Abstract
Background
There has been significant recent emphasis on the use of patient-specific instrumentation (PSI) in shoulder arthroplasty. However, clinical data are lacking to support the increased time and expense associated with PSI. Our purposes were to determine whether PSI significantly improves implantation accuracy during total shoulder arthroplasty (TSA) and to analyze available techniques and correlation with clinical outcomes. We hypothesized that PSI may improve glenoid component position radiographically but without correlation with clinical outcomes.
Methods
The MEDLINE, Scopus, Embase, and Cochrane Library databases were queried. Included articles reported use of any preoperative or intraoperative PSI techniques, models, or guides to assist with TSA prosthesis implantation. The primary outcomes were mean deviation from the preoperative plan in version (in degrees), inclination (in degrees), and entry-point offset on the glenoid (in millimeters).
Results
Among the included articles, 518 TSA procedures (352 anatomic and 166 reverse) were performed. The mean postoperative errors in both version and inclination angles were 5° or less in 20 articles (90.9%) using PSI. Meta-analysis revealed no statistically significant differences in version error (P > .999, I2 = 64.6%), inclination error (P = .702, I2 = 82.2%), or positional offset (P = .777, I2 = 85.7%) between PSI and standard instrumentation. No data regarding patient-reported outcome measures, range of motion, strength, or glenoid component loosening and longevity were reported.
Conclusions
Meta-analysis revealed no significant differences in accuracy between PSI and standard instrumentation. Although PSI may possess the potential to improve TSA techniques, further investigations regarding long-term clinical outcomes, impact on operating room time, and cost-effectiveness are warranted before PSI can be routinely recommended over conventional instrumentation.
Keywords: Patient specific, shoulder arthroplasty, 3-dimensional, accuracy, guide, glenoid, arthroplasty
Although technological advancements have allowed for improved preoperative planning and surgical techniques for anatomic total shoulder arthroplasty (TSA) and reverse TSA (rTSA), accurate placement of the glenoid component remains a challenge for surgeons of all experience levels.7 Variations in preoperative glenoid version, inclination, and amount of bone loss can markedly increase the technical difficulty of accurate glenoid placement.26 Despite substantial improvements in implant design and technique, glenoid component loosening and failure remain the primary cause of clinical failure in TSA patients.25, 31 Glenoid component loosening has been associated with implant malposition, incomplete correction of bony pathology, and persistent subluxation of the humeral head.1, 18, 25
Patient-specific instrumentation (PSI) and preoperative planning have been studied extensively for knee and hip arthroplasty.5, 8, 9, 33, 35, 41 In comparison, investigation of PSI for TSA is at an earlier stage with relatively fewer studies.7, 25, 26 Previous clinical and biomechanical studies have demonstrated that postoperative glenoid component retroversion beyond 10° to 15° is significantly associated with increased rates of osteolysis and high stress at the bone-implant interface.13, 21, 25 The rationale for applying PSI in TSA is that optimal implant alignment has the potential to reduce the risk of premature loosening and implant failure, especially in cases of extreme native glenoid retroversion or inclination.23 Some published evidence suggests that PSI improves accurate positioning of the glenoid component in anatomic TSA and rTSA.7, 23, 25, 38 However, more recent clinical research has disputed these findings, claiming that glenoid component implantation using PSI may not be as accurate as previously reported.26
The methodologies of available studies using PSI in TSA are variable, and no consensus has been established regarding the clinical efficacy for improving implant longevity or patient outcomes, cost-effectiveness, or impact on operative time.24 Despite the paucity of literature to support the clinical efficacy or cost-effectiveness of PSI in TSA, an increasing array of PSI systems for TSA are commercially available for shoulder surgeons, which may be associated with increased costs and delays for creation of instrumentation. As these techniques and devices are developed, it is of paramount importance that research establishes efficacy for improving glenoid component implantation accuracy, patient outcomes, implant longevity, and cost-efficacy. To our knowledge, no comprehensive systematic literature review exists that evaluates the accuracy of PSI in TSA and its potential to improve surgical outcomes compared with standard instrumentation.
The primary purpose of this systematic review and meta-analysis was to determine whether PSI significantly improves the accuracy of component positioning during TSA. Secondary objectives included analyzing PSI techniques available to orthopedic surgeons for differences in methodology and the impact on surgical and clinical patient outcomes. The hypothesis was that PSI may improve glenoid component position radiographically but without correlation with clinical outcomes.
Methods
A systematic review and meta-analysis were performed using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines.
Search strategy
The MEDLINE, Scopus, Embase, and Cochrane Library databases were queried for this literature search. ClinicalTrials.gov and the International Clinical Trials Registry Platform were also used to identify ongoing clinical trials relevant to the topic. The Boolean search used in this study consisted of the following terms: ((“Arthroplasty, Replacement, Shoulder”) OR (“Total Shoulder Replacement”) OR (“Total Shoulder Replacements”) OR (“Shoulder Replacement Arthroplasty”) OR (“Reverse Total Shoulder Replacement”) OR (“Reverse Total Shoulder Arthroplasty”)) AND ((“Patient-Specific Modeling”) OR (“Patient Specific”) OR (“Patient Specific Instrumentation”) OR (“Patient Specific Instrument”) OR (“Patient Specific Guides”) OR (“Patient Specific Guide”) OR (“Guides”) OR (“Guide”) OR (“Drill Guide”) OR (“Implant”) OR (“3D”) OR (“Three-Dimension”) OR (“Three-Dimensional”) OR (“Positioning”)). This search was performed on November 9, 2017, and reviewed on February 15, 2018, for additional articles. References of the included articles were evaluated to capture any potential studies fitting the inclusion criteria for the meta-analysis that were not previously obtained in the original search.
Selection criteria
Included articles were those that reported the use of any preoperative or intraoperative PSI techniques, models, or guides to assist with prosthesis implantation in the setting of anatomic TSA or rTSA. Both cadaveric and clinical studies were included in this review, including any other studies that used PSI on physical or virtual patient models, to ensure all available data regarding evolving PSI technology were captured. Study designs included case series, cohort studies, and randomized controlled trials. Excluded articles were those that involved animals; were not in the English language; did not report preoperative or postoperative evaluations; included other joints besides the shoulder; or involved hemiarthroplasty, joint resurfacing, or any other surgical procedure besides anatomic TSA or rTSA.
Quality evaluation
The MINORS (Methodological Index for Non-randomized Studies) checklist was used to assess the quality of nonrandomized clinical surgical investigations.34 The MINORS criteria include 12 items designed to evaluate study quality. Of the total 12 items, 4 can be applied to comparative studies. The scoring of each item was as follows: 0, not reported; 1, reported but inadequate; or 2, reported and adequate. The maximum score for comparative studies was 24, whereas the maximum score for noncomparative studies was 16. Two authors (B.C.C. and G.L.C.) independently scored each article included in this review and resolved any disagreements to reach a consensus score if necessary.
