Abstract
Background and objective
This study aimed to establish reference equations for spirometry in healthy Taiwanese children and assess the applicability of the Global Lung Function Initiative (GLI)-2012 equations to Taiwanese children.
Methods
Spirometric data collected from 757 healthy Taiwanese children aged 5 to 18 years in a population-based cohort study. Prediction equations derived using linear regression and the generalized additive models for location, scale and shape (GAMLSS) method, respectively.
Results
The GLI-2012 South East Asian equations did not provide a close fit with mean ± standard error z-scores of −0.679 ± 0.030 (FVC), −0.186 ± 0.044 (FEV1), −0.875 ± 0.049 (FEV1/FVC ratio) and −2.189 ± 0.063 (FEF25-75) for girls; and 0.238 ± 0.059, −0.061 ± 0.053, −0.513 ± 0.059 and −1.896 ± 0.077 for boys. The proposed GAMLSS models took age, height, and weight into account. GAMLSS models for boys and girls captured the characteristics of spirometric data in the study population closely in contrast to the linear regression models and the GLI-2012 equations.
Conclusion
This study provides up-to-date reference values for spirometry using GAMLSS modeling in healthy Taiwanese children aged 5 to 18 years. Our study provides evidence that the GLI-2012 reference equations are not properly matched to spirometric data in a contemporary Taiwanese child population, indicating the urgent need for an update of GLI reference values by inclusion of more data of non-Caucasian decent.
Keywords: Asian, Children, Prediction equations, Pulmonary function, Reference values, Spirometry
Abbreviations: GLI, Global Lung Function Initiative; GAMLSS, generalized additive models for location, scale and shape; PATCH, Prediction of Allergies in Taiwanese Children; ATS, American Thoracic Society; ERS, European Respiratory Society; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25–75, forced expiratory flow between 25 and 75% of FVC; PEF, peak expiratory flow rate; LMS, Lambda-Mu-Sigma; BCCG, Box-Cox-Cole-Green; BCPE, Box-Cox-power-exponential; BIC, Bayesian information criterion; LLN, lower limit of normal; MSEs, mean squared errors; SD, standard deviation
Introduction
Pulmonary function tests have been commonly employed in the diagnosis and follow-up of patients with obstructive lung diseases, including asthma and chronic obstructive pulmonary disease.1, 2, 3 Given that pulmonary function varies by age, sex, height, weight, and ethnicity,2, 3, 4, 5, 6 accurate interpretation of pulmonary function results relies on valid reference values to help distinguish between disease and health; and to assess the severity of pulmonary function impairment.7 In 2012, the Global Lung Function Initiative (GLI) has derived multi-ethnic prediction equations for spirometry in subjects aged 3–95 years.2 A major breakthrough of GLI-2012 equations is applying a novel statistical modelling technique, the generalized additive models for location, scale and shape (GAMLSS).2 Since then, the GLI-2012 prediction equations have been endorsed by several international respiratory societies.8,9
Although the GLI-2012 prediction equations have been approved to perform well across several populations,9, 10, 11, 12 the results of validation studies have been rather inconsistent particularly in non-Caucasian populations.13, 14, 15, 16, 17, 18 It is largely unknown whether the GLI-2012 prediction equations are a good fit for healthy children across different Asian regions including Taiwan. Therefore, it is of importance to build up up-to-date reference values for pulmonary function derived from data collected in healthy Taiwanese children. Thus, efforts are required to develop appropriate prediction equations for spirometry using a large representative healthy Taiwanese children population sample, employing state-of-the-art statistical algorithms.19,20
The aims of this study were to assess the applicability of the GLI-2012 prediction equations for South East Asians to 757 healthy Taiwanese children aged 5–18 years in a large population-based cohort study; to establish prediction equations for spirometry in healthy Taiwanese children using both linear regression and the GAMLSS modeling; and to compare performance of prediction equations generated by linear regression and GAMLSS method and the GLI-2012 equations for South East Asians.
