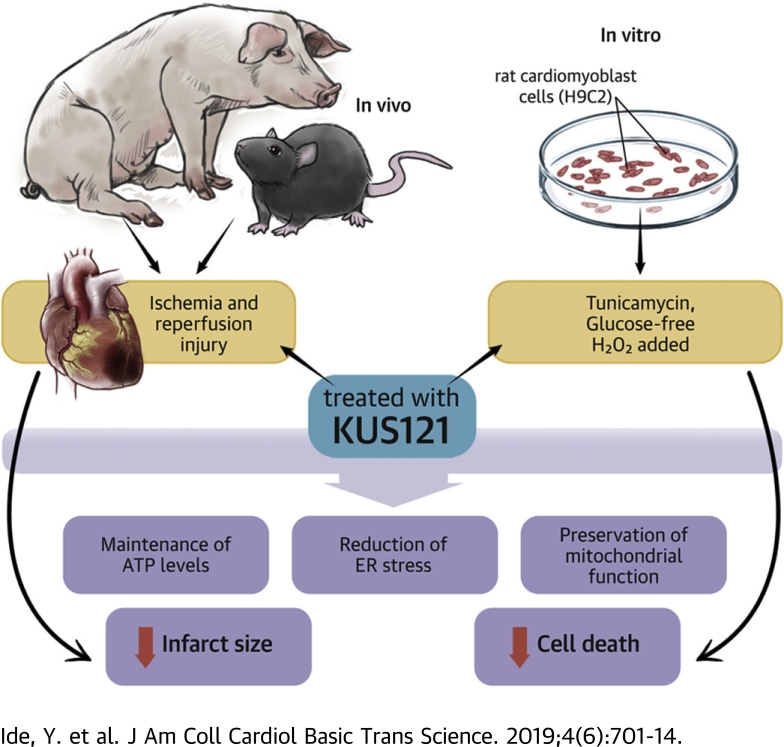
Visual Abstract
Key Words: ATP, ER stress, KUS121, myocardial infarction
Abbreviations and Acronyms: AAR, area at risk; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; BiP, immunoglobulin heavy chain-binding protein; CHOP, C/EBP homologous protein; CMR, cardiac magnetic resonance; EF, ejection fraction; ER, endoplasmic reticulum; FRET, fluorescence resonance energy transfer; FS, fractional shortening; H2O2, hydrogen peroxide; HF, heart failure; I/R, ischemia and reperfusion; IBMPFD, inclusion body myopathy associated with Paget's disease of bone and frontotemporal dementia; IHD, ischemic heart disease; KUS121, Kyoto University Substance 121; LAD, left anterior descending artery; LV, left ventricular/ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention; TTC, triphenyltetrazolium chloride; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; VCP, valosin-containing protein
Highlights
-
•
KUS121 was developed to selectively inhibit the adenosine triphosphatase activity of valosin-containing protein without affecting other cellular functions of valosin-containing protein.
-
•
KUS121 preserved adenosine triphosphate levels, reduced endoplasmic reticulum stress, and suppressed cell death in H9C2 rat cardiomyoblast cells, treated with tunicamycin or hydrogen peroxide, or cultured in glucose-free medium.
-
•
In murine ischemia and reperfusion injury models, KUS121 treatment after reperfusion attenuated the infarcted size and preserves cardiac function by maintaining adenosine triphosphate levels and reducing ER stress.
-
•
In porcine ischemia and reperfusion injury models, intracoronary administration of KUS121 also attenuated the infarcted area in a dose-dependent manner.
-
•
These results indicated that KUS121 is a promising novel therapeutic agent for myocardial infarction.
Summary
No effective treatment is yet available to reduce infarct size and improve clinical outcomes after acute myocardial infarction by enhancing early reperfusion therapy using primary percutaneous coronary intervention. The study showed that Kyoto University Substance 121 (KUS121) reduced endoplasmic reticulum stress, maintained adenosine triphosphate levels, and ameliorated the infarct size in a murine cardiac ischemia and reperfusion injury model. The study confirmed the cardioprotective effect of KUS121 in a porcine ischemia and reperfusion injury model. These findings confirmed that KUS121 is a promising novel therapeutic agent for myocardial infarction in conjunction with primary percutaneous coronary intervention.
Ischemic heart disease (IHD) is a leading cause of death worldwide. Of the 17.5 million cardiovascular deaths in 2012, an estimated 7.4 million deaths were due to IHD (1). In the United States, more than 360,000 people died of IHD in 2015, and IHD accounted for 43.8% of deaths from cardiovascular disease (2). In Japan, IHD accounted for 35.6% of deaths from heart disease in 2016 (3).
In patients with acute myocardial infarction (MI), early reperfusion therapy using primary percutaneous coronary intervention (PCI) is performed to reduce the infarct size and improve outcomes (4). However, when a larger infarct size remains after primary PCI, the rates of all-cause mortality and hospitalization for heart failure (HF) are still high (5). High mortality from HF and rehospitalization for HF are becoming serious concerns from both health care and medical cost perspectives 2, 6.
To further reduce infarct sizes and improve clinical outcomes, new treatments in addition to early reperfusion therapy are needed. Indeed, drugs such as an inhibitor of Na+/H+ exchanger and cyclosporine A were reported to have cardioprotective effects in animal experiments (7) or in small clinical trials (8). However, these effects were not confirmed in multicenter, randomized, double-blind clinical trials 9, 10. Although many other clinical trials of novel therapies for acute MI are ongoing, there are currently no therapeutic agents available to reduce infarct size and improve clinical outcomes (11).
Valosin-containing protein (VCP) is a member of the adenosine triphosphatase (ATPase) associated with diverse cellular activities family, and it is expressed ubiquitously in almost all cell types. As reported previously, in addition to ATPase activity, VCP is involved in various cellular functions, including proteasome-mediated protein degradation, endoplasmic reticulum (ER)–associated degradation, lysosomal protein degradation, autophagy, cell cycle progression, membrane fusion, and so on (12). Gain-of-function mutations in VCP have been reported to cause inclusion body myopathy associated with Paget's disease of bone and frontotemporal dementia (IBMPFD) (13), and IBMPFD-causing mutations in VCP result in elevated ATPase activity (14). The major clinical phenotypes of IBMPFD are myopathy, bone lesions, and dementia, but it is notable that cardiac phenotypes, such as dilated cardiomyopathy, are also manifested in certain IBMPFD patients (15).
