Abstract
Background: Obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) are common conditions; the co-occurrence of these diseases, called the overlap syndrome (OVS), has been associated with poor health outcomes.
Purpose: The purpose of this Official American Thoracic Society Research Statement is to describe pathophysiology, epidemiology, outcomes, diagnostic metrics, and treatment of OVS, as well as to identify important gaps in knowledge and make recommendations for future research.
Methods: Clinicians and researchers with expertise in sleep medicine, pulmonary medicine, or both were invited to participate. Topics were divided among the participants according to their interest and expertise. A literature search was conducted; the search was not a formal systematic review. Evidence was considered and supplemented with the panelists’ nonsystematic clinical observations. Important knowledge gaps were identified.
Results: Recommendations for research to fill existing knowledge gaps were made. The recommendations were formulated by discussion and consensus.
Conclusions: Many important questions about OVS exist. This American Thoracic Society Research Statement highlights the types of research that leading clinicians and researchers believe will have the greatest impact on better understanding the spectrum of disease, improving diagnosis, and optimizing therapy.
Keywords: lung, sleep, COPD, hypoxia, apnea
Contents
Overview
Key Conclusions
Recommendations
Introduction
Methods
Significance of SDB in COPD
Pathophysiology
Diagnosis
Epidemiology
Outcomes
Appraisal of Existing Metrics
Is the AHI Useful in Patients with COPD?
Is “Sleep Apnea” the Appropriate Term in Patients with COPD?
Is REM-related Oxygen Desaturation Predictive of Poor Outcomes in Patients with COPD?
Is Capnometry Valuable in Sleep Studies of Patients with COPD?
Should Supplemental Oxygen Be Withdrawn for Overnight Polysomnography in Patients with Suspected OVS?
Revisiting the Sleep Study
Polysomnographic Features of SDB in COPD
SDB in COPD
Sample Cases
Conclusions
Overview
The overlap syndrome (OVS) is the co-occurrence of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA). This statement summarizes the existing literature, identifies knowledge gaps, and provides guidance regarding future priorities in research for sleep-disordered breathing (SDB) in patients with COPD and other lung diseases.
Key Conclusions
-
•
OVS is common because COPD and OSA are common. There is no definitive evidence that patients with COPD are at increased risk for developing OSA.
-
•
Mortality associated with OVS is higher than mortality associated with either COPD alone or OSA alone. Other outcomes are likely worse too but have not been adequately measured.
-
•
The optimal criteria for confirming OSA in patients with COPD are unclear. The usual criteria for OSA rely on counting apneas and hypopneas, but the predictive values of these respiratory events are unclear in COPD and, therefore, might lead to missed diagnoses.
Recommendations
-
•
We recommend investigations that seek to understand mechanisms underlying impaired respiratory mechanics during sleep and how this understanding might guide therapy to prevent complications.
-
•
We recommend studies that improve our understanding of gas exchange impairment during sleep in COPD.
-
•
We recommend studies that investigate whether there is a direct correlation between the prevalence of OSA and the severity of COPD.
-
•
We recommend observational studies that compare clinical outcomes among patients with OVS to clinical outcomes among patients with OSA alone or COPD alone.
-
•
We recommend randomized trials that compare clinical outcomes among patients with OVS whose OSA is treated to clinical outcomes among patients with OVS whose OSA is untreated.
-
•
We recommend studies that compare the apnea–hypopnea index with other measures of OSA severity (e.g., duration of hypoxemia, nadir of hypoxemia, and area under the oxygen desaturation curve) as predictors of clinical outcomes in patients with COPD.
-
•
We recommend that future research evaluate sleep-disordered breathing (i.e., central sleep apnea, OSA, increased upper airway resistance, hypoventilation, sleep fragmentation, etc.), rather than OSA specifically, in patients with COPD.
-
•
We recommend studies that seek to determine the pathophysiology of oxygen desaturation during REM sleep in patients with COPD.
-
•
We recommend trials that compare bilevel positive airway pressure to both continuous positive airway pressure and no positive airway pressure therapy in patients with COPD who desaturate during REM sleep.
-
•
We recommend trials that compare various methods of measuring gas exchange in patients with OVS. As examples, trials that compare pulse oximetry plus transcutaneous CO2 measurement to pulse oximetry alone, pulse oximetry plus transcutaneous CO2 measurement to arterial blood gas measurement, and pulse oximetry plus transcutaneous CO2 measurement to pulse oximetry plus end-tidal CO2 measurement.
-
•
We recommend trials that compare supplemental oxygen to both continuous positive airway pressure therapy and bilevel positive airway pressure in patients with COPD who exhibit nocturnal oxygen desaturation.
-
•
We recommend research to develop devices that can identify and characterize sleep without EEG. The goal is to improve the assessment of the severity of sleep-disordered breathing in patients with disruptions in sleep architecture.
-
•
We recommend studies that compare various methods to assess and quantify ventilation, the severity of inspiratory and expiratory flow limitation, and the presence of hyperinflation during sleep (e.g., pneumotachography, nasal pressure signaling, and flow sensors) in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major source of morbidity, mortality, and healthcare costs. It is increasing in epidemic proportions because of the aging population, indoor and outdoor air pollution, historical smoking trends, and other factors (1). Efforts to slow the progression of COPD have failed in the majority of patients, and research seeks to identify new therapeutic approaches to improve COPD-related outcomes. Like COPD, obstructive sleep apnea (OSA) has a high prevalence and is associated with adverse health consequences (2–4). Patients with both OSA and COPD are considered to have the overlap syndrome (OVS), which is associated with particularly poor outcomes, including high mortality (5, 6). This observation has led to an increased focus on sleep as an important factor in the care of the patient with COPD.
