Abstract
Purpose
To quantify differences in nonlinear aspects of performance on a seated visual-motor tracking task between clinically asymptomatic males and females with and without a self-reported mild traumatic brain injury history.
Methods
Seventy-three individuals with a self-reported concussion history (age: 21.40 ± 2.25 years, mean ± SD) and 75 without completed the visual-motor tracking task (age: 21.50 ± 2.00 years). Participants pressed an index finger against a force sensor, tracing a line across a computer screen (visual-motor tracking). The produced signal's root-mean-square error (RMSE), sample entropy (SampEn, a measure of regularity), and average power (AvP) between 0 and 12 Hz were calculated.
Results
Males with a history of 0 or 1 concussion had greater RMSE (worse performance) than females with 0 (p < 0.0001) and 1 concussion (p = 0.052). Additionally, females with 2+ concussions exhibited lower SampEn than females with no history (p = 0.001) or a history of 1 concussion (p = 0.026). Finally, females with 2+ concussions had lower 8–12 Hz AvP than males with 2+ concussions (p = 0.031). Few differences were observed in the male participants.
Conclusion
Females with a self-reported history of multiple concussions exhibited lower SampEn in the visual-motor tracking-task force output structure as compared to those with no reported history of concussion and their male counterparts. Lower SampEn and lower power between 8 and 12 Hz indicated persistent impairment in visual processing and feed-forward or predictive motor control systems.
Keywords: Average power, Concussion, Isometric motor control, Nonlinear analyses, Sample entropy, Visual-motor processing
1. Introduction
The Centers for Disease Control and Prevention (CDC) estimate that approximately 1.6–3.8 million mild traumatic brain injuries (mTBIs) occur annually in the US.1 In addition, numerous mTBIs likely go undiagnosed due to inadequate facilities or lack of personnel for proper assessment.2 Furthermore, athletes experiencing multiple mTBIs can experience longer term deficits including memory impairment, balance disorders, and neurologic disease.3 Clinical diagnosis of mTBI typically involves symptom presentation in addition to a multifaceted battery of assessments, which generally includes assessments of cognitive function and balance (i.e., Sport Concussion Assessment Tool-3 (SCAT3) and the Balance Error Scoring System (BESS))4 that may improve with practice5 or expensive equipment that cannot be removed from the clinic (e.g., force plate or Sensory Organization Test (SOT), NeuroCom International Inc., Clackamas, OR, USA),6, 7 which makes objective, informative, and cost-effective “sideline” assessments difficult. In addition, research on concussion recovery indicates that neurocognitive and postural control deficits may persist past symptom resolution7, 8 and clinical measures may not be sensitive to these deficits after the first 10–14 days following injury. This suggests that traditional assessments of mTBI may not fully address the neurocognitive, postural, and motor-related damage incurred following mTBI.9 Furthermore, few studies have identified deficits in the integration of cognitive and motor processes despite the fact that individuals with a history of concussion tend to have increased performance variability relative to individuals with no history of concussion.10, 11, 12 In light of these concerns, there is a clear need for cost-effective, alternative, and screening tools that better assess the impact of mTBI and link behavioral outcomes to specific neural mechanisms of injury.
In this preliminary research, we investigated an alternative mTBI assessment method using nonlinear time series analysis of performance on a seated visual-motor tracking task. Nonlinear analyses quantify changes in behavior over time, yield metrics pertaining to the overall quality of the behavioral time-series (e.g., its regularity), and provide insight into the integration between task-specific physiological components.13, 14 Nonlinear analyses are commonly performed on physiological signals or outcomes from motor behavior over time such as heart rate measurements taken at successive intervals,15, 16 the spatial time-course of postural sway center of pressure,7, 17 and the time-series of taps or step durations.16 In general, more regularity in motor performance has been linked to greater attentional demands, less automaticity and adaptability in movement, and general physiological impairment.13, 16, 18 Pathologic systems (e.g., postural sway and isometric force production in Parkinson's disease) exhibit more regularity (assessed via approximate entropy) than non-pathologic behavior.19 The structure of motor performance may lend more information about the specific deficits resulting from and recovery from mTBI than traditional measures, specifically because nonlinear measures of human behavior reflect collective neural and physiologic processes, as well as multifaceted mechanisms of impairment (e.g., memory, visual-motor coordination, reactive control, and automaticity).20
In addition to regularity, other nonlinear aspects of behavior can lend insight into mechanisms of injury or impairment. Specifically, 3 bandwidths of the power-frequency spectrum of movement output generally represent the slower, middle, and faster frequencies of visual-motor force tracking.21, 22, 23 Frequency bandwidths lower than 4 Hz have been associated with more cognitive processing such as responding to a signal (reaction time; ~200 ms).23 Frequency bandwidths in the higher frequency range (~10 Hz) have been associated with physiological processes such as stiffness or tremor.24 Frequency bandwidths in the middle range (4–8 Hz) have been associated with feedforward or predictive control.25, 26 To date, there has been no examination of changes in power at different frequencies following concussion.
Nonlinear techniques have been used to examine both postural sway7, 17, 27, 28 and visual-motor performance11 following mTBI. For postural sway, previously concussed individuals exhibited increased regularity, relative to healthy individuals, even after standard measures of stability (such as SOT) returned to pre-injury levels.7, 17, 27, 28 While these data provide preliminary support for nonlinear mTBI assessment utility, postural assessments require costly equipment or demanding that the athlete travel to a facility for assessment.
