Abstract
BACKGROUND: Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with high invasive and metastatic potential. We generated a spontaneous PDAC mouse model and examined the therapeutic potential of indirubin 3′-oxime (Indox) against PDAC bearing mouse in vivo. METHODS: Randomized 3-month-old LSL-KrasG12D/+;Trp53flox/+;Pdx-1-cre (KPCflox) mice were intraperitoneally injected with 40 mg/kg Indox (n = 9) or a vehicle (n = 10) twice a week. At the end point, tumor status including proliferation, direct invasion, and distant metastasis was analyzed histopathologically. The inhibitory potentials of Indox for proliferation, migration/invasion, and the phosphorylation of target molecules were determined in KPCflox-derived PDAC cells in vitro. RESULTS: Prolonged survival by Indox via intraperitoneal administration was observed in the KPCflox mice. Indox inhibited tumor proliferation accompanied with low levels of nuclear phosphorylated cyclin-dependent kinase (p-CDK) and cyclin B1 in vivo. Furthermore, Indox inhibited the migration/invasive activities of PDAC via down-regulation of matrix metalloproteinase (MMP)-9 in vitro and in vivo. Antibody array and immunoblotting analysis revealed that Indox inhibited the phosphorylation of multiple molecules, including key upstream proteins of MMP-9 in RAF/extracellular signal-regulated kinase (ERK), AKT, and stress-activated protein kinase/c-Jun-N-terminal kinase (SAPK/JNK) pathways. CONCLUSION: Indox inhibited the proliferative, invasive, and metastatic potentials of PDAC in vitro and in vivo. Therefore, Indox could a therapeutic candidate for treating spontaneously occurring PDAC via blocking the RAF/ERK, AKT and SAPK/JNK pathways.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with a high mortality rate [1], [2]. The 5-year survival rate of PDAC is approximately 8%, which is partially attributed to the difficulty of an early diagnosis, and the post-surgical 5-year survival rate is still around 20%. The poor prognosis in PDAC patients is due to its malignancy such as aggressive invasion and metastasis [3]. Patients with advanced and inoperable PDAC generally receive chemotherapy. A combination chemotherapy regimen using gemcitabine, S-1, and nab-paclitaxel has been demonstrated to prolong the overall survival of PDAC patients [4], [5]. However, there are still issues of partially existing chemoresistant PDAC cells and clinical complications from the side effects of the chemotherapy. Therefore, novel anticancer drugs for the treatment of PDAC are necessary.
Indirubin is an active ingredient found in a traditional Chinese herbal medicine, Danggui Longhui Wan, which is used for the treatment of patients with chronic myelogenous leukemia [6]. Indirubin and its derivatives block the ATP-binding sites in cell cycle-related kinases such as cyclin-dependent kinases (CDKs) [7], [8], [9], [10]. Several studies have shown that indirubin and its derivatives inhibit cell proliferation and partially induce apoptosis by inhibition of CDKs and induction of G2/M arrest in cancer cells [11], [12], [13]. We previously reported that indirubin 3′-oxime (Indox) inhibited the proliferation of PDAC cells by down-regulation of p-CDK1/cyclin B1 in PDAC cells in vitro and in a xenograft mouse model [14]. However, the inhibitory potentials of Indox against the progression stages, direct invasion, and distant metastasis in spontaneously occurring PDAC remain unclear.
Among the many kinds of mouse models generated for the investigation of PDAC [15], LSL-KrasG12D/+;LSL-Trp53R172H/+; Pdx-1-Cre (KPC) mouse is considered as an adequate model for human PDAC patients [16]. KPC mice show hypovascular tumors with abundant stromal reaction (desmoplasia), which is a characteristic phenotype of human PDAC and is considered as a factor in the chemoresistant mechanism in PDAC patients [17]. In the current report, we used LSL-KrasG12D/+;Trp53flox/+;Pdx-1-Cre (KPCflox) mice, which are basically a similar phenotype to the KPC mouse, to determine the potential antitumor effects of Indox in spontaneously occurring PDAC.
Materials and Methods
Anticancer Drugs
The indirubin derivative, Indox, was prepared as described previously [14], [18].
Genetically Engineered Mice and Animal Care
Three individual strains of LSL-KrasG12D/+, Trp53flox/+, and Pdx-1-cre mice were obtained from Jackson Laboratory (Sacramento). We crossed and generated the LSL-KrasG12D/+;Trp53flox/+;Pdx-1-cre (KPCflox) mice in house. All animals were kept in specific pathogen-free housing with abundant food and water under guidelines approved by the Nihon University, School of Medical, Animal Care and Use Committee (AP15M001). At 3 months old, the mice were intraperitoneally injected with 40 mg/kg Indox or a vehicle (a mixture of DMSO/PEG400, 1:1 (v/v)) twice a week until the endpoint. The total pancreatic weight and tumor sizes were measured, and their volumes were calculated using the following formula: width x length x height. All samples were embedded in paraffin followed after fixing in 10% neutral buffered formalin.
Immunohistochemistry
Immunohistochemical staining was performed using antibodies against MMP-9 (1:100; Kyowa Pharma Chemical Co.), Ki-67 antigen, p-CDK1, cyclin B1, K-19 antigen, CD31, LYVE-1, and cleaved caspase-3. The number of positive cells in at least four microscopic fields (200×) in a representative specimen were determined using Image J software.
Mouse PDAC Cell Lines
Murine PDAC cell lines (#146, 147 and 244) were established from the primary site in KPCflox mice (Supplementary Table 1). Therefore, all of these PDAC cells were genetically induced by KrasG12D mutation. All cell lines were maintained at 37°C in 5% CO2 in D-MEM (Wako Pure Chemical Industries, Ltd.) containing 10% fetal bovine serum (Equitech-Bio Inc.) and 1% penicillin/streptomycin.