Kyoto University Substance 121 (KUS121) was developed to selectively inhibit the ATPase activity of VCP without affecting other cellular functions of VCP. Indeed, KUS121 has been shown to maintain cellular adenosine triphosphate (ATP) levels, reduce ER stress, and prevent cell death in vitro without showing any toxic effects (16). KUS121 has also been shown to elicit neuroprotective effects in murine retinitis pigmentosa models, murine glaucoma models, rat retinal ischemic injury models, and murine Parkinson’s disease models 16, 17, 18, 19.
Considering the cardiac phenotype in IBMPFD patients and the neuroprotective effect of KUS121 in vivo, we anticipated that KUS121 may produce a cardioprotective effect in IHD. In this study, we found that KUS121 preserved ATP levels, reduced ER stress, and suppressed cell death in H9C2 rat cardiomyoblast cells. Furthermore, in murine and porcine ischemia and reperfusion (I/R) injury models, KUS121 ameliorated cardiac damage and preserved cardiac function. These results indicated that KUS121 is a promising novel therapeutic agent for MI.
Methods
This study was approved by the Kyoto University Ethics Review Board. Additional detailed methods are available in the Supplemental Appendix.
I/R injury models in mice
In 8-week-old mice, the left anterior descending (LAD) coronary artery was ligated with a PE-10 tube. After 45 min of ischemia, reperfusion was induced by untying the knot and removing the tube. At 7 days after reperfusion, Masson’s trichrome staining was performed to evaluate the infarcted area.
For quantification of heart ATP levels, I/R injury protocols using GO-ATeam2 mice were performed. ATeam biosensors are a series of fluorescence resonance energy transfer (FRET)–based indicators for ATP, which are able to estimate relative ATP levels in live cells in real time (20). Go-ATeam2 mice were developed by genetically integrating the GO-ATeam expression cassette into mice (M. Yamamoto, unpublished data, July 2019) (19); thus, the in vivo orange fluorescent protein and green fluorescent protein FRET ratio depends on the relative cellular ATP levels (21).
Analysis in murine IR injury models was performed by an experimenter who was blinded to treatment groups.
I/R injury models in pigs
In 3-month-old pigs, the LAD was occluded using a 3.0 × 20-mm balloon (Terumo, Tokyo, Japan). After 60 min of occlusion, reperfusion was induced by deflation of the balloon.
At 7 days after reperfusion, to evaluate the infarcted area, gadolinium enhanced cardiac magnetic resonance (CMR) and double staining with triphenyltetrazolium chloride (TTC) and Evans blue were performed, and analyzed by an experimenter who was blinded to treatment groups.
Statistical analysis
Measured data are presented as mean ± SEM. For statistical comparisons between 2 groups, unpaired Student’s t test was used. For statistical analysis of 3 or more groups, 1-way analysis of variance was used. In 1-way analysis of variance, Sidak’s post hoc test was performed to compare all pairs of groups and Dunnett's post hoc test to compare 1 group as a control with the other groups. A p value of <0.05 was considered as statistically significant. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, California).
Results
VCP messenger RNA is expressed in human and murine hearts, especially in cardiomyocytes
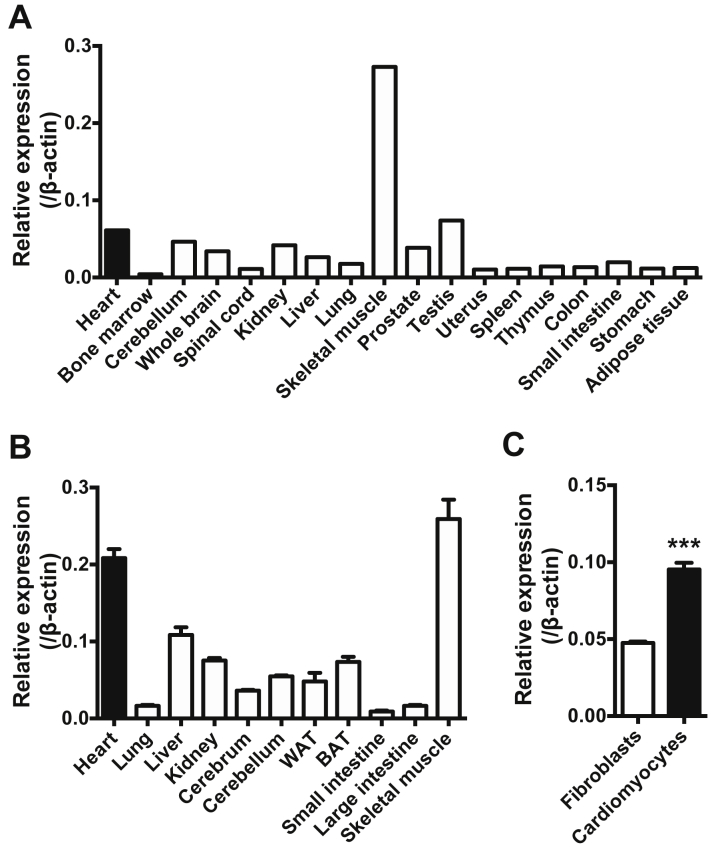
We first analyzed the expression levels of VCP messenger RNAs in various human organs (Figure 1A) and mice (Figure 1B). VCP expression levels in the human heart were comparable to that in central nervous tissues. VCP expression was also confirmed in the mouse heart and its expression in cardiomyocytes was 2-fold higher than in fibroblasts (Figure 1C).
Figure 1.
VCP Is Expressed in Human and Murine Hearts, Especially in Cardiomyocytes
Expression levels of valosin-containing protein (VCP) in various (A) human organs and (B) mouse organs (n = 4). The human samples were from various sources, pooled from 1 or more healthy adults (Human Total RNA Master Panel II, Clontech Laboratories, Mountain View, California). Expression of β-actin was used as an internal control. Black bars indicate expression in hearts. (C) Comparison of VCP expression in cardiomyocytes (n = 4) with that in fibroblasts (n = 4). ***p < 0.001, using unpaired 2-tailed Student’s t test. Data are presented as mean ± SEM. BAT = brown adipose tissue; WAT = white adipose tissue.
