A 49-year-old woman with a history of Roux-en-Y gastric bypass surgery was referred because of elevated liver enzymes, right upper-quadrant abdominal pain, and a dilated common bile duct on MRCP. The options for biliary access were discussed with the patient, including laparoscopy-assisted ERCP, enteroscopy-assisted ERCP, and EUS-directed transgastric ERCP (EDGE). She opted to proceed with an EDGE, in which a lumen-apposing metal stent (LAMS) is used to create a gastrogastrostomy to facilitate ERCP with a duodenoscope. A same-session ERCP was performed because of the patient’s geographic constraints.
A linear echoendoscope was advanced into the gastric pouch and was used to identify the remnant stomach by identifying rugae. A 19-gauge needle was advanced into the remnant stomach, and diluted contrast material was injected to fill the lumen (Fig. 1). A 0.035-inch wire (Jagwire; Boston Scientific, Natick, Mass, USA) was advanced through the needle and coiled within the remnant stomach. A 15-mm electrocautery-enhanced LAMS (AXIOS; Boston Scientific) was deployed over the wire, creating a gastrogastrostomy between the gastric remnant and the gastric pouch. The LAMS was dilated with a 15-mm balloon to accommodate same-session advancement of a diagnostic duodenoscope (JF 140; Olympus Medical Inc, Bethlehem, Pa, USA) (Fig. 2). Two apposing endoscopic sutures (Apollo OverStitch; Apollo Endosurgery, Austin, Tex, USA) were placed to hold the proximal flange of the LAMS within the gastric pouch (Fig. 3).
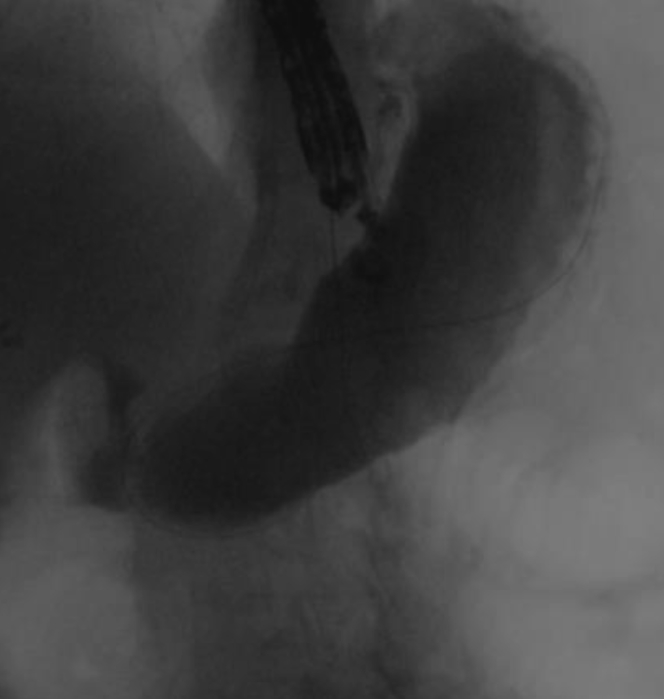
Figure 1.
Remnant stomach filled with contrast material under fluoroscopy.
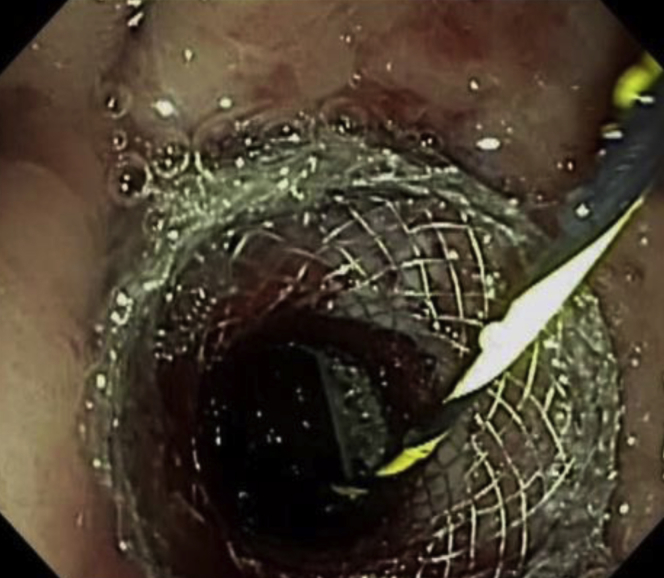
Figure 2.
