Abstract
Gene therapy for severe hemophilia B is advancing and offers sustained disease amelioration with a single treatment. We have reported the efficacy and safety of AMT-060, an investigational gene therapy comprising an adeno-associated virus serotype 5 capsid encapsidating the codon-optimized wild-type human factor IX (WT hFIX) gene with a liver-specific promoter, in patients with severe hemophilia B. Treatment with 2 × 1013 gc/kg AMT-060 showed sustained and durable FIX activity of 3%–13% and a substantial reduction in spontaneous bleeds without T cell-mediated hepatoxicity. To achieve higher FIX activity, we modified AMT-060 to encode the R338L “Padua” FIX variant that has increased specific activity (AMT-061). We report the safety and increased FIX activity of AMT-061 in non-human primates. Animals (n = 3/group) received intravenous AMT-060 (5 × 1012 gc/kg), AMT-061 (ranging from 5 × 1011 to 9 × 1013 gc/kg), or vehicle. Human FIX protein expression, FIX activity, and coagulation markers including D-dimer and thrombin-antithrombin complexes were measured. At equal doses, AMT-060 and AMT-061 resulted in similar human FIX protein expression, but FIX activity was 6.5-fold enhanced using AMT-061. Both vectors show similar safety and transduction profiles. Thus, AMT-061 holds great promise as a more potent FIX replacement gene therapy with a favorable safety profile.
Keywords: AAV, AAV5, AMT-060, AMT-061, adeno-associated virus, factor IX, gene therapy, hemophilia B, non-human primate
Introduction
Hemophilia B is an attractive target for gene therapy as a modest increase in the clotting factor activity can result in clinical benefits and attenuation of the bleeding risk.1 We have previously reported that AMT-060 at doses of 5 × 1012 and 2 × 1013 gc/kg (n = 5) in a phase I/II clinical trial resulted in stable and clinically relevant increases in FIX activity (3%–13%) and a marked reduction in spontaneous bleeds and coagulation factor IX (FIX) usage.2 While AMT-060 was associated with transient elevations in liver enzymes in 3/10 participants, which were treated with corticosteroids, these were not associated with lessening of FIX activity. In addition, there were no detectable cytotoxic immune responses against the capsid.2 Although a clear benefit was observed, achievement of higher FIX activity is likely to offer additional clinical benefits, including protection against traumatic bleeds and a return to a more normal lifestyle for patients. In the current study, we modified the AMT-060 vector to encode a hyper-functional FIX variant, known as “Padua,” that has an 8- to 9-fold increased specific activity compared to wild-type (WT) FIX.3 All other elements of the vector design including the capsid, promoter, and codon optimization remained unchanged. The vector, AMT-061, was designed to have enhanced potency compared to AMT-060 without impacting the favorable safety profile.
Hemophilia B is an X-linked bleeding disorder caused by a deficiency or dysfunction of FIX due to mutations in the FIX gene.4, 5, 6 Patients suffer from spontaneous hemorrhages and are classified as severe, moderate, or mild depending on their residual FIX activity levels (severe: <1%, moderate: 1% to <5%, and mild: 5% to <40%).7 Bleeds in severe hemophilia B patients occur in joints, soft tissues, and muscles8 and result in disabling synovitis, crippling arthropathy, and muscle atrophy.9 Hemophilia B is managed by intravenous injection of purified plasma-derived or recombinant FIX, which is given prophylactically or on demand to manage bleeding episodes.8 Unfortunately, intravenous factor replacement is cumbersome, demanding on peripheral veins, and is very costly. Importantly, factor replacement therapy does not always prevent spontaneous bleeds and may induce immune reactions to the replaced FIX8, 10, 11 and does not completely prevent arthropathy, which may even occur in patients that have never reported a joint bleed.12, 13
Normal FIX activity in people without hemophilia generally ranges 50%–150%; however, the optimal therapeutic range of FIX following gene transfer is unclear. Experience with FIX prophylaxis indicates that achieving trough FIX activity >1% can reduce bleed frequency and improve joint outcomes.14 It is expected that achieving sustained FIX activity in the normal range with gene transfer will further improve bleed reduction and long-term joint outcomes versus prophylaxis. Nevertheless, it is unclear whether the induction of FIX activity in the high normal or above normal range could be associated with increased thrombotic risk. After controlling for potentially confounding variables (age, sex, use of oral contraceptives, and levels of other clotting factors), a level of FIX >129% of normal was associated with a more than 2-fold increase in the risk of developing deep vein thrombosis.15 However, a similar study found that upon further adjustment for body-mass index, association between risk of thrombosis and FIX levels disappeared.16
The efficacy of gene therapy has been tested in animal models (hemophilic mice and dogs) and FIX activity in the plasma correlates well with disease severity.17, 18, 19, 20 Gene therapy will ideally result in steady and long-term FIX expression that is effective in eliminating bleeds and preserves the joint functions, thereby overcoming the limitations of current treatments.21, 22, 23 Given that adeno-associated virus (AAV) is a primate virus, which does not naturally infect non-primates such as mice and dogs, and that non-human primates (NHPs) are evolutionarily closer to humans, NHPs provide the most appropriate pre-clinical animal model for human AAV-mediated gene transfer.24, 25 Therefore, we investigated FIX activity and safety following different doses of AMT-061 in non-human primates. This study demonstrates that the safety profile of AMT-061 is comparable to AMT-060 in NHPs and confirms that AMT-061 displays increased FIX activity that is characteristic for the hyperactive Padua variant.