There exist many important questions about sleep-disordered breathing (SDB) in COPD. Is the pathophysiology of SDB unique/special in COPD? How prevalent is SDB in COPD? Does SDB affect clinical outcomes in COPD? Should different metrics be used to identify SDB in COPD? Are there polysomnographic characteristics of SDB that are unique in COPD? How should positive airway pressure therapy be used to treat SDB in COPD, given the conflicting evidence (7–10)? How should supplemental oxygen be used in patients with SDB and COPD, given the uncertain benefits in patients with COPD and either exertional or nocturnal hypoxemia (11)?
The purpose of this American Thoracic Society research statement is to summarize existing evidence, identify knowledge gaps, and make specific recommendations that pertain to SDB in patients with COPD.
Methods
This research statement was developed according to the rules of the American Thoracic Society. Details are provided in the online supplement.
Significance of SDB in COPD
Pathophysiology
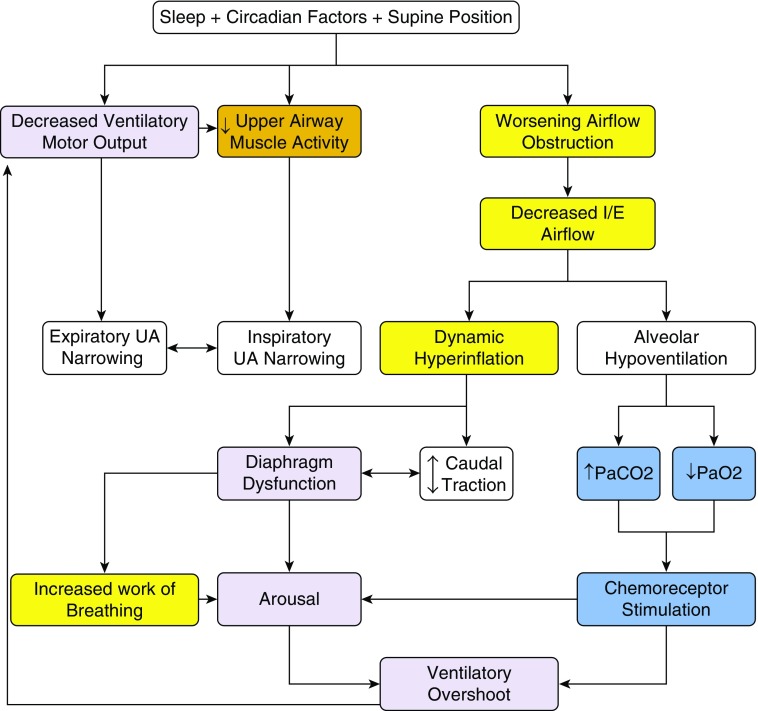
COPD is associated with abnormalities in respiratory mechanics and gas exchange (12). As a result, sleep is not a period of rest for the respiratory system, but rather a physiological challenge for patients with impaired pulmonary mechanics. During sleep, the drive to breathe that is normally provided by wakefulness is lost (Figure 1). This change is associated with impaired load compensation, increased airflow resistance, and hypoventilation (13), even among healthy persons. Individuals with COPD have even greater increases in airflow resistance and elastic loading during sleep because of intrathoracic airway narrowing and hyperinflation, respectively (16), similar to obese patients with a narrow upper airway (14, 15). Although healthy humans are able to preserve stable sleep and adequate alveolar ventilation despite such changes, individuals with abnormal respiratory mechanics may be unable to tolerate the changes and, as a result, may deteriorate during sleep (16).
Figure 1.
Sleep cycle instability in chronic obstructive pulmonary disease. I/E = inspiratory/expiratory; UA = upper airway.
Patients with COPD may be unable to compensate for added loads. The usual compensatory physiologic responses to sustained resistive loading include elevation of the respiratory rate or a shortening of the expiratory time. In patients with COPD, however, these responses worsen dynamic hyperinflation and increase the work of breathing. Patients with COPD have a physiological need to lengthen their expiratory time, but this mechanism compromises ventilation during sleep, particularly in severe COPD (17).
Marked disturbances of the sleep-wake cycle are seen in patients with COPD analogous to those observed in patients with primary sleep disorders, such as OSA, restless legs syndrome, periodic limb movements, and psychiatric disorders (18). A large proportion of the sleep-wake disturbances exhibited by patients with COPD are not well described by existing classification schemes. Sleep disruption may be related to impaired lung function and hyperinflation or, alternatively, to changes in sleep-related behaviors associated with worsening disease (e.g., increased time in bed or daytime napping). The mechanism for these disturbances remains unclear. Alterations in the sleep-wake cycle are associated with elevations in inflammatory markers, yet the long-term effects remain unknown (19).
Patients with COPD may manifest subtle oscillations in ventilation, sleep architecture, and oxygenation that do not meet the 4% detection threshold for hypopnea (20). The physiological effects of resultant chronic intermittent hypoxia-reoxygenation warrant further investigation. Whether these cyclic episodes contribute to adverse outcomes in patients with COPD is yet to be determined. Moreover, there is considerable complexity underlying the biology of sustained versus intermittent hypoxemia and the two in combination, which requires further study.
Recommendation: We recommend investigations that seek to understand mechanisms underlying impaired respiratory function during sleep and how these understandings might guide therapy to prevent complications.
Diagnosis
OVS represents the additive or synergistic effects of COPD and OSA. The diagnosis of OVS is made by full, in-laboratory polysomnography performed on a patient with COPD who presents with sleep-related complaints. The typical polysomnographic features are recurrent episodes of hypopnea alternating with hyperpnea, arousals, and a variable degree of oxygen desaturation. These findings are presumed consequences of upper airway narrowing or obstruction. Although this pattern is also seen in patients who have classic sleep apnea/hypopnea syndrome (i.e., whose risk factors include unfavorable upper airway anatomy and/or an unstable ventilatory control system), in OVS the cycle of instability may be initiated by triggers such as worsening airflow obstruction, hyperinflation, or hypoxia (17, 21). The presence and severity of upper airway narrowing during the hypopnea phase is unclear, and methods to identify the underlying pathophysiology of a hypopnea have not been validated. As a result, SDB in patients with COPD has been classified under the rubric “OSA,” owing to the limitation of qualitative diagnostic tools to identify the cause that initiated the cycle of instability.
