Abstract
Purpose
The aim of this study was to examine systemic responses of oxidant/antioxidant status following 2 training sessions of different intensity in amateur rhythmic gymnasts.
Methods
Before the experimental training, 10 female gymnasts performed a gradually increased exercise test to assess maximal heart rate, maximal oxygen consumption, and anaerobic threshold. They executed 2 intermittent training sessions separated by 48 h of recovery (48 h-post R): the first was performed at low-moderate intensity (LMI) and the second at high intensity (HI). Blood samples were collected immediately pre- and post-training and 48 h-post R. Hydroperoxide level (OxL) and total antioxidant capacity (TAC) were photometrically measured.
Results
OxL was significantly higher in post-training and 48 h-post R following HI than the same conditions after an LMI session (HI vs. LMI post-training: 381.10 ± 46.17 (mean ± SD) vs. 344.18 ± 27.94 Units Carratelli (U.CARR); 48 h-post R: 412.21 ± 26.61 vs. 373.80 ± 36.08 U.CARR). There was no change in TAC between the 2 training sessions investigated. In LMI training, OxL significantly decreased in post-training and increased to reach the baseline at 48 h-post R, whereas TAC increased only at 48 h-post R. In HI training, OxL significantly increased to reach a high oxidative stress 48 h-post R, whereas TAC was lower in post-training than pre-training.
Conclusion
The pattern of OxL and TAC levels implies different regulation mechanisms by HI and LMI training sessions. High oxidative stress induced by an HI protocol might be associated with both insufficient TAC and recovery time at 48 h necessary to restore redox balance.
Keywords: Antioxidant capacity, Exercise physiology, Free radicals, Oxidative stress, Reactive oxygen species, Rhythmic gymnastics, Training intensity
1. Introduction
In the past decade, several studies proved a strict relationship between exercise physiology and cellular oxidant/antioxidant systems, emphasizing that these can influence both sport performance and individual health status,1, 2 respectively. It is well known that the reactive oxygen species (ROS) are physiologically produced during cellular respiration. These can serve as messenger molecules that have an important role in triggering signaling cascades that modulate changes in cell and tissue homeostasis and gene expression.3, 4 However, when the production of ROS is excessive and not counterbalanced by adequate antioxidant reserves, this may lead to a disruption of redox signaling and cause oxidative stress, which has been shown to be a risk factor for the onset of many diseases.1, 3, 4 This phenomenon has been widely observed in both contracting and disuse atrophic muscle and may implicate an oxidative damage to lipids, DNA, and proteins, contributing to muscular aging with consequent loss of muscle mass and function.5, 6, 7 The activity-dependent ROS production may contribute to fatigue during strenuous exercise or modulate the repair process during recovery from high-intensity or damaging exercise.2, 8 Parameters characterizing exercise such as type, intensity, duration, and energy demands have been reported to influence redox homeostasis.9, 10, 11 For instance, a single bout of strenuous exercise as well as acute endurance and intermittent exercise has been shown to increase the markers of lipid and protein peroxidation and reduce the antioxidant enzyme activity.9, 12, 13 Moreover, recently it has been observed that plasma redox status changes according to the practiced sport and also when an athlete is exposed to unaccustomed physical activity.11, 13
Rhythmic gymnastics is an artistic and aesthetic sport that requires high values of strength, flexibility, balance, and technical ability.14 Competition exercises have a short length (from about 1 min and 30 s to 2 min and 30 s) and demand maximal efforts, especially during the execution of jumps and leaps.15 It is also known that in aesthetic sports, female athletes are at high risk for developing the athletic triad characterized by menstrual cycle abnormalities, eating disorders, and premature osteoporosis. These factors may affect the redox state and induce oxidative stress in athletes.16 Several studies indeed reported an altered redox balance in female gymnasts and ballet dancers.17, 18, 19 Nevertheless, little is known about ROS levels and antioxidant capacity in rhythmic gymnasts.