Furthermore, the regularity of target deviations in a circle-tracing task (departures from the target circle) increases immediately after injury and increases in irregularity over the course of the first several weeks to months.11 Though postural sway and circle-tracing are distinct motor tasks and rely on different sensorimotor inputs and outputs, these studies provide cumulative evidence that the nonlinear structure of motor performance is altered acutely by a concussive event7, 11, 27, 29, 30 and may remain altered months to years post-injury.17, 29, 31, 32
In light of findings and the equipment restrictions for assessing the nonlinear structure of postural sway, we developed a visual-motor tracking-task method of assessing the nonlinear structure isometric force production. This test can be administered on site and isolates the visual-motor system from other impairments that could accompany mTBI (e.g., vestibular influences on postural sway, lower extremity injury).
Finally, males and females exhibit differing patterns of performance on visual and motor tasks. Generally, males exhibit better performance on visuospatial tasks than females, while females have better verbal memory and motor processing speed.33, 34 These differences exist both at baseline (e.g., prior to concussion) and following injury. To date, no studies using nonlinear metrics with respect to visual-motor outcomes following concussion have investigated gender differences. Given gender differences in both visual and motor function following concussion, the inclusion of gender is an important consideration with this visual-motor tracking task.
The purpose of this cross-sectional study was to quantify differences in nonlinear aspects of performance on a seated visual-motor tracking task between clinically asymptomatic individuals with a history of mTBI and those with no previous mTBI. We hypothesized that, generally, individuals with a history of mTBI would exhibit greater visual-motor tracking regularity than individuals with no prior history of mTBI. We further hypothesized that individuals with a history of multiple mTBIs would exhibit greater regularity than individuals with no previous mTBI or individuals with only 1 mTBI for both males and females. With respect to the frequencies represented in each individual's output, we hypothesized that individuals with a history of concussion, and in particular those with a history of more than 1 concussion, would exhibit lower power at the higher frequency range of force output (4–12 Hz), typically thought to represent more automatic, complex aspects of motor control.
2. Materials and methods
2.1. Participants
Seventy-five participants without a history of concussion (21.50 ± 2.00 years old; 49 males, 26 females) and 73 participants with a self-reported history of 1 or more concussions diagnosed by a medical professional (21.40 ± 2.25 years old; 51 males, 22 females) were recruited. Participants were recruited from Utah State University varsity athletics and on-campus fliers. Participants with a history of concussion were all more than 6 months post-injury (range: 0.58–15 years) to limit the effects of short-term recovery following concussion. All participants were right-handed. Cumulative self-reported athletic experience (all athletic experience added together) was used as a metric of athletic experience. For multi-sport athletes, this means that cumulative athletic experience may meet or exceed chronological age (e.g., 2 sports played in a single year is 2 years of athletic experience). Both groups had individuals reporting no athletic experience (concussed: n = 2; non-concussed: n = 5). Additional descriptions of the groups divided by gender and number of previously diagnosed concussions are presented in Table 1, as well as numbers of individuals participating in various sports by group in Table 2.
Table 1.
Descriptive variables (mean ± SD).
| Gender | 0 self-reported concussion |
1 self-reported concussion |
2+ self-reported concussion |
|||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| n | 27 | 48 | 13 | 29 | 9 | 22 |
| Number of diagnosed concussionsa | 0 | 0 | 1 | 1 | 3(2–6) | 3(2–8) |
| Age (year) | 21.1 ± 1.4 | 21.7 ± 2.3 | 20.5 ± 2.1 | 21.7 ± 1.9 | 21.3 ± 2.9 | 21.5 ± 2.5 |
| Total athletic experience (year) | 13.5 ± 11.4 | 16.6 ± 11.3# | 14.3 ± 8.8 | 22.5 ± 8.9 * | 23.7 ± 12.2 | 23.2 ± 9.3 |
| Time from last concussion (year) | 0 | 0 | 3.7 ± 3.2 | 4.6 ± 3.2 | 1.3 ± 0.5† | 3.1 ± 3.1* |
Values are provided as median (range).
p < 0.05, compared with females within the same concussion level;
p < 0.05, compared with 1 and 2+ concussion level for males;
p < 0.05, compared with females with 1 concussion.
Table 2.
Sports participation.