Results
Administration of AMT-060 and AMT-061 Results in Dose-Dependent Human Factor IX Protein Expression and FIX Clotting Activity in NHPs
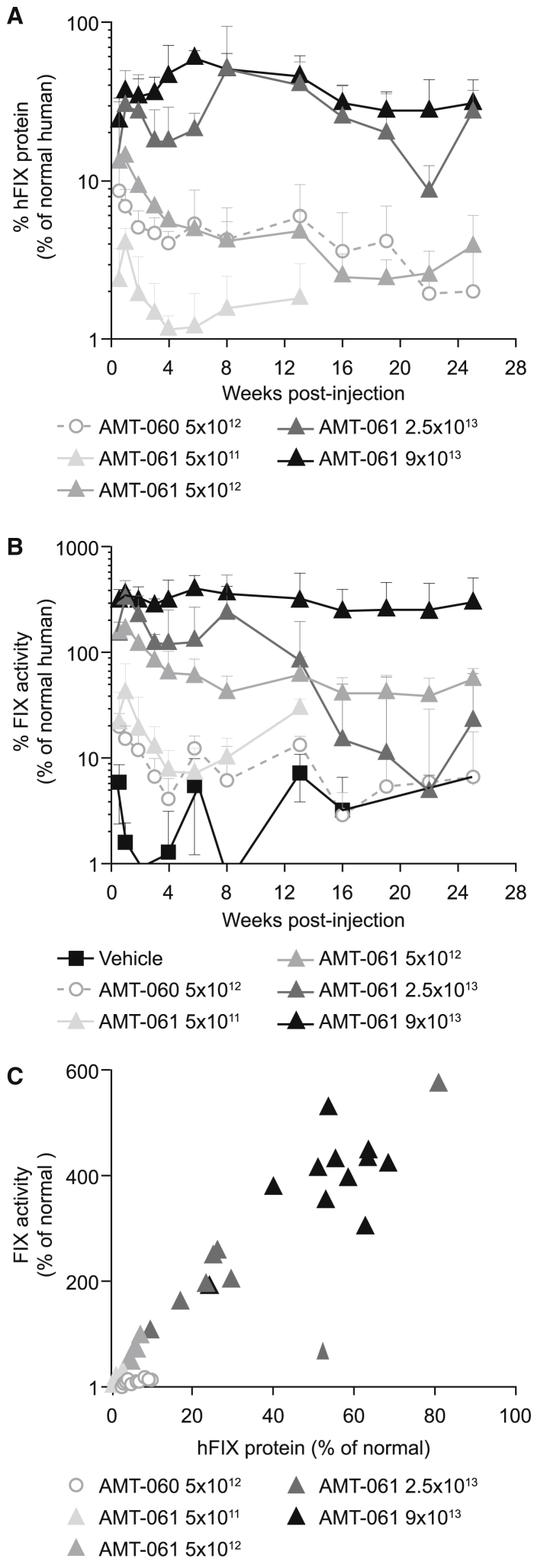
Cynomolgus monkeys were intravenously injected with vehicle, AMT-060 at a dose of 5 × 1012 genome copies/kg (gc/kg) or AMT-061 with several doses (5 × 1011, 5 × 1012, 2.5 × 1013, and 9 × 1013 gc/kg) and followed up for 26 weeks after dosing (n = 3 per group). Two of the doses cover those used in the ongoing AMT-060 clinical trial (NCT02396342). Plasma samples were collected at 13 time points during the study for determination of human FIX (hFIX) protein and FIX activity levels. hFIX protein was not detected in pre-treatment and vehicle treated animal samples confirming the specificity of the ELISA for human FIX. AMT-061 treatment resulted in increasing circulating hFIX protein levels, averaging around 1%–2% of normal human levels in the low dose group up to approximately 60% in the highest dose group (Figure 1A). The hFIX protein expression kinetics of AMT-061 and AMT-060 at 5 × 1012 gc/kg were similar, with levels peaking shortly after dosing followed by a decline to reach stable levels after week 4 throughout the study (Figure 1A). The 9 × 1013 gc/kg group of AMT-061 showed slightly different kinetics, with a rise in hFIX levels up to week 6 followed by stabilization until the end of the study. Cynomolgus antibodies against hFIX were detected after treatment with AMT-060 and AMT-061, independent of the dose (Figure S1). One animal in the AMT-060 group, 1 animal in the 5 × 1012 gc/kg dose group of AMT-061, 3 animals in the 2.5 × 1013 gc/kg dose group of AMT-061, and 1 animal in the high dose (9 × 1013 gc/kg) group of AMT-061 developed antibodies against hFIX. All animals in the 2.5 × 1013 gc/kg dose group of AMT-061 developed anti-hFIX-specific antibodies, which was reflected in a decline of hFIX protein expression at 12 weeks post-treatment (Figure S1B; Figure 1A). Antibody development against the expressed therapeutic transgene has been previously reported in studies using AAV-hFIX and AAV-hFVIII in NHPs.26, 27
Figure 1.
Expression of hFIX in NHPs after Peripheral Administration of AMT-061 and AMT-060
(A) hFIX protein in the monkey plasma was determined at different time points after AAV administration. Mean of n = 3 ± SD, n = 2 for 2.5 × 1013 gc/kg group. (B) FIX activity as measured by activated partial thromboplastin (APTT) assay in the plasma of the NHPs post-treatment after baseline correction. Endogenous baseline FIX activity levels were determined by three pre-treatment clotting activity determinations. Mean of n = 3 ± SD, n = 2 for 2.5 × 1013 gc/kg group. (C) Correlation of the expressed hFIX protein and measured FIX activity from week 4–13.
To compare the clotting activity after transduction with AMT-060 or AMT-061, we performed a FIX-specific one-stage APTT test. Endogenous monkey baseline FIX activity was around 50% of normal human levels and the FIX activity shown is baseline corrected (Figure 1B). On average (weeks 4–13 period) an increase of baseline-corrected FIX activity of 9.1% and 58.9% was detected in the AMT-060 and AMT-061 group (5 × 1012 gc/kg), respectively. There was a non-linear dose-dependent increase in baseline-corrected FIX activity induced by AMT-061 to over 400% in the highest dose group (Figure 1B) and this correlated with the hFIX protein levels (Figure 1C).
Similar hFIX Protein Levels but Higher FIX Activity Level in AMT-061 Group
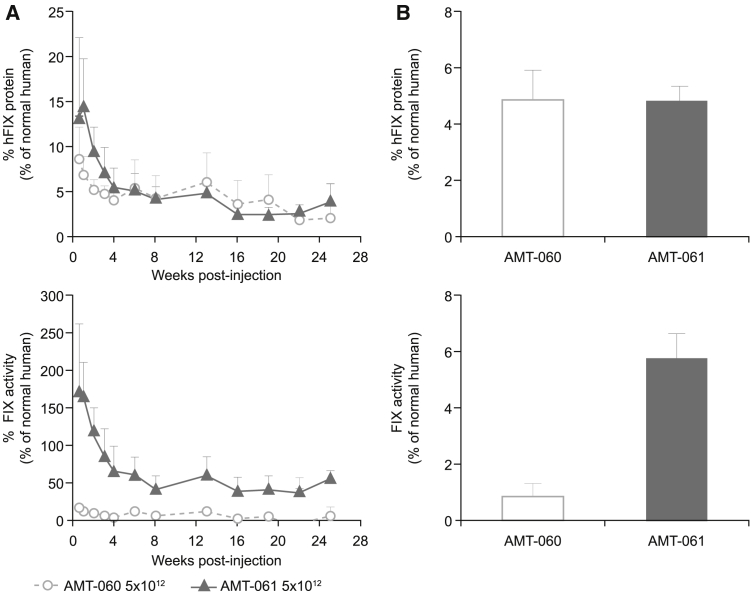
In animals injected with an equal dose of AMT-060 and AMT-061, the hFIX expression pattern over time was identical (Figure 2A), while FIX activity levels were substantially higher in the AMT-061 group, which is 6.5-fold higher compared to the AMT-060 group (Figure 2B).
Figure 2.
Comparison of the hFIX Protein Expression and FIX Activity Level in the Plasma of NHPs that Received AMT-060 and AMT-061 at 5 × 1012 gc/kg
Mean of n = 3 ± SD. (A) hFIX protein expression and FIX clotting activity in the plasma of the NHPs that received 5 × 1012 gc/kg of AMT-061 and AMT-060 during the study. (B) Average hFIX protein expression and FIX clotting activity determined in the NHPs from week 4 to 13.
