Abstract
Rationale: Pulmonary arterial hypertension (PAH) is a vascular remodeling disease with a poor prognosis and limited therapeutic options. Although the mechanisms contributing to vascular remodeling in PAH are still unclear, several features, including hyperproliferation and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs), have led to the emergence of the cancer-like concept. The molecular chaperone HSP90 (heat shock protein 90) is directly associated with malignant growth and proliferation under stress conditions. In addition to being highly expressed in the cytosol, HSP90 exists in a subcellular pool compartmentalized in the mitochondria (mtHSP90) of tumor cells, but not in normal cells, where it promotes cell survival.
Objectives: We hypothesized that mtHSP90 in PAH-PASMCs represents a protective mechanism against stress, promoting their proliferation and resistance to apoptosis.
Methods: Expression and localization of HSP90 were analyzed by Western blot, immunofluorescence, and immunogold electron microscopy. In vitro, effects of mtHSP90 inhibition on mitochondrial DNA integrity, bioenergetics, cell proliferation and resistance to apoptosis were assessed. In vivo, the therapeutic potential of Gamitrinib, a mitochondria-targeted HSP90 inhibitor, was tested in fawn-hooded and monocrotaline rats.
Measurements and Main Results: We demonstrated that, in response to stress, HSP90 preferentially accumulates in PAH-PASMC mitochondria (dual immunostaining, immunoblot, and immunogold electron microscopy) to ensure cell survival by preserving mitochondrial DNA integrity and bioenergetic functions. Whereas cytosolic HSP90 inhibition displays a lack of absolute specificity for PAH-PASMCs, Gamitrinib decreased mitochondrial DNA content and repair capacity and bioenergetic functions, thus repressing PAH-PASMC proliferation (Ki67 labeling) and resistance to apoptosis (Annexin V assay) without affecting control cells. In vivo, Gamitrinib improves PAH in two experimental rat models (monocrotaline and fawn-hooded rat).
Conclusions: Our data show for the first time that accumulation of mtHSP90 is a feature of PAH-PASMCs and a key regulator of mitochondrial homeostasis contributing to vascular remodeling in PAH.
Keywords: cell metabolism, cancer, vascular smooth muscle cells, proliferation, apoptosis
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary arterial hypertension (PAH) is a fatal disease characterized by progressive obliteration of small-caliber pulmonary arteries due, at least in part, to exaggerated proliferation and resistance to apoptosis of pulmonary artery smooth muscle cells. Although great strides have been made in understanding the pathogenesis of PAH, the precise mechanisms accounting for the acquisition of this cancer-like phenotype remains largely unknown.
What This Study Adds to the Field
Our study demonstrates for the first time a mitochondrial accumulation of the molecular chaperone HSP90 (heat shock protein 90) in PAH–pulmonary artery smooth muscle cells, contributing to their proliferation and survival under environmental stresses and thus promoting vascular remodeling in PAH. Finally, we demonstrated that the specific inhibition of the mitochondrial pool of HSP90 improves PAH in rodents.
Pulmonary arterial hypertension (PAH) is characterized by the narrowing of pulmonary arteries due to vasoconstriction, excessive proliferation, and resistance to apoptosis related to metabolic alterations (i.e., Warburg effect) of pulmonary artery (PA) smooth muscle cells (PASMCs), PA endothelial cells, and adventitial fibroblasts (1). Available therapies offer only symptomatic improvements and do not cure PAH (2). Therefore, a better understanding of the pathogenesis of PAH and the identification of new selective therapeutic targets capable of suppressing abnormal growth of PA cells are desperately needed to improve clinical outcomes. Targeting the cancer-like pro-proliferative and apoptosis-resistant phenotype of PAH cells that underpins the pulmonary vascular remodeling represents an attractive strategy for tackling PAH (3). In this regard, apoptosis-based therapies have been tested for the treatment of PAH (4, 5). Although these antitumor drugs have been shown to reverse the thickening of pulmonary arterioles in various animal models, their clinical development has been hampered by their toxicity to normal cells (4, 5). Identifying and validating a specific biochemical feature that PAH-PASMCs possess and their normal counterparts lack is thus mandatory.
As cancer cells, PAH cells experience numerous cellular stresses (DNA damage stress, metabolic stress, oxidative stress, etc.) not experienced by normal cells, pushing PAH cells to develop cytoprotective responses (3). Mitochondrial dysfunction is implicated in the development of PAH (6). Indeed, to deal with these stressful conditions that jeopardize their survival, mitochondria of PAH-PASMCs have been documented to undergo enhanced fission (7), perinuclear clustering (8), membrane hyperpolarization, and metabolism rewiring to a survival mode with enhanced glycolysis over oxidation phosphorylation (OXPHOS), although the latter still contributes to energy production ensuring cell survival (9). In this context, acquired mitochondrial abnormalities play a pivotal role in coping with stress and preserving PAH cell survival (6, 10). Nevertheless, the molecular mechanisms regulating mitochondrial protection in PAH remain elusive.
HSP90 (heat shock protein 90) is an abundantly expressed cytosolic chaperone involved in folding and stability of proteins (11). To deal with stressful conditions leading to the accumulation of damaged proteins harmful for the cells, cancer cells exploit the HSP90 chaperone machinery to protect an array of overexpressed oncoproteins from misfolding and degradation, many of which are implicated in PAH development and progression (12).
In this regard, HSP90 inhibitors have emerged as promising drugs that can simultaneously disable more than one oncogenic mechanism of tumor development, progression, and maintenance. In addition to being highly expressed in the cytosol, HSP90 is also compartmentalized in mitochondria (mtHSP90) of tumor cells but not in normal cells (13–16). The selective recruitment of HSP90 in mitochondria of tumor cells is an adaptive mechanism to counteract proteotoxic stress, suppressing cell death and maintaining energy production (15, 16). Consistent with these findings, selective inhibition of mtHSP90 using Gamitrinib, a mitochondria-targeted HSP90 inhibitor, was associated with anticancer activity in preclinical models with no overt organ or systemic toxicity (14, 17, 18).
Although HSP90 was recently documented to be overexpressed in PAH-PASMCs (19, 20), its implication in PAH remains largely unexplored. The present study demonstrates that compartmentalized HSP90 plays a crucial role in PAH, regulating the cancer-like phenotype of PASMCs. Specifically, we showed that mtHSP90 controls interconnected stress responses that converge to ensure mitochondrial function and PAH-PASMC survival.
Methods
For full experimental details, including information on proliferation and apoptosis measurements, mitochondrial respiration and glycolysis, assessment of mitochondrial DNA (mtDNA) damage and content, subcellular fractionation and protein analyses, histology and immunofluorescence analyses, and immunogold electron microscopy, please see the online supplement.