| Number of self-reported concussions |
||||||
|---|---|---|---|---|---|---|
| 0 |
1 |
2+ |
||||
| Female | Male | Female | Male | Female | Male | |
| Football | 0 | 34 | 0 | 22 | 0 | 16 |
| Basketball | 9 | 21 | 3 | 15 | 4 | 12 |
| Track | 7 | 13 | 3 | 12 | 1 | 8 |
| Soccer | 9 | 5 | 5 | 7 | 4 | 4 |
| Baseball | 0 | 13 | 0 | 8 | 0 | 7 |
| Wrestling | 0 | 5 | 0 | 3 | 0 | 5 |
| Cross country | 3 | 4 | 1 | 1 | 0 | 1 |
| Gymnastics | 4 | 0 | 4 | 0 | 2 | 1 |
| Volleyball | 4 | 2 | 0 | 1 | 2 | 0 |
| Tennis | 4 | 2 | 0 | 0 | 0 | 2 |
| Softball | 2 | 0 | 3 | 0 | 2 | 0 |
| Golf | 2 | 2 | 0 | 0 | 0 | 1 |
| Rugby | 0 | 3 | 0 | 1 | 0 | 1 |
| Snowboard | 1 | 0 | 0 | 1 | 1 | 3 |
| Swimming | 1 | 2 | 0 | 1 | 0 | 0 |
| Dance | 0 | 0 | 2 | 0 | 1 | 0 |
| Lacrosse | 0 | 0 | 1 | 1 | 1 | 1 |
| Swimming | 2 | 3 | 1 | 1 | 0 | 0 |
| Hockey | 0 | 1 | 0 | 1 | 0 | 0 |
| Martial Arts | 1 | 2 | 0 | 0 | 1 | 1 |
| Othera | 1 | 5 | 2 | 5 | 2 | 2 |
Note: Most participants were multi-sports athletes; therefore, the total number of individuals participating in each sport will not necessarily equal to the number of individuals in each gender × concussion group.
Other sports include cheerleading, cricket, cross fit, horse vaulting, motocross, mountain biking, pole vaulting, racquetball, rodeo, roller derby, running, triathlons, ultimate Frisbee, water skiing, and weight lifting.
2.2. Apparatus and task
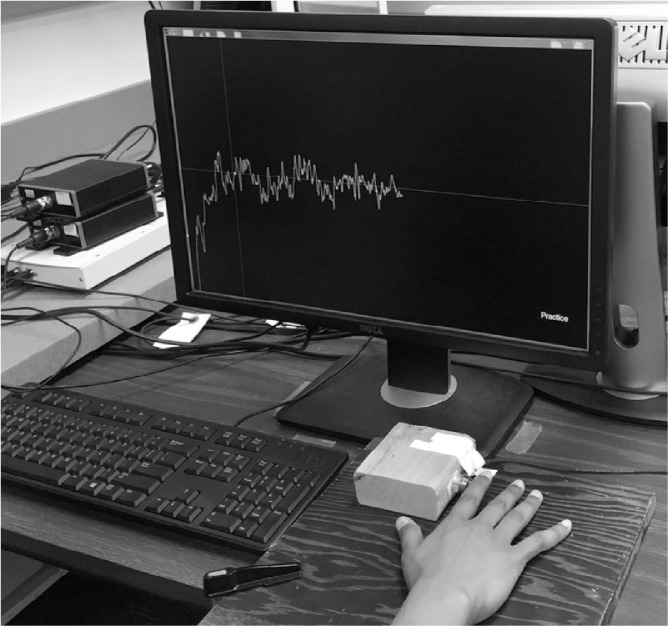
Participants were seated at a table, facing a 22″ (29 cm × 47 cm horizontal and vertical) LCD (Dell Inc., Round Rock, TX, USA) monitor. An ATI Industrial Automation load cell (diameter 1.27 cm; Apex, NC, USA) was affixed to a wooden block and secured. The load cell was mounted just right of the center of the monitor and 35 cm from the bottom edge of the monitor. The participant rested the right hand on the right side of the load cell and pressed the load cell with the lateral side of the right distal interphalangeal joint. Abduction/adduction of the index finger registered force through the load cell. Load cell output was amplified through a National Instruments DAQ board (National Instruments, Austin, TX, USA). A white line displayed on the screen represented the force administered by the index finger. Delay between finger force and output display is unavoidable but was minimized and undetectable by the experimenters (~60 ms). Furthermore, no subject mentioned noticing any delay. A red line was displayed on the screen for each trial and served as the target waveform. The white line, representing the force applied to the load cell, moved across the computer monitor from left to right, leaving a trace of its previous position (Fig. 1). Output force was sampled and displayed at 100 Hz. For each trial, the target line was centered in the middle of the screen and was visible for the entire duration of the trial. The target line was set at a constant 10% of the participant's maximum voluntary contraction (MVC) consistent with previous use of this task, as this value provides a maximal estimate of sample entropy when computed over short-time scales (~30 ms in the present study).35 The entire screen ranged from 0% to 20% of a participant's MVC.
Fig. 1.
Visual-motor tracking task apparatus and display. The horizontal midline is the tracking target line. The vertical line indicates the start of the trial's recording period. The participant's produced trace is shown in white.
2.3. Procedures and instructions
Subsequent to approval by the Utah State University Institutional Review Board for the protection of human subjects, written consent was obtained from each participant, and an acclimation trial (40 s) was performed to familiarize the subject with the apparatus. The subject adjusted his or her finger position and “got a feel for” the load cell and the task display. Following acclimation, 3 trials (5 s) of maximal finger force were performed. The maximal value of these 3 trials was recorded as that participant's MVC. Each participant then performed 1 trial of practice, followed by 10 trials of tracking. Participants were instructed to minimize the difference between the white (participant produced) and red (target) lines. Each trial lasted 20 s. At the end of each trial, the root-mean-square error score (RMSE) was presented on the screen. This score represented the number of pixels, on average, a participant's white line deviated from the red line. Participants were instructed to try to minimize this value.
