Abstract
Glycosylation is a common modification found on numerous proteins and lipids. However, direct detection of glycans on these intact biomolecules has been challenge. Here, utilizing enzymatic incorporation of fluorophore-conjugated sialic acids, dubbed as direct fluorescent glycan labeling, we report the labeling and detection of N- and O-glycans on glycoproteins. The method allows detection of specific glycans without the laborious gel blotting and chemiluminescence reactions used in Western blotting. The method can also be used with a variety of fluorescent dyes.
Keywords: enzymatic labeling, fluorescent labeling, glycan labeling, sialic acid, sialyltransferase
Introduction
Glycosylation is an important posttranslational modification found on most proteins expressed by mammalian cells. Common glycans include N-glycans that are linked to asparagine residues (Stanley et al. 2015) and O-glycans that are linked to serine/threonine residues (Brockhausen and Stanley 2015). Frequently, these glycan structures terminate in sialic acids (Varki et al. 2015).
Sialylation is catalyzed by multiple sialyltransferases (Harduin-Lepers et al. 2005). N-glycan sialylation typically occurs on Gal residues and is mediated by the N-glycan-specific α-2,6-sialyltransferase 1 (ST6Gal1) (Weinstein et al. 1987) and α-2,3-sialyltransferase 4 (ST3Gal4) (Mereiter et al. 2016). O-glycans can also be sialylated on Gal residues by O-glycan-specific α-2,3-sialyltransferase 1 and 2 (ST3Gal1 and ST3Gal2) and on O-GalNAc residues by a family of α-N-acetylgalactosaminide α-2,6-sialyltransferases (ST6GalNAc) (Kitagawa and Paulson 1994; Sewell et al. 2006; Ju et al. 2008). Among all ST6GalNAcs, ST6GalNAc4 is strictly active on sialylated T antigen and is responsible for disialylated T antigen expression (Harduin-Lepers et al. 2000).
Despite the abundance of N- and O-glycans and their important biological functions, research on these glycans has been hampered by the lack of high affinity (Ambrosi et al. 2005) and specific binding reagents (Geisler and Jarvis 2011; Sterner et al. 2016). The emergence of click chemistry (Kolb et al. 2001) has provided a new avenue for glycan labeling (Hsu et al. 2007; Codelli et al. 2008). Subsequently, using enzymatic incorporation of clickable monosaccharides, specific glycan labeling became feasible (Chaubard et al. 2012; Mbua et al. 2013; Wu et al. 2018). More recently, glycan labeling via direct incorporation of biotinylated sialic acids using ST6Gal1 (Capicciotti et al. 2017) and ST6GalNAc4 (Wen et al. 2018) have been reported. Here, we describe a new method for specific glycan labeling we have named direct fluorescent glycan labeling (DFGL), in which fluorophore-conjugated sialic acids are directly attached to target glycans via specific sialyltransferases (Figure 1).
Fig. 1.
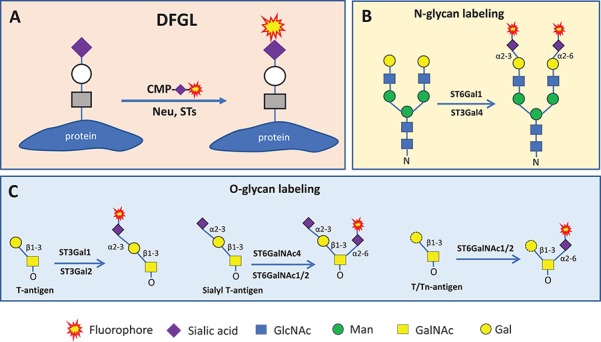
Method of DFGL (A) and labeling reaction on N-glycans (B) and O-glycans (C). In all cases, existing terminal sialic acids can be removed by neuraminidase treatment and replaced with fluorophore-conjugated sialic acids. Neu means neuraminidase; STs, sialyltransferases; CMP, cytidine monophosphate. Monosaccharide symbols follow the Symbol Nomenclature for Glycans system (PMID 26543186, Glycobiology 25: 1323–1324, 2015) details at NCBI.
Results
Three fluorophore-conjugated cytidine monophosphate (CMP)-sialic acids were synthesized by incubating CMP-c5-azido-sialic acid (CMP-N3-SA) and Alexa Fluor® 555-alkyne, Alexa Fluor® 488-alkyne or Cy5-alkyne via copper (I)-catalyzed azide-alkyne cycloaddition (Rostovtsev et al. 2002). The conjugated CMP-sialic acids were then applied to label the glycans on fetal bovine fetuin and asialofetuin using various sialyltransferases, including core-1 O-glycan-specific ST3Gal1 and ST3Gal2, N-glycan-specific ST3Gal4 and ST6Gal1, and O-GalNAc-specific ST6GalNAc1, ST6GalNAc2 and ST6GalNAc4 (Supplementary Table 1). Fetal bovine fetuin is known to contain both N- and O-glycans (Baenziger and Fiete 1979) and has historically been used as a model glycoprotein. The labeled reactions were separated by Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and directly imaged with a traditional protein gel imager with trichloroethanol (TCE) staining and a fluorescent gel imager (Figure 2).