Therefore, the aim of this study was to examine plasma levels of hydroperoxides and antioxidant capacity in amateur rhythmic gymnasts before and after 2 training sessions at different intensities and 48 h post-sessions.
2. Materials and methods
2.1. Subjects
Ten young women (age: 23.80 ± 3.42 years; weight: 52.58 ± 4.57 kg; height: 158.42 ± 2.20 cm; body mass index: 20.88 ± 1.23 kg/m2), practicing rhythmic gymnastics for 13.14 ± 5.40 years, voluntarily participated in this study. They regularly exercise trained about 1 h per session 3 times a week. Before data collection, gymnasts filled out an anamnesis questionnaire to exclude those who smoked, took medications (including contraceptive pills), or had recent musculoskeletal injuries. Moreover, they declared that they were amenorrheic and during the week of experimentation were at Day 23.02 ± 3.16 of their menstrual cycle, corresponding to the luteal phase, in which the lowest ROS production has been reported.20 After a careful explanation of the testing and training protocol and the possible risks and benefits involved, all gymnasts provided their written informed consent for participating in this study. The study was approved by the local Ethical Committee of the University of Palermo. All procedures were done in accordance with the Declaration of Helsinki.
2.2. Study design
Four weeks before measuring pro- and antioxidant capacities, gymnasts were instructed to discontinue any vitamin or antioxidant dietary supplementation. To assess their dietary consumption, they completed a 7-day food diary, from which it emerged that caloric and nutrient ingestion was in accordance with the daily intake levels of nutrients recommended for the Italian population.21 Therefore, all gymnasts were invited to follow the same dietary plan 1 week before testing sessions and during training protocols. The application of dietary instructions by gymnasts was checked with a face-to-face interview before testing. Two weeks before blood sampling, the participants performed a gradually increasing exercise test on a treadmill to measure maximal oxygen consumption (VO2max), maximal heart rate (HRmax), and anaerobic threshold (AT).22 These physiological variables were assessed to quantify the exercise intensity of rhythmic gymnastics sessions as HR was monitored telemetrically in all athletes during the training sessions. Gymnasts were instructed not to perform any physical activity 48 h before testing and training sessions. They executed 2 training sessions in the same week separated by 48 h of recovery (48 h-post R): the first was performed at low-moderate intensity (LMI) and the second at high intensity (HI) (see Session 2.4). Blood samples were taken immediately before and after both training sessions and 48 h after both sessions. In this way, the sample corresponding to 48 h-post R after the first training session was the one corresponding to pre-training for the training session.
2.3. Measurement of VO2max, HRmax, and AT parameters
To define the workload for both protocols of training sessions, each subject was initially assessed for VO2max, HRmax, and AT parameters through a laboratory-graded exercise test performed on a motorized treadmill (COSMED T150; COSMED Srl, Rome, Italy) using a breath-by-breath cardiopulmonary metabolic system (Quark CPET system; COSMED Srl). Gymnasts were previously familiarized to the testing procedures. The test started with a 3-min warm-up (4 km/h and 0% of elevation), followed by an initial speed of 7 km/h with 2% of elevation, and continued with speed increases in 1 km/h per 1-min stage (elevation fixed at 2%) until exhaustion. During the cool-down, subjects walked for 5 min at a speed of 2 km/h and 0% of elevation. The test was stopped when subjects were unable to maintain the required work rate, and VO2max was defined as the highest consecutive 30-s average value achieved during the test. The AT parameter was indirectly detected using V-slope method, a computerized regression analysis of the slopes of CO2 uptake and O2 uptake plots.23
2.4. Training protocols of rhythmic gymnastics sessions
Both training protocols consisted of exercises aimed to develop anaerobic power, muscle strength, flexibility, agility, and body balance and differed from one other in training intensity: 1 session was planned at LMI and the other at HI. The intensity was established according to the number of exercise sets, repetitions, and recovery times among exercises. In the HI session, set and repetition numbers and recovery times were respectively higher and shorter than in the LMI session.