Data extraction and analysis
The following information was collected from each included article: (1) publication information; (2) patient or specimen demographic characteristics; (3) procedures performed; (4) indications; (5) implant systems; (6) surgical techniques and approaches; (7) imaging modalities and protocols; (8) preoperative glenoid measurements and classifications; (9) surgical planning methods; (10) patient-specific instrument designs, costs, and manufacturers; (11) postoperative measurements of implantation accuracy; (12) complications associated with the patient-specific instruments; and (13) intraoperative times.
Statistical analysis
Statistical analysis was performed using the “metafor” package as part of RStudio software (version 1.0.143; R Foundation for Statistical Computing, Vienna, Austria). On the basis of the most consistently reported outcomes in the available literature, the primary outcomes of this analysis were mean deviation from the preoperative plan measured in the postoperative version angle (in degrees), inclination angle (in degrees), and entry-point and/or implant positional offset on the glenoid face (in millimeters). These outcomes were analyzed for all studies that compared preoperative and intraoperative PSI techniques with a standard instrumentation control. The heterogeneity of included studies was measured with the I2 index, with an I2 between 25% and 49% being considered low heterogeneity; between 50% and 74%, moderate; and 75% or greater, high. Because of high levels of heterogeneity, a random-effects model was used to combine available data by meta-analysis. Random-effects DerSimonian-Laird models were used to calculate weighted averages of the transformed values, which were then back-transformed to produce final pooled rates.
Mean deviations from the preoperative plan measured in the postoperative version angle, inclination angle, and entry-point offset were reported with 95% confidence intervals (CIs), and values from the included studies were compiled in a forest plot. Publication bias was evaluated with a funnel chart (study size on the y-axis and estimated effect on the x-axis). If no bias exists, point estimates should produce a symmetrical distribution about the real treatment effect. P < .05 was used uniformly to detect statistical significance.
Results
Study characteristics
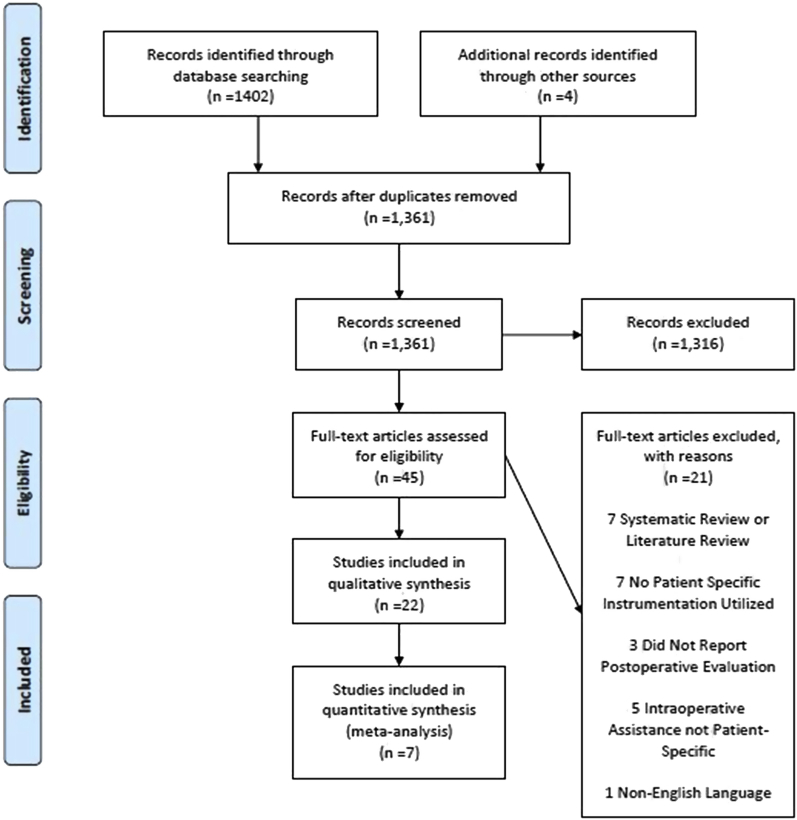
A total of 22 articles met the inclusion criteria. The PRISMA flow diagram summarizing the progression of the literature review is displayed in Figure 1. Regarding the level of evidence, 2 articles (9.1%) were categorized as level I, 1 (4.5%) was categorized as level II, 3 (13.6%) were categorized as level III, and 16 (72.7%) were categorized as level IV. Of the articles, 13 (59.1%) were clinical studies that used patients as subjects, 7 (31.8%) used cadaveric specimens, and 3 (13.6%) used physical or virtual patient models (1 article used both cadaveric specimens and clinical patients). All clinical studies included only primary anatomic TSA or rTSA procedures. The average MINORS score for noncomparative clinical studies was 8.8, whereas the average MINORS score for comparative clinical studies was 18.8. A total of 10 articles (45.5%), including 4 clinical studies and 6 studies using cadaveric or artificial models, compared the accuracy of PSI with a standard instrumentation control.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram illustrating systematic literature review.
Demographic information and surgical techniques
A total of 518 TSA procedures (352 anatomic and 166 reverse) were performed among all included articles. Reported indications for TSA were primary end-stage glenohumeral osteoarthritis, post-traumatic glenohumeral osteoarthritis, and rheumatoid arthritis. Indications for rTSA were rotator cuff tear arthropathy with pseudoparalysis or some other rotator cuff deficiency. A total of 7 articles (31.8%) reported the following patient demographic information: mean age of 72.4 years (range, 44.0-88.0 years) and mean body mass index of 28.3 (range, 21.4-34.1). Insufficient data were reported to calculate pooled standard deviations, and sex was reported for only 118 patients (39 male and 79 female patients). A standard deltopectoral approach was used in all studies for which surgical approach was reported. Seventeen articles reported the prosthesis systems used, which are displayed in Table I.
Table I.