2.4. Data collection and analysis
Prior to analyses, the first 4 s (1 s of the recorded trial following a 3 s “warm-up”) and the last 1 s of force output were removed to account for changes that might occur as an individual acclimates to the task and changes that might occur as an individual completes the task. RMSE as a metric of overall task performance was calculated as follows, where xi is an individual measured value and is the mean of the measured values for a data set.
| Eq. (1) |
Frequency domain characteristics of the force output were calculated using custom written software in MATLAB (Version 2015b; MATLAB, Natick, MA, USA). The Fourier transform of the force output yields the amplitude and phase of the time series in the frequency domain. The power in a given time series is equal to the square of the amplitude. The average power between 3 different frequency bandwidths was examined. The power within-bandwidth was averaged over all the trials of 1 experimental condition and then over all participants within each group.
Force output regularity was quantified using sample entropy (SampEn).15 SampEn quantifies the negative natural log ratio of the conditional probability that a pattern m samples long and some distances apart will repeat when the pattern is m + 1 samples long.15, 36 SampEn is defined as:
| Eq. (2) |
where m is the number of data points in per pattern considered, r is a pre-defined threshold for comparison, and N is the dataset; Bm(r) is the number of matching patterns of length m; and Am(r) is the number of matching patterns of length m + 1. For each m- or (m + 1)-length pattern in the data, a matching pattern is defined as another pattern of equal length whose endpoints are within r of the endpoints of the original pattern.
SampEn is recommended over approximate entropy, which has been used in previous work,7, 17, 27, 32 for its consistency, robustness to data length, as well as avoiding a bias toward regularity through self-matching patterns.15, 36 Consistent with previous work, we used an m of 2, and a criterion of 0.2 multiplied by the time-series standard deviation to calculate SampEn.21 Lower SampEn reflects a greater ratio of repeating patterns in the data ranges from 0 (highly predictable, regular) to 2 (white noise, irregular). Consistent with previous isometric force tracking studies using approximate entropy, SampEn was calculated on the original data.21, 25
2.5. Statistical analyses
All statistics and plotting were done in R Version 3.3.1, (Vienna, Austria). To assess the homogeneity of our groups, we used χ2 tests for group sizes (number of concussions × gender) and individual t tests to identify between-gender differences in age, years of total athletic experience, and years since most recent concussion at each level of number of concussions (0, 1, and 2+) with Holm-adjusted p values for multiple comparisons. Individual t tests were used to identify within-gender differences for years since most recent concussion between those with 1 and 2+ concussions. We additionally used one-way analysis of variances to identify within-gender differences in age and total athletic experience, with Tukey-adjusted post hoc tests as appropriate.
We fit separate analysis of covariances for each outcome measure with main and interaction effects for number of concussions (0, 1, and 2+) and gender (male, female). Total athletic experience and years from the most recent concussion were included as continuous covariates to control for the role of sport and injury recovery in the nonlinear structure of the motor performance. The dependent variables were the average values over all trials for each outcome measure. All dependent variables, except for 0–4 Hz average power, required transformation (y = ln(outcome)) to meet assumptions of normality and homoscedasticity. To provide meaningful comparisons, planned comparisons with Tukey's adjustment were conducted within gender, across number of concussions, and within each level of concussion history between genders. All tests were conducted with significance set at α = 0.05.
3. Results
There were no statistically significant differences in group sizes (χ2 = 0.376, p = 0.829) or between genders for age. Males with a history of 1 concussion had significantly greater total athlete experience (year) than females with 1 concussion (p = 0.033). Additionally, males with 2+ concussions were significantly further from the time of injury than females with 2+ concussions (p = 0.03). Males with no history of diagnosed concussion had significantly fewer total years of athletic experience than those with 1 (p = 0.042) or 2+ concussions (p = 0.036). Females with a history of 1 concussion were significantly further from the time of injury than females with 2+ concussions (p = 0.024) (Table 1).
The main effect of number of concussions was statistically significant for SampEn (F(2,140) = 6.860, p = 0.001, η2 = 0.095) and 8–12 Hz average power (F(2,140) = 3.332, p = 0.039, η2 = 0.074) after controlling for both total athletic experience and time from injury. The main effect of gender was statistically significant for absolute RMSE (F(1,140) = 27.620, p < 0.0001, η2 = 0.172) after controlling for both total athletic experience and time from injury. Total athletic experience did not have a significant effect on any outcome measure. The effect of time from injury was small (mean increase of 2.5% per year post-injury) but significant for SampEn (F(1,140) = 4.142, p = 0.044, η2 = 0.033).