Antibody Array Analysis
The mouse PDAC cell line (#146) was treated with 10 μM Indox for 24 h and then was subjected to protein analysis by the antibody arrays based on the instructions that accompanied the antibody array assay kit (Full Moon BioSystems, Inc.). The processed antibody arrays on slides were scanned by a Microarray Scanner System G2565CA and the data obtained were analyzed with Feature Extraction software (Agilent Technologies, Inc.).
Cell Cycle Analysis
Cell cycle analysis was performed using a Cell-Clock Assay Kit (Biocolor Ltd.) on a murine PDAC cell line (#146) treated with 3 or 10 μM Indox for 24 h.
Migration and Invasion Assays
Migration and invasion assays were performed by the method described previously [19]. Cells (2.5 × 104) were plated into either control or Matrigel-coated invasion chamber inserts (Becton Dickinson) and cultured with or without 10 μM Indox for 24 h.
Immunoblotting
Immunoblotting analysis was performed by the method described previously [19]. PDAC cell lines (#146, #147, and #244) were treated with Indox for 24 h. Antibodies to MMP-2, MMP-9 (1:100; Kyowa Pharma Chemical Co.), B-RAF (1:1000; Abcam); p-B-RAF (Ser446), p-ERK (Tyr204), p-AKT (Thr308), SAPK/JNK, p-SAPK/JNK (Tyr183), and p-c-Jun (Thr91) (1:1000; Cell Signaling Technology); Akt, c-Jun, GAPDH (1:1000; R&D systems); MMP-7 and ERK (1:1000; Santa Cruz) were used.
Statistical Analyses
Results are presented as average ± SD or percentage. Data were analyzed using one-way ANOVA with post-hoc Tukey tests. All statistical analyses were performed using SPSS software (version 25.0, IBM SPSS Statistics). P values of <.05 were classified to be significant.
Results
Indox Inhibits PDAC Proliferation and Prolongs KPCflox Mice Survival
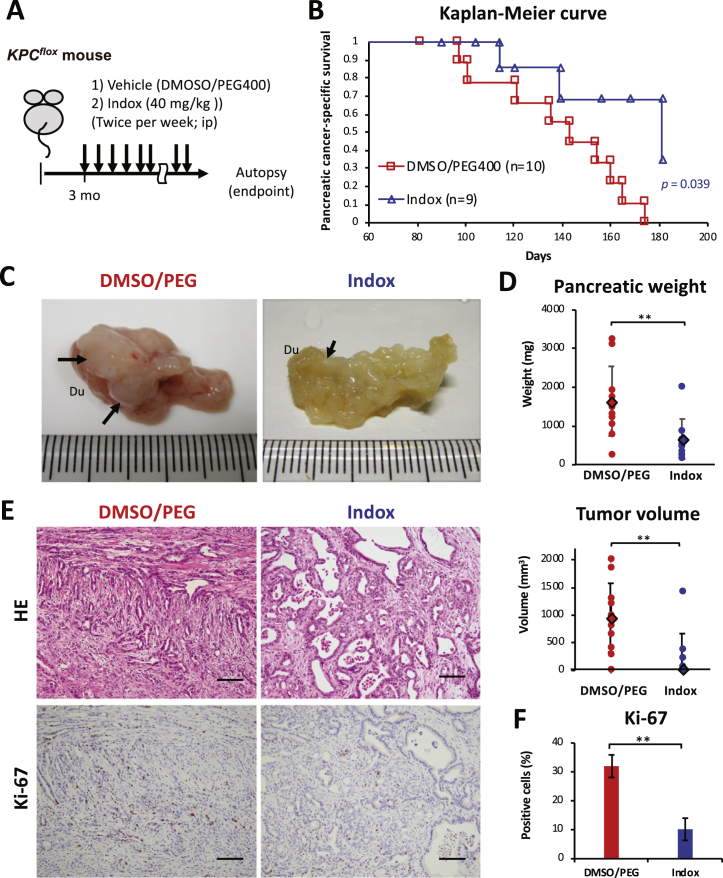
To investigate the antitumor effect of Indox on spontaneous a PDAC bearing mouse model, we generated KPCflox mice and intraperitoneally injected Indox (Figure 1A). Significant prolonged survival (P = .039) was observed by Indox administration (Figure 1B). The pancreatic tumors that occurred in the KPCflox mice were whitish solid nodules with pancreatic atrophy (Figure 1C). Tumor volume and total pancreatic weight were reduced by Indox administration (Figure 1D). A summary of the phenotypes and pancreas analyses of the mice in the treatment groups is shown in Supplementary Table 1. In the KPCflox mice without Indox administration, histopathological evidence of the PDAC differentiated between moderately and poorly with occasional sarcomatoid or anaplastic carcinoma component (Figure 1E, Supplementary Table 1). In contrast, well to moderately differentiated PDAC was frequently observed in the KPCflox mice who received Indox (Supplementary Table 1, Supplementary Table 2). Ki-67-positive cell content in the tumor portions were reduced by Indox-treatment (Figure 1, E and F).
Figure 1.
The effects of indirubin 3′-oxime (Indox) on pancreatic ductal adenocarcinoma (PDAC) occurring in a KPCflox (LSL-KrasG12D/+;Trp53flox/+;Pdx-1-cre) mouse.