For patients with mild to moderate COPD, the panel has had good clinical experience using home sleep testing in their clinical practices, particularly for patients without comorbid insomnia. Although the detection of apnea is reasonably straightforward, hypopneas, as defined by a transient reduction in alveolar ventilation of at least two breaths, are more challenging to assess definitively with home sleep testing, because detection methods rely on the consequences of decreased ventilation, namely oxygen desaturation and arousals. However, it is unclear if the magnitude of oxygen desaturation may be dampened by hyperinflation and/or the presence of carboxyhemoglobin (by shifting the oxyhemoglobin dissociation curve to the left) (22, 23).
Recommendation: We recommend studies that improve our understanding of gas exchange impairment during sleep in COPD. Such studies could help guide definitions of various respiratory events as well as criteria to judge severity of disease in patients with COPD and OSA.
Epidemiology
The epidemiology of SDB in patients with respiratory disorders is not well established, with inconsistent reports in the literature. The inconsistent reports may be due, in part, to changes in diagnostic criteria and improved testing technology during the past 20 years. For example, the diagnosis of OSA can now be made based on hypoxemia, which may exaggerate the association of the two disorders, because patients with COPD readily desaturate in the presence of mild flow limitation.
Both OSA and COPD are common disorders. More than 5% of the United States population (i.e., at least 13.7 million people) and about 5–10% of the world population is burdened by COPD (2, 3), which is a leading cause of morbidity and mortality. Its high prevalence is attributable to high rates of smoking and environmental pollution (41). Among middle-aged (i.e., 50–70 yr) men, the prevalence of moderate to severe OSA is roughly 17%, whereas among middle-aged women, the prevalence is approximately 9% (24). These prevalences exist throughout most of the developed world (25).
The most recent epidemiological studies looking at patients with both OSA and COPD have estimated that OSA and COPD coincide at rates predicted by the epidemiology of each disorder alone (26, 27). About 1 in 10 patients with one disorder will have the other disorder by chance alone; there is no clear evidence that having one disorder predisposes someone to acquiring the other, despite many theories of potential pathophysiological links. An important limitation of these studies, however, is that most have restricted assessment to cohorts with only mild COPD. Therefore, it is unknown whether more severe COPD predisposes to OSA.
The prevalence of SDB in COPD may be higher than typically reported if all severities of COPD are considered, as suggested by a study that demonstrated a 66% prevalence of OSA among patients with moderate to severe COPD (2). Possible pathophysiological explanations for a higher prevalence of OSA among patients with moderate to severe COPD include upper airway narrowing due to edema fluid shifting from the lower extremities to the neck in those with cor pulmonale and upper airway myopathy due to either COPD itself (which can cause a skeletal muscle myopathy) or inhaled or systemic steroid use (28). Arguing against a relationship between COPD severity and OSA is that dynamic hyperinflation may reduce upper airway collapsibility due to tracheal traction (17, 29).
Recommendation: We recommend studies that investigate whether there is a direct correlation between the prevalence of OSA and the severity of COPD.
Outcomes
Mortality
Patients with OVS have been long recognized as having a unique and severe phenotype; however, survival data have only recently become available. One study found that individuals with COPD plus untreated OSA have decreased survival compared with individuals with COPD alone or COPD plus OSA treated with continuous positive airway pressure (CPAP) (5). These findings have been confirmed in other cohorts (30) and make intuitive sense; because OSA and COPD are each known to increase cardiovascular risk (31–33), one would expect the absence of one (i.e., COPD alone) or the treatment of one (i.e., COPD plus treated OSA) to be associated with better survival.
There are several reasons that the survival estimates may be incorrect, however. First, the data do not derive from randomized trials and, therefore, it is possible that they are due to unmeasured confounders. As examples, individuals who used CPAP in the study (2) differed from those who did not in terms of their financial means and willingness to pursue CPAP therapy. The possibility that such factors may be responsible for some of the differences cannot be excluded (34). Second, patients with OVS tend to die from cardiovascular events that are attributed to cardiac disease, with little acknowledgment that underlying OSA or COPD may have contributed to the cardiovascular event (5). Thus, the impact of OVS, COPD, and OSA on survival may be underestimated. The precise mechanisms of increased cardiovascular mortality in OVS have proven elusive.
Few studies have been performed that evaluated the impact of treatment on survival. One observational study of more than 1,000 patients with COPD reported that the duration of CPAP use directly correlated with improved survival (30). Such observational data justify randomized trials to evaluate the treatment of OSA in patients with COPD.
Myocardial abnormalities
Intermittent hypoxemia due to OSA with subsequent pulmonary vasoconstriction confers several undesirable consequences in patients with COPD. The increased right ventricular load created by hypoxemia-induced vasoconstriction can result in hypertrophy, which is capable of doubling the right ventricular mass, as demonstrated by a study that compared patients with OVS to patients with severity-matched COPD alone (35). Myocardial fibrosis can also be induced by hypoxemia. A cardiac magnetic resonance imaging study used a novel, gadolinium-enhancement technique to measure the extracellular matrix of the ventricle and found increased myocardial fibrosis that correlated with worsening hypoxemia (36). Studies looking at the impact of treatment of OSA on right ventricular mass and myocardial fibrosis have not been performed in patients with COPD.
Blood gases
Patients with OVS tend to have worse blood gases than those with either COPD alone or OSA alone, as illustrated by the following clinical observations of the panel. Patients with OVS develop daytime hypercapnia at a relatively preserved lung function, whereas patients with COPD alone develop daytime hypercapnia once lung function becomes severely compromised. Patients with OVS also tend to develop daytime hypercapnia at a lower body mass index and apnea–hypopnea index (AHI) than patients with OSA alone. Hypoxemia is more prolonged and profound in patients with OVS than in those with COPD alone or OSA alone and, perhaps as a result, patients with OVS more frequently have pulmonary hypertension than those with OSA alone (35, 37, 38).
