doi: 10.1182/blood-2018-01-829937. [DOI] [PubMed] [Google Scholar]
- 54.Mead A.J., Mullally A. Myeloproliferative neoplasm stem cells. Blood. 2017;129:1607–1616. doi: 10.1182/blood-2016-10-696005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eaves C.J. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanjuan-Pla A., Macaulay I.C., Jensen C.T. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto R., Morita Y., Ooehara J. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Sun J., Ramos A., Chapman B. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurenti E., Gottgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas S., Trumpp A., Milsom M.D. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22:627–638. doi: 10.1016/j.stem.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Chen E., Schneider R.K., Breyfogle L.J. Distinct effects of concomitant Jak2V617F expression and Tet2 loss in mice promote disease progression in myeloproliferative neoplasms. Blood. 2015;125:327–335. doi: 10.1182/blood-2014-04-567024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKerrell T., Park N., Chi J. JAK2 V617F hematopoietic clones are present several years before MPN diagnosis and follow different expansion kinetics. Blood Adv. 2017;1:968–971. doi: 10.1182/bloodadvances.2017007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med. 2018;24:711–720. doi: 10.1038/s41591-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ridker P.M. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 66.Swirski F.K., Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 67.Jatiani S.S., Baker S.J., Silverman L.R., Reddy E.P. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolach O., Sellar R.S., Martinod K. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10:eaan8292. doi: 10.1126/scitranslmed.aan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savchenko A.S., Borissoff J.I., Martinod K. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123:141–148. doi: 10.1182/blood-2013-07-514992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinod K., Witsch T., Erpenbeck L. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214:439–458. doi: 10.1084/jem.20160530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.