Lumen-apposing metal stent after dilation.
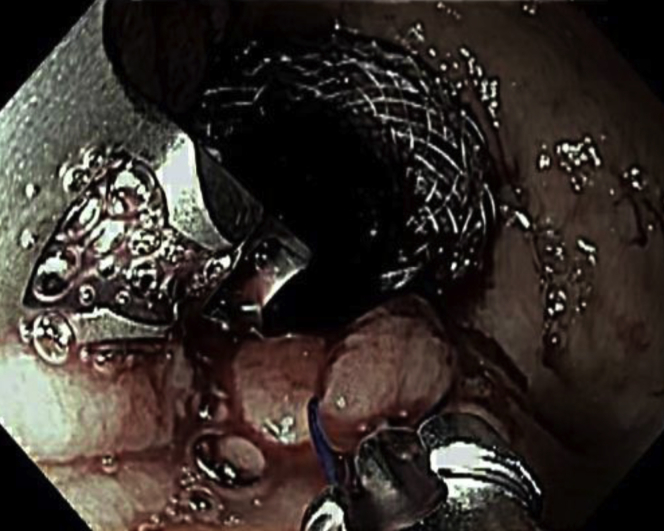
Figure 3.
Two endoscopic sutures placed in an interrupted fashion to hold the lumen-apposing metal stent in position within the gastric pouch.
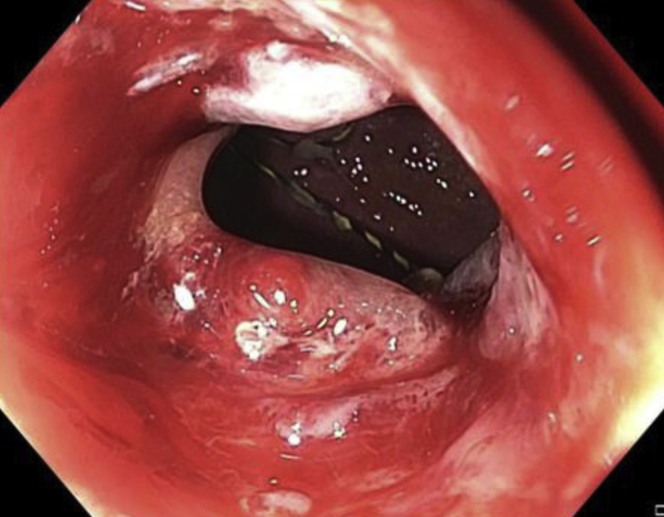
The duodenoscope was advanced through the LAMS into the duodenum, and an ERCP was performed. The cholangiogram showed diffuse dilatation of the common bile