Inflammation and metabolism
Patients with OVS have increased chronic inflammation and, possibly, impaired metabolism. In patients with OSA, sleep fragmentation and chronic intermittent hypoxia facilitate oxidative injury and chronic inflammation. In patients with COPD, impaired gas exchange similarly facilitates oxidative injury and chronic inflammation, which may contribute to extrapulmonary complications of COPD (i.e., loss of skeletal muscle mass, decreased bone mineral density, atherosclerosis, and insulin resistance). The pathways mediating oxidative injury and inflammation are probably similar in COPD and OSA, because the profiles of elevated markers of these processes are similar among patients with these conditions.
Data from the Sleep Heart Health Study (39) demonstrated that sleep-related respiratory events accompanied by oxyhemoglobin oxygen desaturation greater than or equal to 2% are associated with fasting hyperglycemia. This suggests that mild degrees of chronic intermittent hypoxia may predispose individuals to adverse metabolic outcomes. Such mild intermittent hypoxia is common in patients with OVS and can be undetected in conventional polysomnographic analyses.
Recommendations:
1. We recommend observational studies that compare clinical outcomes among patients with OVS to clinical outcomes among patients with OSA alone or COPD alone.
2. We recommend randomized trials that compare clinical outcomes among patients with OVS whose OSA is treated to clinical outcomes among patients with OVS whose OSA is untreated.
3. Clinical outcomes in the preceding recommendations include mortality, cardiovascular-specific mortality, nonfatal cardiovascular events, quality of life, and excessive daytime sleepiness.
Appraisal of Existing Metrics
Is the AHI Useful in Patients with COPD?
The AHI—the sum of apneas (cessations in breathing) plus hypopneas (reductions in breathing) —was designed to assess the severity of sleep apnea, generally in obese men without underlying lung disease (40–42). However, the AHI has many limitations, some of which are particularly relevant to patients with COPD and may affect the detection of OSA.
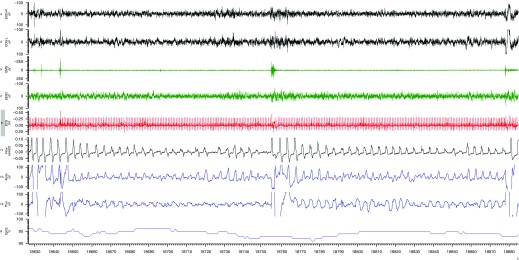
The definition of hypopnea has varied over time and across organizations, but most definitions include oxyhemoglobin oxygen desaturation as a criterion. There have been calls for reductions in airflow with electrocortical arousal to be considered hypopneas even without major oxygen desaturation. However, EEG is usually not performed during home sleep studies and, therefore, the presence or absence of oxygen desaturation remains a key criterion for evaluating hypopneas, as recommended by the United States’ major healthcare payer, Medicare (43). Existing definitions of hypopnea have important flaws. One problem is that the degree and duration of oxygen desaturation are not considered. Thus, an individual with 20 minutes of sustained hypoxemia to the 70–80% range due to hypoventilation would only be counted as having had a single hypopnea (Figure 2). A second problem is that recurrent arousals for any reason (i.e., poor sleep quality, hyperinflation, and nocturnal cough) may result in an irregular breathing pattern that could be interpreted as recurrent hypopneas. Conversely, frequent arousals due to repetitive respiratory events may not exhibit oxygen desaturations and, therefore, would not be scored as hypopneas. Such patients may benefit from therapy, even though they may not meet traditional criteria on the basis of AHI. Finally, smokers may have considerable carbon monoxide circulating in their blood, which can shift the oxyhemoglobin curve to the left, leading to higher saturations for any given PaO2 (22), potentially masking clinically important hypoxemia. Each of these limitations may affect the detection of OSA among patients with COPD.
Figure 2.
Prolonged respiratory events. A representative polysomnograph recording in a patient with sleep-disordered breathing and obstructive lung disease demonstrates how sustained desaturation due to prolonged hypoventilation would be counted as a single respiratory event.
The issue of sustained oxygen desaturation leading to underestimation of the AHI (and, therefore, underestimation of the severity of the condition) is particularly pertinent to patients with COPD, because one of the major findings on polysomnography in patients with COPD is nocturnal oxygen desaturation with sustained hypoxemia, without frequent apneas and hypopneas. There are a variety of reasons for the oxygen desaturation other than upper airway obstruction, including a drop in ventilation that occurs during sleep (especially REM sleep) and ventilation–perfusion mismatch that worsens during sleep. The magnitude of oxygen desaturation may be particularly severe in patients with COPD because of the sigmoidal shape of the oxyhemoglobin oxygen desaturation curve. Those with mild baseline hypoxemia (e.g., oxygen saturation of 90–93%) are uniquely positioned near the steep portion of the oxyhemoglobin dissociation curve, where small changes in PaO2 lead to large changes in oxygen saturation. Thus, even with mild perturbations, sustained and intermittent oxygen desaturations commonly occur.
Taking these observations together, it is clear that the quantification of hypopneas and apneas alone may be insufficient to identify and characterize OSA in patients with COPD. As an example, a patient with partial upper airway obstruction (hypopneas) plus severe COPD and a patient with complete upper airway obstruction (apneas) plus mild COPD will both be diagnosed as having OVS. However, the different pathogenesis of OSA in the patients suggests that different treatment strategies be deployed.
It is unknown whether the AHI predicts clinical outcomes in patients with COPD, including symptoms, cardiovascular consequences, and metabolic complications. In theory, the area under the oxygen desaturation curve or the total hypoxemic load may have better predictive value than the AHI in patients with COPD, as may the nadir oxygen saturation, but this has not been evaluated.