Prosthesis systems cited among included articles
| Prosthesis system | Article(s) cited | No. of patients |
|---|---|---|
| Zimmer Comprehensive Reverse Total Shoulder System (Zimmer Biomet, Warsaw, IN, USA) | Throckmorton et al,38 2015 Lau and Keith,26 2018 Pietrzak,32 2013 Suero et al,37 2013 Throckmorton et al,39 2014 Berhouet et al,2 2017 |
87 |
| Zimmer Comprehensive Total Shoulder System (Zimmer Biomet) | Throckmorton et al, 2015 Lau and Keith, 2018 Pietrzak, 2013 Suero et al, 2013 Throckmorton et al,39 2014 |
60 |
| Global APG Implant or Global STEPTECH APG glenoid component (DePuy Synthes, Warsaw, IN, USA) | Iannotti et al,25 2015 | 46 |
| DJO Surgical Reverse Shoulder Prosthesis (DJO, Austin, TX, USA) | Dallalana et al,7 2016 Levy et al,27 2014 Subramanya and Herald,36 2014 Elliott and Dallalana,11 2017 |
39 |
| Duocentric reverse shoulder prosthesis (Aston Medical) | Trouilloud et al,40 2014 | 30 |
| Biomet TESS Anatomic (Zimmer Biomet) | Heylen et al,20 2016 | 14 |
| Zimmer TM Reverse (Zimmer Biomet) | Heylen et al, 2016 | 14 |
| DJO Surgical Turon modular shoulder system | Dallalana et al, 2016 | 10 |
| Aequalis PerFORM (Wright Medical, Montbonnot-Saint-Martin, France) | Berhouet et al,3 2018 | 10 |
| Biomet TESS Reverse (Zimmer Biomet) | Heylen et al, 2016 | 4 |
| Zimmer Anatomical (Zimmer Biomet) | Heylen et al, 2016 | 4 |
| DELTA XTEND reverse shoulder system (DePuy Synthes) | Suero et al, 2013 | 1 |
The types of prosthesis systems and number of patients for which each respective system was used among the included articles are shown.
Preoperative imaging and evaluation
Of the 22 included articles, 21 (95.5%) reported computed tomography (CT) as the imaging modality used for preoperative patient evaluation of glenoid morphology. Of those 21 articles, 2 used CT scans reformatted to the plane of the scapula as described by Bryce et al.4 In the 1 article that did not use CT scans, patients underwent standard anteroposterior and lateral radiographic imaging to measure glenoid inclination.20 In 5 articles (22.7%), preoperative glenoid morphology was characterized according to the Walch classification system,42 with type A1 in 27 cases, type A2 in 24, type B1 in 10, type B2 in 41, type B3 in 1, and type C in 5. The pooled mean ± standard deviation for preoperative native glenoid version and inclination was –10.40° ± 9.99° (range, –41.0 to 7.69) and 7.97° ± 7.30° (range, –17 to 59.3), respectively. Negative values indicate glenoid retroversion and inferior inclination.14
Preoperative planning
Thirteen different preoperative surgical planning software programs were used across 21 included articles (95.5%) to create a patient-specific plan for insertion of the glenoid guide pin and/or glenoid implant during TSA. Details of the available preoperative surgical planning techniques used in the articles included in this review are outlined in Table II. These programs used 2-dimensional (2D) CT scans in DICOM (Digital Imaging and Communications in Medicine) file format to create 3-dimensional (3D) reconstructions of the patient anatomy. Of the studies, 8 (36.4%) reported automatically determined plans for glenoid guide pin or implant positioning whereas 12 (54.5%) allowed for manual determination of positioning. Plans were automatically determined in that preset goals for degrees of version (0° for TSA and rTSA), degrees of inclination (0° for TSA and between 5° and 10° of inferior inclination for rTSA), and a centered position of the guide pin or implant on the glenoid face were used across all procedures performed in the studies. Manual planning allowed the operating surgeons to set target version and inclination angles, as well as the position on the glenoid face, as they deemed appropriate for each patient.
Table II.
Techniques to determine preoperative surgical plan using PSI technology
| Planning platform | Article(s) cited | Description | Preoperative planning |
|---|---|---|---|
| Mimics Innovation Suite Medical Imaging Software (Materialise) | Eraly et al,12 2016 Heylen et al,20 2016 Lewis et al,28 2015 Suero et al,37 2013 Berhouet et al,2 2017 Nguyen et al,29 2007 |
Import 2D CT DICOM data. Create 3D scapula model. Can import 3D CAD models of prosthesis or other tools. Can characterize native glenoid morphology. |
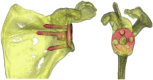 Suero et al, 2013∗ |
| Surgicase Connect (Materialise) | Dallalana et al,7 2016 Levy et al,27 2014 Subramanya and Herald,36 2014 Heylen et al,20 2016 |
Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
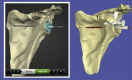 Levy et al, 2014† |
| Glenosys (Imascap, Brest, France) | Gauci et al,16 2016 Walch et al,43 2015 |
Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
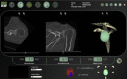 Walch et al, 2015‡ |
| Signature (Biomet, Warsaw, IN, USA) | Throckmorton et al,38 2015 Pietrzak,32 2013 Lau and Keith,26 2018 |
Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
 Lau and Keith, 2018§ |
| OrthoVis (Custom Orthopaedic Solutions, Cleveland, OH, USA) | Iannotti et al,25 2015 Iannotti et al,24 2017 |
Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
 Source: https://customorthopaedics.com |
| Personal Fit (Duocentric Group, Aston Medical) | Trouilloud et al,40 2014 | Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Specific for Duocentric Reverse Prosthesis. Allows planning of humeral implant. |
 Trouilloud et al, 2014‖ |
| ArthroPlan (Cleveland Clinic, Cleveland, OH, USA) | Hendel et al,19 2012 | Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
|
| Rhinoceros 3D (Robert McNeel & Associates, Seattle, WA, USA) | Nguyen et al,7 2007 | Used to measure version and inclination relative to anatomic coordinate system. Coordinate system used to calculate preoperative plan. |
|
| BluePrint 3D Planning Software (Wright Medical) | Berhouet et al,3 2018 | Import 2D CT DICOM data. Simulate positioning of glenoid component on 3D scapula model. Manually refine surgical plan and choose implant appropriate for individual case. |
|
| 3-Matic Finite Element Analysis Software (Materialise) | Lewis et al,28 2015 | CAD program. Work with 3D models produced from Mimics Medical (Materialise). Design patient-specific devices. on complex anatomic shapes. Prepare files for 3D printing. |
 Lewis et al, 2015¶ |
PSI, patient-specific instrumentation; 2D, 2-dimensional; CT, computed tomography; DICOM, Digital Imaging and Communications in Medicine; 3D, 3-dimensional; CAD, computer-aided design.