duct, and a sphincterotomy was performed for presumed papillary stenosis (Fig. 4). To maintain access to the gastric remnant during withdrawal of the endoscope, the 0.035-inch wire (Jagwire; Boston Scientific) was left in place within the lumen of the remnant stomach.
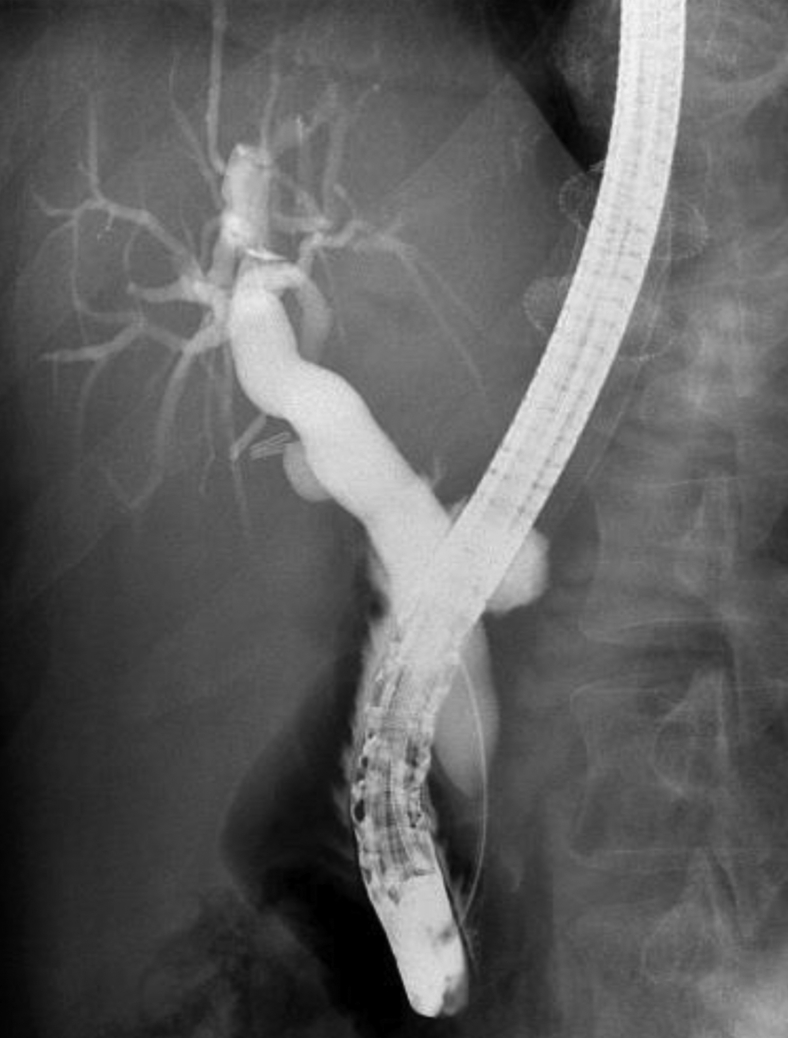
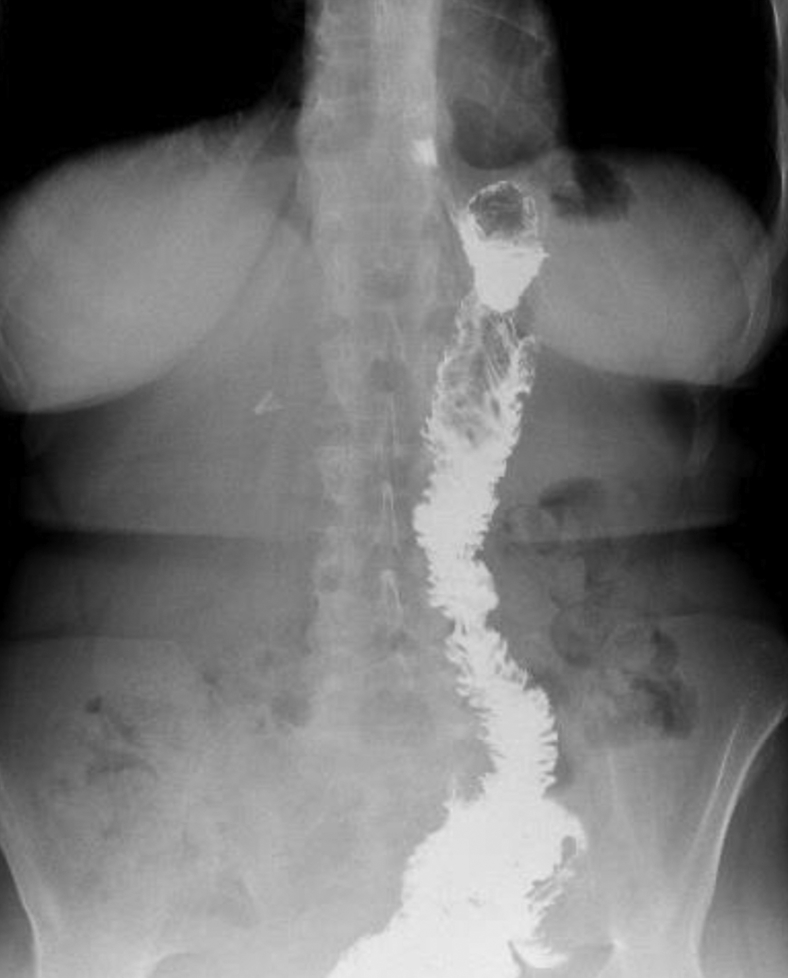
Figure 4.
Diffuse dilatation of the main bile duct on cholangiogram.