Human Tissue Samples
Experimental procedures using human cells conformed to the principles outlined in the Declaration of Helsinki and were performed with the approval of Laval University and the Institut Universitaire de Cardiologie et de Pneumologie de Québec Biosafety and Ethics committees (CER#20273). Tissues were obtained from patients who had previously given signed informed consent. Healthy lung tissues (controls) were obtained during lung resection for tumors. Lung samples were taken at distance from the tumor and demonstrated normal lung parenchyma. All the PAH tissues were from lung explants from transplant or early (“warm”) autopsy. PAH diagnosis was previously confirmed by right heart catheterization (see Table E1 in the online supplement). PAH and control tissues were obtained from Respiratory Health Network tissue bank. PAH and control donors were matched for age and sex. Tissues were used in accordance with the recommendation described in Reference 21.
Animal Models
Experiments were performed according to the guidelines of the Canadian Council on Animal Care and approved by the institutional animal care committee. Male Sprague-Dawley rats weighing approximately 250 g were purchased from Charles River. Monocrotaline (MCT)-induced PAH was developed by a single subcutaneous injection of MCT (60 mg/kg; Sigma), as previously described (22, 23). Control rats received saline. Once PAH was established (2 wk after MCT injection), rats with pulmonary hypertension were randomly divided into two groups (five to nine animals per group) and treated with either the mitochondrial HSP90 inhibitor (Gamitrinib, 10 mg/kg/d as i.p. injections) or vehicle (DMSO) dissolved in 20% cremophor EL (Sigma) for 2 additional weeks. This dose was chosen based on prior experiments (14, 17). In the fawn-hooded rat (FHR) model, Gamitrinib or its vehicle was given (10 mg/kg i.p. with the schedule 1 day on/3 days off during 2 wk) to FHRs with echocardiographically proven PAH (48–50 wk of age). All rats underwent closed-chest right heart catheterization to assess right ventricular systolic pressure (RVSP), mean PA pressure (mPAP), right ventricular cardiac output (CO), and total pulmonary resistance, as previously described (20, 22, 24). All hemodynamic measurements and analyses were performed blinded to the condition.
Cell Culture
PAH-PASMCs (n = 12 cell lines) were isolated from small PAs (<1,000 μm diameter) from patients with PAH. Control PASMCs (n = 9 cells lines) were either purchased from Cell Application or isolated from patients without PAH as previously described (22, 24). Cells were used at passages five to nine for experiments
Statistical Analysis
Statistical analysis between two groups was performed using the unpaired Student’s t test. Statistical analysis for more than two groups was performed using one-way ANOVA followed by a Tukey-Kramer post hoc test. When same cells were used under different conditions, paired analyses were performed among treatments. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using Prism 6 (GraphPad).
Results
Accumulation of mtHSP90 Is a Cardinal Feature of PAH-PASMCs
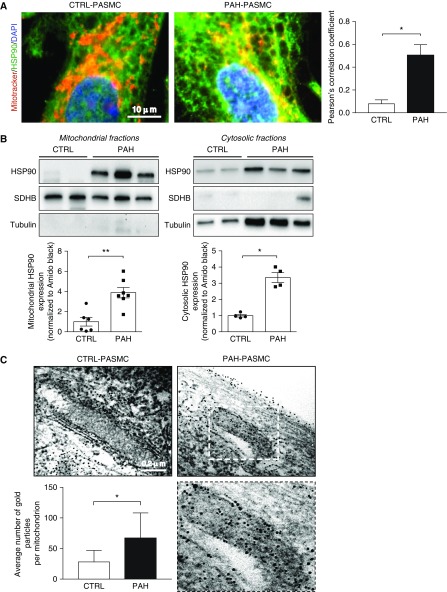
A pool of HSP90 is specifically found in mitochondria of tumor cells suppressing cell death and reprogramming energy metabolism (13, 15, 16). To determine whether this feature also exists in PAH-PASMCs under basal conditions, isolated control PASMCs and PAH-PASMCs were stained with MitoTracker Red and HSP90. Confocal microscopic observation revealed that, contrary to healthy PASMCs, HSP90 accumulates in PAH-PASMC mitochondria, as evidenced by the overlap between the two signals and quantification of Pearson’s correlation coefficient (Figure 1A). To confirm the accumulation of HSP90 inside the mitochondria, we next performed Western blots with proteins extracted from enriched mitochondrial and cytosolic fractions. Assessment of α-tubulin and succinate dehydrogenase complex, subunit B (SDHB) expression was used to validate the purity of the cytosolic and mitochondrial fractions, respectively. In addition to being increased in the cytosolic fraction of PAH-PASMCs, HSP90 was preferentially localized in the mitochondria of PAH-PASMCs compared with their normal counterparts (Figure 1B). To further validate these results, we performed immunogold electron microscopy. In agreement with biochemical results, we found that mitochondria from PAH-PASMCs exhibited more HSP90 labeling, as revealed by electron-dense beads, than did those from control PASMCs (Figure 1C). The accumulation of immunogold particles was detected inside mitochondria of PAH-PASMCs, whereas in control cells, immunogold particles were observed along the surface but rarely within mitochondria. Taken together, these findings clearly demonstrate that mitochondrial HSP90 accumulation is a hallmark of PAH-PASMCs.
Figure 1.
HSP90 preferentially accumulates in mitochondria of pulmonary arterial hypertension–pulmonary artery smooth muscle cells (PAH-PASMCs). (A) Representative images of HSP90 (green) localization in human control and PAH-PASMCs labeled with MitoTracker Red. Quantification of HSP90-MitoTracker Red colocalization is shown in the right panel. (B) Representative Western blotting analysis and corresponding densitometric analyses of mitochondrial and cytosolic fractions obtained from control (n = 4–6) and PAH-PASMC (n = 4–7) lysates and probed for HSP90. To assess fraction purity, antibodies for succinate dehydrogenase complex, subunit B and α-tubulin were used. (C) Representative images of control PASMCs and PAH-PASMCs immunogold labeled with an anti-HSP90 antibody and corresponding quantification of the average number of gold particles per mitochondria in control PASMCs and PAH-PASMCs. The lower right image is an enlargement of the area inside the dashed box in the upper right image. Data are presented as mean ± SEM in A and B or mean ± SD in C. *P < 0.05; **P < 0.01. CTRL = control; SDHB = succinate dehydrogenase complex, subunit B.