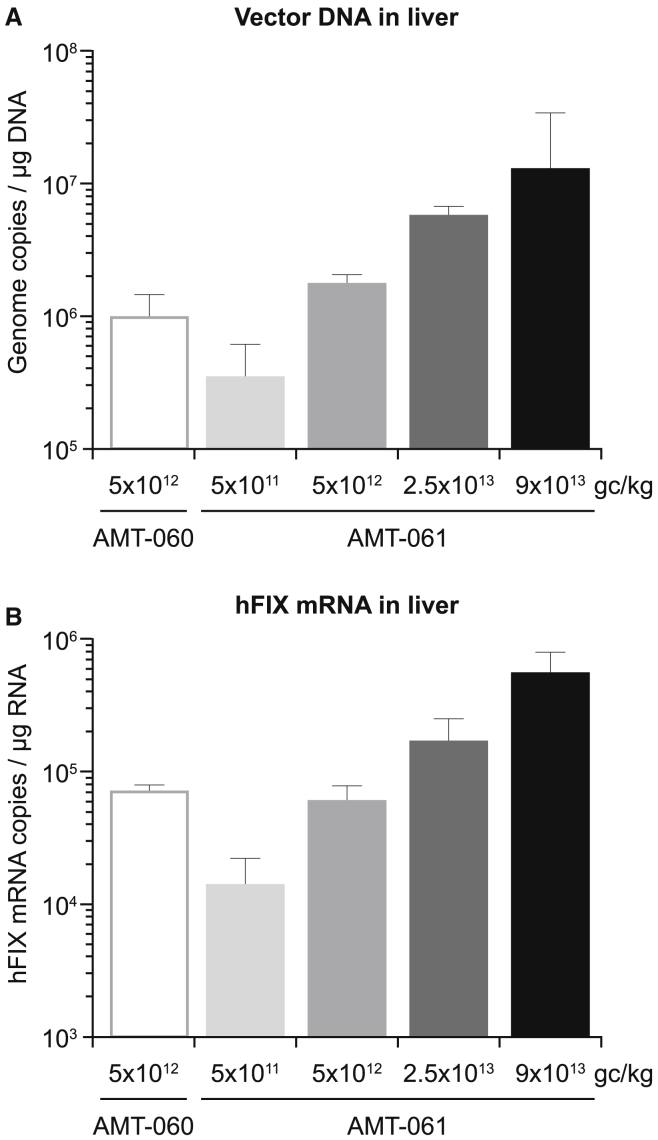
Dose-Dependent Vector DNA Levels and hFIX mRNA Expression in the Liver after AMT-061 Administration
Vector genome copies in liver samples were determined by qPCR. A non-linear dose-dependent increase of liver vector DNA levels was detected in animals that received increasing doses of AMT-061, ranging from 3 × 105 to ∼1 × 107 gc/μg of genomic DNA (Figure 3A) with comparable DNA levels in the AMT-060 and AMT-061 group injected with the same dose. hFIX mRNA expression pattern in the liver was identical to the vector DNA expression and similar hFIX mRNA levels were detected in the livers of NHPs that received equivalent dose of AMT-060 and AMT-061 (Figure 3B). Thus, AMT-060 and AMT-061 administration at equal dose results in similar vector DNA transduction and mRNA expression levels in the liver.
Figure 3.
Detection of hFIX Specific Vector DNA Copies and hFIX mRNA in the Liver of the AAV-Injected NHPs
Mean of n = 3 ± SD. (A) A dose-dependent increase in vector DNA copies and (B) hFIX mRNA expression in the livers of the NHPs that received increasing doses of AMT-061.
Dose-Dependent Vector DNA and FIX mRNA Levels following Transduction with AMT-061 and AMT-060 in the Liver
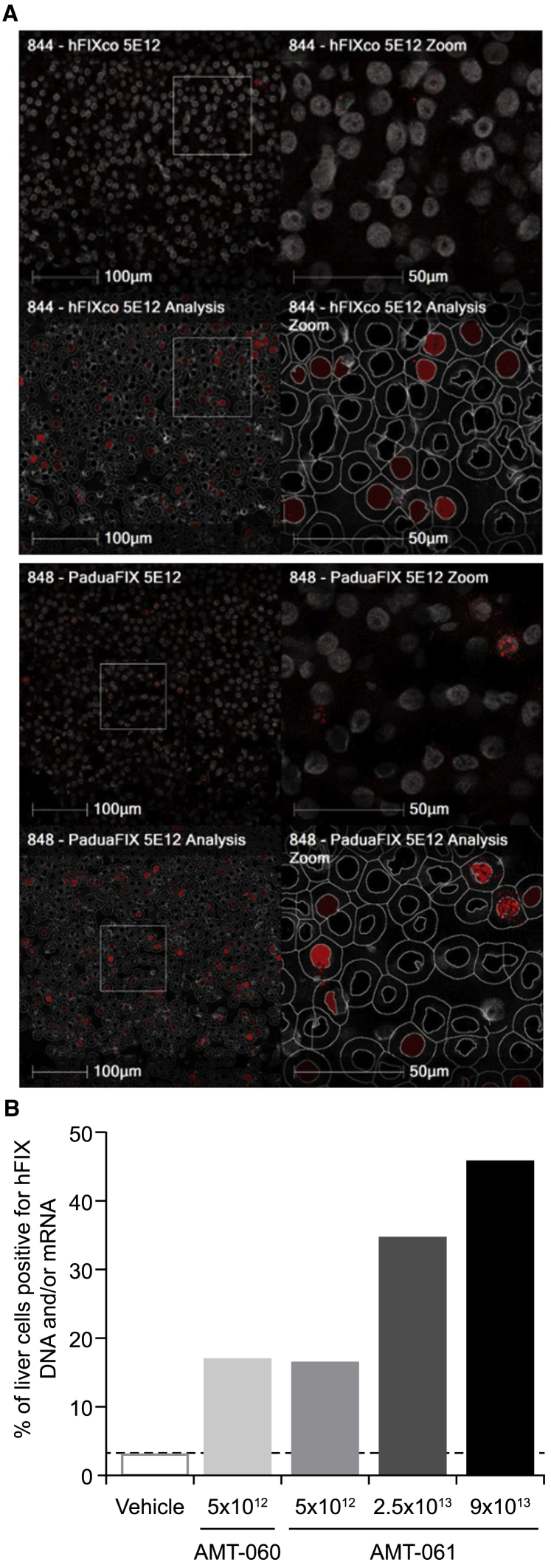
A liver tissue sample from the left median lobe from one animal per treatment group was analyzed for the presence of vector DNA and hFIX transgene mRNA by fluorescent in situ hybridization. The assay was performed with a probe that hybridizes to WT and Padua vector DNA and hFIX mRNA. At similar dose of AMT-060 and AMT-061, the percentage of positive liver cells was comparable (17.1% and 16.7%) (Figure 4A). AMT-061 treated animals showed a non-linear dose-related increase in the percentage of liver cells positive for vector DNA and transgene mRNA ranging from 17.1% to 46% (Figure 4B). Given that these percentages are based on the analysis of a single biopsy for each dose group, these results should be considered as indicative and should not be interpreted as overall values for the total liver. The number of positive liver cells correlated with the levels of vector DNA and transgene mRNA detected in the liver by qPCR in the same liver sample (data not shown).
Figure 4.
Percentage of Liver Transduction after Liver Targeted Delivery of the AAV Vectors
(A) In situ hybridization on liver tissue of NHPs that received AMT-060 and AMT-061 to detect hFIX specific DNA and/or mRNA. (B) The percentage of transduced liver cells was determined by quantification of the in situ hybridized liver tissues derived from the AAV-injected animals.
Pharmacokinetic Profile of AMT-060 and AMT-061
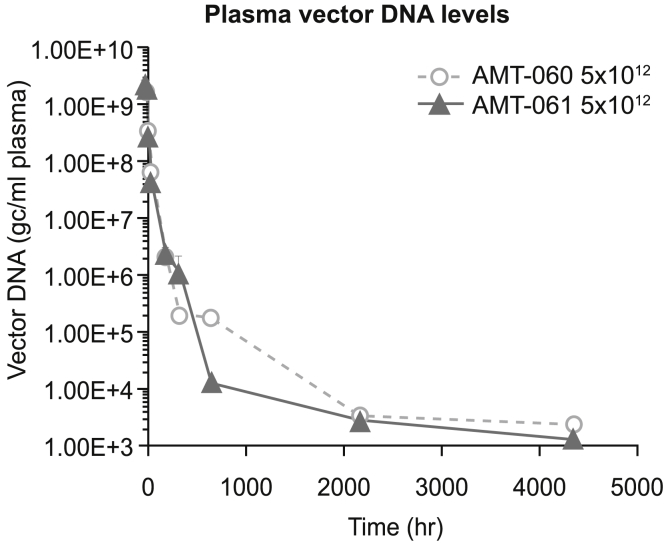
To assess the pharmacokinetic profiles of AMT-060 and AMT-061, vector DNA analyses were performed on plasma samples taken shortly after infusion until the end of the 26-week observation period. Peak vector DNA (Cmax) and plasma vector DNA levels over time in the AMT-060 and AMT-061 treated animals were comparable and consistent with the intravenous infusion dose route (Figure 5). Concentrations declined in a bi-phasic manner up to the end of the sample period (4,224 h or 26 weeks). The area under curve (AUC), a measurement for levels of circulating vector DNA over time, results were comparable (3.22 × 1010 and 3.25 × 1010 gc/ml) for AMT-060 and AMT-061.