Reprinted with permission from Suero et al.37
Reprinted with permission from Levy et al.27
Reprinted with permission from Walch et al.43
Reprinted with permission from Lau and Keith.26
Reprinted with permission from Trouilloud et al.40
Reprinted with permission from Lewis et al.28
PSI techniques
A physical PSI device designed to assist intraoperatively with at least 1 component of the shoulder arthroplasty procedure was used in 21 of the 22 included articles (95.5%). The other article available described a custom software program that allowed for patient-specific planning and virtual implantation of a glenoid prosthesis. Instrument designs were heterogeneous across the articles included in this review. Design details for each patient-specific instrument available are outlined in Table III. Patient-specific devices were outsourced to 6 different commercial manufacturers across 16 studies (72.7%), whereas 3 studies (13.6%) reported the use of custom-machined devices. Two articles did not report the source of their PSI devices. Costs associated with production of the patient-specific instruments were not reported in any of the included articles. A minimum of 10 working days (2 weeks) was necessary for production of the PSI device once the surgical procedure was planned, according to Gauci et al.16 The only other article to document the necessary time for production of the patient-specific device reported a minimum of 5 weeks.40
Table III.
PSI devices and technology used among included articles
| PSI manufacturer | Article(s) cited | Description | PSI |
|---|---|---|---|
| Tornier–Wright Medical | Gauci et al,16 2016 Walch et al,43 2015 Berhouet et al,3 2018 |
Four-pin peripheral support Surgeon introduces onto glenoid. 2.5-mm titanium guide wire passed through central hole. Polyamide; EOSINT P380 selective laser sintering (EOS, Krailling, Germany). |
 Walch et al, 2015∗ |
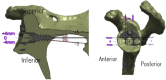
| Materialise (Patient-Specific Shoulder Guide) | Dallalana et al,7 2016 Levy et al,27 2014 Subramanya and Herald,36 2014 Heylen et al,20 2016 |
Removal of soft tissues from anterosuperior glenoid rim and exposure of coracoid base necessary. Guide fitted to stable position on native glenoid. Central guide pin drilled into native glenoid. Polyamide material. Selective laser sintering. |
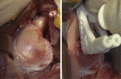 Heylen et al, 2016† |
| Zimmer Biomet (Signature Glenoid Shoulder System) | Throckmorton et al,38 2015 Pietrzak,32 2013 Lau and Keith,26 2018 Throckmorton et al,39 2014 |
TSA: pin trajectory is neutral version and inclination. rTSA: pin trajectory is 10° of inferior tilt and neutral version. Guide applied to anterior glenoid Material not reported. |
 Lau and Keith, 2018‡ |
| Custom Orthopaedic Solutions (Glenoid Intelligent Reusable Instrument System) | Iannotti et al,25 2015 Iannotti et al,23 2014 Iannotti et al,24 2017 |
Reusable—cannulated handle over guide pin. 3 or 4 adjustable peripheral legs fit on glenoid. Each leg specified leg length (1-mm increments). Legs locked by tightening collets after desired length achieved with SmartBone model (Custom Orthopaedic Solutions, Cleveland, Ohio, USA). |
|
| Duocentric Group, Aston Medical (Personal Fit) | Trouilloud et al,40 2014 | Guide pin is passed through guide at chosen center of glenoid. Humeral component fits onto humeral head and cutting guide placed against it. |
 Trouilloud et al, 2014§ |
| Astro Manufacturing & Design, Eastlake, OH, USA (patient-specific stereolithography devices) | Hendel et al,19 2012 | 2 instrument parts. First instrument fits on anterior glenoid. Drill guide places glenoid pin and superior guide pin; superior pin placed into base of coracoid. After glenoid reaming, second patient-specific instrument engages superior pin and sets roll of implant. Stereolithography resin. |
|
| DJO (DJO Surgical Match Point System) | Elliott and Dallalana,11 2017 | Guide used to place initial 2.5-mm bicortical central guide pin. 6-mm threaded tap (4.5-mm shaft width) is inserted along path of pin to act as reaming post. Unspecified 3D printed plastic. |
|
| DTM, Silver Spring, MD, USA | Suero et al,37 2013 | Central hole guides central glenoid guide pin into desired position. Duraform Polyamide (DTM); selective laser sintering. |
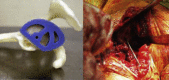 Suero et al, 2013‖ |
| Custom | Eraly et al,12 2016 | Guide interacts with (1) tip of coracoid, (2) base of coracoid, (3) inferior border of glenoid, and (4) roof of acromion Reusable metal cylinder for inserting central glenoid guide pin. Cylinder for each screw hole for implantation. Polyamide PA2201; EOSINT P730 selective laser sintering (EOS). |
 Eraly et al, 2016¶ |
| Custom | Lewis et al,28 2015 | Array of pins with adjustable length according to 3D glenoid reconstruction. Manually pressed against glenoid face. At least 3 pins needed; number of pins and locations changeable. Central hole for drilling of glenoid guide pin. |
 Lewis et al, 2015# |
| Custom | Nguyen et al,29 2007 | Custom-machined mounts to hold drill guide for glenoid guide pin in place. Electromagnetic tracker attached to determine position according to 3D coordinate system.Crystalline plastic material. |
|
PSI, patient-specific instrumentation; TSA, total shoulder arthroplasty; rTSA, reverse total shoulder arthroplasty; 3D, 3-dimensional.
Reprinted with permission from Walch et al.43
Reprinted with permission from Heylen et al.20
Reprinted with permission from Lau and Keith.26
Reprinted with permission from Trouilloud et al.40
Reprinted with permission from Suero et al.37
Reprinted with permission from Eraly et al.12
Reprinted with permission from Lewis et al.28
Accuracy of PSI for glenoid guide pin or component placement
The accuracy of PSI for glenoid component placement was evaluated in all the included studies with a variety of methods that, in general, quantified deviation from a preoperative surgical plan via postoperative radiologic imaging and/or 3D reconstructions. Of the articles, 11 (50.0%) created 3D CT reconstructions from 2D CT images to evaluate deviation from the preoperative surgical plan, whereas 8 articles (36.4%) performed the necessary measurements on 2D CT images. Moreover, 1 article (4.5%) each used anteroposterior or lateral radiographs, a 3D scanner, and a computer-aided grid interface to quantify PSI accuracy.20, 28, 29
Various postoperative measurements were reported as indicators of accurate implantation of the glenoid prosthesis, central guide pin, or some component of the glenoid prosthesis, such as the central screw position for rTSA. The most frequently used postoperative outcomes reported were errors in postoperative version angle (in degrees, 95.5% of articles), inclination angle (in degrees, 100% of articles), and central guide pin entry-point offset or overall implant positional offset on the glenoid face (in millimeters, 54.5% of articles) compared with the preoperative plan. Other less commonly measured outcomes included angular deviation (quantification of both version and inclination angles, 4.5% of articles),12 roll deviation (angle at which the postoperative implant had rolled around its normal compared with the planned implant, 9.1% of articles),12, 19 beta angle between the supraspinatus fossa and the glenoid fossa (4.5% of articles),20 and intraosseous screw length (4.5% of articles).12 Additional outcome measurements were errors in central guide pin or implant positional offset specifically in the superior-inferior, medial-lateral, and anterior-posterior directions on the glenoid face (in millimeters, 31.8% of articles).2, 7, 12, 16, 19, 25, 37 Although version and inclination errors were reported in nearly all articles, the methodologies used to obtain such measurements were heterogeneous. No single post-intervention evaluation methodology was used consistently across all articles.