Methods
Study subjects
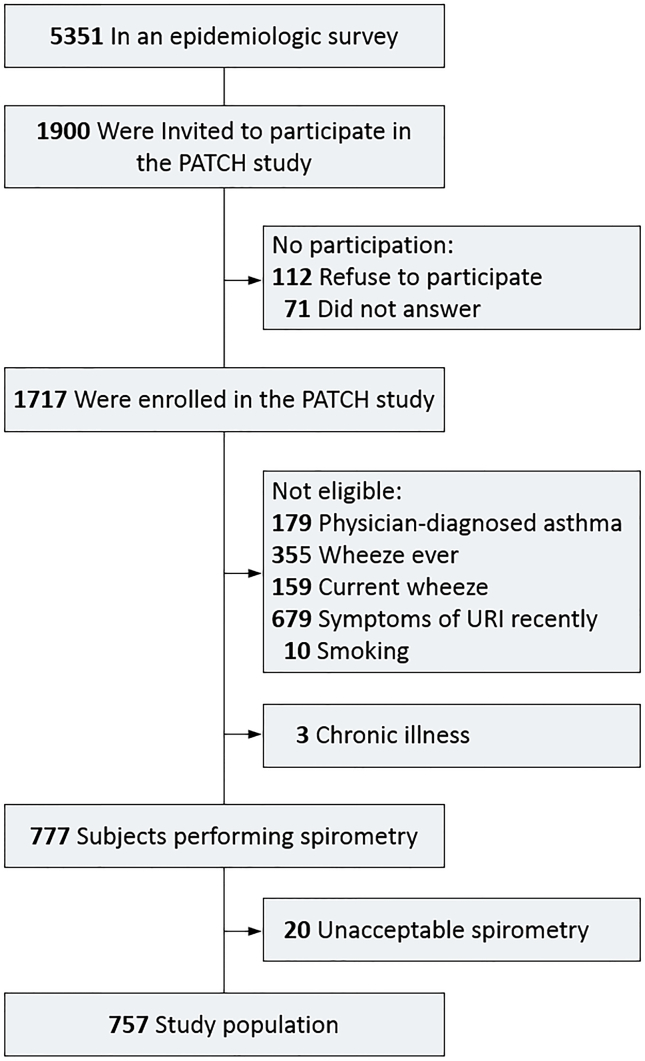
The study sample included a total of 757 healthy Taiwanese children (327 boys and 430 girls), part of the Prediction of Allergies in Taiwanese Children (PATCH) study, a prospective population-based cohort study of 1,717 schoolchildren aged 5–18 years.21, 22, 23, 24 The flow diagram of subject recruitment and selection is shown in Fig. S1. Among a school-based sample of 5,351 children, a random sample of 1,900 children were invited to participate in the PATCH study and 1,717 (90.4%) were enrolled. There was no significant difference in baseline characteristics between these 1,717 cohort participants and the original 5,351 subjects, indicating the sampling cohort was representative of the source population. Healthy children in the current study were selected from the 1,717 PATCH study participants accordingly to the following criteria: specifically, children without history of asthma, no current or past history of wheeze, no symptoms of upper respiratory infection in the past two weeks, no chronic illnesses and never smoked. The Institutional Review Boards of Chang Gung Memorial Hospital approved the study protocol (100–3214A3). The parents or guardians of each participant gave the written informed consent.
Anthropometric measurements
All participants had their weights and heights measured according to a standard protocol. Weight (in kg) was measured to the nearest 0.1 kg with lightweight clothing and height (in cm) was also measured to one decimal without shoes.
Pulmonary function measurements
Pulmonary function was measured using spirometry (Spirolab II, Medical International Research, Roma, Italy) in accordance with the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations.25 Three technically acceptable forced expirations were performed for up to 8 tests. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, forced expiratory flow at 25–75% of FVC (FEF25–75) and peak expiratory flow rate (PEF) were recorded and used in the analyses.