Fig. 2.
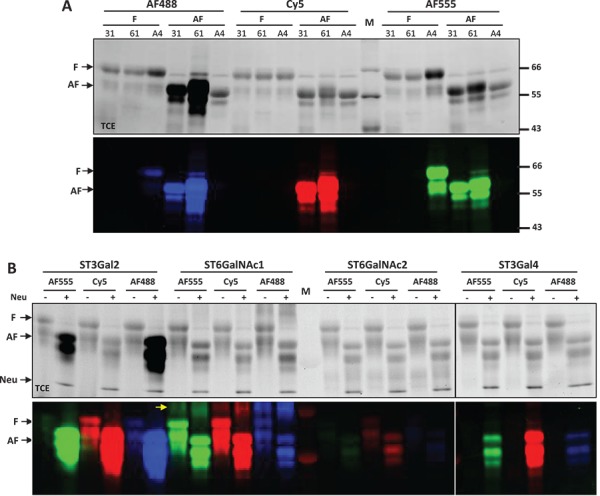
DFGL on fetal bovine fetuin and asialofetuin. All labeled samples were separated on SDS-PAGE and imaged by both TCE staining (top panels) and fluorescent imaging (lower panels). (A) Fetal bovine fetuin (F) and asialofetuin (AF) were labeled by ST3Gal1 (31), ST6Gal1 (61) and ST6GalNAc4 (A4) with Alexa-Fluor®488 (AF488), Cy5 and Alexa-Fluor®555 (AF555). (B) Labeling of fetuin and asialofetuin samples by ST3Gal2, ST6GalNAc1, ST6GalNAc2 and ST3Gal4 with AF555, Cy5 and AF488. Asialofetuin in (A) was purchased from Sigma Aldrich. Asialofetuin in (B) was generated from fetuin by addition of C.p neuraminidase (Neu) to the labeling reactions. ST6GalNAc1 exhibited self-labeling [indicated with arrow in (B)]. Same amount of protein (2.5 μg) was loaded into each lane; however, due to the presence of multiple benzene rings, Alexa Fluor® fluorophore-labeled samples exhibit significantly increased band intensities in TCE images. M represents molecular marker.
Our results indicate that ST3Gal1, ST6Gal1 and ST3Gal4 only labeled asialofetuin; ST3Gal2 not only primarily labeled asialofetuin but also weakly labeled fetuin; ST6GalNAc4 only labeled fetuin; ST6GalNAc1 and ST6GalNAc2 labeled both fetuin and asialofetuin (Figure 2A and B). These results demonstrated that fetal bovine fetuin contains both N- and O-glycans. The strict labeling on asialofetuin by ST6Gal1 and ST3Gal4 also suggests that N-glycans on fetuin are normally fully sialylated. Labeling by ST6GalNAc1, ST6GalNAc2 and ST6GalNAc4 indicated that O-GalNAc residues on fetuin are not fully sialylated. While ST3Gal1 and ST3Gal2 primarily labeled asialofetuin, ST3Gal2 showed some weak labeling on fetuin (Figure 2B), suggesting that the Gal residues on core-1 O-glycan are largely sialylated, and that ST3Gal1 and ST3Gal2 may have slightly different substrate specificities. In addition, the incorporation of Alexa Fluor® fluorophores greatly increased band intensities in TCE images, which is likely due to the presence of multiple benzene rings in these dyes.
Although some of the sialyltransferases tolerated the three fluorophores equally well, some enzymes showed a preference. For example, ST6GalNAc4 showed strong preference for Alexa Fluor® 555 over Cy5, while ST6GalNAc2 showed preference for Cy5 over Alexa Fluor® 488 (Figure 2). The tolerances of the three fluorophores by these sialyltransferases are summarized in Supplementary Table 1.
As a further demonstration of the specificity of labeling by these sialyltransferases, we labeled some representative mucins and integrins with Cy5 using O-glycan-specific ST3Gal1 and ST6GalNAc1 and N-glycan-specific ST6Gal1 and ST3Gal4. Mucins are known to be abundant in O-glycans (Tran and Ten Hagen 2013), and integrins are known to be abundant in N-glycans (Gu and Taniguchi 2004). MUC16 in particular contains both N- and O-glycans (Taniguchi et al. 2017). Indeed, it was found that MUC1 was strictly labeled by ST3Gal1 and ST6GalNAc1; all integrins were strictly labeled by ST6Gal1 and ST3Gal4; and MUC16 was labeled by all four enzymes (Supplementary Figure 1).