Stress Factors Stimulate HSP90 Accumulation within Mitochondria
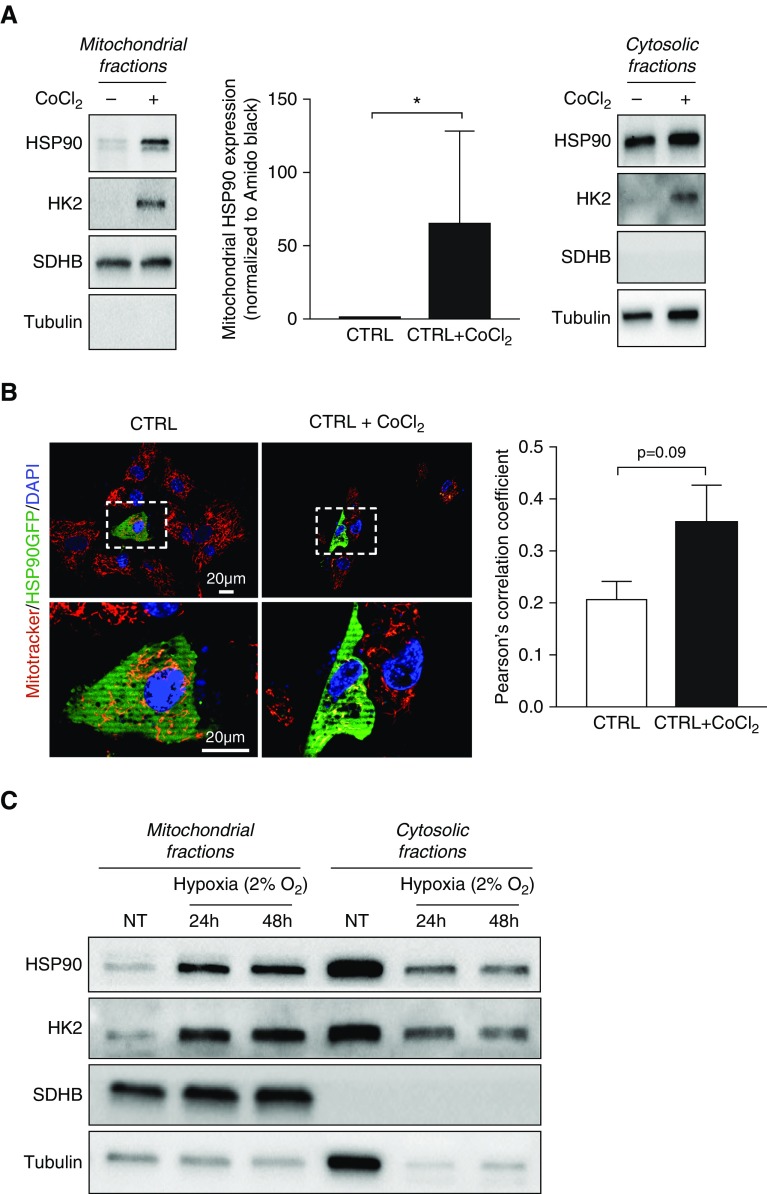
Because HSP90 orchestrates a stress response signaling and mtHSP90 exerts cytoprotection against stress-induced cellular damage and apoptosis in cancer cells (11), we hypothesized that PAH-relevant stress conditions induce a rapid accumulation of HSP90 in the mitochondria. Thus, we investigated whether hypoxia promotes the mitochondrial translocation of HSP90. To address this question, we first exposed, or not, control PASMCs to cobalt chloride (CoCl2), a well-known hypoxia-mimetic agent, for 2 hours. As assessed by immunoblots after subcellular fractionation and dual immunofluorescence microscopy using a plasmid expressing a green fluorescent protein–tagged HSP90, CoCl2 treatment resulted in the recruitment of Hexokinase 2 (HK2) to mitochondria, confirming the hypoxia mimetic effects in cells, and induced a robust mitochondrial translocation of HSP90, recapitulating the feature of PAH-PASMCs (Figures 2A and 2B). To validate this result, control PASMCs were next exposed to hypoxia (2% O2) for 24 or 48 hours, after which mitochondrial fractions were isolated. As observed with CoCl2, hypoxic stimulation triggered recruitment of HK2 to mitochondria as well as mtHSP90 accumulation (Figure 2C). We next studied the ability of mild oxidative damage to induce mitochondrial translocation of HSP90. To this end, control PASMCs were exposed to hydrogen peroxide for 30 minutes and then subjected to immunofluorescence. Similarly to that observed after hypoxic stimulation, oxidative damage promoted the mitochondrial translocation of HSP90 (Figure E1). In contrast, no recruitment of HSP90 to mitochondria was seen when control cells were exposed to pro-PAH growth factors and cytokines (Figure E1). Taken together, our results identified mitochondrial HSP90 translocation as a stress-inducible mechanism.
Figure 2.
Hypoxia induces mitochondrial HSP90 accumulation in control pulmonary artery smooth muscle cells (PASMCs). (A) Representative Western blotting analysis of mitochondrial and cytosolic fractions obtained from control PASMCs (n = 4) exposed or not to 500 μM cobalt chloride (CoCl2) for 2 hours and probed for HSP90. Data are presented as mean ± SEM; *P < 0.05. (B) Representative images of control human PASMCs transfected with a plasmid encoding a green fluorescent protein (GFP)-tagged HSP90 and stimulated or not with CoCl2 (500 μM) for 2 hours. Cells were labeled with MitoTracker Red. The left and right lower images are enlargements of the area inside the dashed boxes in the left and right upper images, respectively. Quantification of GFP-MitoTracker Red colocalization is shown in the right panel. Data are presented as mean ± SEM. (C) Western blotting analysis of mitochondrial and cytosolic fractions obtained from control PASMCs exposed or not to 2% O2 for 24 or 48 hours and probed for HSP90. Immunoblotting with SDHB and with α-tubulin was used to assess purity of the mitochondrial and cytoplasmic fractions, respectively. Hexokinase 2 (recruited to mitochondria under hypoxic stimulation) was used as a positive control of hypoxia exposure or CoCl2 treatment. CTRL = control; HK2 = hexokinase 2; NT = nontreated; SDHB = succinate dehydrogenase complex, subunit B.
Mitochondrial HSP90 Maintains mtDNA Integrity
Maintenance of mtDNA integrity and copy number is essential for cell function, as its impairment is associated with a range of conditions, including neurodegenerative diseases and cancer (25). Moreover, mitochondrial DNA, which encodes subunits of the oxidative phosphorylation complexes subunits essential for cellular respiration, is highly sensitive to oxidative damage, and mtDNA damage may compromise bioenergetic function and cell death if unresolved (25). To study the role of mtHSP90 in mtDNA integrity, Gamitrinib, a well-characterized HSP90 ATPase inhibitor engineered to selectively inhibit mtHSP90 without affecting the cytosolic functions of HSP90 (Figure E2), was used (14).
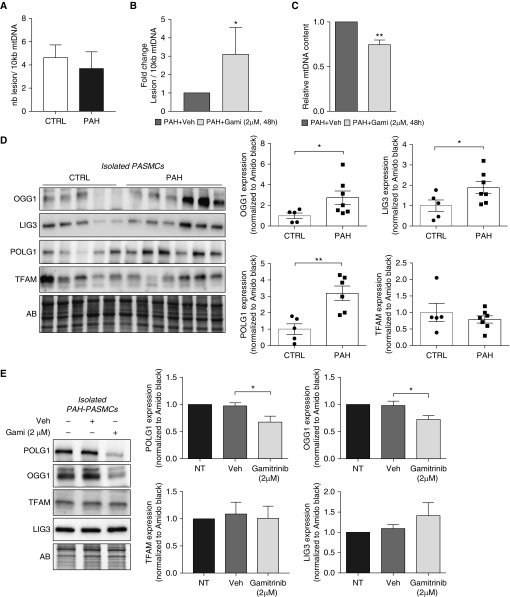
To test whether mtHSP90 inhibition induces mtDNA damage, we used a semi–long-run real-time PCR–based assay (26). Although great variability was observed between different cells, the frequency of mtDNA lesions per 10 kb, taken as a whole, was similar between control PASMCs and PAH-PASMCs at baseline (Figure 3A). In each PAH-PASMC cell line, Gamitrinib induced mtDNA damage, and no effect was found in control cells (Figure 3B and Figure E3). Similar results were observed using cytosolic 8-oxo-deoxyguanosine immunoreactivity (Figure E3), a marker commonly used for assessing oxidative mtDNA damage in mitochondria (27). Then, we asked whether inhibition of mtHSP90 affects mtDNA abundance, essential for maintaining cellular energy requirements. Gamitrinib-treated PAH-PASMCs showed a significant 25% reduction in mtDNA copy number compared with vehicle-treated PAH-PASMCs (Figure 3C), whereas no effect was detected in control PASMCs exposed to the inhibitor (Figure E3).