(A) Schematic of the experimental treatment regimen. Three-month-old KPCflox mice were intraperitoneally injected with 40 mg/kg Indox or vehicle control twice a week until the endpoint. (B) Kaplan–Meier survival analysis of the KPCflox mice by log-rank test (P = .039). (C) Gross appearance of representative tumors (arrows) in the pancreas (6 months old). Du, duodenum. (D) Total pancreatic weights and pancreatic tumor volumes. Moderately to poorly differentiated PDAC by hematoxylin and eosin (HE) staining and Ki-67 proliferative marker in PDAC (E). Scale bars, 100 μm. (F) Quantification of data presented in E. *P < .05; **P < .01 vs. vehicle control by ANOVA Tukey’s test.
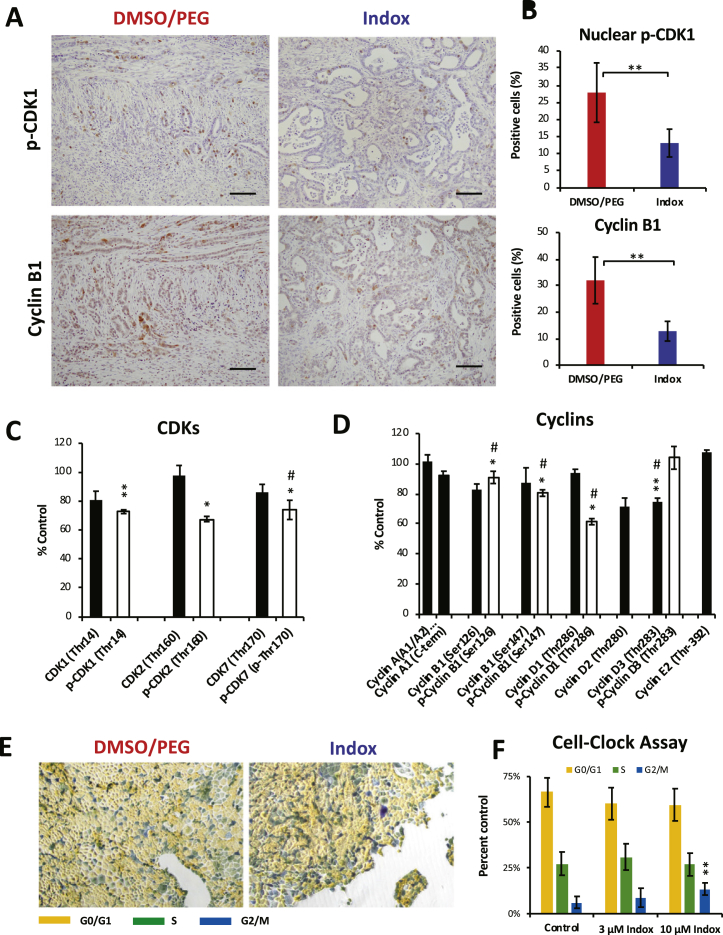
Next, we determined the cell cycle-related molecules. Nuclear p-CDK1- and cyclin B1-positive PDAC cell percentages were immunohistochemically decreased in tumors in the KPCflox mice that received Indox (Figure 2, A and B). Then the effects of Indox on CDKs and cyclins were examined in an established cell line from the PDAC in the KPCflox mice. In this case, the PDAC cells were induced by KrasG12D mutation. The decrease in the p-CDK1 level in the PDAC cells was supported by antibody array analysis (Figure 2C). The array analysis revealed that phosphorylated CDK1, CDK2, and CDK7 levels in the PDAC cells were significantly reduced by Indox treatment in vitro while changes of the non-phosphorylated CDKs levels were insignificant. The intensive suppression of phosphorylation on cyclins by Indox was observed on only cyclin D1 (Figure 2D). Furthermore, Indox reduced the levels of other cell cycle-associated molecules, including p-ATM, p-WEE1, p-cyclin-dependent kinase inhibitor (p-CKIs), and p-ChKs (Supplementary Figure 1). Since the effect of Indox on the cell cycle of PDAC cells is determined to be G2/M arrest by Cell-Clock Assay (Figure 2, E and F), we conclude that Indox inhibits PDAC proliferation by suppression of p-CDK1/p-cyclin B1 levels, thereby inducing G2/M arrest (Figure 5 and Supplementary Figure 2).
Figure 2.
Effects of Indox administration on the proliferation of PDAC in the KPCflox mice.
(A) Cycle-related molecules p-CDK1 and cyclin B1 were markedly decreased in PDAC with the administration of Indox. (B) Quantification of the data presented in A. Levels of phosphorylated CDKs (C) and cyclins (D) in murine PDAC cells (#146) by antibody array (n = 6 each). Microscopic features of Cell-clock assay (E) and the quantification of data presented in E (F). *P < .05; **P < .01; ***P < .001 vs. vehicle control by ANOVA Tukey’s test. #n = 5.
Figure 5.
Inhibition of phosphorylated RAF/ERK, AKT, SAPK/JNK and c-Jun by Indox.
(A) After treatment with indicated concentration of Indox for 24 h, low levels of p-B-RAF (Ser446), p-ERK (Tyr204), p-AKT (Thr308), p-SAPK/JNK (Tyr183) and p-c-Jun (Thr91) in the murine PDAC cell line (#146) were determined by immunoblotting. (B) Quantification of data presented in A. *P < .05; **P < .01; ***P < .001 vs. vehicle control by ANOVA Tukey’s test.