3.1. Difference within gender
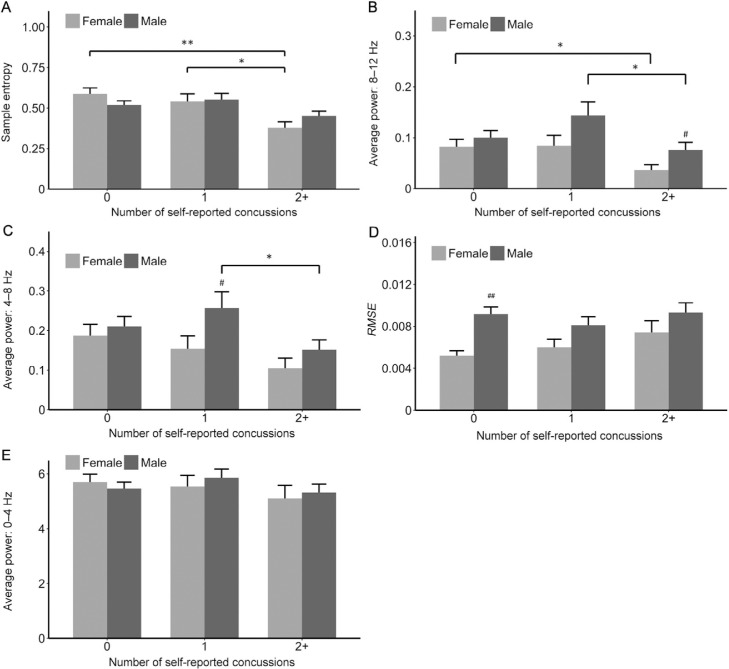
Females with a history of 2 + diagnosed concussions had lower SampEn than females with 0 (p = 0.001) and 1 (p = 0.026) diagnosed concussions (Fig. 2A). Females with 2+ concussions also had lower 8–12 Hz average power than those with no history of concussion (p = 0.043, Fig. 2B). The difference in 8–12 Hz AvP between females with 2+ and 1 concussion was not statistically significant (p = 0.075, Fig. 2B).
Fig. 2.
Outcome measures by number of self-reported concussions and gender. (A) sample entropy; (B) average power: 8–12 Hz; (C) average power: 4–8 Hz; (D) absolute root-mean-square error (RMSE); (E) average power: 0–4 Hz. Error bars are SE. * p < 0.05, ** p ≤ 0.001, significant difference within gender; #p < 0.05, ##p < 0.001, significant difference between gender.
Males with a history of 1 diagnosed concussion had greater 8–12 Hz and 4–8 Hz average power than those with 2+ (8–12 Hz: p = 0.029; 4–8 Hz: p = 0.036, Figs. 2B, 2C, respectively). However, there was no statistically significant difference for SampEn between those with 1 and 2 + concussions (p = 0.055) (Fig. 2A).
3.2. Difference between genders
Females with no history of diagnosed concussion had lower RMSE (better performance) than males with no history of diagnosed concussion (p < 0.001; Fig. 2D). However, the between-gender difference for those with 1 diagnosed concussion was not significant (p = 0.052, Fig. 2D). Females with a history of 1 single concussion had significantly lower 4–8 Hz average power than comparable males (p = 0.042, Fig. 2C). Females with a history of 2+ concussions had lower 8–12 Hz AvP than males with 2+ concussions (p = 0.031) (Fig. 2B). Finally, the between-gender difference in 8–12 Hz AvP for those with a history of 1 concussion was not statistically significant (p = 0.068) (Fig. 2B). There were no significant differences within or between genders for 0–4 Hz average power (Fig. 2E).
4. Discussion
The purpose of this study was to quantify differences in nonlinear aspects of performance on a seated visual-motor tracking task between clinically asymptomatic individuals with a history of mTBI and those with no previous mTBI, as well as to identify gender-related differences in performance on a visual-motor tracking task both prior to and following concussion. Importantly, we did not observe between-gender differences in age for any of the 3 levels of concussion history. We likewise did not observe between-gender differences in total athletic experience for those with 0 and 2+ concussions. Males with a history of a single concussion had significantly greater athletic experience than females with a single concussion, and this difference may partially explain differences in outcome measures at this level. This was included as a covariate in our models to minimize the effect that differences total athletic experience may have played in our outcomes.
We partially confirmed our original hypothesis, that individuals with a history of mTBI would demonstrate greater regularity in motor output than those with no history, as quantified by SampEn. We did observe lower SampEn, which indicates greater regularity, in the females who had sustained 2+ concussions relative to those with 1 or fewer diagnosed concussions. Additionally, males with 2+ concussions demonstrated greater regularity than those with a history of single concussion, though this difference was not statistically significant.
Additionally, females with a 1 or fewer diagnosed concussions had significantly better performance (lower RMSE) than their male counterparts. Furthermore, females with 2+ concussions had significantly poorer performance than females with no history of concussion. Again, we observed no differences between male subgroups. These findings in overall performance on visual-motor tracking agree with the SampEn results and support the visual-motor tracking task as a viable assessment of long-term visual-motor impairment resulting from multiple concussions for females.
Lastly, we observed that 8–12 Hz average power was lower for females with 2+ concussions compared to those who had sustained 0, as well as lower than their male counterparts with 2+ concussions. Generally, individuals performing a continuous tracking task, like the one in the present study, create output incorporating multiple frequencies.14, 37 Decreased average power in the 8–12 Hz frequency band agrees with observed decreases in SampEn and task performance (RMSE). Decreased incorporation of 8–12 Hz frequencies for the 2+ concussion group represents a less complex output signal.13, 14 This decrease in complexity is thus reflected by greater regularity—decreased SampEn. Specific to this task, healthy individuals are expected to incorporate higher frequencies leading to reduced deviation from the target line.37 Under pathologic conditions, individuals are less able to incorporate a diverse range of frequencies.14 Fluctuations 8–12 Hz are associated with processes that operate at higher frequencies, such as visual processing and feed-forward motor control.26, 38 Consequently, the reduction in power suggests persistent impairment in these processes, which could have implications for adaptability of control, and the time-course of motor responses. Furthermore, previously concussed females may be more susceptible to these decrements in 8–12 Hz average power than males.