Both training sessions had a 45-min duration, starting with a 10-min warm-up, followed by a 30-min central training phase and finishing with a 5-min cool-down. During the warm-up, gymnasts performed various types of slow running (frontal, lateral, skipped) and dynamic stretching, especially in the lower limbs. The central training phase contained jumps, leaps, across-the-floor exercises, balances, turns, flexibility movements, and technical-artistic skills of rhythmic gymnastics. Before cooling down, participants executed a competition performance (1 min-and-30 s duration) coordinated to music (4/4 beat) without technical apparatus. At the end, they performed static stretching of various body segments.
During the training sessions, HR was recorded with the Polar Team System (Polar Electro Oy, Kempele, Finland) to assess the training intensity according to the time that gymnasts spent in each HR zone. The latter was divided into 5 ranges expressed as a percentage of HRmax (50–59, 60–69, 70–79, 80–89, 90–100), and HR over anaerobic threshold was also analyzed. Exercise intensity with < 80% and ≥ 80% of HRmax were considered as LMI and HI, respectively.24
2.5. Measurement of oxidant and antioxidant capacity
Oxidative stress evaluation was performed with a portable integrated analytical system composed of a photometer and a mini-centrifuge (FRAS 4 Evolvo; H&D Srl, Parma, Italy) immediately before and after the training session and 48 h after both sessions. Samples of whole capillary blood were centrifuged for 1 min and 30 s immediately after being harvested with a finger puncture, and 10 µL of plasma was used for measuring the concentration of hydroperoxides (using d-ROMs testing; see next paragraph) and antioxidant capacity (using BAP testing; see later).25
The test of active oxygen metabolites (d-ROMs; Diacron International Srl, Grosseto, Italy) measures increases in red color intensity after the addition of a small quantity of plasma to a solution of N,N-diethylparaphenylendiamine (chromogen), buffered to pH 4.8. Such coloring is attributed to the cation radical formation of the amines via oxidation, which is due to alkoxyl and peroxyl radicals. The latter derive from the reaction of the Fe2+ and Fe3+ ions released by proteins in acidic conditions as created in vitro. The results are expressed as Units Carratelli (U.CARR), and it has been experimentally established that 1 U.CARR corresponds to 0.08 mg/dL H2O2. The normal values of a d-ROMs test range from 250 to 300 U.CARR (i.e., between 20 and 24 mg/dL H2O2).26
The test of biological antioxidant power (BAP; Diacron International Srl) measures the capacity of a plasma sample to reduce the iron of a colored complex containing ferric ions to its colorless ferrous derivative. The chromatic change of this reaction was photometrically measured at 505 nm, and the results were expressed in µmol/L of reduced iron using ascorbic acid as a standard. The normal value of a BAP test is >2200 µmol/L. To maintain consistency, the same set of kits was used for all tests, and the same operator using the same calibrated machine carried out all tests.
2.6. Statistical analysis
Data are presented as mean ± SD. Assumption of normality was verified using the Shapiro-Wilk test (W U.CORR = 0.93) (W total antioxidant capacity = 0.96). Subsequently, to determine any significant differences among pre-training, post-training, and 48 h-post R, a within subject one-way analysis of variance was applied for each training type. When a significant F value was found, least-significant difference (Bonferroni) was chosen as the post hoc procedure. A paired t test (two-tailed; information coefficient 0.95%) was adopted to display differences in LMI vs. HI for each stage (pre-training, post-training, and rest). A statistical analysis was performed using the software SPSS Statistic Version 15.0 (SPSS Inc., Chicago IL, USA). Level statistical significance was set at a p < 0.05.