KUS121 preserves ATP levels, reduces ER stress, and suppresses cell death in cultured H9C2 cells
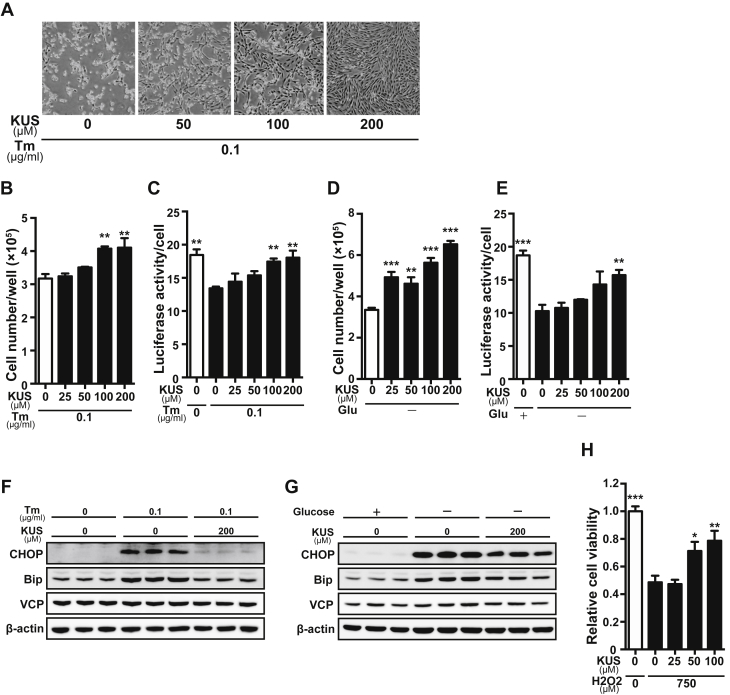
To examine the cell protective effect of KUS121 on cardiomyocytes, H9C2 rat cardiomyoblast cells were treated with tunicamycin or cultured in glucose-free medium to induce cell death. KUS121 suppressed cell death and maintained ATP levels in tunicamycin-treated H9C2 cells in a dose-dependent manner (Figures 2A to 2C). KUS121 also preserved ATP levels and protected H9C2 cells against cell death when the cells were cultured in glucose-free medium (Figures 2D and 2E). KUS121 reduced ER stress, which was determined by the reduction in C/EBP homologous protein (CHOP) and immunoglobulin heavy chain-binding protein (BiP) levels in these conditions, without changing VCP expression levels (Figures 2F and 2G). In addition, KUS121 reduced hydrogen peroxide (H2O2)–induced H9C2 cell death (Figure 2H). KUS121 itself did not affect cell growth or cellular ATP levels, or CHOP, BiP, or VCP expression levels (Supplemental Figures 1A to 1C) in normal culture conditions.
Figure 2.
KUS Preserves ATP Levels, Reduces Endoplasmic Reticulum Stress, and Suppresses Cell Death in Cultured Cells
(A) Representative images of H9C2 rat cardiomyoblast cells cultured with tunicamycin (Tm) (0.1 μg/ml) for 48 h with different concentrations of Kyoto University Substance 121 (KUS) (50, 100, and 200 μM). (B to E) Number and adenosine triphosphate (ATP) levels of H9C2 cells (B, C) cultured with Tm (0.1 μg/ml) for 48 h or (D, E) cultured in glucose-free medium (Glu−) for 48 h, with different concentrations of KUS (25, 50, 100, and 200 μM) (n = 3). (B, C) **p < 0.01 vs. Tm (0.1 μg/ml) without KUS; (D, E) **p < 0.01, ***p < 0.001 vs. Glu− without KUS using 1-way analysis of variance with Dunnett's post hoc test. (F, G) Western blotting analysis of H9C2 cells (F) cultured with Tm (0.1 μg/ml) for 12 h or (G) cultured in Glu− for 24 h, with and without KUS (200 μM, n = 3). (G) Relative viability of H9C2 cells treated with hydrogen peroxide (H2O2) (750 μM) for 24 h with different concentrations of KUS (25, 50, and 100 μM) (n = 7). Viability of cells in normal culture conditions was the reference, indicated as 1. *p < 0.05, **p < 0.01, ***p < 0.001 vs. H2O2 (750 μM) without KUS using 1-way analysis of variance with Dunnett's post hoc test. All data are presented as mean ± SEM. BiP = immunoglobulin heavy chain-binding protein; CHOP = C/EBP homologous protein; VCP = valosin-containing protein.
KUS121 preserves mitochondrial function after tunicamycin treatment
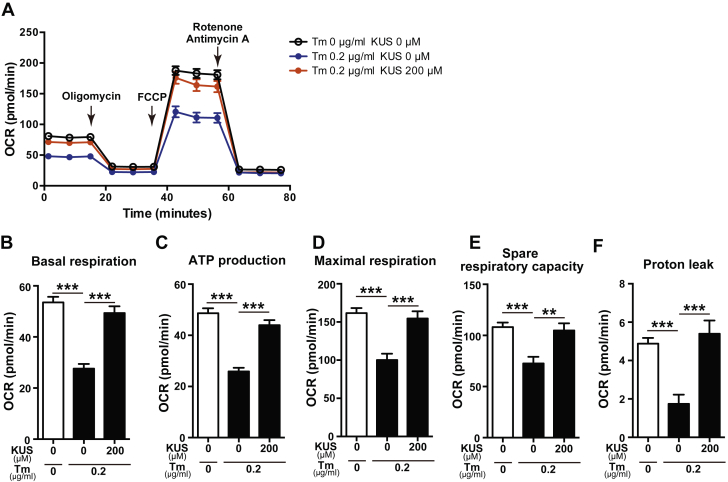
It is known that ER stress and ischemia affect mitochondrial functions 22, 23, 24; therefore, we examined mitochondrial functions using an XF96 extracellular flux analyzer (Agilent Technologies, Santa Clara, California). H9C2 cells were treated with tunicamycin in the absence or presence of KUS121 for 6 h, and the oxygen consumption rate was measured (Figure 3A). The numbers of H9C2 cells in these conditions were similar to that in normal culture (Supplemental Figure 2A). The parameters of mitochondrial respiration, such as basal respiration, ATP production–linked respiration, maximal respiration, spare respiratory capacity, and proton leak were calculated, as shown in Supplemental Figure 2B. The parameters of mitochondrial respiration were lower in H9C2 cells treated with tunicamycin than in control cells (Figures 3B to 3F). However, the parameters of mitochondrial respiration were preserved in tunicamycin and KUS121-treated cells, similar to those in normal cells without tunicamycin treatment. KUS121 also significantly increased mitochondrial respiration in normal culture conditions (Supplemental Figures 2C to 2H). Thus, the protective effects of KUS121 against H9C2 cell death are most likely mediated by ATP preservation, ER stress reduction, and conservation of mitochondrial functions.
Figure 3.
KUS Preserves Mitochondrial Function After Tm Treatment
(A) Oxygen consumption rate (OCR) measured using an XF96 extracellular flux analyzer in H9C2 cells treated with Tm (0.2 μg/ml) and KUS (200 μM). After basal OCR was measured, oligomycin (2 μM), carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) (1 μM), and a mix of rotenone (1 μM) and antimycin A (1 μM), were added sequentially to assess mitochondrial respiration. OCR at each time point was obtained from an average of 10 replicate wells and presented as mean ± SEM. (B to F) Parameters of mitochondrial respiration: (B) basal respiration, (C) adenosine triphosphate (ATP) production–linked respiration, (D) maximal respiration, (E) spare respiratory capacity, and (F) proton leak. **p < 0.01, ***p < 0.001, using 1-way analysis of variance with Sidak’s post hoc test. Data are presented as mean ± SEM. Abbreviations as in Figure 2.