Figure 3.
Mitochondrial HSP90 controls mitochondrial DNA (mtDNA) integrity. (A) Quantification of baseline mtDNA damage per 10-kb DNA by a semi-long quantitative PCR amplification of total DNA isolated from control (n = 4) and pulmonary arterial hypertension–pulmonary artery smooth muscle cells (PAH-PASMCs) (n = 4). (B) Quantification of mtDNA lesions in PAH-PASMCs (n = 4) exposed to 2 μM Gamitrinib or its vehicle (DMSO) for 48 hours. (C) Effects of Gamitrinib on mtDNA copy number in PAH-PASMCs (n = 4). (D) Representative Western blots and corresponding densitometric analyses of OGG1 (8-oxoguanine DNA glycosylase-1), LIG3 (DNA ligase 3), POLG1 (polymerase γ), and TFAM (mitochondrial transcription factor A) expression in PASMCs isolated from control subjects (n = 5) and patients with PAH (n = 7). (E) Representative Western blots and corresponding densitometric analyses of POLG1, OGG1, TFAM, and LIG3 expression in PAH-PASMCs (n = 6) treated or not with Gamitrinib (2 μM) or its vehicle (DMSO) for 48 hours. Protein expression was normalized to Amido black (AB). Data are presented as mean ± SEM; *P < 0.05; **P < 0.01. CTRL = control; Gami = Gamitrinib; nb = number; NT = nontreated; Veh = vehicle.
These findings prompted us to examine whether mtHSP90 inhibition affects expression of key proteins implicated in mtDNA replication and repair (28). We found that POLG1 (DNA polymerase gamma), OGG1 (8-oxoguanine DNA glycosylase-1), and LIG3 (DNA ligase 3) were significantly overexpressed in PAH-PASMCs compared with control cells, underscoring a role of these proteins in the adaptive and protective mitochondrial response to stress (Figure 3D). By contrast, TFAM (mitochondrial transcription factor A) was unchanged. Moreover, we found that Gamitrinib treatment in PAH-PASMCs significantly reduces expression of POLG1 and OGG1, whereas no effect was observed for TFAM and LIG3 (Figure 3E). Together, these data support that mtHSP90 contributes to mtDNA quality control and homeostasis.
Mitochondrial Pools of HSP90 Regulate PAH-PASMC Bioenergetics
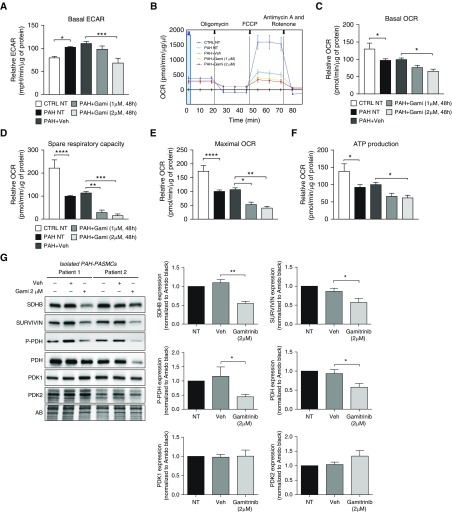
On the basis of the above results and published data demonstrating that PAH-PASMCs undergo metabolic reprogramming to sustain cell growth and proliferation (9), we speculated that mtHSP90 accumulation represents an adaptive mechanism to maintain cellular bioenergetics. To examine the role of mtHSP90 on PAH-PASMC mitochondrial metabolism, cells were treated with Gamitrinib before being subjected to bioenergetics measurements via Seahorse assay. As expected, we found that PAH-PASMCs displayed a significant increase in the glycolytic capacity (Warburg effect) compared with healthy PASMCs, as assessed by extracellular acidification rate. Exposure to Gamitrinib significantly reduced the glycolytic capacity of PAH-PASMCs after 48 hours (Figure 4A). In addition, we found that Gamitrinib diminished basal oxygen consumption rate (representative of the basal mitochondrial OXPHOS activity) and significantly reduced spare respiratory capacity (believed to be an important determinant in coping with cellular stress) in PAH-PASMCs (Figures 4B–4D). Gamitrinib also markedly inhibited maximal respiration and ATP production (Figures 4E and 4F). Collectively, these findings indicate that mtHSP90 maintains multiple sources of energy production in PAH-PASMCs. In contrast, extracellular acidification rate and oxygen consumption rate were not affected in PAH-PASMCs treated with a cytosolic HSP90 (cytHSP90) inhibitor, 17-AAG, known to not affect mtHSP90 (14, 16) (Figure E4). To gain insight into the effect of mtHSP90 inhibition on PAH-PASMC bioenergetics, we analyzed the expression level of Survivin and SDHB, two proteins stabilized by HSP90 and known to enhance cellular respiration as well as survival of tumor cells (29, 30). In agreement with published results conducted in cancer cells (30), we found that inhibition of mtHSP90 in PAH-PASMCs caused a robust diminution of their expression (Figure 4G) and that 17-AAG had no effect on SDHB expression (Figure E2). Furthermore, we found that Gamitrinib substantially reduced the expression of phosphorylated and total forms of pyruvate dehydrogenase (PDH), inhibition of which reduces the conversion of pyruvate to acetyl-CoA for entry into the tricarboxylic acid cycle and oxidative phosphorylation (Figure 4G). Gamitrinib had no effect on the level of PDH upstream kinases, pyruvate dehydrogenase kinases (PDK)-1 and 2 (Figure 4G).
Figure 4.