Recommendation: We recommend studies that compare the AHI with other measures of obstructive sleep apnea severity (e.g., duration of hypoxemia, nadir of hypoxemia, arterial tonometry, and area under the oxygen desaturation curve) as predictors of clinical outcomes in patients with COPD. Various definitions of severity of OVS could be assessed for their predictive value using hard clinical outcomes.
Is “Sleep Apnea” the Appropriate Term in Patients with COPD?
A large proportion of patients with COPD experience some deterioration in gas exchange and respiratory mechanics during sleep. Non-REM sleep is characterized by increased upper airway resistance, impaired load compensation, and blunted chemosensitivity, all of which may disturb gas exchange. REM sleep may further compromise respiratory function because of a propensity for upper airway collapse and skeletal muscle atonia in accessory muscles of respiration (e.g., rib cage intercostal muscles) (44).
These sleep-related changes probably all contribute to the observation that a large proportion of patients with COPD desaturate during sleep, even those who are not hypoxemic during wakefulness. This was illustrated by a study that found that 38% of patients with moderate to severe COPD who do not qualify for home oxygen therapy on the basis of their daytime PaO2 have nocturnal oxygen desaturation without evidence of sleep apnea. Therefore, the assessment of gas exchange during sleep is critical in patients with COPD (45).
SDB and OSA are sometimes conflated. However, we believe that SDB better describes the pathophysiology that occurs in patients with COPD. SDB encompasses all respiratory dysfunction during sleep, including central and obstructive sleep apnea, worsening airflow obstruction, hypoventilation, oxygen desaturation, sleep fragmentation, and other abnormalities that are not necessarily implied by traditional terminology.
Recommendation: We recommend that future research evaluate SDB (i.e., central sleep apnea, OSA, increased upper airway resistance, hypoventilation, sleep fragmentation, etc.), rather than OSA specifically, in patients with COPD. Mechanistic research could help identify endotypes amenable to targeted intervention rather than simply describing different airflow patterns.
Is REM-related Oxygen Desaturation Predictive of Poor Outcomes in Patients with COPD?
REM sleep is defined by skeletal muscle atonia that affects most muscles of the body, with some notable exceptions, including the diaphragm (46). It is characterized by variability in the EEG and other physiological signals. Phasic eye movements during REM sleep have been associated with suppressed upper airway motor tone and reductions in ventilation. Patients with sleep apnea often have more respiratory events during REM sleep. Therefore, oxygen desaturation tends to worsen during REM in patients with OVS.
Controversy exists regarding the clinical importance of respiratory events and gas exchange alterations during REM sleep (47), with most believing that the importance likely depends on the underlying cause of the hypoxemia. Fluctuations in tidal volume during REM sleep are expected, and hypoxemia due to such fluctuations is unlikely to be indicative of major pathology or require therapy (48). This is supported by a study that showed no major symptoms associated with respiratory events occurring during REM sleep (49). However, occasional patients exhibit profound oxygen desaturations during REM sleep that are likely a result of atonia in the accessory muscles of respiration (48). Such derangements in gas exchange are unlikely to be physiologic and, thus, provide rationale for therapy. This view is supported by a study that found important consequences, such as arterial hypertension, associated with REM-related respiratory events (47).
In patients with COPD, data during REM sleep remain sparse, but the panel’s clinical experience suggests that some patients with severe COPD will experience major oxygen desaturations during REM sleep, likely as a result of hypoventilation. Such patients may benefit from bilevel positive airway pressure therapy, although outcome data are lacking. Similarly, volume-assured devices are becoming available but have been minimally studied in this context.
Recommendations:
1. We recommend studies that seek to determine the pathophysiology of oxygen desaturation during REM sleep in patients with COPD.
2. We recommend trials that compare bilevel positive airway pressure to both CPAP and no positive airway pressure therapy in patients with COPD who desaturate during REM sleep.
Is Capnometry Valuable in Sleep Studies of Patients with COPD?
Monitoring gas exchange is critical to a thorough evaluation of sleep and breathing. Traditionally, oxygen saturation has been used as a surrogate for gas exchange under the assumption that most important disturbances would lead to oxygen desaturation, and because of high cost and poor availability of reliable transcutaneous systems. However, oxygen saturation is an imperfect measure, as an occurrence of oxygen desaturation could in theory be related to multiple mechanisms, including hypoventilation, upper airway collapse, ventilation–perfusion mismatch, shunting, and other factors.
The addition of capnometry could be helpful for several reasons. First, capnometry plus pulse oximetry is more sensitive than pulse oximetry alone. Because of the shape of the oxyhemoglobin dissociation curve, important hypoventilation may occur with only minor changes in SaO2. For example, a 10–mm Hg increase in PaCO2 may drop PaO2 from 80 mm Hg to roughly 70 mm Hg, which would not lead to any major change in the saturation. Moreover, patients who are receiving supplemental oxygen may experience marked hypoventilation that could be overlooked because of lack of oxygen desaturation. Capnometry has the potential to detect hypoventilation that pulse oximetry may miss in these situations. Second, pulse oximetry does not provide information about the mechanism underlying oxygen desaturation, but capnometry may in some circumstances. For example, a decrease in oxygen saturation accompanied by an increase in PaCO2 is suggestive of hypoventilation as the underlying mechanism rather than ventilation–perfusion mismatch or other factors. Mechanistic information might help to guide the choice of therapy, such as whether bilevel positive airway pressure or CPAP therapy is more appropriate.
In our clinical practices, we prefer transcutaneous CO2 monitoring over end-tidal CO2 in patients with COPD, particularly if supplemental oxygen is being provided. In patients with unstable breathing patterns, particularly if tidal volume is low, end-tidal Pco2 can be unreliable because of the absence of an alveolar plateau. Transcutaneous CO2 is particularly helpful semiquantitatively, because it is directionally accurate regarding disturbances in gas exchange. Depending on the device being used, however, transcutaneous CO2 changes can be delayed because of slow time constants, making abrupt dynamic changes difficult to assess. Other methods of measuring gas exchange, such as arterial blood gases, have been scarcely studied.