Cumulatively, these findings for our female participants agree with more broad nonlinear analyses of postural sway7, 17, 27, 29, 30, 31, 32 and visual-motor tracking11 following mTBI. Both acutely concussed individuals (within the first 7–10 days of injury)7, 27, 29, 30 and individuals with a history of concussion17, 31, 32 exhibit increased postural sway regularity relative to both pre-injury assessments and healthy individuals. Additionally, visual-motor tracking performance on a circle-tracing task is also more regular shortly after concussion.11 Though specific mechanisms for increases in postural sway and visual-motor regularity following concussion remain unclear, theories include reduced neurophysiological adaptability,13, 17, 31 reduced sensorimotor integration,31 alterations in motor cortex activation,29 and decreases in lower extremity muscle stiffness.29
Regardless of specific mechanisms, our data extend the body of evidence for persistent alterations in the nonlinear structure of motor performance following concussion, despite clinical recovery. It is necessary to make 2 important distinctions between the findings of the present study and previous findings for postural sway. First, visual-motor tracking task performance in the present study is vision-dependent with limited proprioceptive influence,39 whereas postural sway is a function of proprioceptive, visual, and vestibular system integration.40 Therefore, our findings highlight similar changes in nonlinear structure following concussion to those found in postural sway within a separate and distinct motor task. Future research should address the extent to which there are concomitant alterations in nonlinear metrics in both postural sway and isometric motor performance.
Secondly, previous studies have demonstrated increased regularity in concussion groups including both males and females, whereas we observed between-group differences when stratifying by sex and the number of previously sustained concussions. The females in our study who had sustained 2 or more concussions demonstrated greater regularity, and lower SampEn, in the visual-motor tracking task than those with a history of 0 or 1 concussion. Concussed females may, therefore, be more susceptible to lasting alterations in visual-motor performance than males, particularly with increasing numbers of concussions.
Broadly, our findings disagree with previous neurocognitive testing outcomes showing that men's visuospatial task performance exceeds women's, both prior to and after concussion.33, 34 In general, the men in our sample had poorer (greater RMSE) or equivalent task performance than the women, rather than better (Fig. 2D). For men with 0 concussion, nonlinear task characteristics were similar to those of the women despite significantly poorer performance, suggesting that differences in task performance were unrelated to these nonlinear characteristics. These findings cumulatively suggest that between-gender differences in visual memory and visuospatial performance may be task specific. Future research should examine the relationship between nonlinear and task performance on visual-motor tracking tasks, such as the one in the present study, and visual and visuospatial tasks common to neurocognitive testing packages.41, 42, 43
Finally, the general similarity on all outcome measures in the male participants, regardless of concussion history and accounting for both athletic experience and time from injury, is not easily explained. The most plausible reason is that our participants were only asked for the number of previously diagnosed concussions. Non- and underreporting of concussions is common, particularly in football,44, 45 and 73% of the males in our sample had current or prior experience playing competitive football (Table 1). It is, therefore, possible that males with no reported history of concussion had actually sustained 1 or more concussions, but had never been formally diagnosed with concussion. It is also possible that these participants were aware of previous concussions, but reported none to the experimenters.
Another explanation is that, for the males who had previous or current football experience, a history of sub-concussive events—blows to the head that never get reported as full concussions—may cumulatively result in similar visual-motor deficits to 1 or more diagnosed concussions.46 Individuals with a history of participation in contact sports are more susceptible to these sub-concussive events, and therefore might show similar long-term deficits to those with multiple diagnosed concussions. Further research is needed to identify the role of suspected concussions, without formal diagnosis, and sub-concussive impacts on visual-motor tracking-task performance and nonlinear characteristics.
There are several limitations to the present study. First, we relied on self-report for diagnosed concussion history. Consequently, individuals may have deliberately lied about, or forgotten, instances of having been diagnosed with a concussion and without access to medical records, it impossible for us to confirm these diagnoses. However, it is reasonable that, barring lying, participants were able to recall having been diagnosed by a medical professional (e.g., athletic trainer, physician). Furthermore, this self-report may have excluded concussions or concussion-like events that were not diagnosed. However, if individuals who reported no history of concussion either hid previous concussion diagnoses from the experimenters or had sustained concussions that were undiagnosed, then these analyses provide a conservative estimate of the impact of concussions on the nonlinear structure in our visual-motor task. Finally, though our participants reported no lingering symptoms related to their most recent concussive events, we were unable to ascertain specifically when individuals were deemed fully recovered.
Secondly, there was an unavoidable delay between force being registered by the load cell and being reflected on-screen (≤60 ms). With a sampling rate of 100 Hz, SampEn is computed on data points separated by 30 ms. However, the delay was consistent for all individuals and therefore any influence that such visual lag had on task performance should also be consistent across individuals. Furthermore, previous work shows that short delays (<100 ms) have minimal to no effects on task performance or nonlinear metrics.25 Third, there is the possibility that participants learned to perform the task better over the course of the trials and this may have influenced the findings. However, previous research related to this task has demonstrated minimal within-day learning effects for the structure of force output, though there may be an improvement in RMSE.21 Future research should identify the extent to which within-day and across-day learning occurs and alters task performance, temporal structure, and spectral characteristics of visual-motor tracking tasks following concussion. Finally, our task only incorporated a constant level of target force. Other tracing patterns (e.g., sine wave) may identify other patterns of impairment following concussion and should be investigated in future research.