Furthermore, we tested the sensitivity of the labeling regarding both the donor and acceptor substrates and the enzymes themselves. It was found that the lower limits for detection was achieved at micromolar level of CMP-Cy5-SA (Supplementary Figure 2), submicrogram levels of fetuin (Supplementary Figure 3), nanogram levels of MUC1 (Supplementary Figure 4) and submicrogram levels of labeling enzymes (Supplementary Figure 5).
It is also interesting to note that resialylation with fluorophore-conjugated sialic acids did not obviously reduce the mobility of the target protein in SDS-PAGE (Figure 2 and Supplementary Figures 2 and 5). This phenomenon might be explained by the presence of multiple negative charges carried by these fluorophores. The net increase of a protein’s negative charge may result in increased mobility in SDS-PAGE, counteracting the mobility reduction due to the increased molecular mass.
Summary
In this report, we describe the specific labeling of N- and O-glycans using the technique DFGL. Compared to the methods of glycan labeling with azido-sugars previously (Wu et al. 2015, 2016), DFGL has the following advantages:
The method is much more convenient as it involves only a single enzymatic reaction step and allows direct imaging of separated samples on an SDS gel without time-consuming membrane transfer and the chemiluminescence reaction required by Western blotting.
The method has eliminated all side effects caused by click chemistry reagents, such as oxidative cleavage of target proteins by copper ions and nonspecific click reaction, by removal of these reagents before labeling reaction.
Since fluorophores are specifically introduced by enzymatic reactions, the method has virtually eliminated all nonspecific background staining.
In summary, DFGL is a highly sensitive, convenient and user-friendly tool for specific glycan labeling and detection.
Material and methods
CMP-azido-sialic acid, recombinant human ST3Gal1, ST3Gal2, ST3Gal4, ST6Gal1, ST6GalNAc1, ST6GalNAc4, MUC1, MUC16, integrin α1β1, α3β1, α5β1, αVβ3 and Clostridium perfringens Neuraminidase were from R&D Systems (Minneapolis, MN), Bio-Techne. Alexa Fluor® 488 alkyne and Alexa Fluor® 555 alkyne were from Thermo Fisher Scientific (Waltham, MA). Cy5 alkyne, ascorbic acid, fetal bovine fetuin and asialofetuin were from Sigma-Aldrich (St. Louis, MO).
Preparation of fluorophore-conjugated CMP-sialic acid
Fluorophore-conjugated CMP-sialic acids were prepared by incubating equivalent CMP-azido-sialic acid (CMP-N3-SA) and an alkyne-conjugated fluorophore via copper (I)-catalyzed azide-alkyne cycloaddition. In a typical reaction, 5 mM of CMP-N3-SA was mixed with 5 mM of Cy5-alkyne in the presence of 0.1 mM of Cu2+ and 1 mM of ascorbic acid, and the mix was kept at room temperature for 2 h. Final products were purified on a HiTrap® Q HP (GE Healthcare) column, eluted with a 0–100% gradient of NaCl elution buffer (300 NaCl, 25 mM Tris at pH 7.5) and concentrated to >0.1 mM by a speed-vacuum concentrator.
Direct fluorescent glycan labeling
For a typical labeling reaction, 1–5 μg target protein was mixed with 0.2 nmol fluorophore-conjugated CMP-SA, 0.2 μg of a sialyltransferase in a 30 μL buffer of 25 mM Tris pH 7.5, 10 mM MnCl2 and then incubated at 37oC for 30 min. In the case that the preexisting sialic acid of a glycoprotein needed to be removed, 0.1 μg of recombinant C. perfringens Neuraminidase was also added into the reaction. The neuraminidase showed no activity on fluorophore-conjugated sialic acids and was not removed in most cases. The reaction was then separated on an SDS-PAGE and imaged by a traditional protein imaging station via TCE staining and a fluorescent imager (FluorChem M, ProteinSimple of Bio-Techne, San Jose, CA).
Equipment and settings
For taking fluorescent image of an SDS gels using FluorChem M, RGB (red, green and blue) multiple fluorescent channel or single fluorescent channel was selected based on the incorporated fluorescent dyes, and the exposure time was set at Auto.
Supplementary Material
Acknowledgments
We want to acknowledge numerous colleagues who have made contributions to this manuscript through product development, quality control and assurance, and product support.
Abbreviations
- CMP
cytidine monophosphate
- CMP-N3-SA
CMP-azido-sialic acid
- DFGL
direct fluorescent glycan labeling
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TCE
trichloroethanol
Funding
Bio-Techne.
Conflict of interest statement
None declared.