3. Results
3.1. Analysis of training session intensity
VO2max, HRmax, and AT parameters registered during the laboratory test are shown in Table 1. During the LMI training session, HRpeak and HRaverage were 178.33 ± 3.79 beats per minute (bpm) (96.60% ± 2.05% HRmax) and 122.00 ± 8.89 bpm (66.09% ± 4.81% HRmax), respectively; whereas during the HI session, these parameters were 187.33 ± 5.86 bpm (101.48% ± 3.17% HRmax) and 149.00 ± 7.63 bpm (80.71% ± 4.13% HRmax), respectively. The percentage of training time that gymnasts spent in every HR zone is reported in Table 2. Particularly, the total time spent below 80% HRmax was 82.36% ± 4.19% in the LMI training session with 5.53% ± 0.27% over AT, whereas in the HI session 42.34% ± 2.34% of the total time was spent above the 80% HRmax with 24.92% ± 1.47% over AT.
Table 1.
Rhythmic gymnasts' physiological variables at baseline (mean ± SD).
| Variable | Value |
|---|---|
| VO2max (mL/kg/min) | 39.80 ± 6.34 |
| HRmax (bpm) | 184.60 ± 6.02 |
| AT (mL/kg/min) | 31.27 ± 5.09 |
| AT (bpm) | 161.60 ± 11.67 |
Abbreviations: AT = anaerobic threshold (expressed as a VO2 value (mL/kg/min) and an HR value (bpm)); bpm = beats per minute; HRmax = maximum heart rate; VO2max = maximal oxygen consumption.
Table 2.
Gymnasts' workload during training sessions (mean ± SD).
| Training session | HR zone (%HRmax) |
|||||
|---|---|---|---|---|---|---|
| 50–59 | 60–69 | 70–79 | 80–89 | 90–100 | Over AT | |
| LMI | 32.61 ± 1.86 | 31.25 ± 1.62 | 18.50 ± 0.71 | 14.11 ± 0.76 | 3.60 ± 0.15 | 5.53 ± 0.27 |
| HI | 2.90 ± 1.39 | 21.27 ± 1.27 | 33.74 ± 2.02 | 18.96 ± 1.06 | 23.38 ± 1.28 | 24.92 ± 1.47 |
Note: Percentage of minutes spent in every HR zone considering a training total time (from 50% over AT) during LMI and HI training sessions.
Abbreviations: AT = anaerobic threshold; HI = high intensity; HR = heart rate; LMI = low-moderate intensity; % HRmax = percentage of maximal heart rate.
3.2. Evaluation of plasma redox status
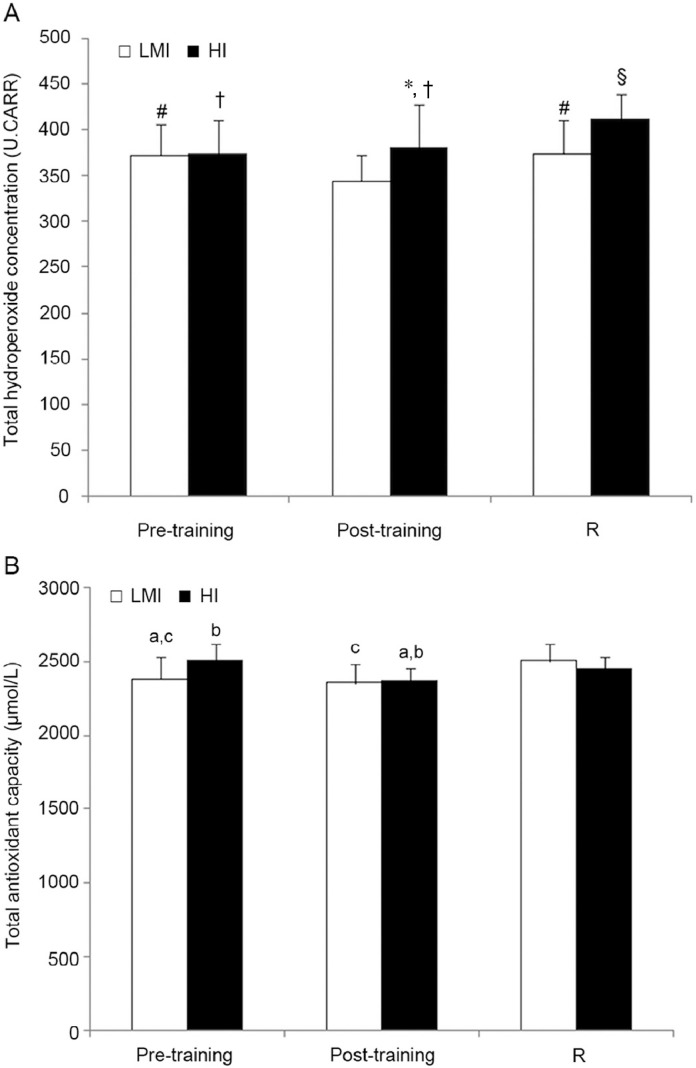