Statistical analysis
Spirometric data from all participants were converted to z-scores according to the GLI-2012 South East Asian equations. If the equations provide a good fit to the data, the spirometry z-scores derived from the GLI-2012 equation are expected to be symmetric around zero.12 We first evaluated whether the GLI-2012 prediction equations for South East Asians were applicable to the 757 healthy Taiwanese children by testing the zero mean of z-scores using one-sample t-test.19 We next developed separate prediction equations including age, height, and weight as potential predictors for boys and girls, respectively. We considered two modeling strategies to build the prediction equations: (i) linear regression model, which assumes normally distributed residuals, additive predictor effects on the spirometric indices in the original scale, and constant variability of spirometric indices across the range of predictors; and (ii) GAMLSS,19 which considers a variety of residual distributions, provides different choices of link functions between predictors and outcome, and allows the incorporation of predictors for each moment parameter (including median, variability, skewness and kurtosis). GAMLSS includes the Lambda-Mu-Sigma (LMS) method, which is widely used to establish reference equations as a special case,2,11,26 and can be implemented in the R package “GAMLSS” by selecting the Box-Cox-Cole-Green (BCCG) distribution for residuals. In the model building process of GAMLSS, we considered normal, BCCG, and the Box-Cox-power-exponential (BCPE) for residual distributions. We considered the log and identity link functions and assessed whether a predictor was required for modeling each moment parameter (i.e., median , coefficient of variation , and skewness ), and if so, whether the predictor should be included in the original or log scale. For modeling median, we considered height, weight, age, age2, and age3 as candidate predictors. For modeling variability and skewness , we considered age as a candidate predictor. When a BCPE residual model was considered, we also estimated the kurtosis parameter . For a given spirometric index and sex group, a total of 6,144 candidate models were fitted, which included 27 possible models for the function (i.e., 2 link functions for , 2 transformation functions [identity or log] for age, for weight and for height, and whether age/transformed age to be included in a polynomial form or not); 4 possible models for the function (i.e., identity or log transformation on age and whether to use a polynomial form on the age/transformed age); 4 possible models for the function (i.e., identity or log transformation on age and whether to use a polynomial form on the age/transformed age); and 3 candidate error distributions (i.e., normal, BCCG, and BCPE). The model with the smallest Bayesian information criterion (BIC), as outputted in the R package “GAMLSS”, was selected as the “best” GAMLSS model. Because of the need of evaluating the predictive performance, we randomly selected 4/5 of the individuals to build the model and used the remaining 1/5 of the individual for model assessment.
Given the linear regression model and the “best” GAMLSS model, we selected the model with smaller BIC between the two as our final prediction model. The final model was used to compute predicted values and the corresponding lower limit of normal (LLN). In addition, we also compared the predictive performance of our final model with the GLI-2012 prediction equations for South East Asians.8 Because it was infeasible to compute BIC values for the GLI-2012 prediction equations, we used mean squared errors (MSEs) to evaluate the performance between the “best” GAMLSS models and the GLI-2012 prediction equations for South East Asians. Specifically, MSEs of the “best” GAMLSS models were computed based on the 1/5 model-assessment samples, and MSEs of the GLI-2012 were computed based on all samples.