Figure 5.
The Pharmacokinetic Profile of the Vector Was Determined by Detecting the Vector DNA Levels in the Plasma of the Animals Over Time
Data shown are mean (SEM).
Safety Evaluation of Intravenous Infusion of AMT-060 and AMT-061
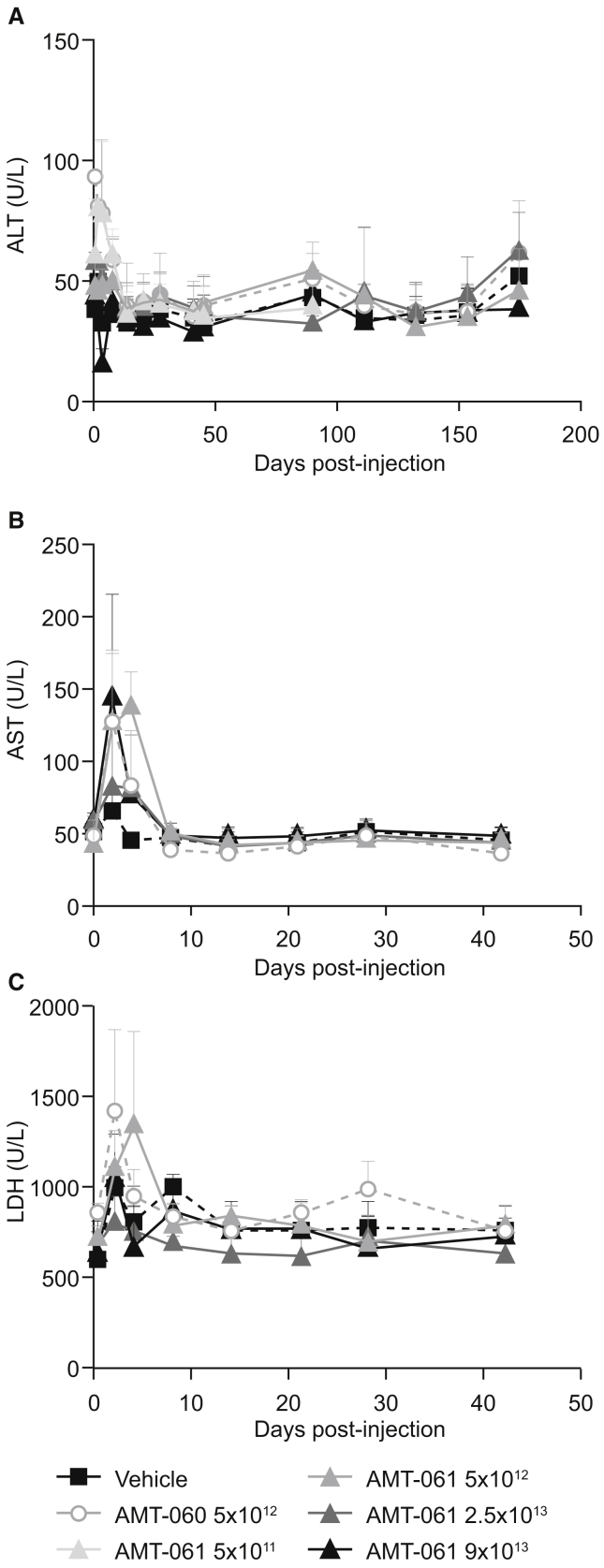
Previously transient asymptomatic elevation of liver transaminases has been observed after administration of AAV vectors in clinical trials for hemophilia B;28, 29 therefore, detection of several liver enzyme levels was included as a general safety measure. At day 2, a mild and transient increase of alanine aminotransferase (ALT; up to 1.90× control) and aspartate aminotransferase (AST; up to 2.22× control) was observed in all animals (Figure 6). By day 4, ALT levels had returned to pre-treatment levels except in a single animal in the low-dose group of AMT-061. AST and LDH levels returned to baseline by day 8 and remained within the normal range until the end of the study. No changes from baseline were observed for any of the other measured liver enzymes and clinical chemistry parameters. The hematological profile and histopathological examination of all major organs revealed no treatment related findings in the animals, supporting the safety profile of AMT-060 and AMT-061.
Figure 6.
Liver Enzyme Profiles of NHP Plasmas following AAV Administration
Mean of n = 3 ± SD. (A) Alanine aminotransferase (ALT). (B) Aspartate aminotransferase (AST). (C) Lactate dehydrogenase (LDH) was determined in the plasma of the animals.
AMT-061 and AMT-060 Treatment Does Not Increase the Prothrombotic Markers: Thrombin-Antithrombin Complex and D-Dimers
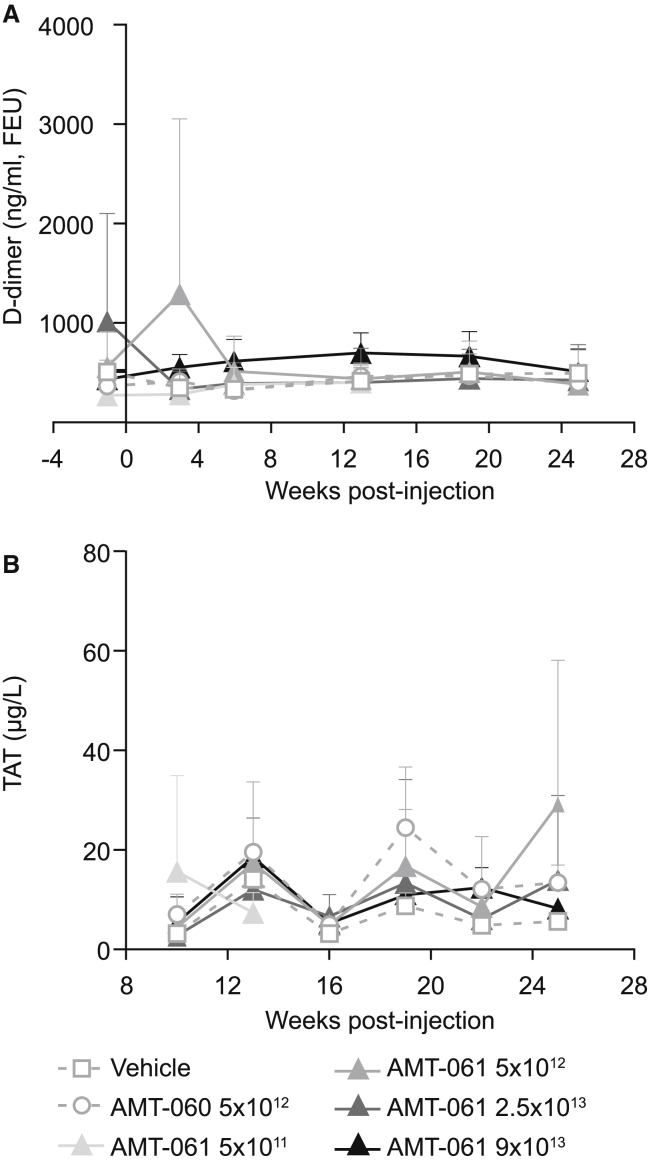
No animals developed spontaneous thrombosis during the study. Nonetheless, the risk of a possible prothrombotic phenotype due to AMT-060 and AMT-061 administration was assessed by determining the levels of plasma thrombin-antithrombin (TAT) complex and D-dimers. At random time points, increased D-dimer levels were observed in pre-treated, post-treated, and in control animals. Statistical analysis showed that there is no significant difference between any of the groups. Treatment with AMT-060 or AMT-061 did not induce a prothrombotic state even at FIX activity up to 500% in hFIX-Padua expressing animals as D-dimer levels (Figure 7A) and the TAT levels stayed constant at 6 different time points post-treatment (Figure 7B).