Gymnasts' pro-oxidant and antioxidant levels obtained in response to both training protocols are shown in Fig. 1. Before both training sessions, gymnasts presented the same amount of hydroperoxides (p > 0.05), corresponding to a condition of middle oxidative stress according to the CARR classification.25, 26 The levels of oxidants significantly decreased after the LMI session and reached the baseline at 48 h-post R. The U.CARR value reached in the LMI after training was at the border of mild oxidative stress. In contrast, the pattern of plasmatic pro-oxidant levels was different in response to the HI training session. In detail, the baseline hydroperoxide level remained unchanged after training and significantly increased to overcome the initial value at 48 h-post R (48 h-post R vs. pre- and post-training, p < 0.01). The reached value corresponds to a condition of high oxidative stress according to CARR classification.25, 26 Comparing pro-oxidant production between the 2 training protocols, a significantly higher value was reached at immediately after training and 48 h-post R following the HI session compared with the value after the LMI session (p < 0.01) (Fig. 1A).
Fig. 1.
Total hydroperoxide concentration (A) and total antioxidant capacity (B) before (pre-training), immediately after (post-training), and 48 h after recovery (R) in response to low-moderate intensity (LMI) and high-intensity (HI) trainings. 1 U.CARR = 20–24 mg/dL H2O2. *p < 0.01, compared with post-training LMI; #p < 0.05, compared with post-training LMI; †p < 0.01, compared with R HI; §p < 0.01, compared with R LMI; ap < 0.05, compared with pre-training HI; bp < 0.05, compared with R HI; cp < 0.05, compared with R LMI.
The plasmatic values of antioxidant capacity detected in both training sessions fell into the normal range according to the CARR classification.25, 26 As exhibited in Fig. 1B, the baseline antioxidant capacity did not change after an LMI session (p = 0.307), whereas it increased at 48 h-post R compared with pre- and post-training (F(1,8) = 3.815, p = 0.035). In the HI training session, we found a lower level of antioxidant capacity in post-training than in pre-training (p = 0.011) and a significant difference between pre- and post-training vs. 48 h-post R (F(1,8) = 5.067, p = 0.014). From the comparison of the 2 training sessions, we detected that antioxidant capacity in the HI pre-training session was significantly higher than in the LMI pre-training session (p < 0.05), whereas there was no significant variation between post-training conditions and between recovery periods according to different training intensities.
