Abstract
Background
Preliminary evidence suggests an association of hypovitaminosis D (hypo.D) with mechanical Low back ache (mLBA).
Aim
This study was designed to 1. Explore the relationship of hypovitaminosis D with mLBA in the absence of other confounding factors 2. Formulate and validate an appropriate treatment protocol and 3. Explore the differences in outcomes with various oral formulations of vitamin D available in Indian market.
Materials & methods
Three randomised groups of patients with mLBA and hypo.D between 18 and 45 years of age without any co morbid conditions were studied for the effectiveness of adjunctive vit.D supplementation of 6,00,000 IUs (60,000 IUs/day for ten consecutive days) in the form of granule or nano syrup or soft gel capsule for the treatment of mLBA. Review evaluation of pain, functional disability and vit.D was done at three weeks and an additional evaluation of vit.D was done at nine months. Evaluation with 3,00,000 IUs of vit.D (60,000 IUs/day for five consecutive days) was done with nano syrup in a different cohort.
Results
High prevalence of hypo.D (96%) was noted in patients with mLBA. Significant improvement was noted after supplementation of vit.D. The subjects of nano syrup group have shown significantly better improvement compared to others (P < 0.000). Non obese and chronic patients have shown significantly better results than their peers. Though there was significant difference in vit.D before treatment, the difference of improvement between the genders, deficiency and insufficiency, in-door and out-door, smokers and non smoker subgroups was not significant. Seasonal variation in vit.D before and after the treatment was significant.
Conclusion
Hypovitaminosis D can be a potential causative factor for mLBA in addition to the other known causes. Proper evaluation and adjunctive vit.D supplementation can effectively break the vicious cycle of low back ache with significant improvement in serum vit.D level, effective relief of pain and significant functional improvement without any adverse effects. Improvement in vit.D was not significantly related to its initial status and obese individuals have shown significantly lesser improvement. The results with nano syrup formulation were significantly better compared to others. Formulation based dosage adjustments assume significance in view of these results.
Keywords: Hypovitaminosis D, Mechanical low back ache, Formulation, Nano syrup
1. Introduction
Mechanical/non neurological low back ache (mLBA) is one of the commonest and expensive ailments of youngsters with ambiguous pathophysiology leading to a significant loss of productivity. 90% of them improve after six to eight weeks of treatment with 60% recurrence in two years to follow.1,2 The dynamic stabilizers of spine are predisposed to acute and chronic strain owing to various modifiable and non modifiable risk factors.1,3 Though hypovitaminosis D (hypo.D) is rampant worldwide, very few studies have reported its prevalence in LBA patients with inherent study limitations of age related degenerative changes and co morbid conditions. Further studies to establish a causal relationship and propose an appropriate evaluation, treatment protocol were recommended.4, 5, 6, 7, 8, 9
Though vitamin D (vit.D) is a proven anabolic hormone for the entire musculoskeletal system, hypo.D is still an overtly underestimated, preventable and correctable etiological factor for mLBA.6,9 In view of the lacunae in literature, this study was designed to explore the relationship of hypo.D with mLBA, formulate an appropriate treatment protocol and explore the outcomes with various formulations of vit.D.
2. Material and methods
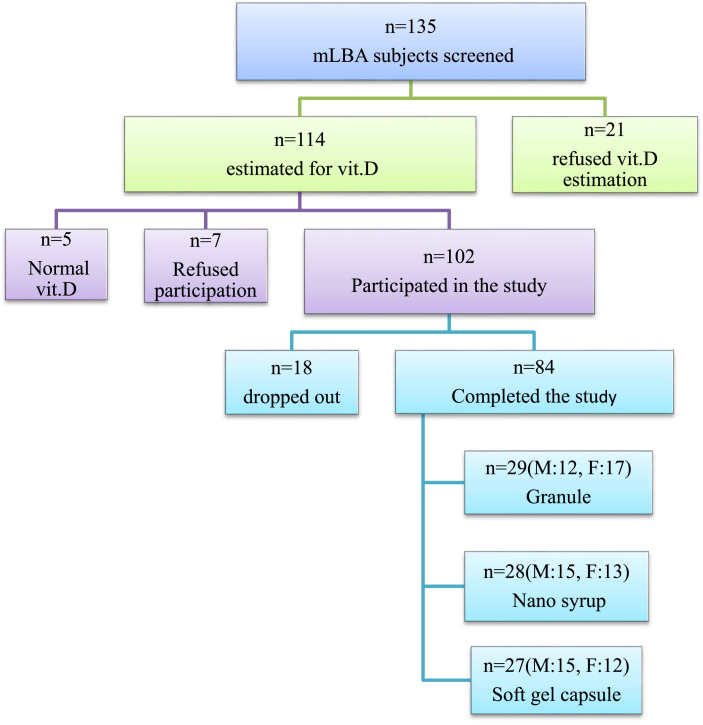
This is a randomized, prospective, open label analytical study of a cohort of patients with mLBA and hypo.D. Patients were sequentially randomized to one of the three treatment subgroups (Granule group, Nano Syrup group and Soft gel capsule group) named after the vit.D formulation they received after establishment of clinical, radiological and biochemical eligibility. Ethical committee approval and informed consent were taken before commencing the study. 135 subjects were screened. 102 subjects were eligible to participate and 84 have completed the study.
Patients of both the genders between 18 and 45 years of age were included. Pregnant and lactating women, patients on vit.D supplements for the past three months, patients on drugs altering vit.D metabolism, medical or surgical disorders affecting vit.D metabolism, pre-existing co morbidities, neurological back ache, congenital or developmental malformations of spine and patients with history of trauma were excluded.
Pain and functional disability were assessed with visual analogue scale (VAS) and Modified Oswestry low back pain disability questionnaire (MODQ) respectively.10 Treatment with analgesic (aceclofenac), muscle relaxant (thiocolchicoside) and antacid (ranitidine) were given to all the patients uniformly for five days. Vit.D analysis was done by Chemiluminescence Immuno Assay method. Vit.D < 30 ng/ml was considered as hypovitaminosis D, 20–29.9 ng/ml as insufficiency, <20 ng/ml as deficiency and 30–100 ng/ml as sufficiency.4,6 Apart from the three treatment subgroups, patients were divided into various groups for comparison of results. Pain beyond three months was considered as chronic.11
Fit for study candidates were allotted to one of the treatment subgroups sequentially as per the randomization chart and vit.D supplementation of 60,000 IUs per dose for ten consecutive days (pulse-D therapy: author proposed nomenclature for high dose daily supplementation of vitamin D in a pulsed manner) was given in the form of granule (1 g sachet) or nano syrup developed using aqueol nano technology (5 mL bottle) or soft gel capsule. Adverse drug reaction recording chart was provided to all patients and was reviewed regularly. Review analysis was done at three weeks to conclude the findings. Additional blood sample was collected from willing subjects after nine months to study the decline of vit.D level.