Next, we evaluated the effect of Indox on apoptosis in PDAC in KPCflox mice. At first we stained the PDAC tissues immunohistochemically using an anti-cleaved caspase-3 antibody and measured the positive areas microscopically. The cleaved caspase-3 positive area was deceased by Indox administration, but it was statistically insignificant (Supplementary Figure 3, A and B). On the other hand, anti-apoptotic p-BCL-xL and pro-apoptotic p-Bad and p-Bid levels determined by the antibody array analysis were decreased in the PDCA cells in vitro by Indox treatment (Supplementary Figure 4A). On the caspases 3, 6, 8, and 9 and cytochrome c (inter) in the PDAC cells, by Indox treatment in vitro, caspases 3 (phosphorylated/non-phosphorylated), 6 (non-phosphorylated), and 8 (non-phosphorylated) and cytochrome c (inter) levels were reduced significantly while caspases 9 (phosphorylated/non-phosphorylated) and 6 (phosphorylated) levels were not changed (Supplementary Figure 4B). This evidence leads us to conclude that the Indox induced cell death of PDAC in KPCflox mice is non-apoptotic and is also not related to the modulation of mitochondrial BCL-2 family members and caspase signaling cascades (Supplementary Figure 5).
Indox Inhibits Migration and Invasion Activities of PDAC Cells via Down-Regulation of MMP-9 In Vitro and In Vivo
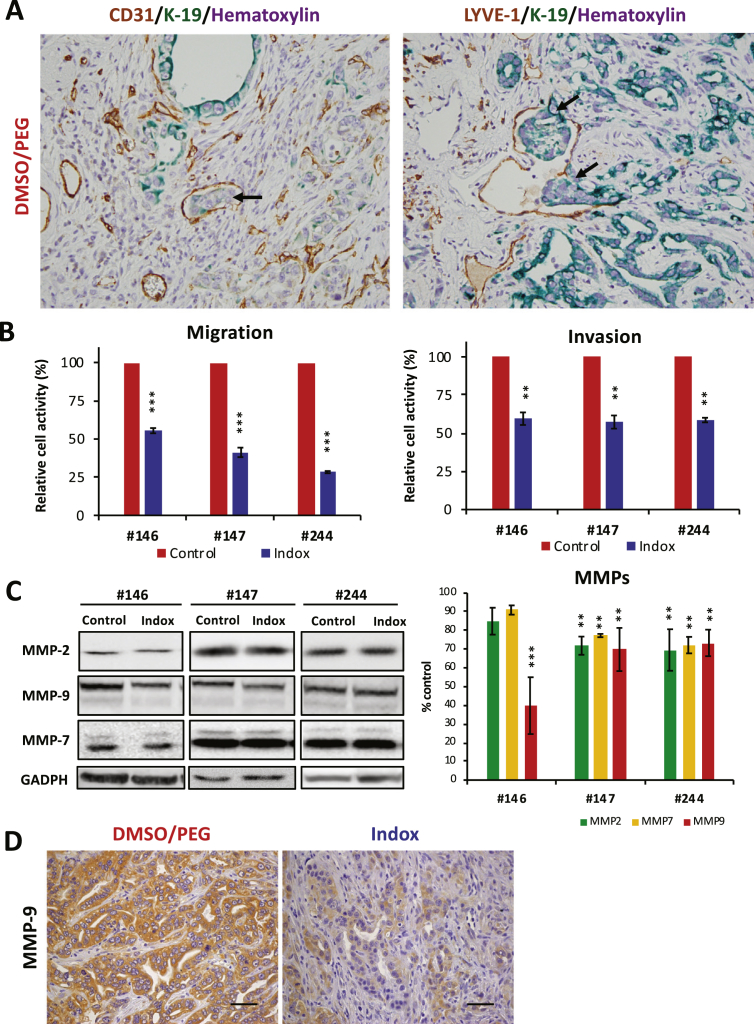
The pathological status of PDAC bearing KPCflox mice is listed in Supplementary Table 2. The invasions of the vascular, lymph, and peripheral organs including the duodenum and common bile duct, metastasis to the lymph nodes, and distant metastasis and/or dissemination in the KPCflox mice were remarkably suppressed by Indox administration in vivo. We thus determined the PDAC cell distribution in pancreatic tissue, microinvasion, in the KPCflox mice by a K-19 antigen as a marker. Microinvasion of the K-19+ PDAC cells into the CD31+ tumor blood vessels and LYVE-1+ lymph vessels were observed in all of the control KPCflox mice (Figure 3A). In contrast, microinvasion was significantly suppressed by Indox administration (Data not shown).
Figure 3.
Effects of Indox on migration/invasion activities in vivo and in vitro.
(A) Microscopic invasion of PDAC into CD31+ veins and LYVE-1+ lymph vessels were analyzed by staining with epithelial marker K-19 antigen (arrows). (B) Migration and invasion activities of mouse PDAC cells. (C) Expression levels of MMP-2, -7 and -9 in murine PDAC cell lines treated with 10 μM Indox for 24 h. (D) Expression of MMP-9 in PDAC cells was inhibited in the KPCflox mice. Scale bars, 100 μm. *P < .05; **P < .01; ***P < .001 vs. vehicle control by ANOVA Tukey’s test.
Next, we performed in vitro migration and invasion assays using the mouse PDAC cell lines (Figure 3B). Consistent with the results in the KPCflox mice, Indox blocked both the migration and invasion activities in the PDAC cells. Since matrix metalloproteinase (MMP) is the major invasion-associated molecule in various cancers, we next determined the expressions of MMPs in mouse PDAC cells by the western blotting method. The expression of MMP-9 in the PDAC cells was reduced by Indox treatment, especially the #146 cells (Figure 3C). Likewise, by Indox administration, the reduction of MMP-9 expression was observed in the PDAC in the KPCflox mice in vivo (Figure 3D). The expressions of MMP-2 and -7 were also partially suppressed by Indox treatment in the PDAC cells with statistical significance. These results suggest there is an inhibitory effect of Indox on PDAC cell invasion via down-regulation of MMP-9.