Mitochondrial pools of HSP90 regulate pulmonary arterial hypertension–pulmonary artery smooth muscle cell (PAH-PASMC) bioenergetics. (A) The cellular aerobic glycolysis was assessed by measures of the extracellular acidification rate from control and PAH-PASMCs treated or not with Gamitrinib or its vehicle (DMSO) using the Seahorse XF96 analyzer. (B) Effects of Gamitrinib on real-time mitochondrial oxygen consumption rate (OCR). Control PASMCs and PAH-PASMCs exposed or not to Gamitrinib or its vehicle (DMSO) for 48 hours were evaluated after sequential injection of the ATPase synthase inhibitor oligomycin (1 μM), uncoupler carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (5 μM), complex I inhibitor rotenone (1 μM), and complex III inhibitor antimycin A (1 μM). (C–F) Effects of Gamitrinib on (C) basal OCR, (D) spare respiratory capacity, (E) maximal OCR, and (F) ATP production. Experiments were performed in four control and four PAH-PASMC cell lines. (G) Representative Western blots and corresponding densitometric analyses of succinate dehydrogenase complex, subunit B, Survivin, phospho–pyruvate dehydrogenase, pyruvate dehydrogenase (PDH), and PDH kinases-1 and -2 expression in PAH-PASMCs (n = 6) treated or not with Gamitrinib (2 μM) or its vehicle (DMSO) for 48 hours. Protein expression was normalized to Amido black. Data are presented as mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. AB = Amido black; CTRL = control; ECAR = extracellular acidification rate; FCCP = carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone; Gami = Gamitrinib; NT = nontreated; PDK = pyruvate dehydrogenase kinase; P-PDH = phospho–pyruvate dehydrogenase; SDHB = succinate dehydrogenase complex, subunit B; Veh = vehicle.
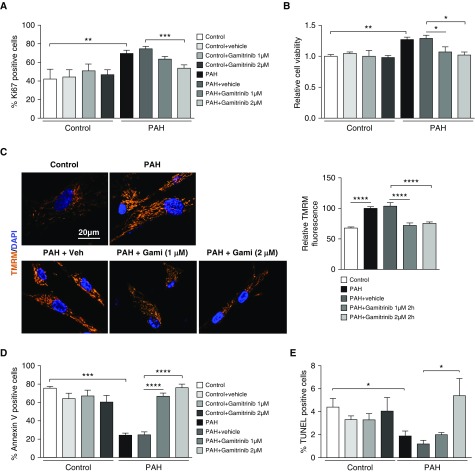
Selective mtHSP90 Inhibition Reduces PAH-PASMC Proliferation and Resistance to Apoptosis without Affecting Control PASMCs
Given that mtDNA integrity and mitochondrial bioenergetics are critical determinants of cell proliferation and survival, which are both impacted by mtHSP90 inhibition, we investigated whether the preferential accumulation of mtHSP90 in PAH-PASMCs can be exploited for therapeutic benefit in vitro to specifically correct the cancer-like phenotype of PAH-PASMCs. We demonstrated that selective inhibition of mtHSP90 using Gamitrinib reduced PAH-PASMC proliferation (as assessed by Ki67 labeling) and viability (as assessed by thiazolyl blue tetrazolium blue [MTT] assay) without affecting control cells, indirectly corroborating our previous results showing that HSP90 is not or is weakly present in mitochondria of control cells (Figures 5A and 5B). Mitochondrial membrane hyperpolarization is a hallmark of PAH-PASMCs contributing to apoptosis resistance (31, 32). Consistently, we found that mtHSP90 inhibition in PAH-PASMCs decreases mitochondrial tetramethylrhodamine, methyl ester (TMRM) fluorescence intensity indicative of mitochondrial depolarization (Figure 5C). Moreover, Gamitrinib treatment of PAH-PASMCs resulted in an increased proportion of cells positive for the apoptotic markers Annexin V and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) with no effect on control cells (Figure 5D and 5E), underscoring the disease specificity and the prosurvival function of mtHSP90 in PAH-PASMCs.
Figure 5.
Selective mitochondrial HSP90 inhibition reduces pulmonary arterial hypertension–pulmonary artery smooth muscle cell (PAH-PASMC) proliferation and resistance to apoptosis without affecting control PASMCs. (A) Proliferation (Ki67) was measured in control PASMCs and PAH-PASMCs treated or not with Gamitrinib or its vehicle (DMSO) for 48 hours. The graph shows the percentage of cells with positive nuclear staining. PAH-PASMCs exhibit a significantly greater proliferation rate than control cells. Gamitrinib reduces PAH-PASMC proliferation without affecting control cells. (B) Cell viability was examined by thiazolyl blue tetrazolium blue assay. (C) Representative images and corresponding quantitative analysis of mitochondrial membrane potential in cells exposed or not to Gamitrinib or its vehicle (DMSO) for 2 hours, detected by tetramethylrhodamine, methyl ester fluorescence. Gamitrinib depolarizes PAH-PASMC mitochondria. (D and E) Apoptosis was evaluated by Annexin V and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays. PAH-PASMCs are more resistant to starvation-induced apoptosis (0 and 1% fetal bovine serum) than control PASMCs. Gamitrinib induces apoptosis in PAH-PASMCs without affecting control cells. Experiments were performed in three control and four PAH-PASMC cell lines. Data are presented as mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Gami = Gamitrinib; TMRM = tetramethylrhodamine, methyl ester; Veh = vehicle.
In parallel, we wished to establish the importance of cytosolic HSP90 in the hyperproliferative and apoptosis-resistant phenotype of PAH-PASMCs. To this end, we treated control PASMCs and PAH-PASMCs with two non–mitochondrially targeted cytHSP90 inhibitors, 17-AAG or AT13387 (33, 34). We found that inhibition of cytHSP90 dose-dependently reduced the expression of numerous client proteins, such as IGF1R (insulin-like growth factor-1 receptor), AKT (protein kinase B), STAT3 (signal transducer and activator of transcription 3), and PIM (proviral integration site of Moloney murine leukemia virus)-1 (Figure E5) overexpressed in PAH-PASMCs (Figure E6) and critically involved in vascular remodeling (12). Cytosolic HSP90 inhibition rescued the pro-proliferative and apoptosis-resistant phenotype of PAH-PASMCs but fails to demonstrate disease specificity, as illustrated by a marked diminution in proliferation and enhanced apoptosis in control cells (Figure E7). These data indicate that cytHSP90 regulates the cell function of both control and disease cells and suggest that its inhibition in experimental PAH models would be associated with serious adverse effects. Collectively, our results indicate that Gamitrinib causes disease-specific mitochondria toxicity leading to suppressed proliferation and cell death.