Figure 7.
Coagulation Activation Markers Were Measured at Different Time Points following AAV Delivery
Mean of n = 3 ± SD. (A) Thrombin-antithrombin (TAT) levels were determined in the plasma of the NHPs. (B) D-dimer levels in the plasma of the animals were measured before and after treatment.
Potential Immunogenicity of the Padua Variant
The potential immunogenicity risk related to the Padua mutation was assessed by an in silico analysis. The full-length WT hFIX protein sequence and the FIX protein sequence with the Padua mutation were evaluated for their global and regional immunogenic potential by use of an in silico platform for epitope identification and prediction (EpiMatrix system developed by Epivax) for class I (all nucleated cells) and class II (antigen-presenting cells) human leukocyte antigen (HLA) (Figure S2).
At both a global and regional level, FIX-Padua did not show a significant change in EpiMatrix hits restricted by class I or class II HLA, with minimal observed changes in EpiMatrix score compared to WT FIX. Altogether, there is no predicted significant difference in immunogenicity between the WT FIX and FIX-Padua.
Discussion
In the current study, we tested an improved vector, AMT-061, that expresses the hyperactive FIX-Padua variant instead of the WT FIX transgene in our previous AMT-060 vector. The safety and efficacy of AMT-060 has been studied in a phase I/II clinical trial that included ten patients with moderate (n = 1) or severe (n = 9) hemophilia B at doses of 5 × 1012 and 2 × 1013 gc/kg (n = 5).2 Treatment was well tolerated and effective in all patients, resulting in discontinuation of FIX prophylaxis for 8 of 9 patients with severe hemophilia. Although the occurrence of bleeding episodes was reduced following AMT-060 treatment relative to pre-treatment history on high-dose prophylactic FIX replacement therapy, it is not expected to completely prevent arthropathy and therefore we have improved the design of AMT-060 by introducing a single amino acid change in the encoded protein to encode the hyperactive FIX-Padua for increased FIX activity.
The present study demonstrates successful intravenous administration of AMT-061 via peripheral vein infusion, which resulted in a dose-dependent expression of hFIX protein and increased FIX activity levels in NHPs. 6.5-fold higher baseline-corrected FIX activity levels were detected in the AMT-061 injected NHPs compared to AMT-060, thereby confirming the higher potency of the FIX-Padua variant as previously reported (Table 1).30, 31, 32, 33, 34 Administration of 5 × 1012 gc/kg of AMT-060 and AMT-061 into NHPs resulted in similar pharmacokinetic profiles in plasma, similar vector DNA levels and similar liver transduction efficiency, which supports the comparable FIX transgene expression in the NHP plasma (Table 1). As the protein expression by both AMT-060 and AMT-061 was similar in NHPs, it can be expected that AMT-061 at a dose of 2 × 1013 gc/kg in humans would result in FIX activity levels in the upper mild into the non-hemophilic ranges.2 Although one expects that levels observed following AMT-060 should reduce joint damage over time,2 more factors can impact the process of developing arthropathy other than the FIX levels. Current treatment using clotting factor has been typically provided in amounts that are just sufficient to limit joint bleeds due to the high costs and limited availability, but subclinical hemarthroses can still occur.12 Despite early prophylaxis, some patients are susceptible to develop joint disease due to unrecognized bleeding episodes that were not treated in a timely matter or due to repeated injury. A gene therapy approach that results in higher FIX activity would ideally result in discontinuation of prophylaxis, reduction in annualized bleed rate, but also prevention of blood-induced arthropathy that is the main cause of morbidity in hemophilia.35
Table 1.
Comparison of AMT-060 and AMT-061
| AMT | Dose (gc/mL) | Plasma AUC (gc/mL) | Liver Vector DNA (gc/mL) | Liver FIX RNA (gc/mL) | Liver Cell Transduction (%) | hFIX Protein Weeks 4–13 (%) | FIX Clotting Activity Weeks 4–13 (%) | Ratio of FIX Activity 061/060 |
|---|---|---|---|---|---|---|---|---|
| 060 | 5.0 × 1012 | 3.20 × 1010 | 1.0 × 106 | 7.3 × 104 | 17.1 | 4.89 | 9.1 | 6.5 |
| 061 | 5.0 × 1012 | 3.25 × 1010 | 1.7 × 106 | 7.3 × 104 | 16.7 | 4.85 | 58.9 |
Expression of hFIX-Padua did not show abnormal coagulation activation in NHPs and the in-life, hematology, and clinical chemistry parameters and full histopathological examinations of major organs were unaffected by treatment with AMT-061. The safety assessment revealed no adverse findings and the No Observed Adverse Effect Level (NOAEL) was the highest dose studied. Our findings are consistent with previous studies in hemophilic dogs showing that FIX-Padua was not associated with pathological coagulation activation or thrombosis.31, 34 Notably, the animals in the highest dose group of AMT-061 reached up to 500% FIX activity levels without any signs of adverse effects. This is not unexpected, considering that thrombosis occurred in the patient with naturally-occurring FIX-Padua at activity levels of >700% of normal, but thrombosis did not occur either in the younger brother that was hemizygous for the gene mutation nor in the mother that had >500% and >300% FIX activity, respectively.3
Immune responses have been a major issue in gene therapy trials for hemophilia using either WT or FIX-Padua. A recent phase I/II trial (NCT01687608) utilized an AAV8 vector expressing FIX-Padua. Seven patients were dosed, 2 received 2 × 1011 gc/kg, 3 received 1 × 1012 gc/kg, and 2 received 3 × 1012 gc/kg of AAV. Peak FIX activity levels of 30%–60% of normal were observed in the highest dose group. However, a sharp decline in expression occurred at around week 6 with a concomitant increase in ALT levels. Only a single subject had sustained FIX activity expression for more than 2.5 years.36 Another trial (NCT02484092) involved 10 patients dosed with AAV expressing FIX-Padua that showed sustained FIX activity levels. Two patients had elevated liver enzymes, one above the normal limit at day 34, that were resolved using prednisone.37 There was no evidence of the development of humoral or T cell mediated immune responses against FIX-Padua. Instead, the increases in liver enzymes corresponded with periods of increased cellular immune responses to AAV detected by interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assay.37 Another clinical trial (NCT02618915) utilized the AAVrh10 capsid encapsidating the WT FIX gene. Two groups of subjects were injected (n = 3) at a dose of 1.6 × 1012 and 5 × 1012 gc/kg. There was a clear dose-dependent response in the FIX expression. Five subjects experienced elevated ALT levels and lost FIX expression to near baseline levels despite prednisone administration.38 With AMT-060, transient increases in liver transaminases were observed that required corticosteroid treatment. These occurred in approximately 30% of participants at around 10 weeks following AMT-060 treatment but did not result in decreases in FIX activity.2 Modest increases in liver transaminases were also observed following gene-transfer in NHP in this study and another study.39
Although the ALT levels in this study were elevated compared with baseline, they were within the normal range described for male cynomolgus macaques (20–177 IU/L),40 did not appear to be dose dependent and returned to pre-treatment levels by day 4 post-gene therapy treatment. For AST, the increases appeared to be greater in the higher doses of the AMT-061 groups, but levels returned to normal by day 8. There was no evidence of FIX expression being affected by the transient increases in liver enzymes. It is interesting that the timing of liver enzyme elevations in the NHP model occurred much earlier (in the first 10 days post gene transfer) compared with human studies and a preclinical study in dogs (4–10 weeks post gene transfer).2, 28, 37 It is possible that in some of the trials liver enzymes were not measured at early time points, however, a preclinical study of FIX liver-directed gene transfer in dogs reported transient elevations of liver enzymes below or just above the upper limit of normal in the first few days following administration.41 ELISPOT assays were not performed in this study so we cannot say whether the acute liver enzyme elevations were associated with markers of cellular immune responses such as interferon-γ. Thus, it is unclear whether the increases in liver enzymes observed in the first 10 days following FIX-Padua administration in this study reflect the same processes as those liver enzyme elevations reported at later time points in other trials, which in the latter case are usually associated with evidence of activation of cellular immunity.