Results
The 757 healthy Taiwanese children (327 boys and 430 girls) aged 5–18 years have a mean age of 10.27 years with standard deviation (SD) 2.54 years (Table 1). The demographic characteristics and pulmonary function of the study population are provided in Table 1.
Table 1.
Demographic characteristics and pulmonary function of 757 healthy children in the PATCH study.
| Variable | All (n = 757) |
Boys (n = 327) | Girls (n = 430) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (year) | 10.27 ± 2.54 | 10.07 ± 2.37 | 10.42 ± 2.66 |
| Weight (kg) | 36.10 ± 2.34 | 36.78 ± 12.38 | 35.59 ± 12.30 |
| Height (cm) | 138.54 ± 4.24 | 138.24 ± 14.04 | 138.78 ± 14.41 |
| BMI (kg/m2) | 18.28 ± 3.3 | 18.75 ± 3.44 | 17.92 ± 3.15 |
| BSA (m2) | 1.17 ± 0.25 | 1.18 ± 0.25 | 1.16 ± 0.25 |
| FVC (L) | 2.04 ± 0.62 | 2.15 ± 0.67 | 1.96 ± 0.56 |
| FEV1 (L) | 1.78 ± 0.53 | 1.86 ± 0.58 | 1.73 ± 0.49 |
| FEV1/FVC ratio (%) | 87.53 ± 5.88 | 86.53 ± 6.00 | 88.30 ± 5.68 |
| FEF25-75 (L/sec) | 2.20 ± 0.74 | 2.23 ± 0.80 | 2.19 ± 0.70 |
| PEF (L/min) | 3.44 ± 1.01 | 3.69 ± 1.10 | 3.26 ± 0.91 |
Abbreviation: PATCH: prediction of allergies in Taiwanese children; SD: standard deviation; BMI: body mass index; BSA: body surface area; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25-75: forced expiratory flow at 25–75% of FVC; PEF: peak expiratory flow
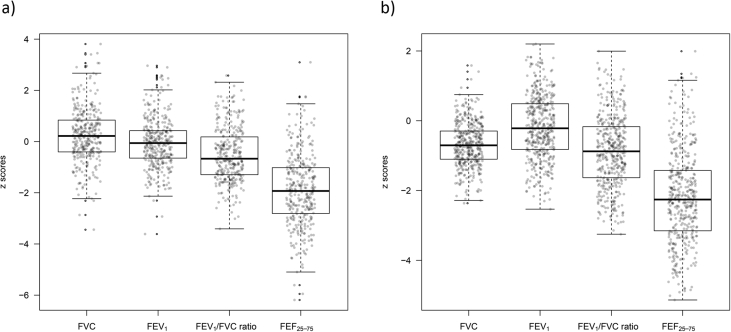
After adjusting for sex, age, and height using the GLI-2012 South East Asian equations, mean ± standard error z-scores for spirometric indices in the healthy Taiwanese children were −0.679 ± 0.030 (FVC), −0.186 ± 0.044 (FEV1), −0.875 ± 0.049 (FEV1/FVC ratio) and −2.189 ± 0.063 (FEF25-75) for girls; and 0.238 ± 0.059, −0.061 ± 0.053, −0.513 ± 0.059 and −1.896 ± 0.077 for boys. We performed Student's t-test to examine the adequacy of applying the GLI-2012 South East Asian equations to our data. Almost all tests resulted in p-value less than 10−3, except for FEV1 in boys (p = 0.25), indicating the disagreement between the observed spirometric data in healthy Taiwanese children and the GLI-2012 predicted values (Table 2 & Fig. 1). The deviations were more pronounced for girls than boys. Specifically, the GLI-2012 South East Asian equations were found to significantly overestimate FEV1/FVC ratio and FEF25-75 in both sexes, and FVC and FEV1 in girls, but significantly underestimate FVC in boys.
Table 2.
Z-scores for spirometric indices according to GLI-2012 South East Asian equations in healthy Taiwanese children.
| Boys (n = 327) |
Girls (n = 430) |
|||||||
|---|---|---|---|---|---|---|---|---|
| zFVC | zFEV1 | zFEV1/FVC ratio | zFEF25-75 | zFVC | zFEV1 | zFEV1/FVC ratio | zFEF25-75 | |
| Mean | 0.238 | −0.061 | −0.513 | −1.896 | −0.679 | −0.186 | −0.875 | −2.189 |
| Standard error | 0.059 | 0.053 | 0.059 | 0.077 | 0.030 | 0.044 | 0.049 | 0.063 |
| P-value* | <10−3 | 0.25 | <10−3 | <10−3 | <10−3 | <10−3 | <10−3 | <10−3 |
Abbreviations: GLI: global lung function initiative; FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC; PEF: peak expiratory flow rate.
*P-value is obtained by testing the zero mean of z-scores using one-sample Student's t-test
Fig. 1.
Spirometry data, adjusted for sex, age, and height based on the Global Lung Function Initiative 2012 equations for South East Asians, in 757 healthy Taiwanese children (327 boys and 430 girls, respectively). a) boys, b) girls. Abbreviation: FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC
Table 3 shows the results of linear regression models, which suggest that age, height, and weight are crucial for establishing prediction equations of four spirometric function measurements (i.e., FVC, FEV1, FEF25-75 and PEF) in Taiwanese healthy children (R2 ranging from 0.57 to 0.84 for boys; and from 0.55 to 0.86 for girls).
Table 3.