KUS121 pretreatment attenuates cardiac damage and preserves cardiac function in murine ischemia and reperfusion injury models
Based on the in vitro cell protective effects on H9C2 cardiomyoblast cells, we next investigated whether KUS121 had protective effects on ischemic hearts by use of a murine I/R injury model. KUS121 was injected intraperitoneally at a dose of 160 mg/kg, which was followed by LAD coronary artery occlusion for 45 min (Supplemental Figure 3A). Subsequently, KUS121 was reinjected at the same dose once every 24 h for 6 days after reperfusion. Using Masson’s trichrome staining at 7 days after reperfusion, the infarcted area-to-left ventricular (LV) area ratios in KUS121-treated mice were significantly lower than those in nontreated mice (control mice hereafter) (Supplemental Figures 3B and 3C). We also measured the infarcted area-to-area at risk (AAR) and AAR-to-LV ratios by double staining with TTC and Evans blue (Supplemental Figure 3D). The infarcted area-to-AAR ratios of KUS121-treated mice were significantly lower than were those in control mice, whereas the AAR-to-LV ratios were indistinguishable, compared with control mice (Supplemental Figures 3E and 3F). The expression of CHOP was significantly reduced in the border and remote zone, but not in the ischemic zone (Supplemental Figure 3G).
By echocardiographic analysis, both ejection fraction (EF) and fractional shortening (FS) in mice with I/R injury without KUS121 were reduced, compared with sham-operated mice, at 7 days after reperfusion. However, the systolic function of KUS121-treated mice was preserved at almost the same level as that in sham-operated mice (Supplemental Figures 4A to 4E). We also performed serial echocardiography at 1, 3, 7, 14, and 28 days after reperfusion (Supplemental Figure 3A). EF, FS, and LV systolic diameters were significantly preserved throughout the time course in KUS121-treated I/R mice, compared with I/R control mice (Supplemental Figures 4F to 4I).
KUS121 treatment after reperfusion also attenuates the infarcted size and preserves cardiac function
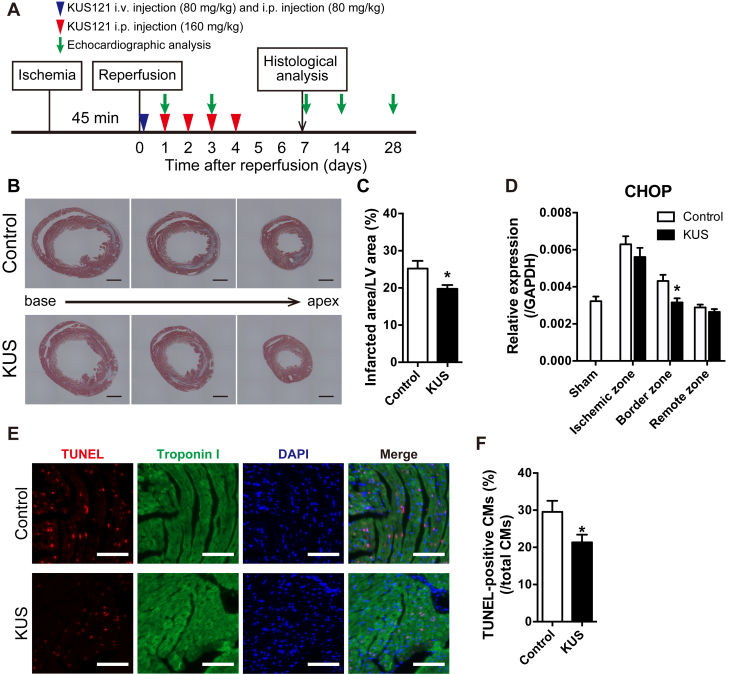
Next, we examined whether KUS121 could produce therapeutic benefits even when administered after I/R injury. Immediately after reperfusion, 80 mg/kg of KUS121 was injected intravenously, and the same amount was administered intraperitoneally (Figure 4A). Subsequently, KUS121 was repeatedly injected intraperitoneally at a dose of 160 mg/kg daily until 4 days after reperfusion. As a result, the infarcted area-to-LV area ratios were significantly reduced in KUS121-treated mice at 7 days after reperfusion compared with control mice (Figures 4B and 4C). The expression of CHOP was significantly reduced in the border zone (Figure 4D). In addition, to investigate the protective effects of KUS121 on cardiomyocyte apoptosis induced by I/R injury, we performed terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays. The rate of apoptotic cardiomyocytes, defined as TUNEL-positive nuclei surrounded by troponin I, was significantly lower in KUS121-treated mice compared with control mice (Figures 4E and 4F).
Figure 4.
KUS121 Treatment After Reperfusion Attenuates Infarct Size and Reduces Cardiomyocyte Apoptosis
(A) Schematic diagram of ischemia and reperfusion (I/R) injury procedures and schedules of Kyoto University Substance 121 (KUS121) administration. (B) Representative images of Masson’s trichrome staining in the short axis of left ventricles (LVs) at 7 days after I/R injury. Black bars indicate 1,000 μm. (C) Quantification of infarcted area in LVs at 7 days after I/R injury (control group, n = 7; KUS121 group, n = 10). *p < 0.05, using unpaired 2-tailed Student’s t test. (D) Expression levels of CHOP in LVs at 1 h after I/R injury. LVs were divided into 3 parts: ischemic zone, border zone, and remote zone (sham group, n = 6; control group, n = 6; KUS121 group, n = 8). The expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. *p < 0.05, using unpaired 2-tailed Student’s t-test. (E) Representative images of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining in LVs at 4 h after reperfusion. White bars indicate 100 μm. (F) Quantification of apoptotic cardiomyocytes (CMs), defined as TUNEL-positive cells surrounded by troponin I (control group, n = 6; KUS121 group, n = 6). *p < 0.05, using unpaired 2-tailed Student’s t-test. All data are presented as mean ± SEM. i.p. = intraperitoneal; i.v. = intravenous; other abbreviations as in Figure 2.