References
- Ambrosi M, Cameron NR, Davis BG. 2005. Lectins: Tools for the molecular understanding of the glycocode. Org Biomol Chem. 3:1593–1608. [DOI] [PubMed] [Google Scholar]
- Baenziger JU, Fiete D. 1979. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 254:789–795. [PubMed] [Google Scholar]
- Brockhausen I, Stanley P. 2015. O-GalNAc Glycans In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH et al., editors. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, p. 113–123. [Google Scholar]
- Capicciotti CJ, Zong C, Sheikh MO, Sun T, Wells L, Boons GJ. 2017. Cell-surface glyco-engineering by exogenous enzymatic transfer using a bifunctional CMP-Neu5Ac derivative. J Am Chem Soc. 139:13342–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubard JL, Krishnamurthy C, Yi W, Smith DF, Hsieh-Wilson LC. 2012. Chemoenzymatic probes for detecting and imaging fucose-alpha(1-2)-galactose glycan biomarkers. J Am Chem Soc. 134:4489–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. 2008. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J Am Chem Soc. 130:11486–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. 2011. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 21:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Taniguchi N. 2004. Regulation of integrin functions by N-glycans. Glycoconj J. 21:9–15. [DOI] [PubMed] [Google Scholar]
- Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. 2005. The animal sialyltransferases and sialyltransferase-related genes: A phylogenetic approach. Glycobiology. 15:805–817. [DOI] [PubMed] [Google Scholar]
- Harduin-Lepers A, Stokes DC, Steelant WF, Samyn-Petit B, Krzewinski-Recchi MA, Vallejo-Ruiz V, Zanetta JP, Auge C, Delannoy P. 2000. Cloning, expression and gene organization of a human Neu5Ac alpha 2-3Gal beta 1-3GalNAc alpha 2,6-sialyltransferase: hST6GalNAcIV. Biochem J. 352:37–48. [PMC free article] [PubMed] [Google Scholar]
- Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH. 2007. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci U S A. 104:2614–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE et al. . 2008. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68:1636–1646. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Paulson JC. 1994. Cloning of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 269:1394–1401. [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. 2001. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 40:2004–2021. [DOI] [PubMed] [Google Scholar]
- Mbua NE, Li X, Flanagan-Steet HR, Meng L, Aoki K, Moremen KW, Wolfert MA, Steet R, Boons GJ. 2013. Selective exo-enzymatic labeling of N-glycans on the surface of living cells by recombinant ST6Gal I. Angew Chem Int Ed Engl. 52:13012–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereiter S, Magalhaes A, Adamczyk B, Jin C, Almeida A, Drici L, Ibanez-Vea M, Gomes C, Ferreira JA, Afonso LP et al. . 2016. Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochim Biophys Acta. 1860:1795–1808. [DOI] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. 2002. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 41:2596–2599. [DOI] [PubMed] [Google Scholar]
- Sewell R, Backstrom M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, Gatgens J, Clausen H, Hansson GC, Burchell J et al. . 2006. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 281:3586–3594. [DOI] [PubMed] [Google Scholar]
- Stanley P, Taniguchi N, Aebi M. 2015. N-Glycans In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH et al., editors. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, p. 99–111. [PubMed] [Google Scholar]
- Sterner E, Flanagan N, Gildersleeve JC. 2016. Perspectives on anti-glycan antibodies gleaned from development of a community resource database. ACS Chem Biol. 11:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, Mauris J, Argueso P. 2017. N-glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem. 292:11079–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DT, Ten Hagen KG. 2013. Mucin-type O-glycosylation during development. J Biol Chem. 288:6921–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schnaar RL, Schauer R. 2015. Sialic acids and other nonulosonic acids In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH et al., editors. Essentials of glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, p. 179–195. [PubMed] [Google Scholar]
- Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. 1987. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 262:17735–17743. [PubMed] [Google Scholar]
- Wen L, Liu D, Zheng Y, Huang K, Cao X, Song J, Wang PG. 2018. A one-step chemoenzymatic labeling strategy for probing sialylated Thomsen–Friedenreich antigen. ACS Cent Sci. 4:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZL, Huang X, Burton AJ, Swift KA. 2015. Glycoprotein labeling with click chemistry (GLCC) and carbohydrate detection. Carbohydr Res. 412:1–6. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Huang X, Burton AJ, Swift KA. 2016. Probing sialoglycans on fetal bovine fetuin with azido-sugars using glycosyltransferases. Glycobiology. 26:329–334. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Person AD, Anderson M, Burroughs B, Tatge T, Khatri K, Zou Y, Wang L, Geders T, Zaia J et al. . 2018. Imaging specific cellular glycan structures using glycosyltransferases via click chemistry. Glycobiology. 28:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