Linear regression equations for spirometric indices in healthy Taiwanese children.
| Variable | Regression coefficients |
Intercept | R2 | BIC | ||
|---|---|---|---|---|---|---|
| Age (20-year) | Height (cm) | Weight (kg) | ||||
| Boys (n = 327) | ||||||
| FVC (L) | –a | 0.039 ± 0.002 | 0.007 ± 0.003 | −3.474 ± 0.242 | 0.841 | 94.230 |
| FEV1 (L) | 0.036 ± 0.013 | 0.032 ± 0.002 | – | −2.952 ± 0.196 | 0.837 | 17.057 |
| FEF25-75 (L/sec) | 0.089 ± 0.029 | 0.029 ± 0.005 | – | −2.5670 ± 0.438 | 0.570 | 437.104 |
| PEF (L/min) | 0.121 ± 0.037 | 0.045 ± 0.006 | – | −3.664 ± 0.547 | 0.650 | 554.083 |
| Girls (n = 430) | ||||||
| FVC (L) | – | 0.027 ± 0.002 | 0.011 ± 0.002 | −2.122 ± 0.171 | 0.859 | −83.777 |
| FEV1 (L) | 0.023 ± 0.008 | 0.019 ± 0.002 | 0.010 ± 0.002 | −1.543 ± 0.191 | 0.856 | −154.369 |
| FEF25-75 (L/sec) | 0.105 ± 0.015 | – | 0.022 ± 0.003 | 0.308 ± 0.103 | 0.552 | 482.488 |
| PEF (L/min) | 0.130 ± 0.019 | – | 0.031 ± 0.004 | 0.798 ± 0.129 | 0.576 | 636.640 |
Abbreviation: FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC; PEF: peak expiratory flow rate; BIC: Bayesian information criterion.
Variables that are not significantly associated with outcomes (i.e., p-value>0.05) are not included in the final linear regression models
To illustrate the process of identifying the best GAMLSS model, we demonstrated the process of building the model for FVC as an example. Table 4a lists the 10 best models (i.e., the 10 models with the bottom 10 small BICs) and the corresponding BICs for boys. As the linear regression model is a special case of GAMLSS, we also include the results of linear regression model (Rank = 1458 in Table 4a) for comparisons. The best model (Rank = 1) of FVC imposes a log link function on median FVC values (), includes height and log-transformed weight as key predictors, and uses the BCPE distribution to model the residuals. The best model suggests that both coefficients of variation (and skewness () do not depend on age and only need an intercept term. That is,
Table 4a.
Development of the GAMLSS model for FVC in boys (n = 327), with separate linear predictors for median μ, variability σ and skewness ν. Each row is a separate model.
| Rank | Distribution | Predictor for |
Predictor for |
Predictor for |
BIC | |||
|---|---|---|---|---|---|---|---|---|
| Link | Height | Weight | Age | Age | Age | |||
| 1 | BCPE | Log | Identity | Log | – | – | – | 13.06 |
| 2 | BCPE | Log | Identity | Log | – | – | Identity | 14.86 |
| 3 | BCPE | Log | Identity | Log | Log | – | – | 15.73 |
| 4 | BCPE | Log | Identity | Log | Identity | – | – | 15.83 |
| 5 | BCPE | Log | Identity | Identity | – | – | – | 16.03 |
| 6 | BCPE | Log | Log | Log | – | – | – | 17.22 |
| 7 | BCPE | Log | Log | Identity | – | – | – | 17.35 |
| 8 | BCPE | Log | Identity | – | – | – | – | 17.59 |
| 9 | BCPE | Log | Identity | Identity | – | – | – | 17.70 |
| 10 | BCPE | Log | Identity | Log | Log | – | Identity | 18.09 |
| 1458 (LR model) | Normal | Identity | Identity | Identity | – | – | – | 94.23 |
Abbreviation: FVC: Forced vital capacity; BIC: Bayesian information criterion; BCPE: Box–Cox–power–exponential distribution, which is an extension of the Box–Cox–Cole–Green (BCCG) distribution to include kurtosis; LR: linear regression; : median; : coefficient of variation; : skewness
In contrast, the second best model of FVC values in boys is similar to the best model, except that the skewness depends linearly on age:
Similarly, Table 4b lists the 10 best GAMLSS models of FVC values for girls, sorted by BICs, as well as the linear regression model (Rank = 599 in Table 4b) for comparisons. The best model of FVC values (Rank = 1) in girls contains different components as the best model of FVC values in boy. Specifically, it considers identity link function on median FVC values (), includes (untransformed) height and weight as key predictors, and uses BCCG distribution to model the residuals. Similar to the best model for boys, the best model for girls also suggests that both coefficients of variation ( and skewness () do not depend on age and only contain an intercept term. That is,
Table 4b.