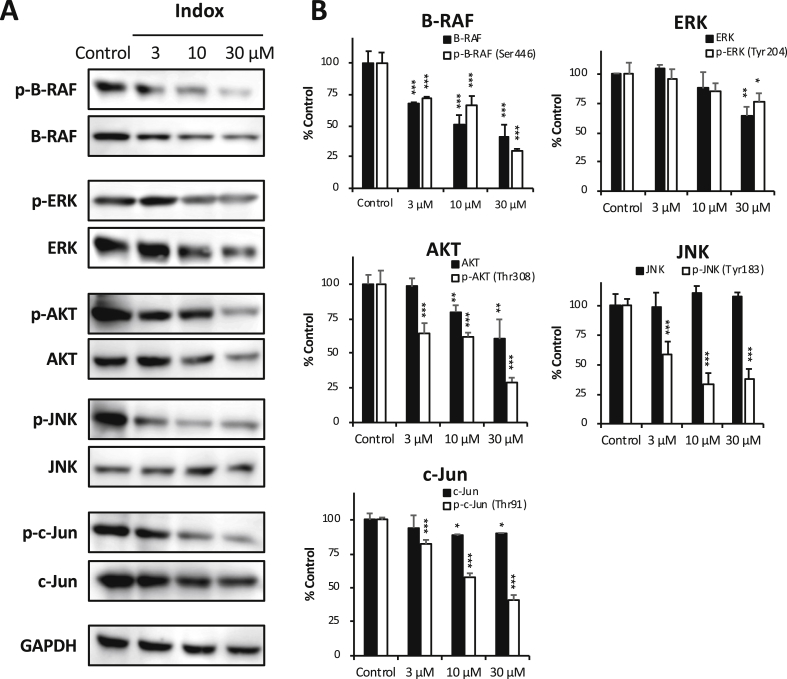
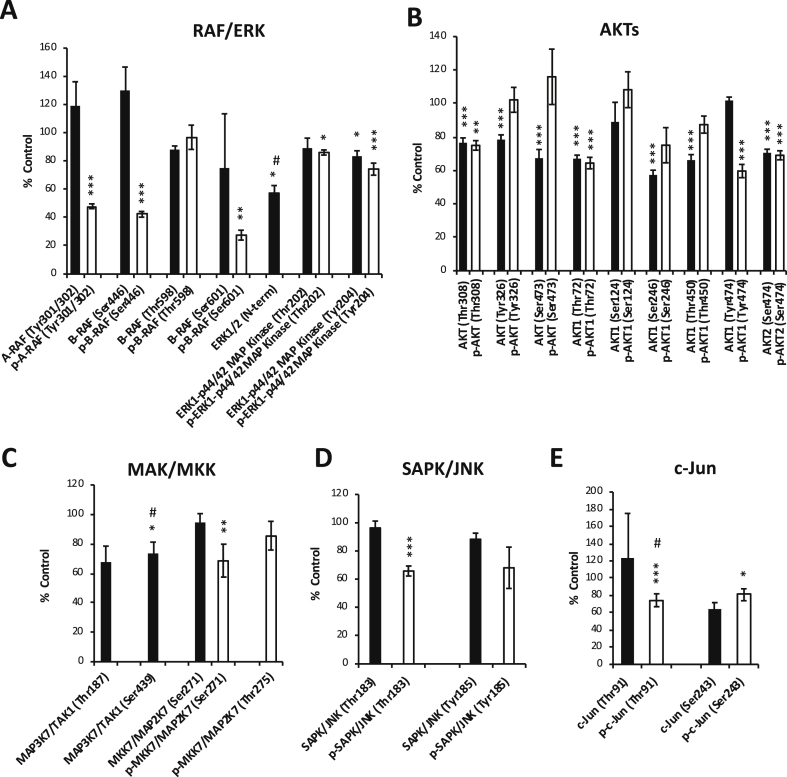
To identify the molecules influenced by Indox treatment in up-stream of MMP-9 expression, we performed antibody array analyses again. The RAF/ERK, AKT, and SAPK/JNK signaling pathways have been reported to be the modulators for MMP-2 and -9 expression in cancer cells [20], [21], [22]. Interestingly, Indox suppressed the phosphorylation of A-RAF and B-RAF significantly in the PDAC cells (Figure 4A). Moreover, Indox slightly reduced the phosphorylated ERK1-p42/44 MAP kinase and p90RSK levels (Figure 4A, Supplementary Figure 4D). Phosphorylated AKT, AKT1/2 and p70S6K levels were reduced by Indox (Figure 5B, Supplementary Figure 4C). Additionally, phosphorylated MKK7/MAP2K7 levels and their down-stream kinases, SAPK/JNK and c-Jun, were also suppressed by Indox (Figure 4, C–E). These results of the antibody array were basically supported by immunoblotting analysis in the mouse PDAC cell lines (Figure 5, A and B, Supplementary Figure 5, A and B). Therefore, these findings suggest that Indox inhibits RAF/ERK, AKT and SAPK/JNK signaling cascades, which most likely leads to the down-regulation of MMP-9 and inhibition of tumor migration and invasion (Figure 6B, Supplementary Figure 6).
Figure 4.
Effects of Indox on the levels of phosphorylated RAF/ERK, AKT, SAPK/JNK pathways.
