Introduction
Adult T-cell leukemia/lymphoma (ATL) is a non-Hodgkin lymphoma caused by the human T-lymphotropic virus type 1. The indolent variant manifests on the skin as pruritic papules and plaques. Current treatment regimens for cutaneous ATL include watchful waiting; skin directed therapy, such as radiation, topical steroids, or ultraviolet light; or the use of oral agents, including interferon-α or zidovudine. Multiagent chemotherapy, notably mogamulizumab, is used for aggressive variantes.1,2 Chemotherapeutic agents used in ATL can be costly and associated with significant adverse effects.
Imiquimod is an immunomodulator approved for actinic keratosis, primary superficial basal cell carcinoma, and genital warts. Off-label use has been efficacious in mycosis fungoides, lentigo maligna melanoma, HIV-negative Kaposi sarcoma, and primary cutaneous anaplastic large-cell lymphoma.3, 4, 5 We present a patient in whom nearly complete resolution of ATL was achieved with topical imiquimod therapy.
Case report
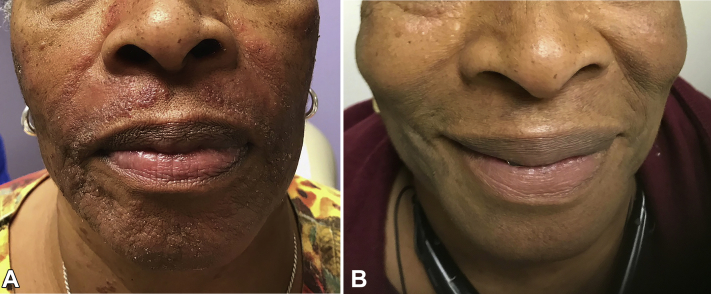
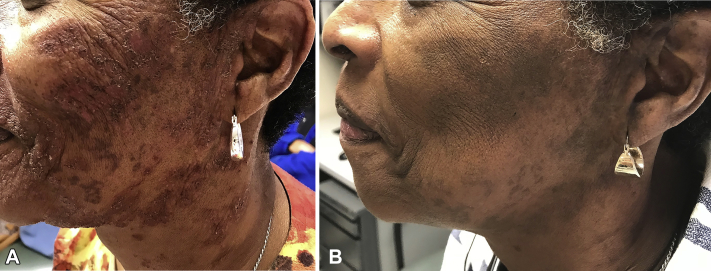
A 66-year-old woman with a 2-year history of indolent ATL presented with a 1-year history of worsening pruritic indurated dark-red papules plaques involving the face (Figs 1, A and 2, A) and upper chest. She noted only minimal improvement with topical steroids, topical pimecrolimus, and phototherapy. A skin biopsy specimen from the right lateral cheek showed dermal lymphoid infiltrate composed of a mixture of small and large cells and interface changes consistent with cutaneous involvement of ATL. Immunohistochemical studies revealed CD3+ T-cells with a predominance of CD4+ over CD8+ cells. CD25 highlighted ∼75% of the infiltrate. Polymerase chain reaction showed monoclonal T-cell receptor beta and gamma gene rearrangements were present.
Fig 1.
Frontal face (A) before treatment and (B) after 2 weeks of imiquimod treatment.
Fig 2.
Left face (A) before treatment and (B) after 2 weeks of imiquimod treatment.
Biannual complete blood count, blood flow cytometry, and computed tomography scans were negative for progressive systemic disease. In addition, the patient denied weight loss, night sweats, and fevers. Given the absence of progressive systemic disease in the face of failure of typical skin-directed remedies and with patient concern of adverse effects of systemic therapy, alternative skin-directed therapies were explored. Imiquimod had a desirable adverse effect profile, with success in other cutaneous lymphomas, and was selected for treatment.
Imiquimod 5% cream applied topically twice weekly was initiated. After 2 weeks, there was nearly complete resolution of the papules and plaques, with only residual postinflammatory hyperpigmented patches without pruritus (Figs 1, B and 2, B). Treatment continued for 6 months, with no new or recurrent cutaneous disease. At the 8-month follow up, the patient noted mild recurrence of lesions and treatment was increased to 3 times weekly with improved control. The patient reported no adverse effects from imiquimod, including irritation, redness, or pruritus at application sites.
At her 11-month follow-up, she reported inability to discontinue the medication due to flaring in its absence. Given the success of the medication in the absence of patient-reported adverse effects, we decided to continue imiquimod treatment 2 to 3 times weekly indefinitely.
Discussion
Imiquimod has profound antiviral and antitumoral action via multiple mechanisms. First, it has shown agonistic activity toward Toll-like receptor 7, leading to activation of multiple cytokines, including interferon-α, tumor necrosis factor-α, interleukin 1, interleukin 6, and eventually the mounting of a T-helper type 1–mediated cellular immune response. Independently of Toll-like receptor 7, imiquimod also interferes with adenosine receptor signaling pathways that enhance the proinflammatory state. Lastly, imiquimod induces apoptosis of tumor cells at higher concentrations that involves caspase activation and B-cell lymphoma 2.3,6
The use of imiquimod in cutaneous T-cell lymphomas has been described in the literature. Topical 5% imiquimod resulted in complete resolution of patch, plaque, and tumor stage mycosis fungoides.7 In addition, it achieved complete resolution in cutaneous anaplastic large-cell lymphoma.4
The present case adds to literature by demonstrating that cutaneous ATL lesions can be successfully cleared by imiquimod. This is especially relevant because ATL frequently requires more intensive chemotherapy. Adverse effects of imiquimod are minimal and limited to the skin, including irritation, redness, and pruritus at the application site.7 Most adverse events resolve after the first weeks of treatment.
In conclusion, imiquimod has cell-mediated immunity-boosting effects that can lead to nearly complete resolution of ATL. The ease of application and minimal adverse effects make imiquimod a desirable therapeutic to patients. Clinicians should consider use of imiquimod in ATL in addition to cutaneous T-cell lymphoma and other skin cancers.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
References
- 1.Katsuya H., Ishitsuka K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J Clin Exp Hematop. 2017;57:87–97. doi: 10.3960/jslrt.17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekine M., Takeuchi M., Toyama T. Mogamulizumab for ATLL in clinical practice. Blood. 2016;128:2998. [Google Scholar]
- 3.Huen A.O., Rook A.H. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr Opin Oncol. 2014;26:237–244. doi: 10.1097/CCO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 4.Kubicki S.L., Park K.E., Aung P.P., Duvic M. Complete resolution of primary cutaneous anaplastic large cell lymphoma with topical imiquimod. J Drugs Dermatol. 2019;18:460–462. [PubMed] [Google Scholar]
- 5.Celestin Schartz N.E., Chevret S., Paz C. Imiquimod 5% cream for treatment of HIV-negative Kaposi's sarcoma skin lesions: a phase I to II, open-label trial in 17 patients. J Am Acad Dermatol. 2008;58:585–591. doi: 10.1016/j.jaad.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Vacchelli E., Eggermont A., Sautes-Fridman C. Trial watch: Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2013;2:e25238. doi: 10.4161/onci.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarabadkar E.S., Shinohara M.M. Skin directed therapy in cutaneous T-cell lymphoma. Front Oncol. 2019;9:260. doi: 10.3389/fonc.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]