The canine FIX-Padua transgene was previously tested in inhibitor prone hemophilic dogs without formation of inhibitory antibodies to FIX-Padua even upon multiple challenges of WT canine FIX protein.34, 42 Importantly, the FIX-Padua transgene has been recently used in a clinical trial where 10 patients received AAV encoding the hyperactive FIX-Padua variant.37 None of the patients developed inhibitors specific to the transgene. In addition, in silico analyses indicate no increased risk of potential immunogenicity for the FIX-Padua protein compared to the WT FIX protein.
Thus, immune responses in hemophilia B clinical trials are more likely caused by the AAV vectors used than the FIX transgene. The AAV5 vector has a lower prevalence of neutralizing antibodies compared to other serotypes and individuals who are seropositive for AAV5 generally have low antibody titers.43, 44 Furthermore, recent findings have indicated that low levels of pre-existing antibodies against AAV5 in human (up to titers of 340) and NHP (up to titers of 1,030) samples have been shown not to impact transduction and expression of FIX using AAV5 vectors.45 Given that the planning for this study predated these findings, NHPs were screened for pre-existing antibodies to AAV5 as part of the entry criteria to the study. Additionally, cytotoxic T cell responses against the AAV5 capsid were not detected in clinical trials,46, 47 whereas cell-mediated immunity against capsid antigens have been observed for AAV2 and AAV8 vectors resulting in loss or decline in transgene expression, which may be controlled with glucocorticoids administration.28, 48
Importantly, the present study demonstrates that our AAV5 gene therapy platform can function as modular components with reproducible characteristics and results. Switching the transgene (FIX-Padua versus WT FIX) in our liver-directed AAV5 resulted in AAV5 vectors having very similar characteristics in terms of liver transduction, protein expression and safety profile.
In conclusion, a 6.5-fold increase in FIX activity in NHPs was achieved upon administration of AMT-061 compared to AMT-060. One-time intravenous administration was well tolerated and resulted in sustained expression up to 26 weeks post-treatment. These promising results of AMT-061 in NHPs combined with the phase I/II study clinical results of AMT-060 support a rapid transition of AMT-061 into clinical studies.
Materials and Methods
Recombinant AAV Vectors
The AAV5 vectors were manufactured in insect cells using a baculovirus expression system. AMT-060 is an AAV5 vector with a gene cassette containing a liver-specific promoter (LP1) and a codon-optimized WT human FIX gene.29, 48 AMT-061 is identical to AMT-060, except that the vector genome harbors a two-nucleotide mutation, resulting in the R338L substitution (Padua).
Animal Experiments
The in-life experimental procedures were in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012, and the study was conducted in compliance with OECD Principles of Good Laboratory Practices (GLP) (ENV/MC/CHEM(98)17). The study was designed to meet the requirements of the following guidelines: European Parliament and Council Directive 2001/83/EC of November 6, 2001 of the Community Code Relating to Medicinal Products for Human Use, OJ L311/67-128, November 28, 2001 as amended Commission Directive 2003/63/EC, OJ L159, June 27, 2003, EMA/CAT/80183/2014- Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products and FDA 2013 Guidance for industry – Preclinical assessment of investigational cellular and gene therapy products.
The animals were housed in cages of stainless-steel framework in trios of the same sex and dose group, except during dosing. The cages were specifically designed to house NHPs in clean and secure conditions. The animal facility is equipped to minimize entry of external biological and chemical agents and to minimize the transference of such agents between rooms. The air supply is filtered fresh air, which was passed to atmosphere and not recirculated. The temperature was within the range of 15–24°C and humidity was 40%–70%. The lighting is artificial with 12 h light and 12 h dark. Each morning, the residue of the food (not including any fruit) from the previous day was removed and weighed before new food was offered. Each animal was offered two biscuit supplements (each weighing 25 g) daily. Potable water from the public supply was made available via automatic valves, which were checked daily, and was not restricted. A detailed weekly physical examination was performed on each animal to monitor general health. A viability check was performed near the start and end of each working day. Animals were isolated or killed for reasons of animal welfare where necessary. No specific contaminations were known that may have interfered with or prejudiced the outcome of the study and therefore no special assays were performed.
Male cynomolgus macaques (Macaca fascicularis; n = 3 per group) older than 2 years of age received a single intravenous administration of AMT-061 (the Padua variant) in different doses: 5 × 1011, 5 × 1012, 2.5 × 1013, and 9 × 1013 gc/kg, and AMT-060 (5 × 1012 gc/kg) or the vehicle control. Two selected doses for AMT-061 cover the doses of AMT-060 in the ongoing clinical trial (NCT02396342). The animals were pre-screened for neutralizing antibodies against AAV5 using a cell-based luciferase assay. Antibodies against hFIX were analyzed at different time points during the study (Supplemental Methods). Blood was collected prior to AAV-injection and throughout the 26-week study (d1, d4, d8, wk2, wk3, wk4, wk6, wk8, wk13, wk16, wk19, wk22, and wk25) or until week 13 for the 5 × 1011 gc/kg group of AMT-061.
ELISA for Quantitating hFIX in NHP Plasma
Human FIX antigen was detected with ELISA using a mouse monoclonal antibody specific to hFIX. Briefly, flat-bottomed 96-well plates were coated with the monoclonal antibody; AHIX-5041 (Haematologic Technologies, Essex Junction, VT, USA) at a dilution of 1:3,000 at 4°C overnight. Next day, wells were emptied and washed three times using PBS/0.05% Tween-20 (wash buffer) and blocked for 1 h at room temperature using PBS/0.05% Tween containing 6% of BSA. Serial dilutions of human plasma in PBS/0.05% Tween containing 2% BSA served as standard. NHP plasma samples were diluted 100-fold in wash buffer loaded into the wells and incubated for 1 h at room temperature. After washing, horseradish peroxidase-conjugated polyclonal goat immunoglobulin G (IgG) against human FIX (Cedarlane Laboratories, Burlington, ON, Canada) was added and incubated for 1 h at room temperature. After the final wash, plates were developed using o-Phenylenediamine (Thermo Fisher Scientific, Waltham, MA, USA) and stopped after 10 min using 2M sulfuric acid (Merck, Darmstadt, Germany). Optical density (OD) was assessed spectrophotometrically at 490 nm using the Versamax Molecular Devices (San Jose, CA, USA). The relation between hFIX levels and OD is determined by a 4-parameter nonlinear regression of the calibrator curve.
Assessment of FIX Clotting Activity Levels by One-Stage Activated Partial Thromboplastin Time Assay
FIX clotting activity levels were determined by one-stage activated partial thromboplastin time (APTT) assay using coagulometric (turbidimetric) principle on the ACL TOP 300 (Instrumentation Laboratory). Plasma samples were mixed with hFIX-deficient plasma and the values were compared with a reference standard consisting of serial dilutions of normal human plasma in hFIX-deficient plasma. Baseline clotting activity was determined in multiple pre-treatment samples and the clotting activity post-treatment was corrected for the endogenous baseline levels.
Measurements of TAT Complexes and D-Dimers
TAT complexes were detected using a sandwich enzyme immunoassay, Enzygnost TAT micro (Siemens Healthcare GmbH, Erlangen, Germany) according to the manufacturer’s protocol.