Antibody array revealed that phosphorylation of RAF/ERK (A), AKTs (B), MAP/MMK (C), SAPK/JNK (D), and c-Jun (E) in the murine cell line (#146) was inhibited by 10 μM Indox for 24 h (n = 6). *P < .05; **P < .01; ***P < .001 vs. vehicle control by ANOVA Tukey’s test. #n = 5.
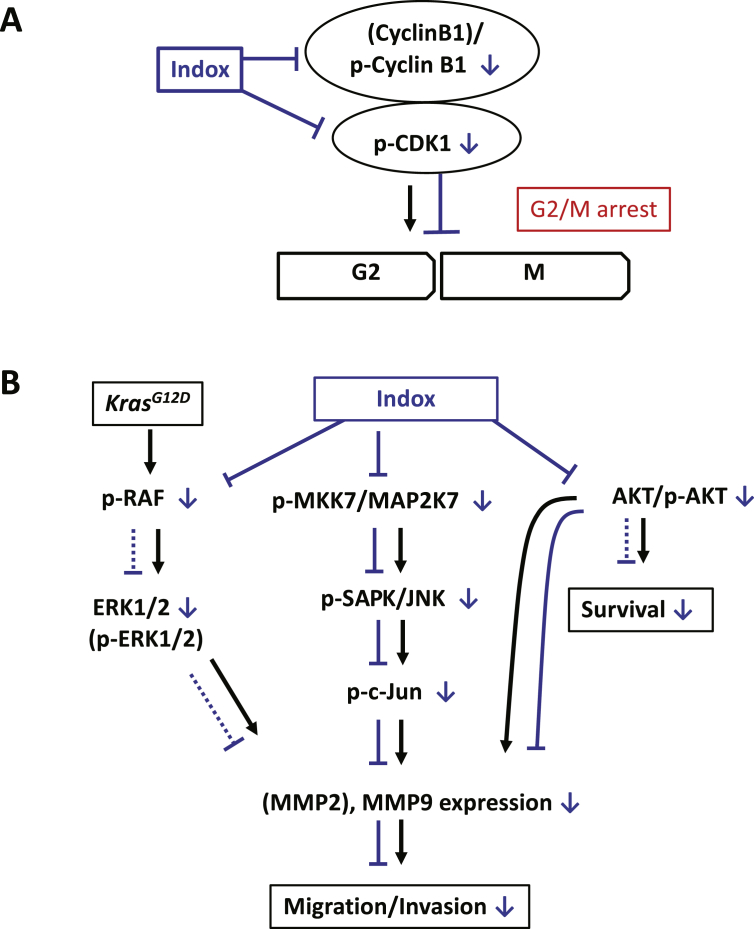
Figure 6.
A schematic diagram of the effect of Indox on the KrasG12D-induced PDAC cells.
(A) Indox inhibits the phosphorylation of CDK1 and cyclin B level, leading to G2/M arrest in mouse PDAC cells. (B) Indox inhibits phosphorylation of B-Raf, and the MMK7/MAP2K7 and AKT pathways, which leads to down-regulation of MMP-9 and decreased migration and invasion activities.
Discussion
Previous reports have demonstrated the antiproliferation effects of indirubin derivatives against PDAC cell lines or cancer stem cells in vitro only [23], [24], [25]. We first show the antitumor effect of Indox to spontaneous PDAC model (KPCflox mice) in vivo in addition to the mice-derived PDAC cell lines in vitro in this report. Most indirubin derivatives have been shown to inhibit CDKs by competitively binding to ATP-binding sites [7], [8], [9] and thereby inducing the G2/M arrest of cancer cells [11], [12], [13]. In this study, antibody array analysis showed that Indox reduced the levels of p-CDK1/2/7 and p-cyclin B1/D1 and other cell cycle-associated molecules such as p-ATM, p-WEE1, p-CKIs, p-CDCs, and p-ChKs. These molecules are involved in complex regulatory mechanisms in other phases of the cell cycle (G1 and S phases) and the checkpoints (G1/S and S/G2). Otherwise, Indox predominantly induced G2/M arrest indicating that the inhibition of p-CDK1/cyclin B1 is a key antiproliferative mechanism of Indox.
The current study first evaluated the anti-migration/invasion effects of Indox on a spontaneous PDAC mouse model and the underlying mechanisms using the mice-derived cell lines. Previous reports demonstrated that indirubin derivatives inhibited the migration and invasion activities in other cancer and endothelial cells [26], [27], [28], [29], [30], [31], [32]. Indox blocked the invasion of the human myeloid cell line KBM-5 by the down-regulation of MMP-9 [26]. In another study, 5-nitroindirubin 3′-oxime (5NO2Indox) inhibited the migration and invasion of salivary gland ductal adenocarcinoma by suppression of beta1 integrin and MMP-2 and -9 [27]. Furthermore, 6-bromoindirubin 3′-oxime (6BrIndox) also blocked the migration and invasion potential of colorectal and invasive urinary bladder carcinomas, accompanied with down-regulation of MMP-2 and -9 [28], [29]. These forms of Indox with different functional groups likely showed similar biological effects but different inhibitory effects due to different affinities to the target molecules.