Development of the GAMLSS model for FVC in girls (n = 430), with separate linear predictors for median μ, variability σ, and skewness ν, where each row is a separate model.
| Rank | Distribution | Predictor for |
Predictor for |
Predictor for |
BIC | |||
|---|---|---|---|---|---|---|---|---|
| Link | Height | Weight | Age | Age | Age | |||
| 1 | BCCG | Identity | Identity | Identity | – | – | – | −111.48 |
| 2 | BCCG | Identity | Identity | Identity | Log | – | – | −111.00 |
| 3 | BCCG | Identity | Identity | Identity | – | – | Identity | −110.38 |
| 4 | BCCG | Identity | Identity | Log | – | – | Identity | −109.80 |
| 5 | BCCG | Identity | Identity | Identity | Identity | – | – | −109.19 |
| 6 | BCCG | Identity | Log | Identity | – | – | Identity | −108.49 |
| 7 | BCCG | Identity | Identity | Log | – | – | – | −108.48 |
| 8 | BCCG | Identity | Log | Identity | Identity | – | – | −108.47 |
| 9 | BCCG | Identity | Log | Identity | Log | – | – | −108.25 |
| 10 | BCCG | Identity | Identity | Log | Identity | – | – | −108.23 |
| 599 (LR model) | Normal | Identity | Identity | – | – | – | – | −83.78 |
Abbreviation: FVC: Forced vital capacity; BIC: Bayesian information criterion; BCCG: Box–Cox–Cole–Green distribution, which is an extension of the Box–Cox–Cole–Green (BCCG) distribution to include kurtosis; LR: linear regression; : median; : coefficient of variation; : skewness
In Table 5, we report the regression coefficients involved in the best GAMLSS models of FVC, FEV1, FEF25-75 and PEF in boys and girls, respectively, including the coefficients of the median model (i.e., ’s), of the variation model (i.e., ’s) and of the skewness model (i.e., ’s), as well as the kurtosis estimate () when a BCPE residual distribution is used.
Table 5.
Best GAMLSS models for spirometric indices in healthy Taiwanese children
| FVC (L) |
FEV1 (L) |
FEF25-75 (L/sec) |
PEF (L/sec) |
FVC (L) | FEV1 (L) | FEF25-75 (L/sec) | PEF (L/sec) | |
|---|---|---|---|---|---|---|---|---|
| Boys | Girls | |||||||
| Distribution | BCPE | BCPE | BCCG | BCCG | BCCG | BCCG | BCPE | BCCG |
| Predictor coefficients for | ||||||||
| Link | Log | Log | Log | Identity | Identity | Log | Identity | Identity |
| Intercept | −1.966 ± 0.067 | −2.117 ± 0.072 | −1.254 ± 0.179 | 1.735 ± 1.283 | −2.050 ± 0.156 | −1.764 ± 0.089 | −8.759 ± 2.938 | −0.062 ± 0.629 |
| Height (cm) | 0.016 ± 0.001 | 0.017 ± 0.001 | 0.012 ± 0.002 | 0.027 ± 0.008 | 0.026 ± 0.002 | 0.016 ± 0.001 | 1.768 ± 0.716a | |
| Weight (kg) | 0.118 ± 0.038a | 0.107 ± 0.042a | 0.703 ± 0.238a | 0.011 ± 0.002 | 0.003 ± 0.001 | 0.433 ± 0.172a | 1.168 ± 0.148a | |
| Ageb | 0.721 ± 0.241 | 16.916 ± 3.128a | 1.311 ± 0.403 | 1.186 ± 0.190a | ||||
| Age2 | 21.113 ± 4.020c | |||||||
| Age3 | 8.752 ± 1.674d | |||||||
| Predictor coefficients for log ( | ||||||||
| Intercept | −2.140 ± 0.057 | −2.142 ± 0.054 | −1.498 ± 0.044 | −1.764 ± 0.044 | −2.260 ± 0.038 | −2.206 ± 0.038 | −1.532 ± 0.032 | −1.702 ± 0.038 |
| Age | ||||||||
| Predictor coefficients for | ||||||||
| Intercept | 0.648 ± 0.495 | 1.051 ± 0.472 | 0.556 ± 0.209 | 0.106 ± 0.273 | 0.905 ± 0.391 | 1.277 ± 0.370 | 0.657 ± 0.163 | 0.900 ± 0.224 |
| Age | ||||||||
| Estimate of | ||||||||
| (for BCPE only) | 0.153 ± 0.112 | 0.280 ± 0.118 | – | – | – | – | 1.025 ± 0.131 | – |
| R2 | 0.859 | 0.846 | 0.566 | 0.663 | 0.867 | 0.850 | 0.560 | 0.586 |
| BIC | 13.06 | −51.09 | 380.03 | 527.24 | −111.48 | −157.24 | 464.71 | 624.68 |
Abbreviation and notation: FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC; PEF: peak expiratory flow rate; BCCG: Box–Cox–Cole–Green; BCPE: Box–Cox–power–exponential; : median; : coefficient of variation; : skewness; : kurtosis.