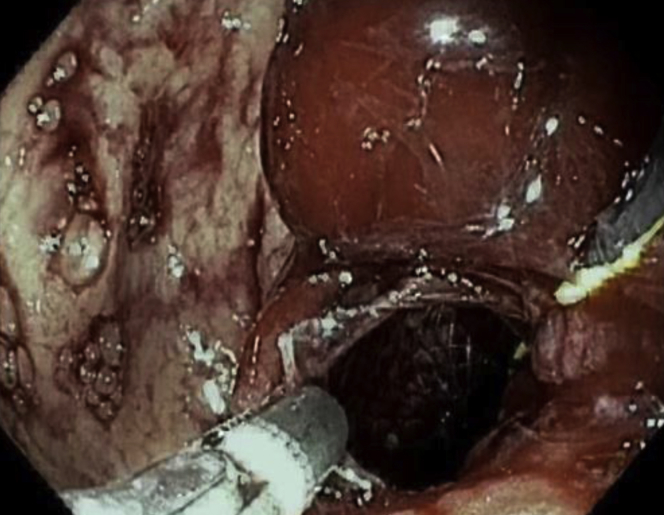
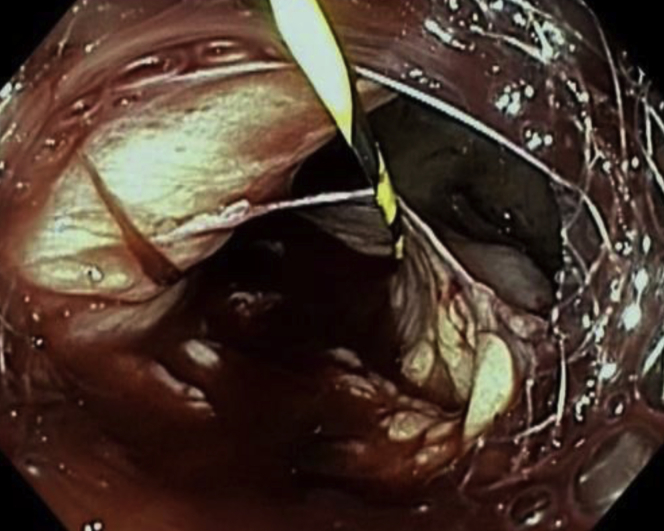
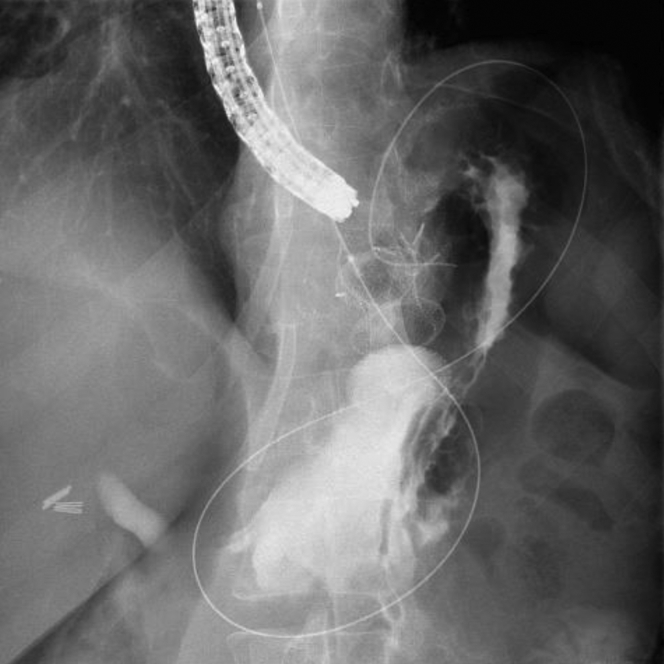
During withdrawal of the duodenoscope, the proximal flange of the LAMS became dislodged from within the gastric pouch and was partially within the peritoneum, with the endoscopic sutures still intact (Video 1, available online at www.VideoGIE.org). We switched to a diagnostic gastroscope for better visualization. The proximal flange of the LAMS was grasped with forceps and pulled back into the gastric pouch (Fig. 5). During these attempts, the distal flange of the LAMS became dislodged from within the gastric remnant and was completely within the peritoneum (Fig. 6), revealing a large separation between the gastric pouch and the remnant stomach. Fortunately, the previously advanced “safety” wire maintained access to the gastric remnant. An attempt at apposition of the remnant and gastric stomach by counterpressure using a fully inflated 20-mm extraction balloon (Cook Endoscopy, Winston-Salem, NC, USA) was not successful. It was then decided that a larger LAMS might help provide enough counterpressure to permit lumen apposition between the gastric remnant and the gastric pouch. A 20-mm LAMS was advanced over the wire into the remnant stomach, through the dislodged LAMS. The distal flange of the LAMS was deployed and then gently pulled under fluoroscopic guidance to appose the gastric remnant and the gastric pouch. Under fluoroscopy, the 2 LAMSs were seen overlapping with apposition of the gastric pouch and the remnant stomach (Fig. 7).