Owing to the difference in results with ten doses of different formulations of vit.D, additional ten cases were analyzed in similar lines with five daily doses of 60,000 IUs of vit.D in nano syrup form.
THEORY: Vit.D can play an important role in pathogenesis and treatment of mLBA. Formulation and modality of supplementation do have an effect on the functional outcome.
CALCULATION: Statistical analysis was done with MedCalc ver.13. Descriptive statistics (n, Mean, Standard Error of Mean (SEM) / Standard Deviation (SD) & Range) were presented for all continuous variables. p value < 0.05 was taken as statistically significant. Paired student T test, Independent sample T test were used for comparisons of two groups and one/two way analysis of variance (ANOVA) was done for multiple comparisons. Nominal variable (VAS) was analyzed by Chi-square test. The prefix “Pre” implies variable before treatment and “Post” implies variable after treatment, suffix “D” implies vit.D. The term improvement/Diff. in vit.D implies “Post.D minus Pre.D”. Total cohort/overall study group (n = 84) implies all the studied patients.
3. Results
Out of the 102 eligible subjects, 84 could complete the study (Fig. 1). Mean age of the total cohort was 31.32 ± 7.02 years and the mean BMI was 23.77 ± 4.18 kg/m2. Highest increase of mean vit.D was noted in nano syrup group i.e. from 16.59 ± 6.34 ng/ml to 96.75 ± 25.74 ng/ml (Table 1).
Fig. 1.
Details of subjects enrolled in the study.
Table 1.
Summary statistics of the study group (n = 84).
| Variable | Total study cohort (n = 84) |
Granule sub group (n = 29) |
Nano syrup sub group (n = 28) |
Soft gel capsule sub group (n = 27) |
||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | |
| Age (years) | 18–45 | 31.32 ± 7.02 | 20–43 | 29.45 ± 6.82 | 18–44 | 30.82 ± 7.19 | 21–45 | 33.85 ± 6.54 |
| BMI (kg/m2) | 16.53–37.66 | 23.77 ± 4.18 | 16.94–37.66 | 24.17 ± 5.17 | 16.53–30.12 | 22.6 ± 3.26 | 16.56–32.74 | 24.58 ± 3.71 |
| Pain (months) | 0.2–60 | 10.84 ± 12.86 | 0.2–36 | 9.59 ± 10.64 | 0.25–60 | 8.23 ± 11.65 | 0.70–47 | 14.3 ± 15.64 |
| Pre-MODQ% | 12–100 | 44.17 ± 15.35 | 12–62 | 38.41 ± 13.92 | 30–100 | 51.29 ± 16.39 | 20–66 | 42.96 ± 13.08 |
| Post-MODQ% | 0–52 | 15.62 ± 12.07 | 0–46 | 17.45 ± 13.19 | 0–28 | 11.64 ± 9.08 | 0–52 | 17.78 ± 12.89 |
| Diff MODQ% | 6–72 | 28.55 ± 16.27 | 8–46 | 20.97 ± 10.33 | 12–72 | 39.64 ± 17.76 | 6–66 | 25.18 ± 13.93 |
| Pre- Vit.D ng/ml | 4.20–28.3 | 15.71 ± 6.62 | 4.20–28.3 | 15.1 ± 7.43 | 7.20–28.30 | 16.59 ± 6.34 | 6.40–27.60 | 15.46 ± 6.11 |
| Post-vit.D ng/ml | 24–150 | 77.47 ± 27.91 | 25.8–150 | 68.92 ± 28.62 | 34–148.30 | 96.75 ± 25.74 | 24–102 | 66.65 ± 17.67 |
| Diff. in vit.D | 14.3–132.9 | 61.75 ± 26.58 | 14.3–132.9 | 53.82 ± 26.99 | 15.2–131.6 | 80.16 ± 24.97 | 15.7–84.8 | 51.19 ± 16.50 |
| BMI= Body Mass Index, Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, MODQ = Modified Oswestry low back pain disability questionnaire (Index in %), Diff = Difference. | ||||||||
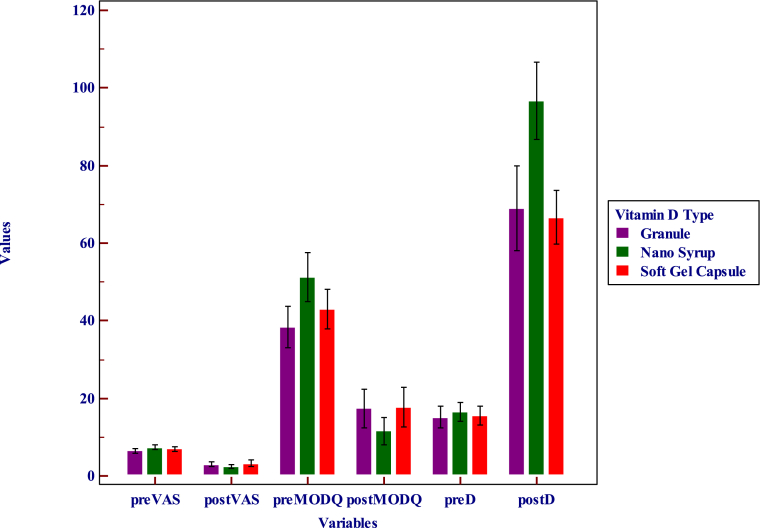
Significant difference in VAS was noted in all the three treatment subgroups and total cohort with adjunctive pulse D therapy (Table 2). The difference in vit.D and MODQ was significant in each of the study groups after treatment (Table 3). The difference in vit.D and MODQ was significant across the three study groups after treatment (Table 4). Significant difference in vit.D was noted between the nano syrup group and the other two groups after treatment (Table 5, Fig. 2).
Table 2.
Statistical data on VAS among different treatment subgroups.
| Study group | Variable | Chi square | Contingency co-efficient | Df | p value |
|---|---|---|---|---|---|
| Total | Pre VAS vs Post VAS | 204.88 | 0.842 | 56 | <0.0001 |
| Granule | Pre VAS vs Post VAS | 137.64 | 0.909 | 105 | = 0.02 |
| Nano syrup | Pre VAS vs Post VAS | 86.15 | 0.87 | 25 | <0.0001 |
| Soft gel capsule | Pre VAS vs Post VAS | 122.51 | 0.905 | 48 | <0.0001 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks,VAS= Visual analogue scale.
Table 3.
Statistical data for Vit.D and MODQ - before versus after treatment.
| STUDY GROUP | VITAMIN D |
MODQ |
||||
|---|---|---|---|---|---|---|
| Paired T test |
Paired T test |
|||||
| t | Df | p | t | Df | p | |
| Total Study cohort | 21.29 | 83 | <0.0001 | −16.08 | 83 | <0.0001 |
| Granule sub group | 10.74 | 28 | <0.0001 | −10.93 | 28 | <0.0001 |
| Nano syrup sub group | 16.99 | 27 | <0.0001 | −11.81 | 27 | <0.0001 |
| Soft gel capsule sub group | 16.12 | 26 | <0.0001 | −9.4 | 26 | <0.0001 |
MODQ = Modified Oswestry low back pain disability questionnaire (Index in %).