The variable is log-transformed.
"Age" values used in the model is the actual age divided by 20, i.e., Age = ”actual age”/20, for assuring the numerical stability when consider Age2 and Age3 as predictors.
The predictor in the model is , i.e., .
The predictor in the model is , i.e., .
Comparing the linear regression models in Table 3 to the GAMLSS models in Table 5, we observed that for FVC data in both boys and girls, the best GAMLSS model has a much smaller BIC than the linear regression model shown in Table 3 (GAMLSS BIC = 13.06 vs. linear regression BIC = 94.23 in boys; GAMLSS BIC = −111.48 vs. linear regression BIC = −83.78 in girls). Similar patterns were also observed with the other three spirometric indices: FEV1 (GAMLSS BIC = −51.09 vs. linear regression BIC = 17.06 in boys; GMALSS BIC = −157.24 vs. linear regression BIC = −154.37 in girls), FEF25-75 (GAMLSS BIC = 380.03 vs. linear regression BIC = 437.10 in boys; GAMLSS BIC = 464.71 vs. linear regression BIC = 482.49 in girls) and PEF (GAMLSS BIC = 527.24 vs. linear regression BIC = 554.08 in boys; GAMLSS BIC = 624.68 vs. linear regression BIC = 636.64 in girls). We hence chose the best GAMLSS models as our final reference equations to compute predictive values for the study population.
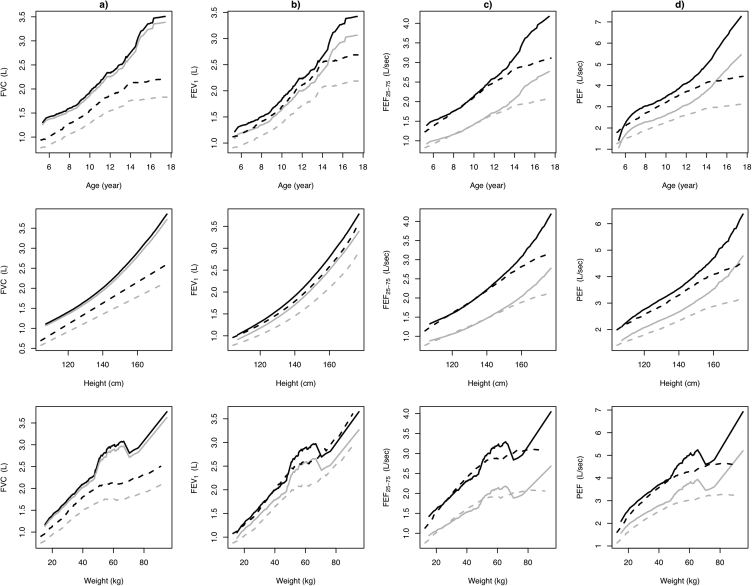
We further illustrated in the Supplementary Material how the results in Table 5 can be used to compute the predictive values, the LLN values and the z-scores of spirometric indices. We also provide a calculator that users can input age, sex, height, and weight and obtain the relevant values. Additionally, we presented the predictive values of spirometric indices including FVC, FEV1, FEF25-75 and PEF, and their corresponding LLN against age, height, and weight, separately; and found that predictive values and the corresponding LLN for all spirometric indices increased non-linearly with the increasing age, height, and weight in both boys and girls (Fig. 2, Fig. S1).
Fig. 2.