Figure 5.
Proximal flange of the lumen-apposing metal stent dislodged from within the gastric pouch and grasped with forceps to bring back into the pouch.
Figure 6.
Distal flange of lumen-apposing metal stent within the peritoneum, with wire maintaining access to remnant stomach.
Figure 7.
Two lumen-apposing metal stents (15 mm and 20 mm) overlapping with apposition of the gastric pouch and remnant stomach.
The patient was admitted after the procedure, was restricted to nothing by mouth, and was given antibiotics. An upper-GI series showed no leak. She was discharged home 3 days after the procedure on a regular diet and on antibiotics. The LAMSs were removed 5 weeks later after endoscopic scissors were used to cut the sutures; a gastrogastric fistula was seen (Fig. 8). Spontaneous gastrogastric fistula closure was allowed and was confirmed by an upper-GI series 8 weeks later (Fig. 9). At a follow-up visit 4 months later, she reported no further right upper-quadrant abdominal pain and no weight regain, and her liver enzymes were normal.
Figure 8.
Gastrogastric fistula seen after lumen-apposing metal stent removal.
Figure 9.
No gastrogastric fistula seen on upper-GI series 8 weeks after lumen-apposing metal stent removal.
This case demonstrates LAMS dislodgment, despite endoscopic suturing and the use of a diagnostic duodenoscope, during same-session ERCP with use of the EDGE technique.1 This adverse event was managed endoscopically with a good outcome, the essential aspect being maintenance of guidewire access to the remnant stomach during withdrawal of the endoscope. The LAMSs were removed, and the gastrogastric fistula closed spontaneously without weight regain.
LAMS dislodgment is not uncommon in same-session EDGE procedures.1, 2, 3, 4, 5 Most cases have been managed endoscopically, with LAMS repositioning or placement of a second LAMS, although surgery has been necessary in a few cases.1, 2, 3, 4, 5 Consideration for ERCP after maturation of the gastrogastric fistula should be made if the indication is of a more elective nature and depending on the patient’s circumstances. Although our case highlights LAMS dislodgment despite endoscopic suturing, a small series of 5 patients who underwent same-session EDGE procedures with endoscopic suturing or over-the-scope clipping of a 15-mm LAMS reported no episodes of stent dislodgment. ERCP timing, the use of suturing and/or over-the-scope clips to secure the LAMS, LAMS size (ie, the use of a larger 20-mm LAMS), and endoscope size may be factors in reducing this adverse event.
Disclosure
Dr Hammad is a consultant for Medtronic. Dr Shah is a consultant and advisory board member for Boston Scientific and a consultant for Cook Endoscopy and Olympus Medical. The other author disclosed no financial relationships relevant to this publication.
Supplementary data
Lumen-apposing metal stent dislodgment during an EUS-directed transgastric ERCP.
References
- 1.Kedia P., Tyberg A., Kumta N.A. EUS-directed transgastric ERCP for Roux-en-Y gastric bypass anatomy: a minimally invasive approach. Gastrointest Endosc. 2015;82:560–565. doi: 10.1016/j.gie.2015.03.1913. [DOI] [PubMed] [Google Scholar]
- 2.Tyberg A., Nieto J., Salgago Endoscopic ultrasound (EUS)-directed transgastric endoscopic retrograde cholangiopancreatography or EUS: mid-term analysis of an emerging procedure. Clin Endosc. 2017;50:185–190. doi: 10.5946/ce.2016.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukhari M., Kowalski T., Nieto J. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc. 2019;89:904–905. doi: 10.1016/j.gie.2018.04.2356. [DOI] [PubMed] [Google Scholar]
- 4.Irani S., Yang J., Khashab M. Mitigating lumen-apposing metal stent dislodgment and allowing safe, single-stage EUS-directed transgastric ERCP. VideoGIE. 2018;3:322–324. doi: 10.1016/j.vgie.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kedia P., Tarnasky P.R., Nieto J. EUS-directed transgastric ERCP (EDGE) versus laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y gastric bypass (RYGB) anatomy: a multicenter early comparative experience of clinical outcomes. J Clin Gastroenterol. 2019;53:304–308. doi: 10.1097/MCG.0000000000001037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lumen-apposing metal stent dislodgment during an EUS-directed transgastric ERCP.