In light of the fact that deficits were observed in our task primarily in females with multiple concussions compared to 0 or 1 concussion, it seems reasonable that this information may be useful in informing the clinician of persistent, subclinical visual-motor impairments in females. Used in conjunction with neurocognitive and balance tests, this visual-motor tracking task could be used to do more than inform clinicians about recovery status following concussion. Future research should investigate this as well as other factors (diagnosed versus suspected or unreported concussion, age of first concussion, time between concussions), which may influence task performance. Doing so may lead to better identification of individuals at risk to have persistent, and potentially detrimental, changes in visual-motor task performance. Additionally, force and pattern tracking tasks may be adapted to mobile-based task (pattern-tracing on a tablet, for example), allowing the clinician to capture metrics of function outside of the clinic or when more sophisticated equipment is unavailable.
5. Conclusion
We observed a significant decrease in performance associated with visual processing and predictive motor control as well as an increase in the regularity of the signal in females with 2 or more concussions relative to individuals with a history of only 1 concussion as well as males with multiple concussions. This indicates persistent, and possibly pathologic, changes in the way in which females with multiple previous concussions use visual information to guide behavior.
Authors' contributions
BES conceptualized and carried out the experimental testing, coordinated and helped draft and finalized the manuscript, and conducted the extraction of the dependent variables; AR drafted the manuscript and conducted the statistical analyses. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Langlois J.A., Rutland-Brown W., Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Buck P.W. Mild traumatic brain injury: a silent epidemic in our practices. Health Soc Work. 2011;36:299–302. doi: 10.1093/hsw/36.4.299. [DOI] [PubMed] [Google Scholar]
- 3.Guskiewicz K.M., McCrea M., Marshall S.W., Cantu R.C., Randolph C., Barr W.B. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. J Am Med Assoc. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 4.McCrory P., Meeuwisse W.H., Aubry M., Cantu R.C., Dvořák J., Echemendia R.J. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, 2012. Br J Sports Med. 2013;47:1–12. [Google Scholar]
- 5.Valovich T.C., Perrin D.H., Gansneder B.M. Repeat administration elicits a practice effect with the Balance Error Scoring System but not with the Standardized Assessment of Concussion in high school athletes. J Athl Train. 2003;38:51–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Guskiewicz K.M., Ross S.E., Marshall S.W. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–273. [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh J.T., Guskiewicz K.M., Giuliani C., Marshall S., Mercer V.S., Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39:805–811. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrea M., Barr W.B., Guskiewicz K.M., Randolph C., Marshall S.W., Cantu R. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 9.Bigler E.D. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- 10.Dalecki M., Albines D., Macpherson A., Sergio L.E. Prolonged cognitive-motor impairments in children and adolescents with a history of concussion. Concussion. 2016;1:CNC14. doi: 10.2217/cnc-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelty-Stephen D.G., Qureshi Ahmad M., Stirling L. Use of a tracing task to assess visuomotor performance for evidence of concussion and recuperation. Psychol Assess. 2015;27:1379–1387. doi: 10.1037/pas0000122. [DOI] [PubMed] [Google Scholar]
- 12.Stuss D.T., Stethem L.L., Hugenholtz H., Picton T., Pivik J., Richard M.T. Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiatry. 1989;52:742–748. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsitz L.A., Goldberger A.L. Loss of “complexity” and aging. Potential applications of fractals and chaos theory to senescence. J Am Med Assoc. 1992;267:1806–1809. [PubMed] [Google Scholar]
- 14.Vaillancourt D.E., Newell K.M. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23:1–11. doi: 10.1016/s0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 15.Richman J.S., Moorman J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–49. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 16.Rhea C.K., Kiefer A.W. Gait biometrics: basic patterns, role of neurological disorders and effects of physical activity. Nova Science Publishers; Hauppauge, NY: 2014. Patterned variability in gait behavior: how can it be measured and what does it mean; pp. 17–44. [Google Scholar]
- 17.Sosnoff J.J., Broglio S.P., Shin S., Ferrara M.S. Previous mild traumatic brain injury and postural-control dynamics. J Athl Train. 2011;46:85–91. doi: 10.4085/1062-6050-46.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulf G., McNevin N., Shea C.H. The automaticity of complex motor skill learning as a function of attentional focus. Q J Exp Psychol A. 2001;54:1143–1154. doi: 10.1080/713756012. [DOI] [PubMed] [Google Scholar]
- 19.Vaillancourt D.E., Newell K.M. The dynamics of resting and postural tremor in Parkinson's disease. Clin Neurophysiol. 2000;111:2046–2056. doi: 10.1016/s1388-2457(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 20.Kloos H., Van Orden G. Voluntary behavior in cognitive and motor tasks. Mind Matter. 2010;8:19–43. [Google Scholar]