Compared with the corresponding preoperative plans, the mean postoperative errors in both version and inclination angles were reported to be 5° or less in 20 individual articles (90.9%) after use of PSI technology during shoulder arthroplasty. Only 1 article reported a mean postoperative glenoid implant version error (± standard deviation) beyond 5° (8° ± 10°) after 11 shoulder arthroplasties (7 TSAs and 4 rTSAs).26 The mean postoperative errors in positional offset on the glenoid face after shoulder arthroplasty with PSI technology were 3 mm or less in 14 of the 15 articles (93.3%) that reported such measurements. The other article reported a mean glenoid implant positional offset (± standard deviation) of 3.4 ± 1 mm after 10 shoulder arthroplasties (6 TSAs and 4 rTSAs).37
In 4 studies (18.2%), significantly reduced frequencies of glenoid component malpositioning (defined as version error ≥ 10°, inclination error ≥ 5° to 10°, and positional offset ≥ 3 mm) were reported with PSI technology compared with standard instrumentation controls.19, 20, 25, 38 Use of PSI technology also resulted in significantly reduced errors in postoperative implant version and inclination in more severely retroverted glenoids compared with standard instrumentation.19, 24, 28
Clinical outcomes and glenoid component longevity with PSI for TSA
The impact of PSI technology on intraoperative time was not evaluated in any of the articles included in this review. Cost-effectiveness data were not reported in any articles. No articles reported changes in patient-reported outcome measures, postoperative range of motion, or strength or performed any evaluation of the impact of PSI on glenoid component loosening rates and longevity. No intraoperative complications associated with the use of patient-specific instruments were reported.
Meta-analysis
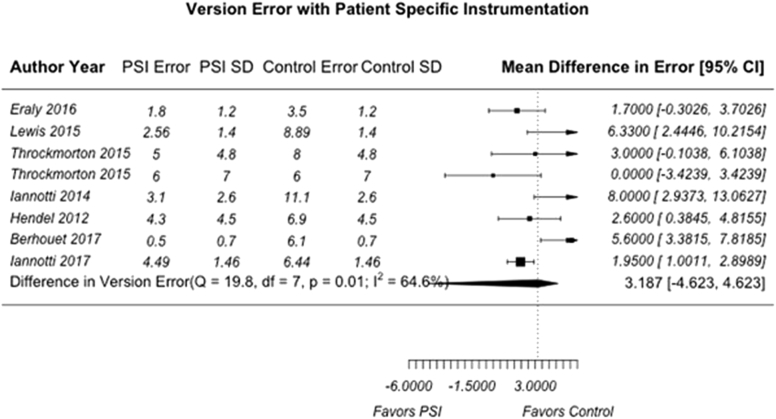
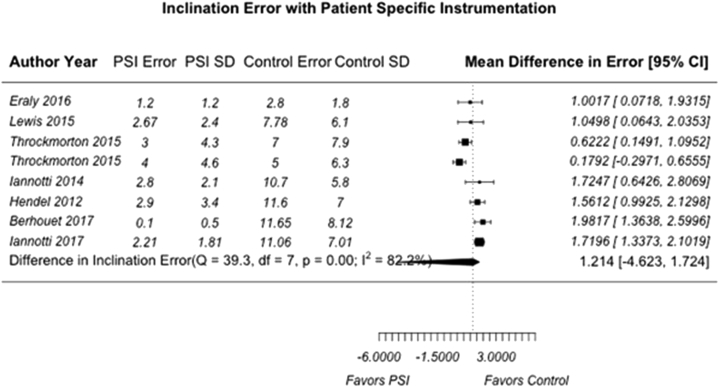
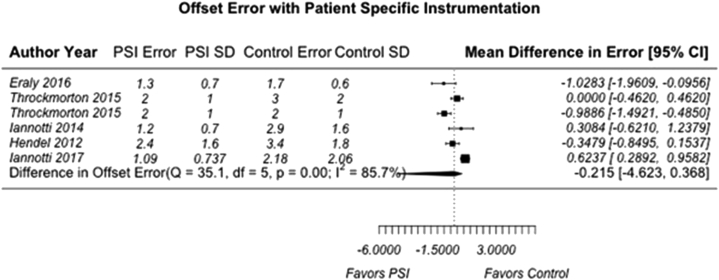
A total of 7 individual articles (31.8%) reported quantifiable comparisons in deviation from the predetermined surgical plan in version angle, inclination angle, and positional offset compared with a standard instrumentation control group.2, 12, 19, 23, 24, 28, 38 We believed it was only appropriate to include studies with the same reported outcome parameters and comparisons to control groups in our meta-analysis to limit the heterogeneity of PSI methodologies and preoperative and/or postoperative evaluation techniques. For the purpose of this analysis, these deviations were termed “version error,” “inclination error,” and “offset error.” Of the 7 included articles, 2 were clinical studies with human subjects who underwent anatomic TSA or rTSA whereas 5 used either cadaveric specimens or physical and/or virtual anatomic models. Publication bias was assessed with a funnel plot compiled from pooled version errors from analyzed articles (Fig. 2). Studies with larger effect sizes are displayed higher along the y-axis and allowed less deviation from the pooled mean along the x-axis. Overall, the pooled mean differences for all 3 analyzed parameters were 3.19° for version error (95% CI, –4.62° to 4.62°) (Fig. 3), 1.21° for inclination error (95% CI, –4.62° to 1.72°) (Fig. 4), and 0.215 mm for offset error (95% CI, –4.62 to 0.368 mm) (Fig. 5). All pooled mean differences were greater in standard control cases compared with PSI cases; however, none were statistically significant (version error, P > .999; inclination error, P = .702; and positional offset error, P = .777).
Figure 2.
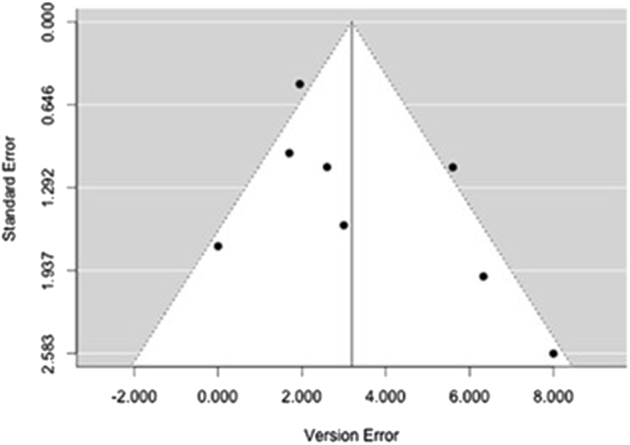
Funnel plot of publication bias for studies in meta-analysis.