Table 4.
Statistical data on vit.D and functional disability (MODQ) across three treatment subgroups.
| Variable | ANOVA |
|
|---|---|---|
| F Ratio | p value | |
| Pre MODQ | 5.71 | 0.005* |
| Post MODQ | 2.36 | 0.101 |
| Diff. in MODQ (Post minus Pre) | 86.56 | <0.001* |
| Pre.D | 0.38 | 0.684 |
| Post.D | 12.98 | <0.001* |
| Diff. in Vit.D (Post minus Pre) | 13.09 | <0.001* |
* Postohoc analysis revealed p<0.05 for pair wise comparisons (Syrup vs Capsule, Syrup vs Granule, Capsule vs Granule). Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, D or Vit.D = Vitamin D, MODQ = Modified Oswestry low back pain disability questionnaire (Index in %), Diff = Difference.
Table 5.
Statistical data on vit.D across different treatment subgroups before and after treatment.
| Comparison | Independent sample T test |
|
|---|---|---|
| Pre.D | Post.D | |
| Nano syrup vs Soft gel capsule | t = − 0.67, Df = 53, p = 0.506 | t = −5.04, Df = 53, p < 0.0001 |
| Nano syrup vs Granule | t = −0.81, Df = 55, p = 0.421 | t = −3.85, Df = 55, p = 0.0003 |
| Soft gel capsule vs Granule | t = 0.20, Df = 54, p = 0.844 | t = 0.35, Df = 54, p = 0.725 |
Fig. 2.
Clustered multiple variable graph comparing VAS, MODQ and Vit. D levels before and after treatment among the three treatment subgroups.
There was no significant difference between the genders in pain (VAS) before and after treatment (Table 6). Women had significantly lower vit.D before treatment and men had significantly better functional improvement after treatment (Table 7).
Table 6.
Statistical data on VAS (before and after treatment) between male and female cohorts.
| Male vs. Female | Chi square | Contingency Co-efficient | Df | p value |
|---|---|---|---|---|
| Pre VAS | 52.2 | 0.74 | 49 | 0.35 |
| Post VAS | 62.65 | 0.774 | 49 | 0.09 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks,VAS= Visual analogue scale.
Table 7.
Statistical data on Vit.D and MODQ before and after treatment between the genders.
| Gender | Vit.D before treatment in ng/ml (Mean ± SD) | Vit.D after treatment in ng/ml (Mean ± SD) |
|---|---|---|
| Males | 17.4 ± 7.28 | 80.97 ± 27.39 |
| Females | 14.03 ± 5.47 | 73.97 ± 28.30 |
| Male vs. Female Significance (ANOVA) | F ratio = 5.77, p = 0.02 | F ratio = 1.33, p = 0.25 |
| Gender | MODQ before treatment | MODQ after treatment |
| Males | 45.7% | 12.52% |
| Females | 42.5% | 18.71% |
| Male vs. Female Significance (ANOVA) | F ratio = 0.906, p = 0.34 | F ratio = 5.85, p = 0.02 |
MODQ = Modified Oswestry low back pain disability questionnaire (Index in %).
The difference in vit.D between deficiency vs. insufficiency groups after treatment was not significant (Table 8). Subjects living indoors had lower vit.D and subjects with chronic mLBA had significantly better improvement with pulse D therapy (Table 9). Significant difference in vitamin D was noted among various season groups (Table 10).
Table 8.
Vit.D between deficiency and insufficiency groups across different treatment subgroups.
| Variable | Number & Significance | Total cohort | Granule | Nano Syrup | Soft gel Capsule |
|---|---|---|---|---|---|
| Deficiency | n = | 60 (71.42%) | 20 | 20 | 20 |
| Pre.D vs Post.D (Paired T test) | t = 18.77, Df = 59, p < 0.0001 | t = 9.72, Df = 19, p < 0.0001 | t = 13.9, Df = 19, p < 0.0001 | t = 13.49, Df = 19, p < 0.0001 | |
| Insufficiency | n = | 24 (28.58%) | 9 | 8 | 7 |
| Pre.D vs Post.D (Paired T test) | t = 10.14, Df = 23, p < 0.0001 | t = 4.838, Df = 8, p = 0.0013 | t = 9.257, Df = 7, p < 0.0001 | t = 8.606, Df = 6, p=0.0001 | |
| Deficiency vs Insufficiency | Post.D – Pre.D (Improvement) Independent sample T test | t = − 0.603, Df = 62, p = 0.548 | t = −0.908, Df = 27, p = 0.372 | t = 0.197, Df = 26, p = 0.845 |
t = −0.504, Df = 25, p = 0.619 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, D = Vitamin D.
Table 9.
Statistical data on vitamin D between various groups.
| Total cohort | Before treatment (Mean ± SD of vitamin D in ng/ml) | After treatment (Mean ± SD of vitamin D in ng/ml) |
|---|---|---|
| Acute vs Chronic | ||
| Acute n = 26 (30%) | 14.67 ± 6.85, 95% CI = 11.90 to 17.43 | 68.15 ± 23.95, 95% CI = 51.48to 85.56. |
| Chronic n = 58 (70%) | 16.18 ± 6.52, 95% CI = 14.47 to 17.89 | 81.59 ± 28.75, 95% CI = 74.03 to 89.15 |
| Independent sample T test | t = 0.97, Df = 82, p = 0.34 | t = 2.08, Df = 82, p = 0.04. |
| Indoor vs Outdoor | ||
| Indoor n = 60 (71%) | 14.47 ± 6.08, 95% CI = 12.90 to 16.04 | 75.85 ± 28.52, 95% CI = 68.48 to 83.22 |
| Outdoor n = 24 (29%) | 19.07 ± 6.73, 95% CI = 16.22 to 21.91. | 81.39 ± 26.53, 95% CI = 70.18 to 92.59 |
| Independent sample T test | t = 3.03, Df = 82, p = 0.003 | t = 0.82, Df = 82, p = 0.42. |
| Non smokers vs Smokers | ||
| Non smokers n = 80 (95%) | 15.71 ± 6.60, 95% CI = 14.25 to 17.18 | 76.29 ± 27.13, 95% CI = 70.25 to 82.33 |
| Smokers n = 4(5%) | 15.67 ± 8.15, 95% CI = 2.71 to 28.64 | 101.02 ± 37.18, 95% CI = 41.86 to 160.19 |
| Independent sample T test | t = −0.012, Df = 82, p = 0.99. | t = 1.75, Df = 82, p = 0.08 |
Chronic: Group of subjects who had back pain for more than 3 months duration.