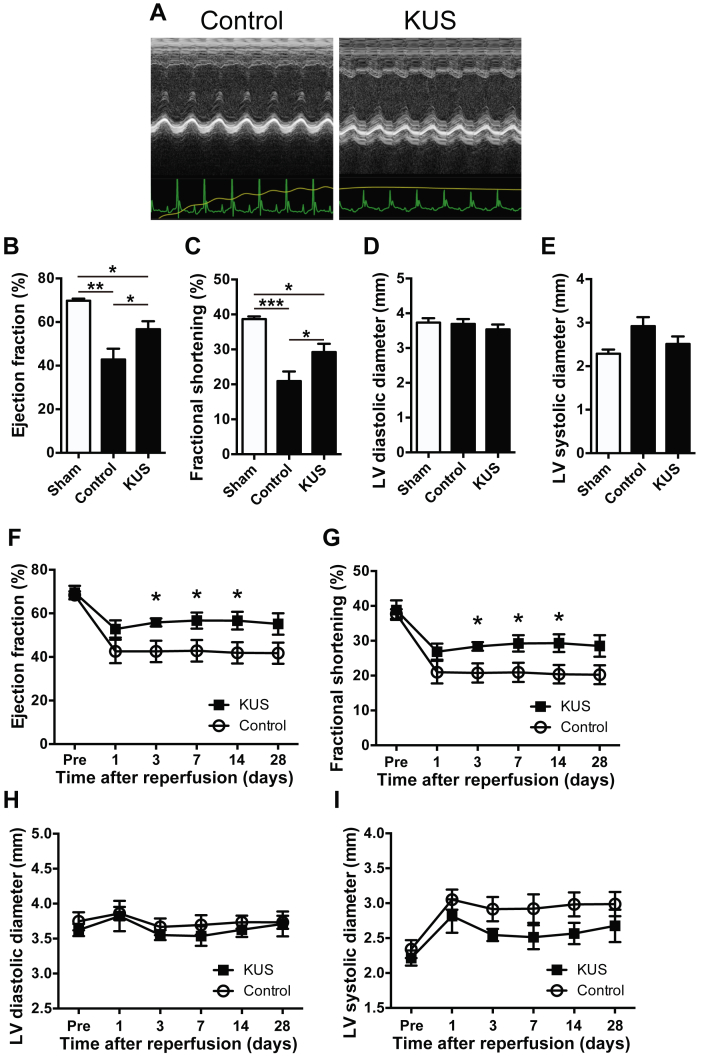
Echocardiographic analysis at 7 days after reperfusion showed that both the EF and FS of KUS121-treated mice were better preserved than those of control mice (Figures 5A to 5E). Similar results were obtained in serial echocardiographic analyses of cardiac functions (Figures 4A and 5F to 5I).
Figure 5.
KUS121 Treatment After Reperfusion Preserves Cardiac Function in I/R Injury Models
(A) Representative images of M-mode echocardiogram of control group and KUS-treated animals at 1 week after I/R injury. (B to E) Echocardiographic data 1 week after I/R injury. (B) Ejection fraction (EF), (C) fractional shortening (FS), (D) LV diastolic diameter, and (E) LV systolic diameter were measured (sham group, n = 4; control group, n = 6; KUS121 group, n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, using 1-way analysis of variance with Sidak’s post hoc test. (F to I) Echocardiographic data at the indicated time points after I/R injury. *p < 0.05, using unpaired 2-tailed Student’s t-test. All data are presented as mean ± SEM. Abbreviations as in Figures 2, 3, and 4.
KUS121 maintains ATP levels in ischemia and reperfusion injury models
To investigate whether KUS121 preserved ATP levels in vivo, we created I/R injury models using GO-ATeam2 mice. In the images, high FRET ratios (i.e., higher ATP levels) were pseudo-colored using warmer colors and low FRET ratios (i.e., lower ATP levels) using cooler colors (Supplemental Figure 5A). ATP levels in infarcted areas of KUS121-treated mice were significantly higher from 0 to 60 min after reperfusion (Supplemental Figures 5A and 5B) than were those of control I/R mice, when KUS121 was administered before ischemia, as shown in Supplemental Figure 3A. In noninfarcted areas of the LV, ATP levels in KUS121-treated mice were also higher than those of control I/R mice (Supplemental Figure 5C). Although ATP levels in the right ventricle of KUS121-treated mice were similar to those of control mice (Supplemental Figure 5D), the relative ATP ratio in the infarcted area to that in the right ventricle was higher in KUS121-treated mice than in control mice (Supplemental Figure 5E).
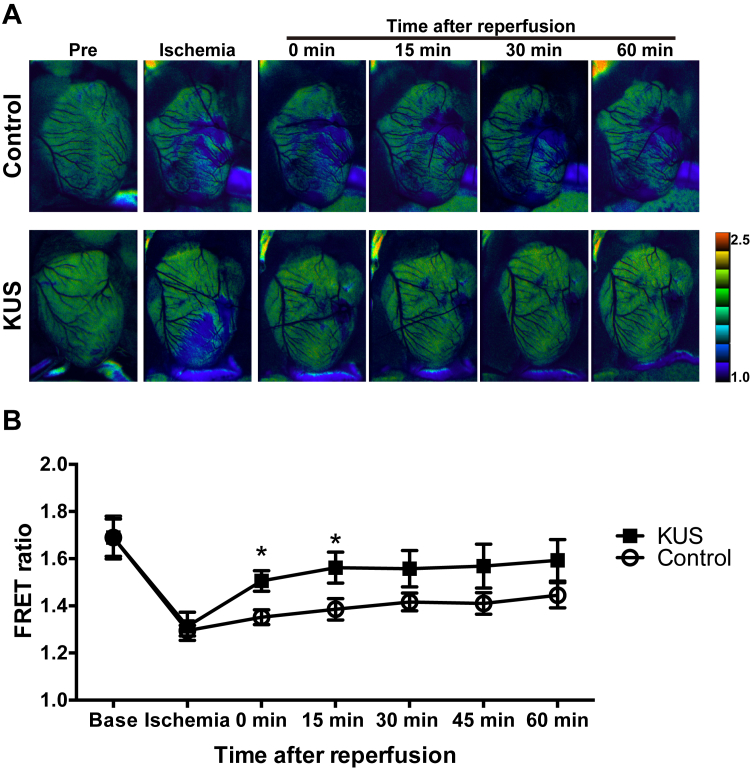
Next, we examined whether KUS121 administration after reperfusion could also preserve ATP levels. As shown in Figure 4A, we administered the same amount of KUS121 immediately after reperfusion in I/R injury models of GO-ATeam2 mice. ATP levels in infarcted areas of KUS121-treated mice were the same as those of control mice during ischemia. However, ATP levels of KUS121-treated mice recovered immediately and significantly after KUS121 administration compared with control mice (Figures 6A and 6B). These data indicate that KUS121 was able to preserve ATP levels in I/R models in vivo.