Figure 3.
Mean difference in version error (in degrees) for patient-specific instrumentation (PSI) vs. standard control. The random-effects DerSimonian-Laird model was used for meta-analysis. Assessment of heterogeneity showed the following: Q value = 19.8, df = 7, P < .001, and I2 = 64.6.8% (overall, moderate). The test for overall effect showed Z = 1.35 (P > .999). No statistically significant difference in mean version error (in degrees) was found between PSI and standard instrumentation. SD, standard deviation; CI, confidence interval.
Figure 4.
Mean difference in inclination error (in degrees) for patient-specific instrumentation (PSI) vs. standard control. The random-effects DerSimonian-Laird model was used for meta-analysis. Assessment of heterogeneity showed the following: Q value = 39.3, df = 7, P < .001, and I2 = 82.2% (overall, high). The test for overall effect showed Z = 1.35 (P = .702). No statistically significant difference in mean inclination error (in degrees) was found between PSI and standard instrumentation. SD, standard deviation; CI, confidence interval.
Figure 5.
Mean difference in positional offset error (in millimeters) for patient-specific instrumentation (PSI) vs. standard control. The random-effects DerSimonian-Laird model was used for meta-analysis. Assessment of heterogeneity showed the following: Q value = 35.1, df = 5, P < .001, and I2 = 85.7% (overall, high). The test for overall effect showed Z = 1.35 (P = .777). No statistically significant difference in mean positional offset error (in millimeters) was found between PSI and standard instrumentation. SD, standard deviation; CI, confidence interval.
Discussion
This systematic review and meta-analysis evaluated the impact of PSI on the accuracy of implant positioning in TSA. Overall, the available literature suggests that accuracy improves with PSI technology. However, pooled analysis did not demonstrate a significant difference in accuracy between PSI and standard instrumentation on direct comparison, although most comparative studies used cadaveric specimens. In addition, no study to date has demonstrated a correlation between improved clinical outcomes and the use of PSI.
The vast majority of all available published articles using PSI for shoulder arthroplasty (90.9%) reported mean postoperative version and inclination errors of 5° or less compared with preoperative plans.2, 3, 7, 11, 12, 16, 19, 20, 23, 24, 25, 27, 28, 29, 32, 36, 37, 38, 40, 43 In addition, all 10 articles that reported comparisons of PSI vs. a standard instrumentation control found either significantly reduced errors or a significantly increased likelihood of achieving the planned presurgical positions with PSI.2, 12, 19, 20, 23, 24, 25, 28, 29, 38 Given that implants are considered malpositioned if they exhibit a postoperative version error of 10° or greater, inclination error of 5° to 10° or greater, and positional offset of 3 mm or greater, these results suggest that PSI helps reduce the percentage of cases beyond an acceptable error and, thus, the frequency of malpositioned implants. However, despite the differences between PSI and standard instrumentation found in most individual studies, our meta-analysis revealed significant heterogeneity in study methodologies and no statistically significant differences in version error, inclination error, or positional offset between PSI and standard instrumentation. These findings precluded drawing definitive conclusions regarding the impact of PSI on implantation accuracy.
One potential explanation for the lack of significant differences between PSI and standard instrumentation accuracy is senior surgeon operative experience. Several senior surgeons who were involved in these studies regularly perform shoulder arthroplasties at particularly high volumes.23, 25, 38, 43 Because of increased operative experience, they may have had higher accuracy and reduced variability with standard instrumentation. Thus, the discrepancy between PSI and standard instrumentation may have been reduced in their hands. The study by Iannotti et al23 found that implantation accuracy was significantly impacted by surgeon expertise when using PSI, with the less experienced surgeon producing more accurate results. This finding suggests that PSI can be particularly advantageous for novice or low-volume shoulder surgeons. Given that TSA still involves a number of surgeon-dependent steps despite the use of PSI, including achieving an adequate reaming depth and screw placement, the relationship between surgeon expertise and accuracy with PSI should be studied more extensively.17 A second possible explanation is that many of the trials may have been performed on glenoids with less severe glenoid pathology than what would normally be encountered in clinical practice, particularly those performed using cadavers. Standard instrumentation performed significantly worse than PSI in glenoids with more severe retroversion and inclination.19, 24, 28 However, most articles (55%) did not report preoperative quantification of glenoid morphology. PSI may be particularly helpful in cases of severely deformed glenoid morphology, yet this benefit cannot be fully understood because of a lack of available data. Finally, patient-specific 3D templating or planning may have significantly influenced accuracy vs. the actual patient-specific instruments themselves. One study found no significant differences between 3D planning and PSI, although both improved accuracy significantly more than 2D CT imaging.25 Three-dimensional preoperative planning can possibly make surgeons more cognizant of deformity such that they make subtle adjustments based on their plan that actually increase surgical accuracy vs. the use of a physical guide. Comparisons in the literature are extremely scarce, and further investigation to elucidate the nature of these relationships is warranted.
Heterogeneity of study methodology was not limited solely to the articles included in our meta-analysis but was present across all available literature. No single validated methodology for preoperative planning, intraoperative technique, or postoperative evaluation was used consistently among included articles. The most frequently cited programs used to determine goals for implant positioning were Mimics Innovation Suite and Surgicase Connect (Materialise, Leuven, Belgium).2, 7, 12, 20, 27, 28, 29, 36, 37 Mimics Innovation Suite allows for 3D segmentation of various 2D radiologic imaging modalities (CT, magnetic resonance imaging, and so on) via DICOM data and has been used throughout the orthopedic and non-orthopedic literature.6, 9, 15, 22, 44 Surgicase Connect is a case management system in which surgeons create individually templated plans for surgical cases and send these plans to an outsourced manufacturer for the production of a patient-specific instrument. Although manufacturers market these and other programs as tools that can streamline and simplify the surgical planning process, no current comparisons in the literature exist that evaluate the accuracy and efficacy of these programs or assess their impact on clinical and patient-reported outcomes.