4. Discussion
This study investigated the blood responses of the redox system to 2 training sessions typical of rhythmic gymnastics performed at different intensities. The first interesting result showed that the baseline levels of hydroperoxides corresponded to middle oxidative stress. This might be due to insufficient levels of plasma antioxidant capacity and/or an adaptive response to chronic exercise training, which determines an enhanced tolerance to exercise-induced stress. In our study, although the baseline antioxidant capacity was in the normal range, it might be not have been enough for athletes subjected to high-intensity exercises. Other authors found an altered antioxidant enzyme profile in competitive rhythmic gymnasts compared with their sedentary peers.17 In regard to the increased amount of oxidants at baseline, our data are confirmed by a previous study in which rhythmic gymnasts showed higher lipid peroxidation than untrained healthy female adolescents.18 This value might increase as a result of a lowering of the susceptibility threshold of redox sensors due to a training-adaptation process and a higher ability of gymnasts to adapt to oxidative stress.27 Different basal lipid peroxidation levels were also found in the blood of top-level athletes in rowing, cycling, and taekwondo, proposing a sport-specific adaptation for ROS production.11 These data imply a crucial role of ROS as mediator of some adaptive responses of skeletal muscle to contractile activity through the specific activation of redox-sensitive transcription factors, as also shown by other studies.28
In this study, we reproduced 2 training sessions separated by 48 h of recovery, which were usually performed by gymnasts in the same week, to collect data useful to coaches for establishing a healthy plan for training sessions. These protocols differed in intensity and had significantly dissimilar effects on pro-oxidant/antioxidant status. After the percentage of time spent at HR above the AT was analyzed, it was determined that the LMI session could be considered an intermittent training protocol with a predominant aerobic component, whereas the HI session was intermittent training with a prevalent anaerobic component. The HI session induced a higher production of hydroperoxides than the LMI session following training and even 48 h after recovery. This might be explained by a larger presence of an anaerobic metabolic contribution and/or eccentric and isometric muscle contractions in the HI compared to the LMI protocol, as suggested by other studies.9, 10, 19, 27 The mechanisms by which this increased ROS production can occur might be ischemia-reperfusion phenomena and inflammatory responses related to muscle damage induced by exercise.19, 27, 29 Some products of oxidative reactions may not be elevated directly after exercise and can reach their maximal levels hours, or even days, after the end of exercise.9, 27
In the present study, oxidant levels increased not immediately after HI training but rather 48 h after recovery, compared with baseline levels. These levels were associated with a high oxidative stress that might contribute to fatigue.2 Our results are confirmed by other studies in which pro-oxidant blood `biomarkers (thiobarbituric acid reactive substances, malondialdehyde, and protein carbonyls) increased throughout the recovery period, particularly 24 h, 48 h, and 72 h after HI exercises.9, 19, 27 In these studies, the increase in oxidative activity was associated with an increase in creatine kinase activity through 48 h of recovery, implying exercise-induced skeletal muscle damage. Therefore, these findings suggest that in our study elevated levels of hydroperoxides found 48 h after HI training might result from an inflammatory response induced by exercise. Moreover, the decline in total antioxidant capacity after HI training might contribute to an increased plasma hydroperoxide concentration in gymnasts, as reported in other studies.6, 19, 27 Because antioxidant capacity found 48 h after the HI session approached the baseline value, we speculated that more recovery time should be necessary to restore redox balance. Comparing both training protocols, it appears that the training intensity does not affect antioxidant capacity, although its trend is different in response to LMI and HI sessions. This might mean that there is an elevated adaptability of the antioxidant system to chronic exercise.30 The significantly higher level of antioxidant capacity in HI compared to LMI at pre-training suggests that an LMI session can positively influence the antioxidant activity of a following training session when the intersession duration is 48 h.
Different from the HI protocol, the responses of the oxidant/antioxidant system to LMI training are quite dissimilar. In detail, immediately after the LMI training session, gymnasts showed an improvement in oxidative stress ranging from moderate to mild, although this returned to the middle baseline condition 48 h after training. On the other hand, total antioxidant capacity did not change after training, whereas it increased more than pre-training after 2 days of recovery. In this case, LMI training appears to have a short- and long-term positive effect on hydroperoxide and antioxidant activity levels, respectively, suggesting different regulation mechanisms. In this regard, it has been stated that several antioxidant enzymes may be rapidly activated following acute oxidative stress caused by infections, toxins, and metabolic disturbances (such as cold, hypoxia, and ischemia), whereas other genes are upregulated in a slower fashion in response to chronic oxidative stresses such as aging, disease, environmental changes, and energy demands (e.g., endurance training).31 Therefore, in the present study, the upregulation of antioxidant capacity after recovery in the LMI session might be the result of chronic oxidative stress. The fact that despite an improved antioxidant capacity, an increase in ROS levels occurred at 48 h-post R might mean that this amount is not toxic for cells and its production is controlled and involved in molecular mechanisms by which physiological adaptations to exercise training are carried out.32 For example, it has been shown that in skeletal muscle, low levels of ROS are required for normal force production during basal conditions. A modest ROS increase is related to an increase in force production, whereas higher ROS concentrations lead to its decrease in both a time- and dose-dependent manner.7 Therefore, the antioxidant cellular network plays a fundamental role in maintaining ROS below the physiologically compatible threshold level, which allows ROS to function as a signaling molecule and avoid toxic effects.32
Given the large number of eccentric muscle contractions and strong muscle stretches in the training programs of rhythmic gymnasts, future investigations might address whether titin, a sarcomeric protein involved in muscle assembly, elasticity, and stability and modulated by exercise,33 is sensitive to ROS-mediated oxidation.