Acute: Group of subjects who had back pain for less than 3 months duration.
Indoor: Group of subjects who were not exposed to adequate sunlight.
Outdoor: Group of subjects who were exposed to adequate sunlight.
Non smokers: Group of subjects who never smoked cigarettes.
Smokers: Group of subjects who have a habit of smoking cigarettes.
Table 10.
Vitamin D in cohorts of different seasons.
| SEASON | n | Vitamin D before treatment |
Vitamin D after treatment |
|---|---|---|---|
| Mean ± SD ng/ml | Mean ± SD ng/ml | ||
| Summer (April–June) | 10 | 10.71 ± 4.21 | 58.08 ± 26.57 |
| Monsoon (July–September) | 33 | 15.40 ± 7.18 | 73.53 ± 23.72 |
| Autumn (October–November) | 30 | 18.02 ± 5.96 | 83.66 ± 32.14 |
| Winter (December–March) | 11 | 14.91 ± 6.14 | 90.02 ± 17.79 |
| ANOVA (Analysis of variance) | F ratio = 3.48, p = 0.020 | F ratio = 3.32, p = 0.024 | |
Majority of the studied subjects were in normal BMI category and the gender variation of BMI was insignificant (Table 11). The difference in vit.D before treatment was insignificant for different grades of BMI. The difference in vit.D after treatment was significant for different grades of BMI in nano syrup group. BMI grade vs. duration of pain was insignificant (Table 12). Improvement in vit.D was higher in lower BMI grades across the three treatment subgroups (Table 13).
Table 11.
BMI & Gender related statistics.
| Description | BMI grade | Percentage (%) |
|---|---|---|
| BMI: <18 kg/m2, Under weight(n = 7) | 1 | 8.3% |
| BMI: 18–24.9 kg/m2, Normal (n = 47) | 2 | 56% |
| BMI: 25–29.9 kg/m2, Overweight (n = 23) | 3 | 27.4% |
| BMI: 30 kg/m2 and above, Obese (n = 7) | 4 | 8.3% |
| Gender | Mean BMIinkg/m2 | |
| Male | 23.99 ± 3.80 | |
| Female | 23.56 ± 4.57 | |
| ANOVA | F ratio = 0.22, p = 0.64. | |
BMI= Body Mass Index, ANOVA = Analysis of variance.
Table 12.
Statistical data on BMI grade versus vit.D & duration of pain.
| Variable | Total Study Group |
Granule sub group |
Nano syrup sub group |
Soft gel capsule sub group |
||||
|---|---|---|---|---|---|---|---|---|
| ANOVA (Analysis of Variance) | ||||||||
| F Ratio | p value | F Ratio | p value | F Ratio | p value | F Ratio | p value | |
| BMI grade vs Pre Vit.D | 0.282 | 0.889 | 0.69 | 0.608 | 0.175 | 0.91 | 1.8 | 0.17 |
| BMI grade vs Post Vit.D | 5.58 | 0.001 | 2.54 | 0.066 | 3.79 | 0.023 | 1.668 | 0.202 |
| BMI grade vs Duration of Pain | 1.17 | 0.33 | 1.114 | 0.37 | 1.25 | 0.31 | 2.15 | 0.12 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks,BMI= Body Mass Index, Vit.D = Vitamin D.
Table 13.
Statistical data on the improvement of vit.D across different BMI grades and treatment subgroups.
| Body mass Index grade | Vitamin D Formulation | n | Two way ANOVA |
||
|---|---|---|---|---|---|
| Estimated marginal mean (vitamin D in ng/ml) | SEM | 95% CI | |||
| 1 | Granule | 3 | 87.2333 | 11.8289 | 63.6413 to 110.8254 |
| Nano syrup | 3 | 97.6667 | 11.8289 | 74.0746 to 121.2587 | |
| Soft gel capsule | 1 | 59.1 | 20.4883 | 18.2374 to 99.9626 | |
| 2 | Granule | 15 | 56.184 | 5.29 | 45.6333 to 66.7347 |
| Nano syrup | 19 | 85.2921 | 4.7003 | 75.9176 to 94.6666 | |
| Soft gel capsule | 15 | 51.6733 | 5.29 | 41.1227 to 62.2240 | |
| 3 | Granule | 8 | 35.2875 | 7.2437 | 20.8404 to 49.7346 |
| Nano syrup | 5 | 54.16 | 9.1626 | 35.8857 to 72.4343 | |
| Soft gel capsule | 8 | 55.8125 | 7.2437 | 41.3654 to 70.2596 | |
| 4 | Granule | 1 | 22.7 | 20.4883 | −18.1626 to 63.5626 |
| Nano syrup | 1 | 60.2 | 20.4883 | 19.3374 to 101.0626 | |
| Soft gel capsule | 3 | 33.8 | 11.8289 | 10.2080 to 57.3920 | |
Significant negative correlation was noted between BMI and improvement in vit.D in nano syrup group (Table 14). Insignificant negative correlation was noted between age and improvement in vit.D in nano syrup group (Table 15). Analysis of the drug content in all the three formulations of vit.D was done in an independent accredited laboratory. 129.40, 118.10 and 149.05% of drug for granule, nano syrup and soft gel capsule respectively per unit was noted (Table 16).
Table 14.
Correlation statistics of BMI and vit.D.
| Variable | BMI vs Pre.D |
BMI vs Improvement in vit.D |
||||
|---|---|---|---|---|---|---|
| r | 95% CI | p | r | 95% CI | p | |
| Total study group | −0.066 | −0.28 to 0.15 | 0.55 | −0.35 | −0.53 to −0.15 | 0.001 |
| Granule sub group | −0.16 | −0.38 to 0.35 | 0.93 | −0.26 | −0.57 to 0.12 | 0.18 |
| Nano syrup sub group | −0.137 | −0.48 to 0.25 | 0.49 | −0.44 | −0.72 to −0.08 | 0.018 |
| Soft gel capsule sub group | −0.03 | −0.40 to 0.36 | 0.89 | −0.23 | −0.56 to 0.17 | 0.254 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, Prefix improvement = Pre minus Post, BMI= Body Mass Index, vit.D = Vitamin D.
Table 15.
Correlation statistics of Age and vit.D.
| Variable | Age vs Pre.D |
Age vs Improvement in vit.D |
||||
|---|---|---|---|---|---|---|
| r | 95% CI | p | r | 95% CI | p | |
| Total study cohort | −0.011 | −0.22 to 0.20 | 0.92 | −0.276 | −0.46 to −0.06 | 0.01 |
| Granule sub group | 0.08 | −0.29 to 0.44 | 0.66 | −0.41 | −0.67 to −0.05 | 0.028 |
| Nano syrup sub group | −0.04 | −0.41 to 0.34 | 0.84 | −0.129 | −0.479 to −0.26 | 0.51 |
| Soft gel capsule sub group | −0.114 | −0.47 to 0.28 | 0.57 | −0.36 | −0.65 to −0.03 | 0.07 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, Prefix improvement = Pre minus Post, BMI= Body Mass Index, D or vit.D = Vitamin D.