D-dimer levels prior and post-treatment were measured using the ASSERACHROM D-DI ELISA kit (Diagnostica Stago, Parsippany, NJ, USA) according to the manufacturer’s instructions. Two dilutions of plasma samples were measured in duplicate.
Description Statistics TAT and D-Dimer
Statistical parametric analysis was performed on log-transformed data as Bartlett’s test for variance homogeneity49 was significant at the 1% level for the data but not for log transformed data. For comparisons involving two groups only, vehicle versus AMT-060, and AMT-060 versus AMT-061, both at a dose of 5 × 1012 gc/kg, t tests were applied. For dose response testing of AMT-061, monotonicity of dose-response was tested with the F1 approximate test, which was not significant at the 1% level, where after Williams’ test50, 51 for a monotonic trend was applied.
Safety Assessments
The GLP study was designed according to guidelines for Gene Therapy Medicinal Products (EMEA/CHMP/GTWP/125459/2006) and included endpoints; e.g., clinical observations, hematology, and clinical chemistry, and histopathological examinations. Routine hematology parameters were measured using the Bayer Advia 120. Serum chemistry analyses and levels of various liver enzymes: ALT, AST, gamma-glutamyl transferase, glutamate dehydrogenase, sorbitol dehydrogenase, and lactate dehydrogenase were analyzed on the Roche P modular. Coagulation profile (prothrombin time, activated partial thromboplastin time, and Clauss fibrinogen) was assessed by an ACL series analyzer. After necropsy, 31 organs were examined macro and microscopically for full histopathological evaluation. The potential for immunogenicity related to FIX-Padua was assessed by in-silico analysis (Supplementary Methods). 52, 53
Detection of hFIX DNA in NHP Livers
hFIX and the Padua FIX AAV vector genomes were quantified using a real-time PCR assay in DNA extracted from monkey samples derived from the livers. The following hFIX specific primers were used: F: 5′-CAAGTATGGCATCTACACCAAAGTCT-3′ and R: 5′-GCAATAGCATCACAAATTTCACAAA-3′. DNA Integrity was determined by amplifying the cynomolgus macaque’s Porphobilinogen deaminase (PBGD) gene using the following primers: F: 5′-GATGCACGGCTCTAGATTTAGTGA-3′ and R: 5′-AATGAAAGGACCACGTCTGTGTAG-3′.
Detection of hFIX mRNA Copies in NHP Livers
Total RNA was isolated from monkey livers using TRIzol Reagent (Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA samples were treated with DNase (Thermo Fisher Scientific, Carlsbad, CA, USA), reverse transcribed, and amplified using the Power SYBR Green RNA-to-CT 1-Step Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) with the following FIX specific primers: F: 5′-GCAAGGTGGATGCCTTCTGT-3′ and R: 5′-CAGTGGGCAGCAGTCACAAT-3′. qRT-PCR reactions without reverse transcriptase served as controls for proper DNase treatment. As controls for RNA integrity and loading, RT-QPCR was performed on the samples using primers specific for NHP PBGD transcripts (primers: F: 5′-CCTGCCCACAGTGCTTCCT-3′ and R: 5′-GGTTTTCCCGCTTGCAGAT-3′).
Fluorescent In Situ Hybridization and Images Analysis
Fluorescent in situ hybridization (FISH) was used to visualize AAV vector DNA and transgene mRNA in frozen liver sections with a fluorescent probe recognizing hFIX AAV vector DNA and transgene mRNA (ACD-Bio, Newark, CA, USA). Tissue samples from the left median liver lobe from one animal per dose group were analyzed. Multiple images were acquired with a confocal microscope (Leica, SP8 confocal laser scanning microscope) and analyzed with the HALO image analysis software (Indicalabs, Corrales, New Mexico, USA). On average, images of 4,000 cells were analyzed. To assess the quality of the tissue and procedure, we used liver-positive (Ubiquitin C) and -negative (DapB) control probes. The percentage of cells positive for hFIX AAV vector DNA and transgene mRNA was analyzed.
Author Contributions
E.A.S. collected data, analyzed and interpreted data, and wrote the manuscript. Y.P.L. designed research, performed research, analyzed and interpreted data, and wrote the manuscript. J.L. H.P., and S.J.V.D. contributed to the design of the conducted preclinical evaluation of the AAV vectors in NHPs and reviewed the manuscript. E.E., S.G., P.M.-M., B.N., and V.F. provided AAV vector material for testing, performed analyses of the plasma and liver samples, collected data, and analyzed and interpreted data. M.D.H. managed the conduction of the study, designed the NHP study, collected data, and analyzed and interpreted data.
Conflicts of Interest
E.A.S., Y.P.L., J.L., E.E., S.G., P.M.-M., V.F., and S.J.V.D. are employees and shareholders at uniQure. B.N. and H.P. were employees and shareholders at uniQure when the study was conducted. M.D.H. is an independent Senior Non-Clinical Consultant.
Acknowledgments
The authors would like to thank Bas Bosma, Francois Du Plessis, Vanessa Zancanella, Elina Hessels, Rude de Laat, Lukas Schwarz, Saskia Haast, Corina van der Kruijssen, Shrijana Tripathi, Tamar Grevelink, Maroeska Oudshoorn-Dickmann, Christina Markopoulou, Betty Au, and Lisanne Schulte for technical assistance. Special thanks to Pavlina Konstantinova and Eileen Sawyer for critically reading the manuscript and Danielle Day for supporting the submission of the manuscript. Mike Lappin, PhD, of GK Pharmacomm, funded by uniQure, assisted with the preparation of the manuscript for submission to Molecular Therapy–Methods and Clinical Development.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.09.005.