Intraoperative PSI techniques and postoperative evaluation were also highly variable. We found 10 different designs for PSI devices among the articles included in this review, including 6 whose production was outsourced to manufacturers.3, 7, 11, 16, 19, 20, 23, 24, 25, 26, 27, 32, 36, 38, 39, 40, 43 The overall purpose of the designs was similar: to provide a template based on the individual patient's anatomy for accurate drilling of the central glenoid guide pin that will later be used for reaming and implantation. However, the mechanisms of fixation on the glenoid face differed, with some instruments requiring single vs. multiple points of contact on the glenoid rim and other instruments requiring contact with additional anatomic structures such as the coracoid base or acromion.7, 12, 16, 19, 38 Although design variation did not seem to significantly impact overall accuracy, the performances of the individual devices were not compared with one another in any instance, which limits the capacity of a surgeon to make a well-informed decision if considering adopting 1 of these techniques. Lack of uniform postoperative evaluation also makes directly comparing PSI device performance difficult, as deviation from preoperatively planned positioning was measured on radiographs, 2D CT imaging, and 3D post-implant reconstructions depending on author preference.16, 20, 26 Although version error, inclination error, and overall positional offset were the most commonly used error quantification parameters in the literature, no consensus exists regarding which parameters are most accurate or appropriate in TSA. Some articles described innovative measurement techniques designed to better quantify implant positioning errors in 3D space, such as angulation deviation, roll deviation, and implant positional offset in 3 dimensions using radiologic markers.12, 19 However, this practice was not widespread in the available literature. Further testing and validation of more sophisticated techniques that better evaluate positional errors in 3D space may assist in determining whether PSI technology significantly improves accurate implantation in TSA compared with standard instrumentation. In any case, however, the goal of using PSI is to improve patient outcomes and reduce the risk of component loosening. Thus, studies must include a robust analysis of clinical outcome measures to prove the efficacy of PSI.
The strengths of this study lie in the exhaustive literature review and in-depth analysis of preoperative planning tools, intraoperative PSI technology, and postoperative evaluation of PSI systems in shoulder arthroplasty. Such analysis does not exist in the available literature yet is clinically relevant given the increasing number of shoulder arthroplasties and volume of medical marketing for PSI systems.30 That being said, several clinically important questions remain unanswered based on limitations in the available data. First, production cost was not reported in any article. The time necessary for production was not reported consistently, and available production times ranged from 2 to 5 weeks at minimum.16, 40 Surgeons may not be willing to wait over a month for device production, which could potentially influence case timing or overall volume or could limit the applicability of PSI to certain elective cases.17 For surgeons to properly evaluate the practicality and investment value of PSI technology, objective and readily available information on associated costs and time of production is critical. Another limitation was the fact that the impact of PSI on intraoperative time was left completely unaddressed. Patient-specific cutting blocks have been suggested to increase operating room efficiency and save costs in knee arthroplasty, although more recent research has indicated that this relationship is not exactly well defined.10, 33 Investigation into this area for shoulder arthroplasty would provide useful information applicable to surgeon decision making. Furthermore, although no studies described any intraoperative complications associated with PSI devices, no clinical or patient-reported outcomes were reported for patients who underwent arthroplasty with the assistance of PSI technology compared with standard instrumentation. No available evidence to date suggests that the improvements in accuracy achieved with PSI translate to improved postoperative outcomes or increased glenoid component longevity compared with standard instrumentation. Although it would be inherently difficult to establish this relationship given the excellent current implant survivorship of 10 years or longer, further study into these areas is warranted.
Conclusion
A variety of PSI systems for shoulder arthroplasty have individually been shown to offer significant improvements in glenoid component implantation accuracy. Despite these findings, our meta-analysis revealed significant heterogeneity in study methodologies and no statistically significant differences in accuracy between PSI and standard instrumentation. To date, there is no consistently validated method for PSI preoperative surgical planning, intraoperative technique, or postoperative evaluation. Although PSI may have the potential to improve TSA techniques, further investigations regarding long-term clinical outcomes, impact on operating room time, and cost-effectiveness are warranted before PSI can be routinely recommended over conventional instrumentation.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
No institutional review board approval was required for this systematic review.
References
- 1.Antuna S.A., Sperling J.W., Cofield R.H., Rowland C.M. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10:217–224. doi: 10.1067/mse.2001.113961. [DOI] [PubMed] [Google Scholar]
- 2.Berhouet J., Gulotta L.V., Dines D.M., Craig E., Warren R.F., Choi D. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2017;103:407–413. doi: 10.1016/j.otsr.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Berhouet J., Rol M., Spiry C., Slimane M., Chevalier C., Favard L. Shoulder patient-specific guide: first experience in 10 patients indicates room for improvement. Orthop Traumatol Surg Res. 2018;104:45–51. doi: 10.1016/j.otsr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Bryce C.D., Davison A.C., Lewis G.S., Wang L., Flemming D.J., Armstrong A.D. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am. 2010;92:692–699. doi: 10.2106/jbjs.i.00177. [DOI] [PubMed] [Google Scholar]
- 5.Cavaignac E., Pailhe R., Laumond G., Murgier J., Reina N., Laffosse J.M. Evaluation of the accuracy of patient-specific cutting blocks for total knee arthroplasty: a meta-analysis. Int Orthop. 2015;39:1541–1552. doi: 10.1007/s00264-014-2549-x. [DOI] [PubMed] [Google Scholar]
- 6.Costello J.P., Olivieri L.J., Su L., Krieger A., Alfares F., Thabit O. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit Heart Dis. 2015;10:185–190. doi: 10.1111/chd.12238. [DOI] [PubMed] [Google Scholar]
- 7.Dallalana R.J., McMahon R.A., East B., Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10:59–66. doi: 10.4103/0973-6042.180717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniilidis K., Tibesku C.O. A comparison of conventional and patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38:503–508. doi: 10.1007/s00264-013-2028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vloo R., Pellikaan P., Dhollander A., Vander Sloten J. Three-dimensional analysis of accuracy of component positioning in total knee arthroplasty with patient specific and conventional instruments: a randomized controlled trial. Knee. 2017;24:1469–1477. doi: 10.1016/j.knee.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 10.DeHaan A.M., Adams J.R., DeHart M.L., Huff T.W. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29:2065–2069. doi: 10.1016/j.arth.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Elliott R.S., Dallalana R.J. Patient-specific instrument-assisted structural glenoid bone grafting in reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2017;18:145–150. doi: 10.1097/BTE.0000000000000123. [DOI] [Google Scholar]