Table 16.
Drug content analysis report.
| Formulation | Average % of vitamin D in each unit |
|---|---|
| Granule | 129.4% |
| Nano syrup | 118.10% |
| Soft gel capsule | 149.05% |
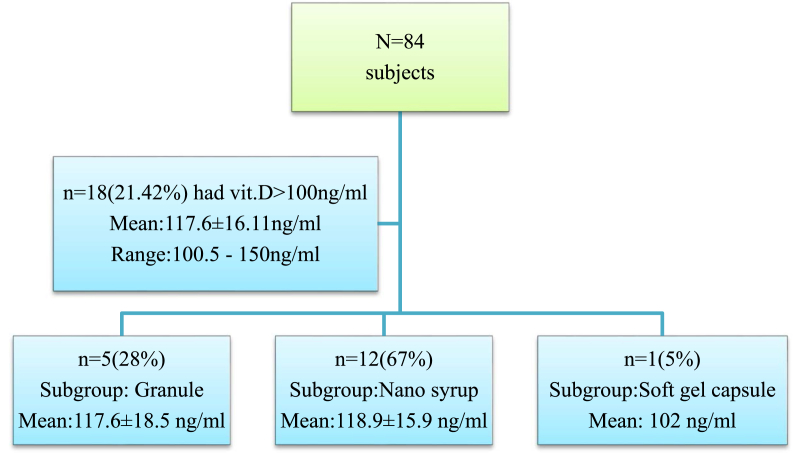
There were no adverse effects attributable to pulse-D therapy. Eighteen subjects had Post.D > 100 ng/ml (Fig. 3). Only two of them consented for the estimation of serum calcium levels as none of them had any complaints of vit.D toxicity and both of them had normal serum calcium levels (9.6, 9.7 mg/dl respectively). Out of n = 84, one patient had puffiness of face and the other one had abdominal discomfort. Both of them responded well after replacing the analgesic with paracet amol.
Fig. 3.
Statistics of subjects with Post.D > 100 ng/ml.
Out of the 84 cases studied, 31 cases were followed up for nine months. For these 31 cases, the difference in vit.D among the three treatment subgroups at 3 weeks post treatment was significant (ANOVA). The same was insignificant before treatment and at nine months after treatment (Table 17).
Table 17.
Statistics of 9 months follow up cohort.
| Group | n = | Mean ± SD: vit.D level at 9 months (ng/ml) | Mean ± SD: Post.D (ng/ml) | Mean ± SD: Pre.D (ng/ml) |
Paired T test (vit.D at 9 months vs Post. D) | Paired T test (vit.D at 9 months vs Pre. D) |
|---|---|---|---|---|---|---|
| 9 months follow up cohort | 31 | 21.84 ± 8.57 | 80.27 ± 24.75 | 16.60 ± 6.19 | t = −12.61, p < 0.0001 |
t = 2.690, p = 0.012 |
| Granule | 10 | 20.59 ± 9.79 | 70.09 ± 22.15 | 16.53 ± 6.31 | t = −07.53, p < 0.0001 |
t = 1.020, p = 0.335 |
| Nano syrup | 13 | 23.79 ± 10.05 | 96.06 ± 24.18 | 16.05 ± 6.85 | t = −08.84, p < 0.0001 |
t = 2.296, p = 0.040 |
| Soft gel capsule | 8 | 20.25 ± 2.47 | 66.31 ± 13.45 | 17.57 ± 5.55 | t = −10.11, p < 0.0001 |
t = 1.450, p = 0.190 |
ANOVA (Granule vs. Nano syrup vs. Soft gel capsule).
Post.D: F ratio = 6.26, p = 0.006.
Pre.D: F ratio = 0.14, p = 0.87.
Vit.D at 9 months follow up: F ratio = 0.56, p = 0.57.
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks,D or vit.D = Vitamin D, ANOVA = Analysis of variance.
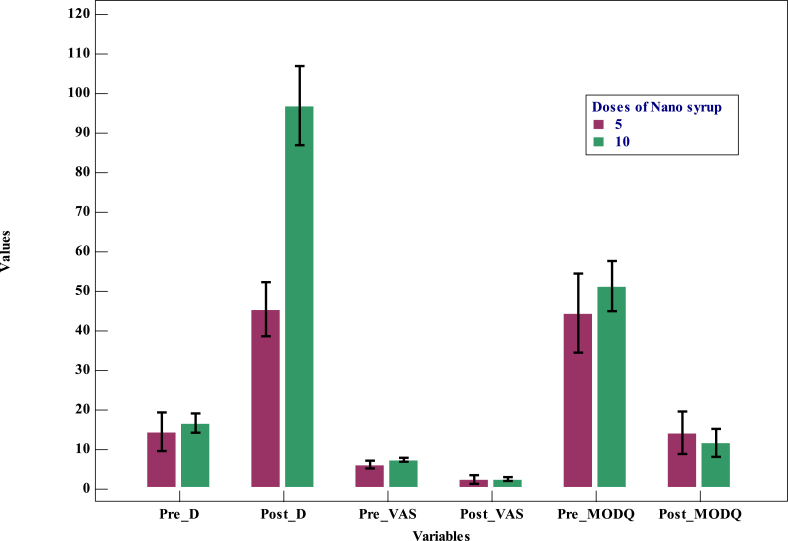
Ten additional cases were studied with 5 daily doses of 60,000 IUs of nano syrup. In these cases, the mean vit.D has increased from 14.3 ± 6.80 ng/ml to 45.4 ± 9.57 ng/ml. There was no significant difference in pain measured by VAS before and after treatment. Significant difference was noted in vit.D and MODQ before and after treatment (Table 18). Significant difference in vit.D after treatment was noted between the group treated with five doses of nano syrup and the three subgroups treated with 10 doses of their respective vit.D formulation (Table 19,Fig. 4).
Table 18.
Statistics of additional subjects (n = 10) analyzed with 5 doses of nano syrup.
| Variable | Range | Mean ± SD | 95% CI for Mean |
|---|---|---|---|
| Age in years | 18–41 | 34.6 ± 7.29 | 29.38 to 39.81 |
| BMI (kg/m2) | 19.38–30.07 | 24.44 ± 3.03 | 22.28 to 26.61 |
| Pain months | 0.16–36 | 8.20 ± 10.69 | 0.56 to 15.85 |
| Pre MODQ% | 22–62 | 44.4 ± 13.88 | 34.47 to 54.33 |
| Post MODQ% | 0–24 | 14.2 ± 7.51 | 8.83 to 19.57 |
| Diff. in MODQ% | 10–48 | 30.2 ± 13.45 | 20.58 to 39.82 |
| Pre.D (ng/ml) | 6.3–24.5 | 14.3 ± 6.80 | 9.43 to 19.17 |
| Post.D (ng/ml) | 33.2–67.2 | 45.4 ± 9.57 | 38.56 to 52.24 |
| Diff. in vit.D (ng/ml) | 17.2–47.6 | 31.1 ± 10.31 | 23.72 to 38.48 |
VAS before and after treatment: (chi square = 21.11, Df = 20, p = 0.391).