Recommendation: We recommend trials that compare various methods of measuring gas exchange in patients with OVS, for example, trials that compare pulse oximetry plus transcutaneous CO2 measurement to pulse oximetry alone, pulse oximetry plus transcutaneous CO2 measurement to arterial blood gas measurement, and pulse oximetry plus transcutaneous CO2 measurement to pulse oximetry plus end-tidal CO2 measurement.
Should Supplemental Oxygen Be Withdrawn for Overnight Polysomnography in Patients with Suspected OVS?
Many patients with COPD are given supplemental oxygen on a long-term basis because of demonstrable abnormal gas exchange or for comfort. However, supplemental oxygen may mask hypopneas during overnight polysomnography by blunting oxygen desaturation due to hypoventilation. For this reason, we routinely remove supplemental oxygen before polysomnography, unless the oxygen saturation during relaxed wakefulness is less than 88%.
It is important that oxygen desaturation be detected and the response to positive airway pressure titration be assessed during polysomnography, because positive airway pressure alone may eliminate oxygen desaturation, eliminating the need for nocturnal supplemental oxygen with its expense and risks. Supplemental oxygen alone is generally considered ineffective for the treatment of OSA and can worsen hypercapnia (11, 50, 51), although the patients enrolled in these studies differed from our patients of interest (i.e., rather than COPD with confirmed nocturnal oxygen desaturation, the studies enrolled patients with COPD with exercise-induced oxygen desaturation or patients with OSA with or without COPD).
Recommendation: We recommend trials that compare supplemental oxygen to both CPAP therapy and bilevel positive airway pressure in patients with COPD who exhibit nocturnal oxygen desaturation.
Revisiting the Sleep Study
Polysomnographic Features of SDB in COPD
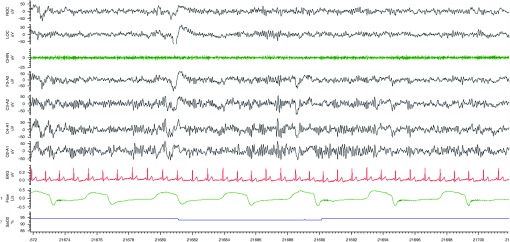
Polysomnography in patients with COPD is adequate to categorize sleep apnea and sleep-related respiratory events (e.g., central versus obstructive sleep apnea, hypopneas versus apneas), as well as to quantify the severity of sleep apnea (e.g., number of events per hour, duration of events, degree of hypoxia with each event, and percentage of events with and without arousal). This approach allows the detection of SDB in most cases. However, the severity of the SDB may be underestimated by polysomnography in patients with COPD, particularly those who require supplemental oxygen or who have disturbances in sleep architecture due to depression or insomnia (both increase alpha intrusion and make it difficult to detect hypopneas terminating in arousals from sleep) (Figure 3).
Figure 3.
Alpha intrusion. A representative polysomnograph recording in a patient with sleep-disordered breathing and obstructive lung disease demonstrates an increase in alpha intrusion and illustrates how it can be difficult to detect hypopneas terminating in arousals from sleep.
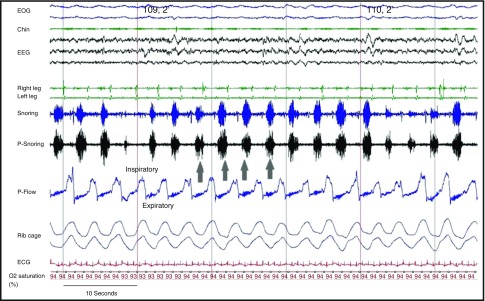
Polysomnography is not intended to detect COPD-specific respiratory disturbances, such as expiratory snoring with associated flow limitation (52, 53) (Figure 4) or dynamic hyperinflation (see Figures E2–E4 in the online supplement). In one study, expiratory snoring had 11-times higher odds of being associated with airflow obstruction (FEV1/FVC < 70%) than the absence of expiratory snoring. Additional measurements that quantify ventilation and/or respiratory effort are required to detect SDB in COPD. For example, a pneumotachograph affixed to a mask would best serve to quantify ventilation, the severity of inspiratory and expiratory flow limitation, and the presence of hyperinflation during sleep. However, conventional pneumotachographs are of limited use in patients with COPD during sleep, because they are too cumbersome and impose excessive dead space and resistive loads on the respiratory system (54). Alternative flow surrogates include an unfiltered nasal pressure signal at a high sampling rate (preferably >200 Hz) or flow sensors with negligible resistance and dead space, both of which can be readily integrated into conventional polysomnographic units. Similarly, esophageal catheters would be best suited to quantify respiratory loads but are rarely used in clinical sleep studies. Inductive plethysmography or intercostal EMG activity might be good surrogates for detecting changes in inspiratory or expiratory effort.
Figure 4.
Expiratory flow limitation and expiratory snoring in chronic obstructive pulmonary disease. A representative polysomnograph recording during non-REM sleep in a patient with sleep-disordered breathing and obstructive lung disease. Arrows point to the expiratory flow limitation and expiratory snoring. Chin = chin electromyogram; EOG = electrooculogram; P-Flow = pressure flow; P-Snoring = snoring tracing obtained by pressure flow sensor; snoring = snoring tracing obtained by chin microphone (53).
Recommendations:
1. We recommend research to develop and/or validate devices that can identify and characterize sleep without EEG. The goal is to improve the assessment of the severity of SDB in patients with disruptions in sleep architecture.
2. We recommend studies that compare various methods to quantify ventilation, the severity of inspiratory and expiratory flow limitation, and the presence of hyperinflation during sleep (e.g., pneumotachograph, nasal pressure signaling, and flow sensors) in patients with COPD.