Indirubin and its derivatives are known to be multi-kinase inhibitors, but the target molecules might depend on cell type. In our study, Indox inhibited the B-RAF/ERK and SAPK/JNK signaling pathways as well as the AKT pathways in the PDAC cells in vitro. In mouse fibroblast NIH/3T3, Indox inhibited the fibroblast growth factor-induced phosphorylation of ERK and AKT, but did not inhibit the phosphorylation of JNK and p38 MAPK [33]. Meanwhile, Indox has been reported to directly inhibit the activity of JNK1 and JNK3 in neuronal cells [34]. Similar to our findings, 5NO2Indox showed inhibitory effects on the phosphorylation of RAF-1, ERK, JNK, and c-Jun, thereby blocking the neoplastic transformation of mouse epidermal cells [35]. Similarly, 5NO2Indox inhibited the metastatic ability of head and neck cancer cells by blocking the AKT pathway [36]. In carcinomas, MMP-2 and -9 expressions were modulated by the activation of the RAF/ERK, AKT, and SAPK/JNK signaling pathways [20], [21], [22]. Therefore, Indox might inhibit the migration/invasion and metastatic potentials of PDAC via down-regulation of MMP-9 modulated by the inhibition of the B-RAF/ERK, AKT and SAPK/JNK signaling pathways.
Recent clinical studies have shown that kinase inhibitors such as Vemurafenib and Dabrafenib were useful compounds for the treatment of malignant melanoma and thyroid papillary carcinoma with mutant B-RAF [37]. Interestingly, Indox strongly inhibited the phosphorylation of B-RAF (Ser446 and Ser601) in the KrasG12D-induced PDAC cells in vitro. Although future investigations are still needed to determine the effects on the B-RAF pathway, Indox may be an effective candidate for treating various cancers though the activation of the B-RAF signaling pathway.
Conclusions
Indox inhibited the proliferation, invasion and metastatic activities of PDAC cells in vitro and spontaneous PDAC model KPCflox mice in vivo. Treatment with Indox could be a new therapeutic candidate for the treatment of advanced and aggressive PDAC.
The following are the supplementary data related to this article.
Pathological phenotype in Kras(G12D), Trp53(flox/+), Pdx1-cre mice with/without administration of Indox
Pathological status of KPC(flox) mice with/without administration of Indox
Supplementary figures
Acknowledgments
Acknowledgments
The authors would like to thank Ms. Yukari Hirotani, Department of Pathology, Nihon University School of Medicine for excellent tissue sectioning and staining and the members in the Animal Laboratory for caring for the animals. Antibody array analysis was performed at the Medical Research Support Center, Graduate School of Medicine, Kyoto University. The authors also wish to thank Prof. Eric M. Skier from the School of Pharmacy, Nihon University, for proofreading.
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI) of Japan Society for the Promotion of Science to MS (17K08772) and Kinjo Gakuin University-Parent Teacher Association Research Grant to YI.
Conflict of Interest Statement
None declared.
Contributor Information
Yoshimi Ichimaru, Email: y-ichimaru@kinjo-u.ac.jp.
Makoto Sano, Email: sano.makoto@nihon-u.ac.jp.
Ichie Kajiwara, Email: kajiwara.ichie@nihon-u.ac.jp.
Takao Tobe, Email: tobet@kinjo-u.ac.jp.
Hiroki Yoshioka, Email: hiroki.yoshioka@uth.tmc.edu.
Kazuhiko Hayashi, Email: hayashi@kinjo-u.ac.jp.
Hideaki Ijichi, Email: hideijichi-gi@umin.ac.jp.
Shinichi Miyairi, Email: miyairi.shinichi@nihon-u.ac.jp.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Sakamitsu K. Director-General for statistics and Information Policy, Ministry of Health, Labour and Welfare; Tokyo: 2018. Vital Statistics in Japan; pp. 18–22. [Google Scholar]
- 3.Bardeesy N., DePinho R.A. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 4.Uesaka K., Boku N., Fukutomi A., Okamura Y., Konishi M., Matsumoto I. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao Z., Hao Y., Liu B., Qian L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk Lymphoma. 2002;43:1763–1768. doi: 10.1080/1042819021000006295. [DOI] [PubMed] [Google Scholar]
- 7.Polychronopoulos P., Magiatis P., Skaltsounis A.L., Myrianthopoulos V., Mikros E., Tarricone A. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47:935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.J., Lee J.E., Jeong S.Y., Im I., Lee S.D., Lee E.J. 5,5′-substituted indirubin-3′-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity. J Med Chem. 2010;53:3696–3706. doi: 10.1021/jm100080z. [DOI] [PubMed] [Google Scholar]
- 9.Myrianthopoulos V., Kritsanida M., Gaboriaud-Kolar N., Magiatis P., Ferandin Y., Durieu E. Novel inverse binding mode of indirubin derivatives yields improved selectivity for DYRK kinases. ACS Med Chem Lett. 2013;4:22–26. doi: 10.1021/ml300207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer L., Skaltsounis A.L., Magiatis P., Polychronopoulos P., Knockaert M., Leost M. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Hoessel R., Leclerc S., Endicott J.A., Nobel M.E., Lawrie A., Tunnah P. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 12.Marko D., Schatzle S., Friedel A., Genzlinger A., Zankl H., Meijer L. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer. 2001;84:283–289. doi: 10.1054/bjoc.2000.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc S., Garnier M., Hoessel R., Marko D., Bibb J.A., Snyder G.L. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 14.Sano M., Ichimaru Y., Kurita M., Hayashi E., Homma T., Saito H. Induction of cell death in pancreatic ductal adenocarcinoma by indirubin 3′-oxime and 5-methoxyindirubin 3′-oxime in vitro and in vivo. Cancer Lett. 2017;397:72–82. doi: 10.1016/j.canlet.