Vit.D before and after treatment: (t = 9.53, Df = 9, p < 0.0001).
MODQ before and after treatment: (t = −7.10, Df = 9, p = 0.001).
BMI= Body Mass Index, Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, MODQ = Modified Oswestry low back pain disability questionnaire (Index in %), D or vit.D = Vitamin D, Diff = Difference.
Table 19.
Statistical data on pair wise comparisons across treatment sub groups.
| Comparison | Independent sample T-test |
|||
|---|---|---|---|---|
| Pre Vit.D | Post Vit.D | Pre MODQ | Post MODQ | |
| Granule 10 doses Vs. Nano syrup 5 doses |
t = 0.3, Df = 37, p = 0.766 | t = 2.53, Df = 37, p = 0.016 | t = −1.17, Df = 37, p = 0.248 |
t = 0.735, Df = 37, p = 0.467 |
| Nano syrup 10 doses Vs. Nano syrup 5 doses |
t = 0.961, Df = 36, p = 0.343 | t = 6.114, Df = 36, p < 0.0001 | t = 0.904, Df = 36, p = 0.372 |
t = −0.797, Df = 36, p = 0.431 |
| Soft gel capsule 10 doses Vs. Nano syrup 5 doses |
t = 0.499, Df = 35, p = 0.621 | t = 3.59, Df = 35, p = 0.001 | t = −0.29, Df = 35, p = 0.772 |
t = 0.823, Df = 35, p = 0.416 |
Prefix Pre = Variable before treatment, Prefix Post = Variable after treatment measured at 3 weeks, MODQ = Modified Oswestry low back pain disability questionnaire (Index in %), vit.D = Vitamin D.
Fig. 4.
Multiple variables bar graph comparing the cohorts treated with 5 and 10 doses of nano syrup.
4. Discussion
Vitamin D is essential for growth, development and maintenance of multiple organs in our body and its deficiency will profoundly affect the musculoskeletal system.4,6, 7, 8,12 Modic changes in the disc have been reported in patients with hypo D and LBA.13 Vit.D has a proven role in the improvement of muscle strength, neuromuscular coordination, pain, sleep and mood modulation.4,5,7,14,15
Paraspinal muscles are the dynamic stabilizers of spine and any effect on them will adversely affect the physiology of lower back leading to back ache.2,3 Non surgical active therapeutic interventions aimed at strengthening the support systems of spine and early return to work have proven to be superior.1 Vit.D has a direct role in the pathogenesis and treatment of mLBA along with analgesics and muscle relaxants in the absence of any discernible objective cause. The causal relationship and usefulness of acute correction of hypo.D was not clearly proven in the available literature.6, 7, 8, 9,16,17
Al Faraj S et al. reported high prevalence (83%) of hypo.D in patients with chronic low back pain (cLBP) and all of them had normal vit.D by three months of oral 5000 to 10,000 IUs of vit.D/day with 95% LBA recovery.9
Ghai B et al. reported high prevalence (86%, 82%) of hypo.D in patients with cLBP with mean age of 43.8, 44 years and mean vit.D level of 18.4 ng/ml,12.8 ng/ml in their respective studies.6,8 66% attained normal vit.D after weekly dosing of 60,000 IUs of vit.D for eight weeks with mean vit.D of 36.07 ng/ml and significant clinical improvement in VAS and MODQ at two, three and six months.8
In the present study, 96% of the screened mLBA patients had hypo.D with a mean vit.D level of 15.71 ng/ml. Majority (71.42%) had vit.D deficiency. Only 4% of mLBA patients had normal vit.D (mean = 34.6 ng/ml) and were therefore excluded from the study. The difference of mean vit.D between the two (i.e. hypo.D and normal cohort) was significant (p < 0.001). These findings indicate a strong association between hypo.D and mLBA apart from the other established causes and warrants effective screening of patients with mLBA for hypo.D. In view of significant improvement in pain and functional status after rectification of hypo.D across all the treatment subgroups, adjunctive supplementation of vit.D can be considered as a means for effective treatment of mLBA. This finding is concurrent and additive to the available literature.6, 7, 8, 9
The differential results of various formulations, dose and dosing patterns of vit.D used as an adjunct for individualized management of mLBA were not studied in the past. Nano engineered delivery systems for lipophilic molecules have shown enhanced stability, water solubility and bioavailability.18,19 Significantly better improvement in vit.D and functional outcome with nano syrup in this study proves that the absorption, assimilation and outcome potential is comparatively better with nano formulation developed with aqueol technology for any given dose. Hence, dose adjustments have to be considered for a given formulation in light of these results.
Low dose daily (1000–4000 IUs) and high dose (60,000 IUs) weekly, monthly treatment with oral vit.D was reported by many authors for correction of hypo.D with contradictory results.8,9,14,17,20,21 In a study, twenty weeks of daily supplementation with 5500 and 11000 IU of vit.D lead to a peak increase of 64 and 88 ng/ml of vit.D.22 Similarly, 43.48% of studied patients remained hypo D after eight weeks of weekly 60,000 IUs of vit.D supplementation.20 Prolonged treatment time, loss of compliance, inadequate improvement were the main hurdles for effective treatment in low dose daily and high dose weekly and monthly regimens.8,20,23, 24, 25 A safe cumulative dose of 6,00,000 IUs of vit.D and slower response with divided weekly oral dosing was reported for the treatment of vit.D deficiency.26 Mega single dose (6,00,000 IUs) of intramuscular vit.D was reported to be effective after eight weeks in 35% of studied subjects with a peak at four months.27,28 Similar oral dose had a peak vit.D restoration by three days to one month and decline by three months.28,29 Hence, oral treatment rapidly restores vit.D than intramuscular route.28 Mega single dose of oral 6,00,000 IUs of vit.D (stoss therapy) preparation was not available in Indian market and was not considered as a safety measure.4,28,30
Few studies have reported the outcomes of vit.D supplementation baring daily administration of high dose vit.D.25,26,31 In the present study, Pulse-D therapy (60,000 IUs of vit.D given daily) for ten days was studied for its comparative effectiveness and safety. In conjunction with analgesics and muscle relaxants, it has shown better dose response relationship, faster rectification of deficiency, quicker restoration of muscle strength and effective relief of LBA. It has proven to be a better means for prompt correction of hypo D in mLBA cases. Significant functional improvement with adjunctive pulse-D therapy was not established earlier.