Figure 6.
KUS121 Treatment After Reperfusion Also Maintains ATP Levels in Ischemia and Reperfusion Injury Models
(A) Representative pseudocolor ratiometric fluorescence resonance energy transfer (FRET) images of whole hearts in I/R injury models of Go-ATeam2 mice. Pseudocolor images were obtained at various time points in ischemia and after reperfusion. In pseudocolor images, warmer colors represent high FRET ratios and cooler colors represent low FRET ratios. (B) Quantification of ATP levels in the infarcted area by FRET ratio (control group, n = 7; KUS121 group, n = 8). *p < 0.05, using unpaired 2-tailed Student’s t-test. Data are presented as mean ± SEM. Abbreviations as in Figures 2, 3, and 4.
Beneficial effects are observed with a single administration of KUS121 after reperfusion
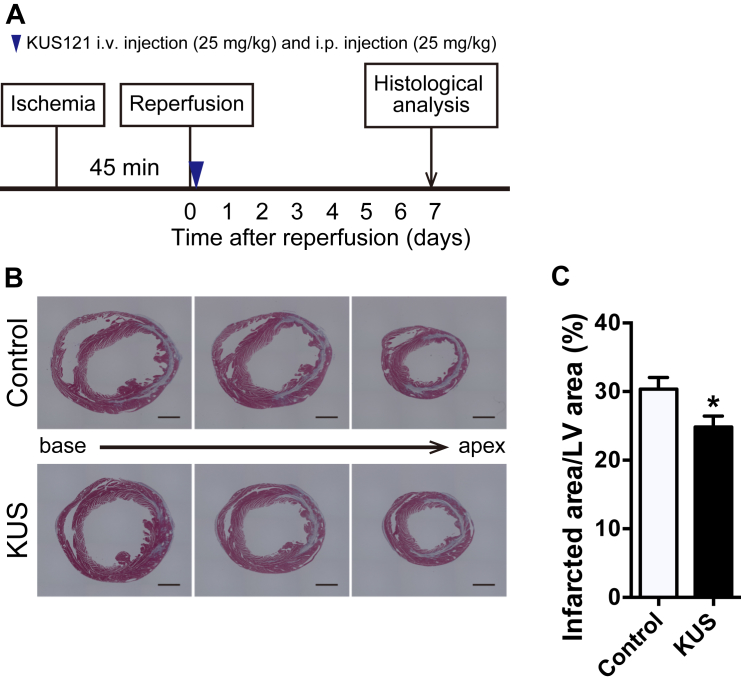
We further examined whether a single KUS121 administration after reperfusion could benefit the infarcted area or not. As shown in Figure 7A, 25 mg/kg of KUS121 was injected intravenously, and the same amount was injected intraperitoneally immediately after reperfusion. The infarcted area-to-LV area ratio was significantly reduced in KUS121-treated mice at 7 days after reperfusion compared with control mice (Figures 7B and 7C). However, a dose of 16 mg/kg KUS121 (Supplemental Figure 6A), failed to produce a beneficial effect (Supplemental Figures 6B and 6C).
Figure 7.
Beneficial Effect of a Single Administration of KUS121 After Reperfusion
(A) Schematic diagram of I/R injury procedures and schedules of KUS121 administration. (B) Representative images of Masson’s trichrome staining in the short axis of LVs at 7 days after I/R injury. Black bars indicate 1000 μm. (C) Quantification of infarcted area in LVs at 7 days after I/R injury (control group, n = 14; KUS121 group, n = 15). *p < 0.05, using unpaired 2-tailed Student’s t-test. Data are presented as mean ± SEM. Abbreviations as in Figures 2, 3, and 4.
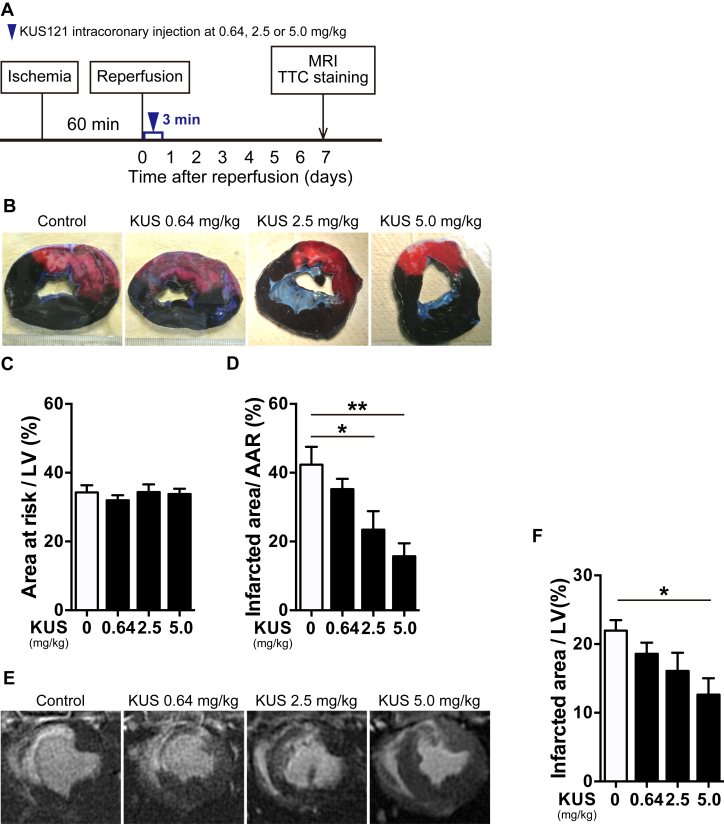
KUS121 attenuates the infarcted area in porcine I/R injury models
Finally, we examined whether KUS121 could produce therapeutic effects in porcine I/R injury models. Reperfusion was induced after 60 min of endovascular LAD coronary artery occlusion, and KUS121 was administered by intracoronary injection at a dose of 0.64, 2.5, or 5.0 mg/kg for 3 min through the wire lumen of a balloon catheter immediately after reperfusion (Figure 8A). Using double-staining with TTC and Evans blue, the infarcted area-to-AAR ratios of KUS121-treated pigs were found to be significantly lower than were those of the control pigs in a dose-dependent manner, although the AAR-to-LV ratios of KUS121-treated pigs were the same as those of control pigs (Figures 8B to 8D). In addition, we confirmed that the appearance of infarcted areas, as determined using Masson's trichrome staining, was almost the same as that evaluated using TTC staining (Supplemental Figures 7A and 7B). We also evaluated the infarcted area by late gadolinium enhancement with CMR (Figure 8E). The infarcted area-to-LV area ratios in KUS121-treated pigs were also significantly lower than were those of control pigs (Figure 8F). These data demonstrate that KUS121 intracoronary administration provides significant benefits in the presence of cardiac damage in porcine I/R injury models.