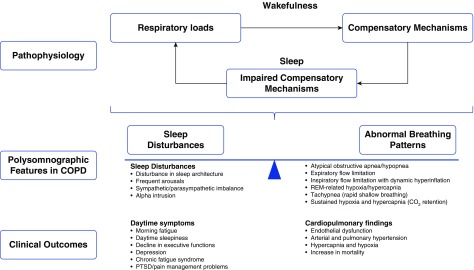
SDB in COPD
COPD is associated with alterations in respiratory mechanics and gas exchange, leading to dynamic hyperinflation and/or hypoventilation (55). During wakefulness, patients compensate for expiratory flow obstruction by adopting specific compensatory mechanisms to minimize hyperinflation (e.g., by prolonging expiratory time or pursed lip breathing) and preserve alveolar ventilation. Sleep is associated with changes in respiratory control that interfere with these compensatory mechanisms (Figure 5) and lead to a cascade of events (Figure 1). Most notably, loss of the ventilatory drive leads to significant reductions in tidal volume. An increase in inspiratory time would seemingly be an effective compensatory mechanism to mitigate the reduction in tidal volume. However, increased inspiratory time reduces expiratory time, which may lead to dynamic hyperinflation in patients with COPD. Similarly, an increase in the respiratory rate would seemingly be another compensatory mechanism, but this also reduces expiratory time. In either case, respiratory patterns during sleep in COPD may worsen dynamic hyperinflation by shortening the expiratory time. In addition, an inspiratory flow limitation is commonly observed during sleep, and even mild degrees of inspiratory flow limitation lengthen inspiration and further compromise expiratory time. Thus, normal sleep-induced alterations in breathing pattern can be detrimental in patients with COPD.
Figure 5.
Sleep-disordered breathing in chronic obstructive pulmonary disease (COPD). PTSD = post-traumatic stress disorder.
Sample Cases
We use a series of cases to illustrate the spectrum of novel COPD-specific events for which both pneumotachograph and esophageal pressure signals are required to detect SDB events. Although these methods have not been widely adopted for diagnostic purposes in most sleep centers, these signals can illuminate breathing disturbances during sleep that can be easily detected by currently used surrogate signals. The cases are provided in the online supplement.
Conclusions
Polysomnographic features in COPD are a consequence of sleep-induced respiratory loads. Patients with COPD may respond to increased respiratory loads with marked sleep disturbances or, alternatively, they may have minimal sleep disturbance but a highly abnormal breathing pattern with or without severe alterations in gas exchange. The same principles described in this statement for COPD may also apply to other respiratory diseases characterized by lower airway obstruction, such as asthma and cystic fibrosis. Depending on which polysomnographic features predominate, patients may present with a variety of daytime symptoms and clinical outcomes, ranging from insomnia to hypersomnia with and without cardiopulmonary complications. We strongly advocate for further research into SDB in patients with COPD, to understand better the spectrum of disease, optimize the definitions and equipment used to assess patients, and determine the optimal mode of therapy.
Acknowledgments
This official research statement was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology.
Members of the subcommittee are as follows:
Atul Malhotra, M.D. (Chair)
M. Safwan Badr, M.D.
Pamela DeYoung, R.P.S.C.-T.
MeiLan K. Han, M.D.
Nadia N. Hansel, M.D., M.P.H.
Robert L. Owens, M.D.
Hartmut Schneider, M.D.
Alan R. Schwartz, M.D.
Jadwiga A. Wedzicha, M.D.
Kevin C. Wilson, M.D.
Michelle R. Zeidler, M.D.
Footnotes
This official Research Statement of the American Thoracic Society was approved December 2017
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: A.M. practices at University of California San Diego, which received a philanthropic donation from ResMed in support of their sleep center. M.K.H. served as a consultant for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion; and received research support and served as a speaker for Novartis. N.N.H. received research support and served on an advisory committee for AstraZeneca and GlaxoSmithKline; and received research support from Boehringer Ingelheim. R.L.O. received honorarium and travel reimbursement from ResMed and Itamar; and served on a scientific advisory board for Novartis. H.S. holds a patent (U.S. 200800922898) issued to RespEQ for a disposable sleep and breathing monitor. A.R.S. served as a consultant for inSleep Health and Respicardia; received research support from ImThera Medical, inSleep Health, and Maxis; and holds stock, stock options, or other ownership interests in Discover Medical and RespEQ. J.A.W. served as a speaker and on an advisory committee for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis; received research support from GlaxoSmithKline, Johnson and Johnson, Novartis, OM Pharma, Takeda, and Vifor Pharma; served on an advisory committee for Johnson and Johnson; served as a speaker for Chiesi; and had meeting expenses covered by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, and Teva. M.S.B., P.D., K.C.W., and M.R.Z. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the ATS Assembly on Sleep and Respiratory Neurobiology
References
- 1.Rice MB, Malhotra A. The air we breathe and lung disease. J Thorac Dis. 2015;7:E245–E247. doi: 10.3978/j.issn.2072-1439.2015.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soler X, Gaio E, Powell FL, Ramsdell JW, Loredo JS, Malhotra A, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohli P, Sarmiento K, Malhotra A. Update in sleep medicine 2012. Am J Respir Crit Care Med. 2013;187:1056–1060. doi: 10.1164/rccm.201302-0315UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayas NT, Owens RL, Kheirandish-Gozal L. Update in sleep medicine 2014. Am J Respir Crit Care Med. 2015;192:415–420. doi: 10.1164/rccm.201503-0647UP. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 6.Jen R, Li Y, Owens RL, Malhotra A. Sleep in chronic obstructive pulmonary disease: evidence gaps and challenges. Can Respir J. 2016;2016:7947198. doi: 10.1155/2016/7947198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64:561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 8.Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152:538–544. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 9.Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc. 1996;71:533–542. doi: 10.4065/71.6.533. [DOI] [PubMed] [Google Scholar]
- 10.Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Asin J, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69:826–834. doi: 10.1136/thoraxjnl-2014-205126. [DOI] [PubMed] [Google Scholar]