- 12.Eraly K., Stoffelen D., Vander Sloten J., Jonkers I., Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25:837–845. doi: 10.1016/j.jse.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Farron A., Terrier A., Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15:521–526. doi: 10.1016/j.jse.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Frankle M.A., Teramoto A., Luo Z.P., Levy J.C., Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18:874–885. doi: 10.1016/j.jse.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Frydrychowicz A., Francois C.J., Turski P.A. Four-dimensional phase contrast magnetic resonance angiography: potential clinical applications. Eur J Radiol. 2011;80:24–35. doi: 10.1016/j.ejrad.2011.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauci M.O., Boileau P., Baba M., Chaoui J., Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-b:1080–1085. doi: 10.1302/0301-620x.98b8.37257. [DOI] [PubMed] [Google Scholar]
- 17.Gomes N.S. Patient-specific instrumentation for total shoulder arthroplasty. EFORT Open Rev. 2016;1:177–182. doi: 10.1302/2058-5241.1.000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan S.S., Leith J.M., Campbell B., Kapil R., Smith K.L., Matsen F.A. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11:431–441. doi: 10.1067/mse.2002.125806. [DOI] [PubMed] [Google Scholar]
- 19.Hendel M.D., Bryan J.A., Barsoum W.K., Rodriguez E.J., Brems J.J., Evans P.J. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94:2167–2175. doi: 10.2106/jbjs.k.01209. [DOI] [PubMed] [Google Scholar]
- 20.Heylen S., Van Haver A., Vuylsteke K., Declercq G., Verborgt O. Patient-specific instrument guidance of glenoid component implantation reduces inclination variability in total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:186–192. doi: 10.1016/j.jse.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Ho J.C., Sabesan V.J., Iannotti J.P. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95:e82. doi: 10.2106/jbjs.l.00336. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y., Yuan Z.S., Kepler C.K., Albert T.J., Yuan J.B., Dong W.X. Deviation analysis of C1-C2 transarticular screw placement assisted by a novel rapid prototyping drill template: a cadaveric study. J Spinal Disord Tech. 2014;27:E181–E186. doi: 10.1097/bsd.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 23.Iannotti J., Baker J., Rodriguez E., Brems J., Ricchetti E., Mesiha M. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am. 2014;96:e71. doi: 10.2106/jbjs.l.01346. [DOI] [PubMed] [Google Scholar]
- 24.Iannotti J.P., Walker K., Rodriguez E., Patterson T.E., Jun B.J., Ricchetti E.T. Three-dimensional preoperative planning and patients specific instrumentation improve glenoid component positioning. J Shoulder Elbow Surg. 2017;26:e321–e323. doi: 10.1016/j.jse.2017.06.011. [DOI] [Google Scholar]
- 25.Iannotti J.P., Weiner S., Rodriguez E., Subhas N., Patterson T.E., Jun B.J. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am. 2015;97:651–658. doi: 10.2106/jbjs.n.00493. [DOI] [PubMed] [Google Scholar]
- 26.Lau S.C., Keith P.P.A. Patient-specific instrumentation for total shoulder arthroplasty: not as accurate as it would seem. J Shoulder Elbow Surg. 2018;27:90–95. doi: 10.1016/j.jse.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Levy J.C., Everding N.G., Frankle M.A., Keppler L.J. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1563–1567. doi: 10.1016/j.jse.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 28.Lewis G.S., Stevens N.M., Armstrong A.D. Testing of a novel pin array guide for accurate three-dimensional glenoid component positioning. J Shoulder Elbow Surg. 2015;24:1939–1947. doi: 10.1016/j.jse.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen D., Ferreira L.M., Brownhill J.R., Faber K.J., Johnson J.A. Design and development of a computer assisted glenoid implantation technique for shoulder replacement surgery. Comput Aided Surg. 2007;12:152–159. doi: 10.3109/10929080701374315. [DOI] [PubMed] [Google Scholar]
- 30.Padegimas E.M., Maltenfort M., Lazarus M.D., Ramsey M.L., Williams G.R., Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473:1860–1867. doi: 10.1007/s11999-015-4231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadonikolakis A., Neradilek M.B., Matsen F.A. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95:2205–2212. doi: 10.2106/jbjs.l.00552. [DOI] [PubMed] [Google Scholar]
- 32.Pietrzak W.S. Biomet Orthopaedics; Warsaw, IN: 2013. Shoulder alignment obtained with the Signature Glenoid Guide System: a cadaver study; pp. 1–3. [Google Scholar]
- 33.Sassoon A., Nam D., Nunley R., Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473:151–158. doi: 10.1007/s11999-014-3804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 35.Small T., Krebs V., Molloy R., Bryan J., Klika A.K., Barsoum W.K. Comparison of acetabular shell position using patient specific instruments vs. standard surgical instruments: a randomized clinical trial. J Arthroplasty. 2014;29:1030–1037. doi: 10.1016/j.arth.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Subramanya S., Herald J. Reverse shoulder arthroplasty with patient-specific glenoid implant positioning guides. Tech Shoulder Elbow Surg. 2014;15:122–129. [Google Scholar]
- 37.Suero E.M., Citak M., Lo D., Krych A.J., Craig E.V., Pearle A.D. Use of a custom alignment guide to improve glenoid component position in total shoulder arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2860–2866. doi: 10.1007/s00167-012-2177-1. [DOI] [PubMed] [Google Scholar]
- 38.Throckmorton T.W., Gulotta L.V., Bonnarens F.O., Wright S.A., Hartzell J.L., Rozzi W.B. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24:965–971. doi: 10.1016/j.jse.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Throckmorton T.W., Vogt W., Wasmaier J., Hurst J.M., Frostick S., Sperling J.W. Patient-specific targeting guides for glenoid component placement in shoulder arthroplasty: technique and initial clinical experience. Tech Shoulder Elbow Surg. 2014;15:103–108. doi: 10.1097/BTE.0000000000000029. [DOI] [Google Scholar]
- 40.Trouilloud P., Gonzalvez M., Martz P., Charles H., Handelberg F., Nyffeler R.W. Duocentric(R) reversed shoulder prosthesis and Personal Fit(R) templates: innovative strategies to optimize prosthesis positioning and prevent scapular notching. Eur J Orthop Surg Traumatol. 2014;24:483–495. doi: 10.1007/s00590-013-1213-2. [DOI] [PubMed] [Google Scholar]
- 41.Van Leeuwen J., Snorrason F., Rohrl S.M. No radiological and clinical advantages with patient-specific positioning guides in total knee replacement. Acta Orthop. 2018;89:89–94. doi: 10.1080/17453674.2017.1393732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walch G., Badet R., Boulahia A., Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 43.Walch G., Vezeridis P.S., Boileau P., Deransart P., Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24:302–309. doi: 10.1016/j.jse.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Wesseling M., De Groote F., Meyer C., Corten K., Simon J.P., Desloovere K. Subject-specific musculoskeletal modelling in patients before and after total hip arthroplasty. Comput Methods Biomech Biomed Engin. 2016;19:1683–1691. doi: 10.1080/10255842.2016.1181174. [DOI] [PubMed] [Google Scholar]