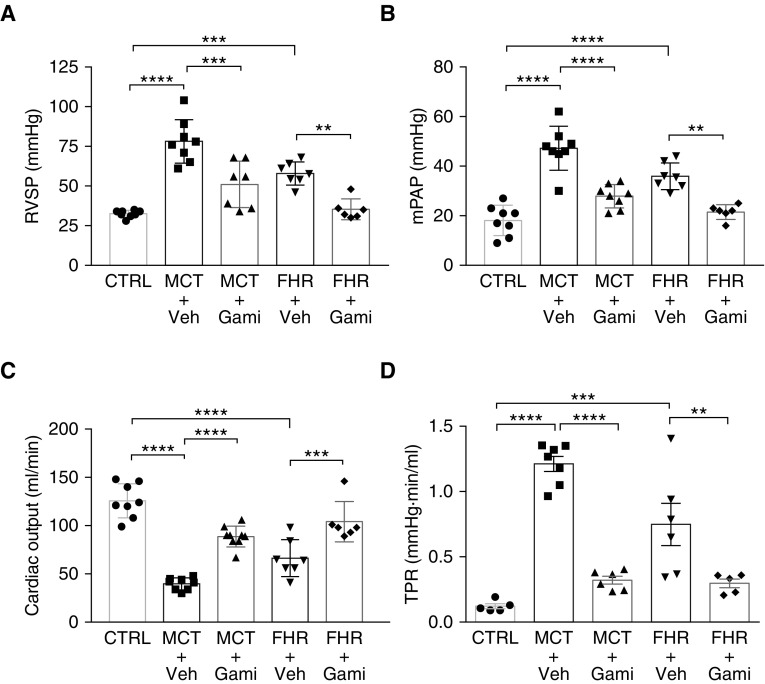
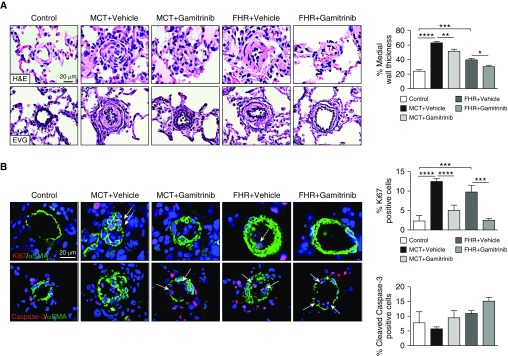
Targeted Inhibition of mtHSP90 Reverses PAH in Rats
On the basis of our results showing that HSP90 is preferentially expressed in mitochondria of PAH-PASMCs and mediates cell survival, we hypothesized that inhibition of mtHSP90 may provide a fresh and valuable avenue to tackle established PAH in vivo. We first evaluated whether Gamitrinib improves MCT-induced pulmonary hypertension in rats. Treatment with Gamitrinib from Day 14 to Day 28 after MCT injection resulted in a significant reduction in mPAP and RVSP compared with vehicle-treated rats (Figures 6A and 6B). In addition, Gamitrinib-treated rats exhibited an increased CO, leading to decreased total pulmonary resistance (Figures 6C and 6D). No signs of unwanted side effects were seen. To determine whether reduced pulmonary vascular remodeling of distal PAs accounts for improved mPAP in Gamitrinib-treated rats, hematoxylin and eosin and Elastica van Gieson staining were performed for morphometric analysis. As expected, pulmonary hypertension in MCT rats was associated with an increase in media wall thickness of distal PAs, which was significantly attenuated after Gamitrinib treatment (Figure 7A). We next examined whether Gamitrinib reverses proliferation and resistance to apoptosis of PASMCs. We found that, compared with control rats, the proportion of Ki67-positive cells in distal PAs was higher in the MCT rats. The regression of pulmonary vascular remodeling after mtHSP90 inhibition was associated with a marked diminution in the proportion of proliferative PASMCs and a trend for increased apoptosis (Figure 7B). As observed in vitro, Gamitrinib effects were associated with a reduced expression of OGG1, POLG1, SDHB, and Survivin (Figure E8). To further validate mtHSP90 inhibition as a therapeutic strategy in PAH, the FHR, which spontaneously develops pulmonary hypertension due to increased HIF-1α (hypoxia-inducible factor 1-α) activation, SOD2 (superoxide dismutase-2) downregulation, and associated mitochondrial-metabolic abnormalities, was used as a second model (35). Before initiating the treatment, establishment of PAH in rats aged 48 to 50 weeks was confirmed by Doppler echocardiography (data not shown). Because FHRs exhibited mild PAH, the schedule of Gamitrinib injection was adjusted accordingly. As observed in the MCT model, treatment with Gamitrinib improved hemodynamic parameters in FHRs, as indicated by a marked attenuation of mPAP and RVSP and a higher CO associated with a lower total pulmonary resistance (Figure 6). As in the MCT model, treatment with Gamitrinib reduced vascular remodeling as well as proliferation of distal PASMCs in the FHR, whereas PASMC apoptosis tended to increase at the end of the treatment protocol (Figure 7).
Figure 6.
Gamitrinib improves pulmonary hemodynamics in monocrotaline (MCT)-induced pulmonary arterial hypertension and fawn-hooded rats (FHRs). (A) Right ventricular systolic pressure, (B) mean pulmonary artery pressure, (C) cardiac output, and (D) total pulmonary resistance were measured in control, MCT + vehicle, MCT + Gamitrinib, FHR + vehicle, and FHR + Gamitrinib; n = 6 to 8 rats/group. Data are presented as mean ± SEM; **P < 0.01; ***P < 0.001; ****P < 0.0001. CTRL = control; Gami = Gamitrinib; mPAP = mean pulmonary artery pressure; RVSP = right ventricular systolic pressure; TPR = total pulmonary resistance; Veh = vehicle.
Figure 7.
Mitochondrial HSP90 inhibition using Gamitrinib improves vascular remodeling in monocrotaline (MCT)-induced PAH and fawn-hooded rats (FHRs). (A) Representative images of distal pulmonary vessels stained with hematoxylin and eosin or Elastica von Gieson and corresponding quantification of vascular remodeling. (B) Proliferation (Ki67) and apoptosis (cleaved Caspase-3) were evaluated in lungs of control, MCT + vehicle, MCT + Gamitrinib, FHR + vehicle, and FHR + Gamitrinib rats. Representative images of distal pulmonary vessels labeled with Ki67 (top) and cleaved Caspase-3 (bottom) in red. Vascular smooth muscle cells were labeled using α-smooth muscle actin staining (green). Graphs on the right represent the percentage of cells positive for Ki67 or cleaved Caspase-3 in distal pulmonary vessels. Arrows indicate positive cells. Data are presented as mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. αSMA = α-smooth muscle actin; EVG = Elastica von Gieson; H&E = hematoxylin and eosin; PAH = pulmonary arterial hypertension.
Discussion
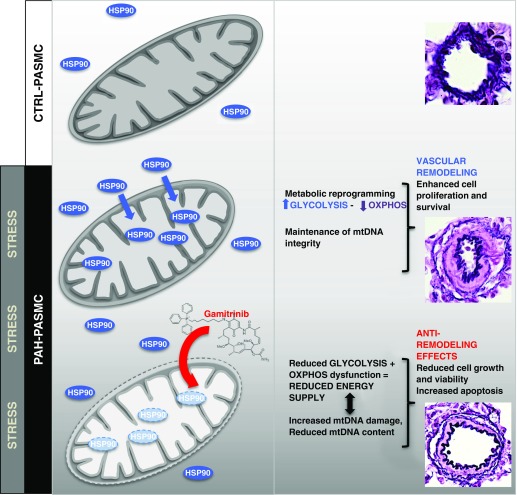
It was demonstrated that PAH-PASMCs exhibit a cancer-like metabolic-dependent pro-proliferative and antiapoptotic phenotype leading to the development of progressive pulmonary artery remodeling (9, 10). We showed for the first time that HSP90 is not only overexpressed in PAH-PASMCs (19) but also disease-specifically localized in distinct subcellular compartments, actively participating in many aspects of this abnormal phenotype (Figure 8). More importantly, we documented that inhibition of mtHSP90 reversed the PAH phenotype in vitro and in vivo in two clinically relevant PAH animal models.