Figure 8.
KUS121 Attenuates Infarcted Areas in Porcine I/R Injury Models
(A) Schematic diagram of I/R injury procedures in pigs. (B) Representative images of double-staining with triphenyltetrazolium chloride (TTC) and Evans blue in the short axis of LVs at 7 days after reperfusion. (C, D) Quantification of infarcted area-to-area at risk (AAR) ratio and AAR-to-LV ratio in double-staining with TTC and Evans blue (control group, n = 7; KUS121 group at 0.64 mg/kg, n = 4; at 2.5 mg/kg, n = 5; at 5.0 mg/kg, n = 5). *p < 0.05, **p < 0.01 vs. control group, using 1-way analysis of variance with Dunnett's post hoc test. (E) Representative images of late gadolinium enhancement on cardiac magnetic resonance (CMR) at 7 days after reperfusion. (F) Quantification of the infarcted area-to-LV ratio using CMR. *p < 0.05 vs. control group, using 1-way analysis of variance with Dunnett's post hoc test. All data are presented as mean ± SEM. Abbreviations as in Figures 2 and 4.
Discussion
In this study, we investigated the cardioprotective effects of KUS121 using murine I/R injury models that mimic reperfusion therapy for MI, and we showed that KUS121 administration even after reperfusion was able to maintain ATP levels and attenuate the infarcted area. Echocardiographic evaluation revealed that KUS121 administration preserved cardiac function at levels similar to those of normal mice. Notably, similar beneficial effects of KUS121 were also confirmed in a porcine I/R injury model using a single administration into the coronary artery in a dose-dependent manner.
This study demonstrated that KUS121 had cardioprotective effects in vivo by the same mechanisms as in in vitro analyses. Additionally, we also confirmed that the immediate cardio-protective effects of KUS121 within 24 h after IR injury were responsible for reducing the infarct size. Using Go-ATeam2 mice, we found that KUS121 treatment even after reperfusion maintained ATP levels in an I/R injury model, most likely due to the inhibition of ATP consumption by VCP or preservation of mitochondrial function observed in H9C2 cells. As reported previously (25), we examined the expression of CHOP in I/R injury and demonstrated that KUS121 treatment after reperfusion significantly reduced CHOP expression in border zone. This also suggested that KUS121 reduced ER stress in vivo and rescued the injured myocardium from cell death. KUS121 treatment also reduced cardiomyocyte apoptosis induced by I/R injury. In our in vitro experiment, KUS121 protected H9C2 cells from H2O2-induced cell death. As reported previously (26), H2O2 is considered to induce necrotic cell death rather than apoptotic cell death. Moreover, ER stress was also reported to induce necrotic cell death (27). This indicates that KUS 121 may protect cardiomyocytes from necrotic cell death by reducing ER stress. Thus, KUS121 is presumed to attenuate the infarct size by reducing both apoptotic and necrotic cell death.
KUS121 was developed to selectively inhibit the ATPase activity of VCP without affecting its other cellular functions, and previous studies demonstrated that KUS121 can maintain cellular ATP levels, reduce ER stress, and prevent cell death in vitro when challenged with many cell-death-inducing insults in many cell types 16, 17, 18, 19. Consistent with the ability of KUS121 to maintain ATP levels and reduce ER stress, KUS121 has been reported to have neuroprotective effects in vivo (e.g., in models of murine retinitis pigmentosa, murine glaucoma, rat retinal ischemic injury, and murine Parkinson’s disease) 16, 17, 18, 19. In this study, we further demonstrated the close link among ER stress, decreased ATP levels, and cell death in the heart.
To the best of our knowledge, the cardioprotective mechanisms of KUS121 are quite different from other drugs that have been tested for the treatment of MI 7, 28, 29, 30, making KUS121 virtually unique in its ability to maintain ATP levels in animal I/R injury models. Recently, LV mechanical support using Impella (Abiomed, Danvers, Massachusetts) was reported to reduce infarct size after I/R injury (31). This was possibly due to a reduction in excessive myocardial energy demand relative to supply, which would be similar in principle to KUS121 administration in our in vivo models.
It is especially noteworthy that KUS121 is able to reduce infarct size by administration even after reperfusion, especially through intracoronary injection. This can be easily performed after primary PCI in everyday clinical practice. Thus, we concluded that KUS121 is a promising compound that could be used in conjunction with primary PCI for the treatment of MI.
Study limitations
We showed that KUS121 reduced the infarct size in murine and porcine I/R injury models by maintaining ATP levels and reducing ER stress, but the detailed mechanism of the link among ER stress, decreased ATP levels, and cell death remains to be elucidated. Further investigations are needed to fully explore the complex signaling mechanisms that are at play during MI and reperfusion.
In porcine models, we evaluated the infarct size only at 7 days after reperfusion, but we did not evaluate the effect of KUS121 at later time points. Moreover, we did not confirm the detailed safety of KUS121, although no obvious toxicity of KUS121 was observed in our study. We need to perform further studies with long-term follow-up to confirm the cardiac protective efficacy and safety of KUS121.
As previously reported 16, 32, KUS121 was confirmed to inhibit the ATPase activity of recombinant VCP in vitro with half-maximal inhibitory concentration value of 330 nM, which is much lower than that of a VCP inhibitor, DBeQ (1 μM). However, we did not evaluate the specificity and off-target effects of KUS 121 in vivo. Further studies are need to elucidate them in vivo by analysis of the pharmacokinetics and pharmacodynamics of KUS121.
Conclusions
Here, we have shown that in I/R injury models, KUS121 reduced infarct size and preserved cardiac function by maintaining ATP levels and reducing ER stress. We also showed that this effect can be achieved by the administration of KUS121 only once after reperfusion, which was confirmed in porcine I/R models. Our study indicates that KUS121 is a promising therapeutic agent for MI in conjunction with primary PCI. Progression of studies leading to a clinical trial of KUS121 is expected in the near future.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although primary PCI is the only therapy for MI to reduce infarct size and improve outcomes, a larger infarction, even after primary PCI, results in a poorer prognosis. However, there are currently no therapies additional to primary PCI to further reduce infarct size and improve clinical outcomes. Our study demonstrated that KUS121 attenuated the infarct size in murine and porcine I/R injury models. These results indicate that KUS121 may be a novel therapy for MI in conjunction with primary PCI.