The mean age of subjects in our study was 31.32 years with insignificant difference between the treatment subgroups. Selection of younger subjects without any objective evidence of spine disorders and preexisting co morbid conditions was useful in establishing the one to one relationship of LBA and hypo.D. Negative correlation of age and pre.D, though insignificant, was comparable with the reported literature.5,12,17 Significant negative correlation of age and vit.D after treatment barring nano syrup group indicates that the improvement in vit.D with nano syrup formulation was constant for age unlike the other two formulations.
Significant difference in mean pre.D between the genders with females having lower vit.D than males in our study was similar to the earlier reports and the insignificant difference after treatment was contrary to the reported literature.8,32 There was neither significant difference nor correlation in BMI and pain before and after treatment between the genders. Significantly better functional improvement in males reported in this study was not reported earlier.
The increment of vit.D after treatment was not significantly related to the initial status of vit.D (deficiency or insufficiency). This was contrary to the available literature.5,12,14,33,34
Hypo.D was reported to be associated with chronic pain.7 Though the subjects in acute and chronic groups did not differ significantly before treatment; the improvement in vit.D was significantly higher in the chronic group in our study. This difference was not reported earlier.
Inadequate exposure to sunlight is the major cause of hypo D.4 Full body exposure to sunlight in light pigmented individuals under ideal conditions for 10 to 15 min would produce about 10,000 to 20,000 IUs of vit.D within 24 hrs.35 In our study, patients of indoor group had significantly lesser Pre.D than the outdoor peers akin to the available literature.12 Vit.D after treatment did not differ significantly between the indoor and outdoor groups. This finding was not reported in the past.
Majority of the patients in our study were non smokers and the difference of mean vit.D before and after treatment between the smokers and non smokers was insignificant. This finding was contrary to the available literature.6
Majority of our subjects were enrolled in the autumn and monsoon seasons. The mean vit.D level before treatment was highest in autumn and lowest in summer. This may be due to decreased exposure to sunlight in hot summer in our region. The mean vit.D level after treatment was highest in winter and lowest in summer. The difference of vit.D across different seasons before and after treatment was significant. This finding was contrary to the reported literature.5,6,8,14
Obese adults require two to three times more vit.D than their peers.5 Significant negative correlation between BMI and improvement of vit.D in our study was in consensus with the available literature.4,6,8,12,34 Though nano syrup subgroup had better outcome, the negative correlation with BMI was profound. This may be attributed to the effective transportation of vit.D into body fat in obese compared to the other two formulations. The duration of pain was not significantly related to BMI grade.
An upper limit of 100 ng/ml of serum vit.D was considered as a safe margin for toxicity and 300 ng/ml has been proven to be truly toxic. Hypercalcemia was reported to occur after 150–200 ng/ml barring patients with chronic granulomatous diseases.4,36 No adverse reaction necessitating the stoppage of treatment was noted with pulse-D therapy. This was in consensus with the available literature on high dose vit.D supplementation.15,25,26,29,36,37 Having known the requirement and formulation based dose response relationship from this study, the total dose and dosing pattern for a given subject can be tailor made for optimum results without vit.D toxicity.
Goswami et al. demonstrated the decline of vit.D to suboptimal levels after one year of stoppage of treatment with 60,000 IUs/week for eight weeks.20 Einarsdottir K et al., reported a decline to just above the starting point by twelve months after single injection of 6,00,000 IUs of vit.D.38 Single oral mega dose of 6,00,000 IUs of vit.D was reported to have declined over three months.39 The decline of vit.D overtime in our study was comparable with weekly oral and single intramuscular dosing reported in the literature. This finding gives an insight about the need for frequent vit.D administration and maintenance protocol.
Supplementation with five sequential doses of 60,000 IUs of vit.D in nano syrup form has also shown significant improvement in vit.D and functional disability barring pain. Though the difference in vit.D after treatment with five doses of nano syrup was significantly different when compared with ten doses of three formulations, the difference in functional disability and pain was insignificant. Apart from the usefulness of pulse-D therapy, these findings give an insight into the dose response relationship. Further randomised studies with larger cohorts in this context will be helpful.
5. Conclusion
Hypovitaminosis D can be a potential causative factor for mLBA in addition to the other known causes. Proper evaluation and adjunctive pulse-D therapy can effectively break the vicious cycle of low back ache with significant improvement in serum vit.D level, effective relief of pain and significant functional improvement without any adverse effects. The improvement in vit.D was not significantly related to its initial status. Obese individuals have shown significantly lesser improvement in vit.D when compared to their peers. The results with nano syrup formulation were significantly better when compared to others. In view of these results, frequency of administration and formulation based dosage adjustments of vit.D will assume significance in the management of patients with mLBA. Regular supplementation or booster correction with ten dose pulse-D therapy at nine months can be considered to avoid recurrence.
6. Limitations of the study
Limited number of subjects from a single tertiary institute and inability to collect bi/tri monthly samples from enrolled subjects to know the time bound decline of vit.D levels after complete correction were the limiting factors. Further randomised controlled studies with special focus upon these limitations can be promising.
Acknowledgements
We thank Mr. Mallesh Kothapally, CRA for helping us in meticulously carrying out the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2019.06.018.
Contributor Information
Maheshwar Lakkireddy, Email: maheshwar.ortho@gmail.com.
Madhu Latha Karra, Email: madhu.harini123@gmail.com.
Chandrasekhar Patnala, Email: pcsnims@yahoo.com.
Raju Iyengar, Email: rajuayengar@rediffmail.com.
Nagesh Cherukuri, Email: cherukuri.ashwini@yahoo.com.
K.S. Asif Hussain, Email: asifks@yahoo.com.
Lalith Mohan Chodavarapu, Email: drscolex@gmail.com.
Koppolu Kranthi Kiran Kumar, Email: drkkirank@yahoo.com.
Sundeep Kund Aluka, Email: drkund@gmail.com.
Arvind Kumar Bodla, Email: bodla_arvind@yahoo.com.
Raja Ramesh Badavath, Email: rajabadavath@yahoo.com.
Shravan Kumar Peddamadyam, Email: drshra1@gmail.com.
Financial disclosure
Financial assistance to the institute was provided by M/s. pulse Pharmaceuticals Pvt.Ltd, Hyderabad, India (PBAC No:1171/16) for carrying out this study.
Conflict of interest
Nil.