5. Conclusion
The present study showed that in moderately trained rhythmic gymnasts, the plasma levels of hydroperoxides vary according to the training intensity differently from what happens for the total antioxidant capacity. Moreover, the pattern of pro- and antioxidant levels implies different regulation mechanisms during HI and LMI training sessions. It was our intention to reproduce the same training sessions that amateur gymnasts usually perform in 1 week to collect data useful to coaches for establishing safe and healthy planning of training sessions. In light of our results, 48 h of recovery following an HI training session is not sufficient to restore redox balance. A diet rich in antioxidants, increased recovery times, and LMI sessions after HI training should be recommended for amateur gymnasts. Given the early age at which gymnasts begin to train, it becomes relevant for coaches to apply this knowledge to limit oxidative stress-induced damages, such as muscle aging, and to avoid injuries, over-reaching, and altered performance in gymnasts. Nevertheless, even though this result could be of interest for amateur training, it is not applicable to highly trained athletes who perform exercise training much more frequently, even with multiple sessions a day. In that regard, it would be appropriate for oxidant/antioxidant levels to be quantified in elite athletes.
Acknowledgments
The authors wish to thank Drs Marco Petrucci and Angelo Cataldo for their kind collaboration in this study. This study was supported by the University of Palermo (FFR 2012-13).
Authors' contributions
MB was the main researcher and was responsible for study design, interpretation of data, and draft of manuscript; AB carried out the acquisition of data and analyses of exercise session intensity; GB conceived the study and participated in the acquisition of data; MSN participated in the subject recruitment and carried out the exercise protocols; GC conceived the study and participated in its design; JP carried out the statistical analysis; KC helped to draft the manuscript; APaoli participated in the interpretation of data; and APalma conceived the study, participated in its design and coordination. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Banerjee A.K., Mandal A., Chanda D., Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2003;253:307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 2.Reid M.B. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 4.Turrens J.F. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers S.K., Talbert E.E., Adhihetty P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finaud J., Lac G., Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36:327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niess A.M., Simon P. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Front Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanis G.C., Stavrinou P., Fatouros I.G., Philippou A., Chatzinikolaou A., Draganidis D. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol. 2013;61:171–177. doi: 10.1016/j.fct.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Bloomer R.J., Goldfarb A.H., Wideman L., McKenzie M.J., Consitt L.A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res. 2005;19:276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 11.Cubrilo D., Djordjevic D., Zivkovic V., Djuric D., Blagojevic D., Spasic M. Oxidative stress and nitrite dynamics under maximal load in elite athletes: relation to sport type. Mol Cell Biochem. 2011;355:273–279. doi: 10.1007/s11010-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso A.M., Bagatini M.D., Roth M.A., Martins C.C., Rezer J.F., Mello F.F. Acute effects of resistance exercise and intermittent intense aerobic exercise on blood cell count and oxidative stress in trained middle-aged women. Braz J Med Biol Res. 2012;45:1172–1182. doi: 10.1590/S0100-879X2012007500166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djordjevic D.Z., Cubrilo D.G., Puzovic V.S., Vuletic M.S., Zivkovic V.I., Barudzic N.S. Changes in athlete's redox state induced by habitual and unaccustomed exercise. Oxid Med Cell Longev. 2012;2012:805850. doi: 10.1155/2012/805850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu D. Strength exercise specific to gymnastics. J Strength Cond Res. 1994;8:95–102. [Google Scholar]