TRANSLATIONAL OUTLOOK: We expect that KUS121 will one day be used in clinical practice. However, further studies are needed to investigate the pharmacokinetics and pharmacodynamics of KUS121, especially those after intracoronary KUS121 administration. Additionally, we need to verify the detailed safety of KUS121. If these are confirmed, the cardioprotective effects of KUS121 may be evaluated in clinical trials.
Acknowledgments
The authors thank Naoya Sowa for providing technical assistance. Extracellular flux analyses using XF96 were performed at the Medical Research Support Center, Graduate School of Medicine, Kyoto University. The authors acknowledge Prof. James Hejna (Kyoto University) for critical reading of the manuscript.
Footnotes
This work was supported by Ministry of Education, Culture, Sports, Science and Technology and Japan Society for the Promotion of Science Grant Nos. 18K15888 (to Dr. Ide), 17K09860 (to Dr. Horie), JP1605297 (to Dr. Kimura), 16H05151 (to Dr. Kakizuka), and 17H04177 and 17H05599 (to Dr. Ono), and a visionary research grant (Step) from the Takeda Science Foundation (to Dr. Ono). In relation to this manuscript, Kyoto University has applied for a patent (Tokugan 2018-078272). Drs. Ide, Horie, Saito, Kimura, Kakizuka, and Ono are named as inventors on the patent. Dr. Kakizuka owns stock in Kyoto Drug Discovery and Development Co., Ltd., a start-up company for the development of VCP modulators. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Akira Kakizuka, Email: kakizuka@lif.kyoto-u.ac.jp.
Koh Ono, Email: kohono@kuhp.kyoto-u.ac.jp.
Appendix
References
- 1.World Health Organization . WHO Press; Geneva, Switzerland: 2014. Global Status Report on Noncommunicable Diseases 2014. [Google Scholar]
- 2.Benjamin E.J., Virani S.S., Callaway C.W. Heart Disease and Stroke Statistics—2018 Update: a report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labour and Welfare Vital statistics in Japan—trends up to 2016. https://www.mhlw.go.jp/english/database/db-hw/dl/81-1a2en.pdf Available at: Accessed November 2018.
- 4.Levine G.N., Bates E.R., Blankenship J.C. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Circulation. 2016;113:1135–1147. [Google Scholar]
- 5.Stone G.W., Selker H.P., Thiele H. Relationship between infarct size and outcomes following primary PCI patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 6.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 7.Aihara K., Hisa H., Sato T. Cardioprotective effect of TY-12533, a novel Na(+)/H(+) exchange inhibitor, on ischemia/reperfusion injury. Eur J Pharmacol. 2000;404:221–229. doi: 10.1016/s0014-2999(00)00613-0. [DOI] [PubMed] [Google Scholar]
- 8.Piot C., Croisille P., Staat P. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K., Nakao K., Shibata Y. Randomized controlled trial of TY-51924, a novel hydrophilic NHE inhibitor, in acute myocardial infarction. J Cardiol. 2016;67:307–313. doi: 10.1016/j.jjcc.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Cung T.-T., Morel O., Cayla G. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 11.Spath N.B., Mills N.L., Cruden N.L. Novel cardioprotective and regenerative therapies in acute myocardial infarction: a review of recent and ongoing clinical trials. Future Cardiol. 2016;12:655–672. doi: 10.2217/fca-2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer H., Weihl C.C. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014;127:3877–3883. doi: 10.1242/jcs.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts G.D.J., Wymer J., Kovach M.J. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 14.Manno A., Noguchi M., Fukushi J., Motohashi Y., Kakizuka A. Enhanced ATPase activities as a primary defect of mutant valosin-containing proteins that cause inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Genes Cells. 2010;15:911–922. doi: 10.1111/j.1365-2443.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- 15.Hübbers C.U., Clemen C.S., Kesper K. Pathological consequences of VCP mutations on human striated muscle. Brain. 2007;130:381–393. doi: 10.1093/brain/awl238. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda H.O., Sasaoka N., Koike M. Novel VCP modulators mitigate major pathologies of rd10, a mouse model of retinitis pigmentosa. Sci Rep. 2014;4:5970. doi: 10.1038/srep05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano N., Ikeda H.O., Hasegawa T. Neuroprotective effects of VCP modulators in mouse models of glaucoma. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata M., Ikeda H.O., Kikkawa C. KUS121, a VCP modulator, attenuates ischemic retinal cell death via suppressing endoplasmic reticulum stress. Sci Rep. 2017;7:44873. doi: 10.1038/srep44873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano M., Imamura H., Sasaoka N. ATP maintenance via 2 types of ATP regulators mitigates pathological phenotypes in mouse models of Parkinson’s disease. EBioMedicine. 2017;22:225–241. doi: 10.1016/j.ebiom.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura H., Huynh Nhat K.P., Togawa H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano M., Imamura H., Nagai T., Noji H. Ca2+regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol. 2011;6:709–715. doi: 10.1021/cb100313n. [DOI] [PubMed] [Google Scholar]
- 22.Vannuvel K., Renard P., Raes M., Arnould T. Functional and morphological impact of ER stress on mitochondria. J Cell Physiol. 2013;228:1802–1818. doi: 10.1002/jcp.24360. [DOI] [PubMed] [Google Scholar]
- 23.Lebeau J., Saunders J.M., Moraes V.W.R. The PERK Arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep. 2018;22:2809–2817. doi: 10.1016/j.celrep.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong J.Q., Molkentin J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015;21:206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki Y., Kaikita K., Endo M. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 26.Ali M.A.M., Kandasamy A.D., Fan X., Schulz R. Toxicology in vitro Hydrogen peroxide-induced necrotic cell death in cardiomyocytes is independent of matrix metalloproteinase-2. Toxicol Vitr. 2013;27:1686–1692. doi: 10.1016/j.tiv.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Ullman E., Fan Y., Stawowczyk M., Chen H.M., Yue Z., Zong W.X. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008;15:422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malka A., Ertracht O., Bachner-Hinenzon N., Reiter I., Binah O. The cardioprotective efficacy of TVP1022 against ischemia/reperfusion injury and cardiac remodeling in rats. Pharmacol Res Perspect. 2016;4:1–15. doi: 10.1002/prp2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki K., Chen L., Ikeno F. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.-S. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. AJP Hear Circ Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 31.Saku K., Kakino T., Arimura T. Left ventricular mechanical unloading by total support of Impella in myocardial infarction reduces infarct size, preserves left ventricular function, and prevents subsequent heart failure in dogs. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004397. [DOI] [PubMed] [Google Scholar]
- 32.Chou T.-F., Brown S.J., Minond D. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc Natl Acad Sci U S A. 2011;108:4834–4839. doi: 10.1073/pnas.1015312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.