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Hruban R.H., Adsay N.V., Albores-Saavedra J., Anver M.R., Biankin A.V., Boivin G.P. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 17.Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H., Tabata K., Hanada S., Kanda Y., Suzuki T., Miyairi S. Synthesis of methoxy- and bromo-substituted indirubins and their activities on apoptosis induction in human neuroblastoma cells. Bioorg Med Chem Lett. 2011;21:5370–5373. doi: 10.1016/j.bmcl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Sano M., Driscoll D.R., DeJesus-Monge W.E., Quattrochi B., Appleman V.A., Ou J. Activation of WNT/beta-catenin signaling enhances pancreatic cancer development and the malignant potential via up-regulation of Cyr61. Neoplasia. 2016;18:785–794. doi: 10.1016/j.neo.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y.J., Neoh C.A., Tsao C.Y., Su J.H., Li H.H. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci. 2015;16:16469–16482. doi: 10.3390/ijms160716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo C.L., Lai K.C., Ma Y.S., Weng S.W., Lin J.P., Chung J.G. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-kappaB, Ras and matrix metalloproteinase-2 and -9. Oncol Rep. 2014;32:355–361. doi: 10.3892/or.2014.3209. [DOI] [PubMed] [Google Scholar]
- 22.Yang J.L., Lin J.H., Weng S.W., Chen J.C., Yang J.S., Amagaya S. Crude extract of Euphorbia formosana inhibits the migration and invasion of DU145 human prostate cancer cells: The role of matrix metalloproteinase-2/9 inhibition via the MAPK signaling pathway. Mol Med Rep. 2013;7:1403–1408. doi: 10.3892/mmr.2013.1380. [DOI] [PubMed] [Google Scholar]
- 23.Cheng X., Kim J.Y., Ghafoory S., Duvaci T., Rafiee R., Theobald J. Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Mol Oncol. 2016;10:806–824. doi: 10.1016/j.molonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam S., Wen W., Schroeder A., Herrmann A., Yu H., Cheng X. Dual inhibition of Janus and Src family kinases by novel indirubin derivative blocks constitutively-activated Stat3 signaling associated with apoptosis of human pancreatic cancer cells. Mol Oncol. 2013;7:369–378. doi: 10.1016/j.molonc.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu Y.L., Jung K.H., Son M.K., Yan H.H., Kim S.J., Shin S. Anticancer activity of HS-527, a novel inhibitor targeting PI3-kinase in human pancreatic cancer cells. Cancer Lett. 2014;353:68–77. doi: 10.1016/j.canlet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Sethi G., Ahn K.S., Sandur S.K., Lin X., Chaturvedi M.M., Aggarwal B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 2006;281:23425–23435. doi: 10.1074/jbc.M602627200. [DOI] [PubMed] [Google Scholar]
- 27.Yoon J.H., Kim S.A., Kim J.I., Park J.H., Ahn S.G., Yoon J.H. Inhibition of invasion and migration of salivary gland adenocarcinoma cells by 5′-nitro-indirubinoxime (5′-NIO) Head Neck. 2010;32:619–625. doi: 10.1002/hed.21230. [DOI] [PubMed] [Google Scholar]
- 28.Liu K., Li J., Wu X., Chen M., Luo F., Li J. GSK-3beta inhibitor 6-bromo-indirubin-3′-oxime promotes both adhesive activity and drug resistance in colorectal cancer cells. Int J Oncol. 2017;51:1821–1830. doi: 10.3892/ijo.2017.4163. [DOI] [PubMed] [Google Scholar]
- 29.Braig S., Kressirer C.A., Liebl J., Bischoff F., Zahler S., Meijer L. Indirubin derivative 6BIO suppresses metastasis. Cancer Res. 2013;73:6004–6012. doi: 10.1158/0008-5472.CAN-12-4358. [DOI] [PubMed] [Google Scholar]
- 30.Williams S.P., Nowicki M.O., Liu F., Press R., Godlewski J., Abdel-Rasoul M. Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 2011;71:5374–5380. doi: 10.1158/0008-5472.CAN-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cockle J.V., Picton S., Levesley J., Ilett E., Carcaboso A.M., Short S. Cell migration in paediatric glioma; characterisation and potential therapeutic targeting. Br J Cancer. 2015;112:693–703. doi: 10.1038/bjc.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Ligr M.., McCarron J.P., Daniels G., Zhang D., Zhao X. Natura-alpha targets forkhead box m1 and inhibits androgen-dependent and -independent prostate cancer growth and invasion. Clin Cancer Res. 2011;17:4414–4424. doi: 10.1158/1078-0432.CCR-11-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhen Y., Sorensen V., Jin Y., Suo Z., Wiedlocha A. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–6385. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y., Liu Y., Ma C., Yuan Z., Wang W., Zhu Z. Indirubin-3′-oxime inhibits c-Jun NH2-terminal kinase: anti-apoptotic effect in cerebellar granule neurons. Neurosci Lett. 2004;367:355–359. doi: 10.1016/j.neulet.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Khanal P., Choi H.K., Namgoong G.M., Ahn S.G., Yoon J.H., Sohn H. 5′-Nitro-indirubinoxime inhibits epidermal growth factor- and phorbol ester-induced AP-1 activity and cell transformation through inhibition of phosphorylation of Pin1. Mol Carcinog. 2011;50:961–971. doi: 10.1002/mc.20761. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.A., Kwon S.M., Kim J.A., Kang K.W., Yoon J.H., Ahn S.G. 5′-Nitro-indirubinoxime, an indirubin derivative, suppresses metastatic ability of human head and neck cancer cells through the inhibition of Integrin beta1/FAK/Akt signaling. Cancer Lett. 2011;306:197–204. doi: 10.1016/j.canlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Holderfield M., Deuker M.M., McCormick F., McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14:455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pathological phenotype in Kras(G12D), Trp53(flox/+), Pdx1-cre mice with/without administration of Indox
Pathological status of KPC(flox) mice with/without administration of Indox
Supplementary figures