Figure 8.
Proposed model depicting the molecular mechanisms by which mitochondrial HSP90 (mtHSP90) promotes pulmonary artery smooth muscle cell (PASMC) survival and vascular remodeling in pulmonary arterial hypertension (PAH). PAH-PASMCs exposed to stress conditions accumulate HSP90 in mitochondria to exert interconnected cytoprotective responses, including maintenance of mitochondrial DNA (mtDNA) integrity (mtDNA abundance and repair) and metabolism. Inhibition of the mitochondrial pool of mtHSP90 using Gamitrinib compromises adaptive mitochondrial bioenergetic function in PAH-PASMCs. Loss of bioenergetics capacity reduces cell growth and viability, leading to antiremodeling effects and significant improvement in cardiopulmonary hemodynamics. CTRL = control; OXPHOS = oxidation phosphorylation.
As in cancer cells, PAH cells including PAH-PASMCs exhibit a metabolic reprogramming often referred as the Warburg effect and characterized by enhanced glycolysis over OXPHOS to promote growth, survival, and proliferation (1, 9). As a consequence, restoration of oxidative glucose metabolism was proposed as a therapeutic avenue in PAH. Dichloroacetate, a specific inhibitor of PDK, was shown to improve established PAH in various models by stimulating OXPHOS at the expense of glycolysis (31, 36, 37). Nonetheless, although the balance between glycolysis and OXPHOS is shifted toward glycolysis in cancer and PAH cells, relationships between glycolysis and OXPHOS are cooperative rather than competitive, as OXPHOS still contributes to energy production in cancers (38, 39). Consistent with previous reports showing that HSP90 exists in a subcellular pool compartmentalized in the mitochondria of tumor cells, but not in most normal tissues in vivo (13, 15, 16), we evidenced that HSP90 resides specifically in mitochondria of PAH-PASMCs, maintaining energy production and survival.
Moreover, although our data demonstrate that hypoxia and oxidative damage promote mtHSP90 accumulation in PASMCs (Figure 2), further experiments are required to fully understand the molecular mechanisms for this translocation in pathological conditions. Not surprisingly, we and others (12, 24, 40) showed that cytosolic HSP90 stimulates cell growth by stabilizing key signaling proteins involved in PAH development and progression. Although our results support the fact that cytHSP90 inhibition can simultaneously disable more than one mechanism promoting the cancer-like phenotype of PAH-PASMCs and corroborate published data showing that cytHSP90 inhibition alleviates the progress of PAH in the MCT model (19), its in vitro inhibition in healthy cells was associated with toxic effects due to impairment of normal cell function and thus raising potential safety concerns. Furthermore, it is well established that cytHSP90 activity contributes to maintaining endothelial nitric oxide synthase (eNOS) expression (41, 42), and endothelial dysfunction commonly associated with eNOS uncoupling due to disruption of HSP90/eNOS interaction (41, 42) is believed to precede vascular remodeling in PAH. The lack of HSP90 expression in mitochondria of PAH–PA endothelial cells, and, accordingly, the absence of Gamitrinib effect on eNOS expression in these cells (Figure E9), indicates that mtHSP90 inhibition is likely to have minimal adverse effects on endothelial cells.
We also found that mtHSP90 inhibition using Gamitrinib results in a marked diminution of Survivin, SDHB, and PDH expression and subsequent impairment of OXPHOS (Figure 4). Moreover, Gamitrinib significantly depressed glycolysis (Figure 4). Our results are consistent with those obtained in cancer demonstrating that HSP90 associates with components of the permeability transition pore, notably Cyclophilin D, and antagonizes its function by promoting the mitochondrial recruitment of the glycolytic enzyme HK2 (15). In this context, HSP90 accumulation in mitochondria of PAH-PASMCs likely represents a general adaptive strategy used to ensure OXPHOS and glycolysis maintenance. In direct relation with its role in metabolism, we showed that mtHSP90 inhibition leads to POLG1 depletion known to carry out mtDNA replication (43). Because of limited capability for repair, the lack of protective histone, and its close proximity to the reactive oxygen species–generating electron transport chain, mtDNA is more vulnerable than nuclear DNA to stress-induced damage (25). Nonetheless, mitochondrial DNA integrity is crucial for mitochondrial homeostasis, and mtDNA depletion is usually associated with defective OXPHOS and increased susceptibility to stress (44). POLG1, OGG1, and LIG3 (all known as major factors governing mtDNA repair [28]) protein expressions are significantly increased in PAH-PASMCs compared with healthy cells and thus may represent a defense mechanism to cope with stress and to preserve mtDNA integrity, contributing to the established resistance of PAH-PASMCs against stress-induced apoptosis compared with normal cells. As such, the PAH-PASMC–specific effect of Gamitrinib-induced depletion of POLG1 and OGG1 may contribute to reduced PAH-PASMC survival by reducing both mtDNA content and repair capacity (Figure 3), thus explaining in part how Gamtrinib increases stress-induced apoptosis in PAH-PASMCs (Figure 5). This is consistent with the increase in apoptosis observed in POLG1 (45) or OGG1 (46) mutant mice. In addition to POLG1 and OGG1, we show that Gamitrinib represses SDHB and Survivin in PAH-PASMCs. Interestingly, both factors are critically implicated in cancer-cell survival, and their inhibition is associated with increased apoptosis (30). Survivin is an important prosurvival factor implicated in PAH-PASMC resistance to apoptosis and is a critical component of mitochondrial respiration, along with SDHB and PDH (30, 36, 47); thus, their reduced expression by Gamitrinib is likely implicated in PAH-PASMC apoptosis as well.
Limitations
Similarly to cytHSP90, the mitochondrial pool of mtHSP90 is an important signaling node stabilizing a wide range of proteins involved in cell proliferation and survival. Because of its pleiotropic effects on a variety of identified and as-yet-unidentified factors not exclusively expressed in the mitochondria, the precise mechanisms by which mtHSP90 inhibition exerts its mitochondriotoxic effect remain elusive. For instance, inhibition of mtHSP90 with Gamitrinib was reported to compromise expression of proteins associated with the mitoribosome, such as MRPLs (mitochondrial ribosomal large subunit proteins) and ERAL1 (era-like 12S mitochondrial ribosomal RNA chaperone 1) (16), and critical for mtDNA-encoded gene expression and maintenance of OXPHOS capacity (48). Although our study supports a role for POLG1, OGG1, PDH, SDHB, and Survivin in the PAH-PASMC Gamitrinib response, it is virtually impossible to appreciate the relative contributions of each factor to its antiproliferative and prodeath effects. In a therapeutic perspective, we demonstrated that targeted inhibition of mtHSP90 with Gamitrinib reversed pulmonary vascular remodeling and improved cardiac output in two PAH models without noticeable toxicity. However, our demonstration that hypoxia stimulates mtHSP90 accumulation in PASMCs suggests similar effects in other cell types and raised questions about the safety of Gamitrinib in hypoxic models. Indeed, these models may introduce a bias into experiments, favoring the accumulation of mtHSP90 in nonrelated PAH cell types and subsequently inducing cell death after Gamitrinib treatment. This possibility needs to be examined before clinical translation.