- 11.Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr, Criner GJ, et al. Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol (1985) 2009;107:309–314. doi: 10.1152/japplphysiol.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. [Discussion p. 31.]. [PubMed] [Google Scholar]
- 14.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 15.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skatrud JB, Dempsey JA, Badr S, Begle RL. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol (1985) 1988;65:1676–1685. doi: 10.1152/jappl.1988.65.4.1676. [DOI] [PubMed] [Google Scholar]
- 17.Biselli P, Grossman PR, Kirkness JP, Patil SP, Smith PL, Schwartz AR, et al. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol (1985) 2015;119:266–271. doi: 10.1152/japplphysiol.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soler X, Diaz-Piedra C, Ries AL. Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD. 2013;10:156–163. doi: 10.3109/15412555.2012.729622. [DOI] [PubMed] [Google Scholar]
- 19.Kohli P, Pinto-Plata V, Divo M, Malhotra A, Harris RS, Lazaar A, et al. Functional capacity, health status, and inflammatory biomarker profile in a cohort of patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2015;35:348–355. doi: 10.1097/HCR.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 22.Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 1986;65:1186–1188. [PubMed] [Google Scholar]
- 23.Chowdhuri S, Quan SF, Almeida F, Ayappa I, Batool-Anwar S, Budhiraja R, et al. ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea. An Official American Thoracic society research statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193:e37–e54. doi: 10.1164/rccm.201602-0361ST. [DOI] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma B, Feinsilver S, Owens RL, Malhotra A, McSharry D, Karbowitz S. Obstructive airway disease and obstructive sleep apnea: effect of pulmonary function. Hai. 2011;189:37–41. doi: 10.1007/s00408-010-9270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11:259–270. doi: 10.5664/jcsm.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teodorescu M, Xie A, Sorkness CA, Robbins J, Reeder S, Gong Y, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10:183–193. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis. 1990;141:854–860. doi: 10.1164/ajrccm/141.4_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 30.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9:767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhuri S, Crook ED, Taylor HA, Jr, Badr MS. Cardiovascular complications of respiratory diseases. Am J Med Sci. 2007;334:361–380. doi: 10.1097/MAJ.0b013e3180a7269e. [DOI] [PubMed] [Google Scholar]
- 32.Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD--a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi: 10.2147/copd.s3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 34.Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, et al. Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect. Chest. 2010;137:102–108. doi: 10.1378/chest.09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma B, Neilan TG, Kwong RY, Mandry D, Owens RL, McSharry D, et al. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2013;10:4–10. doi: 10.3109/15412555.2012.719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neilan TG, Bakker JP, Sharma B, Owens RL, Farhad H, Shah RV, et al. T1 measurements for detection of expansion of the myocardial extracellular volume in chronic obstructive pulmonary disease. Can J Cardiol. 2014;30:1668–1675. doi: 10.1016/j.cjca.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resta O, Foschino Barbaro MP, Brindicci C, Nocerino MC, Caratozzolo G, Carbonara M. Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath. 2002;6:11–18. doi: 10.1007/s11325-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 38.Sajkov D, Wang T, Saunders NA, Bune AJ, Neill AM, Douglas Mcevoy R. Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am J Respir Crit Care Med. 1999;159:1518–1526. doi: 10.1164/ajrccm.159.5.9805086. [DOI] [PubMed] [Google Scholar]
- 39.Stamatakis K, Sanders MH, Caffo B, Resnick HE, Gottlieb DJ, Mehra R, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep. 2008;31:1018–1024. [PMC free article] [PubMed] [Google Scholar]
- 40.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 41.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 42.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, UCLA; 1968. NIH Publication 204; [Google Scholar]
- 43.Pillar G, Bar A, Shlitner A, Schnall R, Shefy J, Lavie P. Autonomic arousal index: an automated detection based on peripheral arterial tonometry. Sleep. 2002;25:543–549. [PubMed] [Google Scholar]
- 44.Orem J.Central respiratory activity in rapid eye movement sleep: augmenting and late inspiratory cells [corrected] Sleep 199417665–673.[Published erratum appears in Sleep 18:137.] [DOI] [PubMed] [Google Scholar]
- 45.Lacasse Y, Sériès F, Vujovic-Zotovic N, Goldstein R, Bourbeau J, Lecours R, et al. Evaluating nocturnal oxygen desaturation in COPD--revised. Respir Med. 2011;105:1331–1337. doi: 10.1016/j.rmed.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol (1985) 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 47.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 49.Chami HA, Baldwin CM, Silverman A, Zhang Y, Rapoport D, Punjabi NM, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malhotra A, Schwartz DR, Ayas N, Stanchina M, White DP. Treatment of oxygen-induced hypercapnia. Lancet. 2001;357:884–885. doi: 10.1016/s0140-6736(05)71817-1. [DOI] [PubMed] [Google Scholar]
- 52.Alchakaki A, Riehani A, Shikh-Hamdon M, Mina N, Badr MS, Sankari A. Expiratory snoring predicts obstructive pulmonary disease in patients with sleep-disordered breathing. Ann Am Thorac Soc. 2016;13:86–92. doi: 10.1513/AnnalsATS.201507-413OC. [DOI] [PubMed] [Google Scholar]
- 53.Owens RL, Edwards BA, Malhotra A, Wellman A. Expiratory resistance increases end-expiratory lung volume during sleep. Am J Respir Crit Care Med. 2012;185:e10–e11. doi: 10.1164/rccm.201105-0912IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson PL, Edwards N, Burgess KR, Sullivan CE. Detection of increased upper airway resistance during overnight polysomnography. Sleep. 2005;28:85–90. doi: 10.1093/sleep/28.1.85. [DOI] [PubMed] [Google Scholar]
- 55.Verbraecken J, McNicholas WT. Respiratory mechanics and ventilatory control in overlap syndrome and obesity hypoventilation. Respir Res. 2013;14:132. doi: 10.1186/1465-9921-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]