- 15.Douda H.T., Toubekis A.G., Avloniti A.A., Tokmakidis S.P. Physiological and anthropometric determinants of rhythmic gymnastics performance. Int J Sports Physiol Perform. 2008;3:41–54. doi: 10.1123/ijspp.3.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Sanborn C.F., Horea M., Simmers B.J., Dieringer K.I. Disordered eating and the female athlete triad. Clin Sports Med. 2000;19:199–213. doi: 10.1016/s0278-5919(05)70199-x. [DOI] [PubMed] [Google Scholar]
- 17.Alshammari E., Shafi S., Nurmi-Lawton J., Taylor A., Lanham-New S., Ferns G. Altered antioxidant and trace-element status in adolescent female gymnasts. Int J Sport Nutr Exerc Metab. 2010;20:291–298. doi: 10.1123/ijsnem.20.4.291. [DOI] [PubMed] [Google Scholar]
- 18.Guerra A., Rego C., Laires M.J., Castro E.M., Silva D., Monteiro C. Lipid profile and redox status in high performance rhythmic female teenagers gymnasts. J Sports Med Phys Fitness. 2001;41:505–512. [PubMed] [Google Scholar]
- 19.Rodrigues-Krause J., Krause M., Cunha Gdos S., Perin D., Martins J.B., Alberton C.L. Ballet dancers cardiorespiratory, oxidative and muscle damage responses to classes and rehearsals. Eur J Sport Sci. 2014;14:199–208. doi: 10.1080/17461391.2013.777796. [DOI] [PubMed] [Google Scholar]
- 20.Cornelli U., Belcaro G., Cesarone M.R., Finco A. Analysis of oxidative stress during the menstrual cycle. Reprod Biol Endocrinol. 2013;11:74. doi: 10.1186/1477-7827-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianco A., Mammina C., Thomas E., Bellafiore M., Battaglia G., Moro T. Protein supplementation and dietary behaviours of resistance trained men and women attending commercial gyms: a comparative study between the city centre and the suburbs of Palermo, Italy. J Int Soc Sports Nutr. 2014;11:30. doi: 10.1186/1550-2783-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padulo J., Chamari K., Ardigò L.P. Walking and running on treadmill: the standard criteria for kinematics studies. Muscles Ligaments Tendons J. 2014;4:159–162. [PMC free article] [PubMed] [Google Scholar]
- 23.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 24.Paoli A., Pacelli Q.F., Moro T., Marcolin G., Neri M., Battaglia G. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis. 2013;12:131. doi: 10.1186/1476-511X-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmieri B., Sblendorio V. Oxidative stress tests: overview on reliability and use. Part II. Eur Rev Med Pharmacol Sci. 2007;11:383–399. [PubMed] [Google Scholar]
- 26.Trotti R., Carratelli M., Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002;44:37–40. [PubMed] [Google Scholar]
- 27.Fatouros I.G., Chatzinikolaou A., Douroudos I.I., Nikolaidis M.G., Kyparos A., Margonis K. Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. J Strength Cond Res. 2010;24:3278–3286. doi: 10.1519/JSC.0b013e3181b60444. [DOI] [PubMed] [Google Scholar]
- 28.Jackson M.J., McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol. 2011;589:2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollaard N.B., Shearman J.P., Cooper C.E. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Med. 2005;35:1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ji L.L. Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic Biol Med. 2008;44:142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Ji L.L., Gomez-Cabrera M.C., Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri E., Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct. 2012;2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellafiore M., Cappello F., Palumbo D., Macaluso F., Bianco A., Palma A. Increased expression of titin in mouse gastrocnemius muscle in response to an endurance-training program. Eur J Histochem. 2007;51:119–124. [PubMed] [Google Scholar]