Taken together, our findings indicate that HSP90 orchestrates a stress response signaling in PAH-PASMCs contributing to adaptive mitochondrial plasticity. In particular, we identified and validated mtHSP90 accumulation as a hallmark of PAH-PASMCs and its pharmacological inhibition as a promising avenue to improve the clinical outcomes of patients with PAH. On the basis of similarities in pathogenic mechanisms and histological features between PAH and several disorders, including other types of pulmonary hypertension, in-stent restenosis, and atherosclerosis, it can be speculated that accumulation of mtHSP90 represents a common denominator of vascular remodeling diseases and thus a weakness to exploit.
Acknowledgments
Acknowledgment
The authors thank the Respiratory Health Network Tissue Bank as well as Drs. Pasquale Ferraro and Emmanuelle Brochiero from the Centre Hospitalier de l’Université de Montreal Research Centre for their help in obtaining PAH lung tissues.
Footnotes
S.B. holds a Canadian research chair. This work was supported by the Entelligence Young Investigator Award (O.B.), a Heart and Stroke Foundation of Canada grant (S.B.), and Canadian Institutes of Health Research grants (S.B. and S.P.).
Author Contributions: Conceptualization: O.B., R.P., S.P., and S.B.; investigation and validation: O.B., T.P., A.B., V.N., S.B.-B., S.B.-M., F.P., J.M., S.C., C.L., E.T., and G.S.; writing—original draft: O.B. and S.B.; writing—review and editing: O.B., D.C.A., G.S., E.D.M., R.P., S.P., and S.B.; funding acquisition: O.B., S.P., and S.B.; resources: Y.C.C., D.C.A., and G.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201708-1751OC on February 2, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 2.Lajoie AC, Lauzière G, Lega JC, Lacasse Y, Martin S, Simard S, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 3.Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ. 2017;7:285–299. doi: 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim YF, Wong CM, Pavlickova L, Liu L, Trasar L, Bansal G, et al. Mechanism of the susceptibility of remodeled pulmonary vessels to drug-induced cell killing. J Am Heart Assoc. 2014;3:e000520. doi: 10.1161/JAHA.113.000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Ibrahim YF, Das D, Zungu-Edmondson M, Shults NV, Suzuki YJ. Carfilzomib reverses pulmonary arterial hypertension. Cardiovasc Res. 2016;110:188–199. doi: 10.1093/cvr/cvw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang Y-H, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fessel JP, West JD. Redox biology in pulmonary arterial hypertension (2013 Grover Conference Series) Pulm Circ. 2015;5:599–609. doi: 10.1086/683814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 12.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, et al. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae YC, Caino MC, Lisanti S, Ghosh JC, Dohi T, Danial NN, et al. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell. 2012;22:331–344. doi: 10.1016/j.ccr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae YC, Angelin A, Lisanti S, Kossenkov AV, Speicher KD, Wang H, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat Commun. 2013;4:2139. doi: 10.1038/ncomms3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang BH, Siegelin MD, Plescia J, Raskett CM, Garlick DS, Dohi T, et al. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin Cancer Res. 2010;16:4779–4788. doi: 10.1158/1078-0432.CCR-10-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang BH, Tavecchio M, Goel HL, Hsieh CC, Garlick DS, Raskett CM, et al. Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease. Br J Cancer. 2011;104:629–634. doi: 10.1038/bjc.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GK, Li SH, Zhao ZM, Liu SX, Zhang GX, Yang F, et al. Inhibition of heat shock protein 90 improves pulmonary arteriole remodeling in pulmonary arterial hypertension. Oncotarget. 2016;7:54263–54273. doi: 10.18632/oncotarget.10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucherat O, Chabot S, Paulin R, Trinh I, Bourgeois A, Potus F, et al. HDAC6: a novel histone deacetylase implicated in pulmonary arterial hypertension. Sci Rep. 2017;7:4546. doi: 10.1038/s41598-017-04874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 23.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay È, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation. 2015;132:932–943. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 24.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 25.Kim HR, Won SJ, Fabian C, Kang MG, Szardenings M, Shin MG. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann Lab Med. 2015;35:1–14. doi: 10.3343/alm.2015.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothfuss O, Gasser T, Patenge N. Analysis of differential DNA damage in the mitochondrial genome employing a semi-long run real-time PCR approach. Nucleic Acids Res. 2010;38:e24. doi: 10.1093/nar/gkp1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 28.Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 29.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivadeneira DB, Caino MC, Seo JH, Angelin A, Wallace DC, Languino LR, et al. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Sci Signal. 2015;8:ra80. doi: 10.1126/scisignal.aab1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solit DB, Zheng FF, Drobnjak M, Münster PN, Higgins B, Verbel D, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- 34.Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, et al. Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-dihydroisoindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem. 2010;53:5956–5969. doi: 10.1021/jm100060b. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 36.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 37.Guignabert C, Tu L, Izikki M, Dewachter L, Zadigue P, Humbert M, et al. Dichloroacetate treatment partially regresses established pulmonary hypertension in mice with SM22alpha-targeted overexpression of the serotonin transporter. FASEB J. 2009;23:4135–4147. doi: 10.1096/fj.09-131664. [DOI] [PubMed] [Google Scholar]
- 38.Caino MC, Altieri DC. Molecular pathways: mitochondrial reprogramming in tumor progression and therapy. Clin Cancer Res. 2016;22:540–545. doi: 10.1158/1078-0432.CCR-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janiszewska M, Suvà ML, Riggi N, Houtkooper RH, Auwerx J, Clément-Schatlo V, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulin R, Courboulin A, Barrier M, Bonnet S. From oncoproteins/tumor suppressors to microRNAs, the newest therapeutic targets for pulmonary arterial hypertension. J Mol Med (Berl) 2011;89:1089–1101. doi: 10.1007/s00109-011-0788-5. [DOI] [PubMed] [Google Scholar]
- 41.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, et al. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–L1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- 42.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lestienne P. Evidence for a direct role of the DNA polymerase gamma in the replication of the human mitochondrial DNA in vitro. Biochem Biophys Res Commun. 1987;146:1146–1153. doi: 10.1016/0006-291x(87)90767-4. [DOI] [PubMed] [Google Scholar]
- 44.Singh KK, Ayyasamy V, Owens KM, Koul MS, Vujcic M. Mutations in mitochondrial DNA polymerase-gamma promote breast tumorigenesis. J Hum Genet. 2009;54:516–524. doi: 10.1038/jhg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 46.Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, et al. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J Biol Chem. 2014;289:6165–6176. doi: 10.1074/jbc.M113.515130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo JH, Skinner JP, Bird MJ, Formosa LE, Zhang JG, Kluck RM, et al. A role for the mitochondrial protein Mrpl44 in maintaining OXPHOS capacity. PLoS One. 2015;10:e0134326. doi: 10.1371/journal.pone.0134326. [DOI] [PMC free article] [PubMed] [Google Scholar]