- 21.Studenka B.E., King A.C., Newell K.M. Differential time scales of change to learning frequency structures of isometric force tracking. J Exp Psychol Hum Percept Perform. 2014;40:1629–1640. doi: 10.1037/a0037113. [DOI] [PubMed] [Google Scholar]
- 22.Sosnoff J.J., Valantine A.D., Newell K.M. The adaptive range of 1/f isometric force production. J Exp Psychol Hum Percept Perform. 2009;35:439–446. doi: 10.1037/a0012731. [DOI] [PubMed] [Google Scholar]
- 23.Slifkin A.B., Newell K.M. Variability and noise in continuous force production. J Mot Behav. 2000;32:141–150. doi: 10.1080/00222890009601366. [DOI] [PubMed] [Google Scholar]
- 24.Deuschl G., Raethjen J., Lindemann M., Krack P. The pathophysiology of tremor. Muscle Nerve. 2001;24:716–735. doi: 10.1002/mus.1063. [DOI] [PubMed] [Google Scholar]
- 25.Sosnoff J.J., Newell K.M. Age-related loss of adaptability to fast time scales in motor variability. J Gerontol B Psychol Sci Soc Sci. 2008;63:344–352. doi: 10.1093/geronb/63.6.p344. [DOI] [PubMed] [Google Scholar]
- 26.Desmurget M., Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh J.T., Guskiewicz K.M., Giuliani C., Marshall S., Mercer V.S., Stergiou N. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley T.A., Oldham J.R., Caccese J.B. Postural control deficits identify lingering post-concussion neurological deficits. J Sport Health Sci. 2016;5:61–69. doi: 10.1016/j.jshs.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fino P.C., Nussbaum M.A., Brolinson P.G. Decreased high-frequency center-of-pressure complexity in recently concussed asymptomatic athletes. Gait Posture. 2016;50:69–74. doi: 10.1016/j.gaitpost.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Gao J., Hu J., Buckley T., White K., Hass C. Shannon and Rényi entropies to classify effects of mild traumatic brain injury on postural sway. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024446. e24446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quatman-Yates C.C., Bonnette S., Hugentobler J.A., Médé B., Kiefer A.W., Kurowski B.G. Postconcussion postural sway variability changes in youth: the benefit of structural variability analyses. Pediatr Phys Ther. 2015;27:316–327. doi: 10.1097/PEP.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Beaumont L., Mongeon D., Tremblay S., Messier J., Prince F., Leclerc S. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–240. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Covassin T., Swanik C.B., Sachs M., Kendrick Z., Schatz P., Zillmer E. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med. 2006;40:923–927. doi: 10.1136/bjsm.2006.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Covassin T., Elbin R.J., Harris W., Parker T., Kontos A.P. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40:1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- 35.Vieluf S., Temprado J.J., Berton E., Jirsa V.K., Sleimen-Malkoun R. Effects of task and age on the magnitude and structure of force fluctuations: insights into underlying neuro-behavioral processes. BMC Neurosci. 2015;16:12. doi: 10.1186/s12868-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yentes J.M., Hunt N., Schmid K.K., Kaipust J.P., McGrath D., Stergiou N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann Biomed Eng. 2013;41:349–365. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slifkin A.B., Vaillancourt D.E., Newell K.M. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- 38.Sosnoff J.J., Newell K.M. Intermittent visual information and the multiple time scales of visual motor control of continuous isometric force production. Percept Psychophys. 2005;67:335–344. doi: 10.3758/bf03206496. [DOI] [PubMed] [Google Scholar]
- 39.Hu X., Newell K.M. Visual information gain and task asymmetry interact in bimanual force coordination and control. Exp Brain Res. 2011;212:497–504. doi: 10.1007/s00221-011-2760-6. [DOI] [PubMed] [Google Scholar]
- 40.Oie K.S., Kiemel T., Jeka J.J. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cogn Brain Res. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 41.Maroon J.C., Lovell M.R., Norwig J., Podell K., Powell J.W., Hartl R. Cerebral concussion in athletes: evaluation and neuropsychological testing. Neurosurgery. 2000;47:659–672. doi: 10.1097/00006123-200009000-00027. [DOI] [PubMed] [Google Scholar]
- 42.Kaushik T., Erlanger D.M. The headminder concussion resolution index. In: Echemendia R.J., editor. Sports neuropsychology: assessment and management of traumatic brain injury. Guilford Press; New York, NY: 2006. pp. 216–239. [Google Scholar]
- 43.Makdissi M., Collie A., Maruff P., Darby D.G., Bush A., McCrory P. Computerised cognitive assessment of concussed Australian Rules footballers. Br J Sports Med. 2001;35:354–360. doi: 10.1136/bjsm.35.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr Z.Y., Register-Mihalik J.K., Kroshus E., Baugh C.M., Marshall S.W. Motivations associated with nondisclosure of self-reported concussions in former collegiate athletes. Am J Sports Med. 2016;44:220–225. doi: 10.1177/0363546515612082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCrea M., Hammeke T., Olsen G., Leo P., Guskiewicz K.M. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Breedlove K.M., Breedlove E.L., Robinson M., Poole V.N., King I.I.I.J.R., Rosenberger P. Detecting neurocognitive and neurophysiological changes as a result of subconcussive blows among high school football athletes. Athl Train Sports Health Care. 2014;6:119–127. [Google Scholar]