Supplemental Information
References
- 1.White G.C., 2nd, Rosendaal F., Aledort L.M., Lusher J.M., Rothschild C., Ingerslev J., Factor VIII and Factor IX Subcommittee Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 2.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simioni P., Tormene D., Tognin G., Gavasso S., Bulato C., Iacobelli N.P., Finn J.D., Spiezia L., Radu C., Arruda V.R. X-linked thrombophilia with a mutant factor IX (factor IX Padua) N. Engl. J. Med. 2009;361:1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 4.Aggeler P.M., White S.G., Glendening M.B., Page E.W., Leake T.B., Bates G. Plasma thromboplastin component (PTC) deficiency; a new disease resembling hemophilia. Proc. Soc. Exp. Biol. Med. 1952;79:692–694. doi: 10.3181/00379727-79-19488. [DOI] [PubMed] [Google Scholar]
- 5.Biggs R., Douglas A.S., MacFarlane R.G., Dacie J.V., Pitney W.R., Merskey Christmas disease: a condition previously mistaken for haemophilia. BMJ. 1952;2:1378–1382. doi: 10.1136/bmj.2.4799.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasper C.K., Osterud B., Minami J.Y., Shonick W., Rapaport S.I. Hemophilia B: characterization of genetic variants and detection of carriers. Blood. 1977;50:351–366. [PubMed] [Google Scholar]
- 7.Mannucci P.M., Tuddenham E.G. The hemophilias--from royal genes to gene therapy. N. Engl. J. Med. 2001;344:1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava A., Brewer A.K., Mauser-Bunschoten E.P., Key N.S., Kitchen S., Llinas A., Ludlam C.A., Mahlangu J.N., Mulder K., Poon M.C. Guidelines for the management of hemophilia. Haemophilia. 2013;19 doi: 10.1111/j.1365-2516.2012.02909.x. e1–47. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Merchan E.C. Musculoskeletal complications of hemophilia. HSS J. 2010;6:37–42. doi: 10.1007/s11420-009-9140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gater A., Thomson T.A., Strandberg-Larsen M. Haemophilia B: impact on patients and economic burden of disease. Thromb. Haemost. 2011;106:398–404. doi: 10.1160/TH11-03-0193. [DOI] [PubMed] [Google Scholar]
- 11.Polack B., Calvez T., Chambost H., Rothschild C., Goudemand J., Claeyssens S., Borel-Derlon A., Bardoulat I., Maurel F., Woronoff-Lemsi M.C., EQOFIX Study Group EQOFIX: a combined economic and quality-of-life study of hemophilia B treatments in France. Transfusion. 2015;55:1787–1797. doi: 10.1111/trf.13016. [DOI] [PubMed] [Google Scholar]
- 12.Manco-Johnson M.J., Abshire T.C., Shapiro A.D., Riske B., Hacker M.R., Kilcoyne R., Ingram J.D., Manco-Johnson M.L., Funk S., Jacobson L. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 13.Roosendaal G., Lafeber F. Prophylactic treatment for prevention of joint disease in hemophilia--cost versus benefit. N. Engl. J. Med. 2007;357:603–605. doi: 10.1056/NEJMe078098. [DOI] [PubMed] [Google Scholar]
- 14.Collins P.W., Fischer K., Morfini M., Blanchette V.S., Björkman S., International Prophylaxis Study Group Pharmacokinetics Expert Working Group Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. doi: 10.1111/j.1365-2516.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 15.van Hylckama Vlieg A., van der Linden I.K., Bertina R.M., Rosendaal F.R. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000;95:3678–3682. [PubMed] [Google Scholar]
- 16.Cushman M., O’Meara E.S., Folsom A.R., Heckbert S.R. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konkle B.A., Huston H., Nakaya Fletcher S. Hemophilia B. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; 1993. [Google Scholar]
- 18.Evans J.P., Brinkhous K.M., Brayer G.D., Reisner H.M., High K.A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc. Natl. Acad. Sci. USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu R.K., Sangiorgi F., Wu L.Y., Kurachi K., Anderson W.F., Maxson R., Gordon E.M. Targeted inactivation of the coagulation factor IX gene causes hemophilia B in mice. Blood. 1998;92:168–174. [PubMed] [Google Scholar]
- 20.Wang L., Zoppè M., Hackeng T.M., Griffin J.H., Lee K.F., Verma I.M. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc. Natl. Acad. Sci. USA. 1997;94:11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbasi J. Hemophilia Gene Therapies Show Promise. JAMA. 2018;319:539. doi: 10.1001/jama.2018.0524. [DOI] [PubMed] [Google Scholar]
- 22.George L.A. Hemophilia gene therapy comes of age. Blood Adv. 2017;1:2591–2599. doi: 10.1182/bloodadvances.2017009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miesbach W., Sawyer E.K. Practical Implications of Factor IX Gene Transfer for Individuals with Hemophilia B: A Clinical Perspective. Hum. Gene Ther. Clin. Dev. 2018;29:80–89. doi: 10.1089/humc.2017.253. [DOI] [PubMed] [Google Scholar]
- 24.Sabatino D.E., Nichols T.C., Merricks E., Bellinger D.A., Herzog R.W., Monahan P.E. Animal models of hemophilia. Prog. Mol. Biol. Transl. Sci. 2012;105:151–209. doi: 10.1016/B978-0-12-394596-9.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh J., Lenting P.J., Rosales C., Lee D., Rabbanian S., Raj D., Patel N., Tuddenham E.G., Christophe O.D., McVey J.H. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 29.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantore A., Nair N., Della Valle P., Di Matteo M., Màtrai J., Sanvito F., Brombin C., Di Serio C., D’Angelo A., Chuah M. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120:4517–4520. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 31.Finn J.D., Nichols T.C., Svoronos N., Merricks E.P., Bellenger D.A., Zhou S., Simioni P., High K.A., Arruda V.R. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120:4521–4523. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monahan P.E., Sun J., Gui T., Hu G., Hannah W.B., Wichlan D.G., Wu Z., Grieger J.C., Li C., Suwanmanee T. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum. Gene Ther. 2015;26:69–81. doi: 10.1089/hum.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suwanmanee T., Hu G., Gui T., Bartholomae C.C., Kutschera I., von Kalle C., Schmidt M., Monahan P.E., Kafri T. Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIX restore normal hemostasis in Hemophilia B mice. Mol. Ther. 2014;22:567–574. doi: 10.1038/mt.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crudele J.M., Finn J.D., Siner J.I., Martin N.B., Niemeyer G.P., Zhou S., Mingozzi F., Lothrop C.D., Jr., Arruda V.R. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffini L., Manno C. Modern management of haemophilic arthropathy. Br. J. Haematol. 2007;136:777–787. doi: 10.1111/j.1365-2141.2007.06490.x. [DOI] [PubMed] [Google Scholar]
- 36.Monahan P.E., Walsh C.E., Powell J.S., Konkle B.A., Josephson N.C., Escobar M. Update on phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy for program for hemophilia B. J. Thromb. Haemost. 2015;13:87. [Google Scholar]
- 37.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calcedo R., Kuri-Cervantes L., Peng H., Qin Q., Boyd S., Schneider M. Immune Responses in 101HEMB01, a Phase 1/2 Open-Label, Single Ascending Dose-Finding Trial of DTX101 (AAVrh10FIX) in Patients with Severe Hemophilia B. Blood. 2017;130:3333. [Google Scholar]
- 39.Majowicz A., Salas D., Zabaleta N., Rodríguez-Garcia E., González-Aseguinolaza G., Petry H., Ferreira V. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1. Mol. Ther. 2017;25:1831–1842. doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park H.K., Cho J.W., Lee B.S., Park H., Han J.S., Yang M.J., Im W.J., Park D.Y., Kim W.J., Han S.C., Kim Y.B. Reference values of clinical pathology parameters in cynomolgus monkeys (Macaca fascicularis) used in preclinical studies. Lab. Anim. Res. 2016;32:79–86. doi: 10.5625/lar.2016.32.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Calcedo R., Nichols T.C., Bellinger D.A., Dillow A., Verma I.M., Wilson J.M. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 42.French R.A., Samelson-Jones B.J., Niemeyer G.P., Lothrop C.D., Jr., Merricks E.P., Nichols T.C., Arruda V.R. Complete correction of hemophilia B phenotype by FIX-Padua skeletal muscle gene therapy in an inhibitor-prone dog model. Blood Adv. 2018;2:505–508. doi: 10.1182/bloodadvances.2017015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 44.Halbert C.L., Miller A.D., McNamara S., Emerson J., Gibson R.L., Ramsey B., Aitken M.L. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX Activity Achieved after Single AAV5-hFIX Treatment in Hemophilia B Patients and NHPs with Pre-existing Anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 47.D’Avola D., López-Franco E., Sangro B., Pañeda A., Grossios N., Gil-Farina I., Benito A., Twisk J., Paz M., Ruiz J. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 2016;65:776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartlett M.S. Properties of sufficiency and statistical tests. Proceedings of the Royal Society. Series A. 1937;160:268–282. [Google Scholar]
- 50.Williams D.A. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–117. [PubMed] [Google Scholar]
- 51.Williams D.A. The comparison of several dose levels with a zero dose control. Biometrics. 1972;28:519–531. [PubMed] [Google Scholar]
- 52.De Groot A.S., Martin W. Reducing risk, improving outcomes: bioengineering less immunogenic protein therapeutics. Clin. Immunol. 2009;131:189–201. doi: 10.1016/j.clim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Koren E., De Groot A.S., Jawa V., Beck K.D., Boone T., Rivera D., Li L., Mytych D., Koscec M., Weeraratne D. Clinical validation of the “in silico” prediction of immunogenicity of a human recombinant therapeutic protein. Clin. Immunol. 2007;124:26–32. doi: 10.1016/j.clim.2007.03.544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.