Ethical committee approval
Approved.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Deyo R.A., Cherkin D., Conrad D., Volinn E. Cost, controversy, crisis: low back pain and the health of the public. Annu Rev Public Health. 1991;12(1):141–156. doi: 10.1146/annurev.pu.12.050191.001041. [DOI] [PubMed] [Google Scholar]
- 2.Wong David A., Transfeldt Ensor., editors. Macnab's Backache. fourth ed. Philadelphia Lippincot Williams and Wilkins; 2007. Epidemiology and natural history of spondylogenic backache; pp. 92–94. [Google Scholar]
- 3.Cho K.H., Beom J.W., Lee T.S., Lim J.H., Lee T.H., Yuk J.H. Trunk muscles strength as a risk factor for nonspecific low back pain: a pilot study. Annals of rehabilitation medicine. 2014;38(2):234–240. doi: 10.5535/arm.2014.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick M.F.1, Binkley N.C., Bischoff-Ferrari H.A. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. [DOI] [PubMed] [Google Scholar]
- 5.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 6.Ghai B., Bansal D., Kapil G., Kanukula R., Lavudiya S., Sachdeva N. High prevalence of hypovitaminosis D in Indian chronic low back patients. Pain Physician. 2015;18(5):E853–E862. [PubMed] [Google Scholar]
- 7.Shipton E.A.1, Shipton E.E.1. Vitamin D and pain: vitamin D and its role in the Aetiology and maintenance of chronic pain states and associated comorbidities. Pain Res Treat. 2015;2015:904967. doi: 10.1155/2015/904967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghai B., Bansal D., Kanukula R. Vitamin D supplementation in patients with chronic low back pain: an open label, single Arm clinical trial. Pain Physician. 2017;20(1):E99–E105. [PubMed] [Google Scholar]
- 9.Al Faraj S., Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28(2):177–179. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hudson-Cook N., Tomes-Nicholson K., Breen A. A revised Oswestry disability questionnaire. In: Roland M.O., Jenner J.R., editors. Back Pain: New Approaches to Rehabilitation and Education. Manchester University Press; New York, NY: 1989. pp. 187–204. [Google Scholar]
- 11.Merskey H. Classification of chronic pain: description of chronic pain syndromes and definitions of pain terms. Pain. 1986;3:216–221. [Google Scholar]
- 12.Dawson-Hughes B.1, Mithal A., Bonjour J.P. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 13.Johansen J.V., Manniche C., Kjaer P. Vitamin D levels appear to be normal in Danish patients attending secondary care for low back pain and a weak positive correlation between serum level Vitamin D and Modic changes was demonstrated: a cross-sectional cohort study of consecutive patients with non-specific low back pain. BMC Muscoskelet Disord. 2013;14:78. doi: 10.1186/1471-2474-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra N., Mithal A., Gupta S., Shukla M., Godbole M. Effect of vitamin D supplementation on bone health parameters of healthy young Indian women. Arch Osteoporos. 2009;4(1-2):47–53. doi: 10.1007/s11657-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 16.Rkain H., Bouaddi I., Ibrahimi A. Relationship between vitamin D deficiency and chronic low back pain in postmenopausal women. Curr Rheumatol Rev. 2013;9(1):63–67. doi: 10.2174/1573397111309010011. [DOI] [PubMed] [Google Scholar]
- 17.Dalle Carbonare L., Valenti M.T., Del Forno F., Caneva E., Pietrobelli A. Vitamin D: daily vs. Monthly use in children and elderly-what is going on? Nutrients. 2017;24(7):9. doi: 10.3390/nu9070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClements D.J., Decker E.A., Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72(8):R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 19.Kiani A., Fathi M., Ghasemi S.M. Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int J Food Prop. 2017;20(11):2466–2476. [Google Scholar]
- 20.Goswami R., Gupta N., Ray D., Singh N., Tomar N. Pattern of 25-hydroxy vitamin D response at short (2 month) and long (1 year) interval after 8 weeks of oral supplementation with cholecalciferol in Asian Indians with chronic hypovitaminosis D. Br J Nutr. 2008;100(3):526–529. doi: 10.1017/S0007114508921711. [DOI] [PubMed] [Google Scholar]
- 21.Papaioannou A., Kennedy C.C., Giangregorio L. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: no advantage of loading doses over daily supplementation. BMC Muscoskelet Disord. 2011;(12):135. doi: 10.1186/1471-2474-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Barger-Lux M.J. Human serum 25- hydroxyl cholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 23.Ish-Shalom S., Segal E., Salganik T., Raz B., Bromberg I.L., Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 24.Chel V., Wijnhoven H.A., Smit J.H., Ooms M., Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int. 2008;19:663–671. doi: 10.1007/s00198-007-0465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kearns M.D., Alvarez J.A., Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341–351. doi: 10.4158/EP13265.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondal K., Seth A., Marwaha R.K. A Randomized controlled trial on safety and efficacy of single intramuscular versus staggered oral dose of 600 000IU Vitamin D in treatment of nutritional rickets. J Trop Pediatr. 2014;60(3):203–210. doi: 10.1093/tropej/fmt105. [DOI] [PubMed] [Google Scholar]
- 27.Khan A.H., Rohra D.K., Saghir S.A., Udani S.K., Wood R., Jabbar A. Response of a single ‘mega intramuscular dose’ of vitamin D on serum 25OHD and parathyroid hormone levels. J Coll Phys Surg. 2012;22(4):207–212. Doi.04.2012/JCPSP.207212. [PubMed] [Google Scholar]
- 28.Munns C.F., Shaw N., Kiely M. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani C., Romagnoli E., Scillitani A. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95(10):4771–4777. doi: 10.1210/jc.2010-0502. [DOI] [PubMed] [Google Scholar]
- 30.Shah B.R., Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994;125(3):487–490. doi: 10.1016/s0022-3476(05)83303-7. [DOI] [PubMed] [Google Scholar]
- 31.Sanders K.M., Stuart A.L., Williamson E.J. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. Jama. 2010;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 32.Kuchay M.S., Jevalikar G.S., Mithal A., Mishra S.K., Dang N. Efficacy and safety of a single monthly dose of cholecalciferol in healthy school children. J Pediatr Endocrinol Metab. 2016;29(4):413–416. doi: 10.1515/jpem-2015-0187. [DOI] [PubMed] [Google Scholar]
- 33.Zittermann A., Ernst J.B., Gummert J.F., Börgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr. 2014;53(2):367–374. doi: 10.1007/s00394-013-0634-3. [DOI] [PubMed] [Google Scholar]
- 34.Heaney R.P., Armas L.A., Shary J.R., Bell N.H., Binkley N., Hollis B.W. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions–. Am J Clin Nutr. 2008;87(6):1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 35.Wagner C.L., Greer F.R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 36.Jones G.1. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 37.Vieth R. Vitamin D and cancer mini-symposium: the risk of additional vitamin D. Ann Epidemiol. 2009;19(7):441–445. doi: 10.1016/j.annepidem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Einarsdottir K., Preen D.B., Clay T.D., Kiely L., Holman C.D., Cohen L.D. Effect of a single ‘megadose’ intramuscular vitamin D (600,000 IU) injection on vitamin D concentrations and bone mineral density following biliopancreatic diversion surgery. Obes Surg. 2010;20(6):732–737. doi: 10.1007/s11695-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 39.Cipriani C.1, Romagnoli E., Pepe J. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98(7):2709–2715. doi: 10.1210/jc.2013-1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.