Relationships between predicted values (black lines) and lower limits of normal (fifth percentile, gray lines) for spirometric indices and age (top panel), height (middle panel) and weight (bottom panel), respectively, grouped by boys (black and gray solid lines) and girls (black and gray dashed lines). a) FVC, b) FEV1, c) FEF25-75, and d) PEF. Abbreviation: FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC; PEF: peak expiratory flow rate
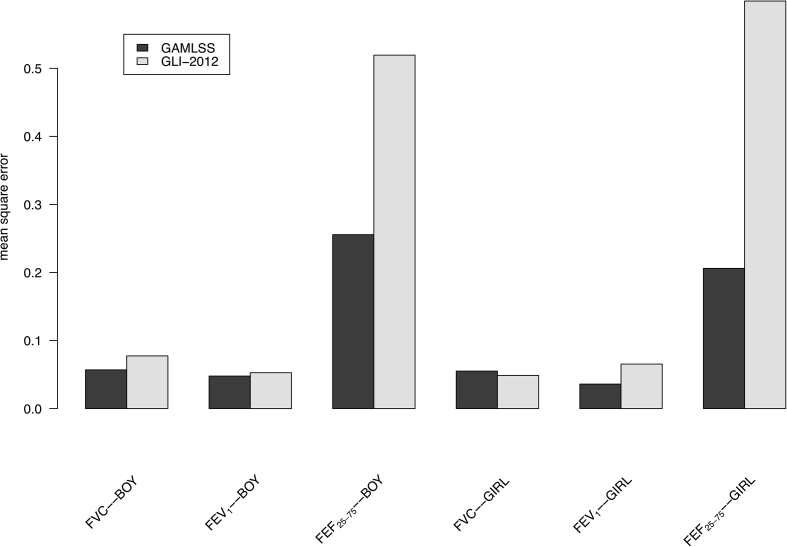
Next we compared the predictive performance in terms of MSEs between our best GAMLSS models (Table 5) and the GLI-2012 South East Asian equations. We focused on FVC, FEV1, and FEF25-75 as the GLI-2012 does not provide prediction equations for PEF. Smaller MSEs were observed for three spirometric indices (FVC, FEV1, and FEF25-75) in our GAMLSS models than those from the GLI-2012 equations in both boys and girls, expect for the MSEs of FVC in girls which is slightly smaller in the GLI-2012 equations (Fig. 3), suggesting better predictive results from our best GAMLSS models.
Fig. 3.
Mean square errors of spirometric indices from the best GAMLSS models and the GLI-2012 South East Asian equations. Abbreviation: GAMLSS: generalized additive models for location, scale and shape; GLI: Global Lung Function Initiative; FVC: Forced vital capacity; FEV1: forced expiratory volume in 1 s; FEF25–75: forced expiratory flow at 25–75% of FVC
Discussion
This is one of the largest study assessing the applicability of GLI-2012 prediction equations for South East Asians to healthy Taiwanese children. This population-based cohort study of 757 healthy Taiwanese children aged 5–18 years presents the following main findings. First, the present study results demonstrate that the GLI-2012 prediction equations for South East Asians are not properly matched to spirometric data in a contemporary Taiwanese children population. Second, we have established prediction equations for spirometry in healthy Taiwanese children aged 5–18 years using the conventional linear regression modeling and the GAMLSS method. Third, comparison of GAMLSS models, linear regression models and the GLI-2012 prediction equations for South East Asians provides supportive evidence that our GAMLSS models outperform linear regression models and the GLI-2012 equations in the prediction of spirometric data in healthy Taiwanese children.
When applying the GLI-2012 prediction equations for South East Asians to our study cohort of healthy Taiwanese children, the results showed that the GLI-2012 prediction equations overestimated most examined spirometric indices in both sexes, but underestimated FVC in boys. The observed results might be due to certain extent of ethnic heterogeneity in the GLI-2012 prediction equations for South East Asians since the GLI-2012 prediction equations were derived based on data collected from different ethnic populations, specifically, subjects from various regions of China (including Hong Kong and Shenzhen), Taiwan and Thailand,2 while the Taiwanese data was obtained from a study aiming to provide reference values for spirometry in adults which therefore had scant data in subjects less than 20 years of age.27 In addition, the Taiwanese data contributed to establish the GLI-2012 prediction equations were collected between 1990 and 1993, which might not reflect contemporary Taiwanese child populations and introduce overestimated bias probably because of different study protocols. The significant deviations from the GLI-2012 prediction equations for spirometry in the current study of healthy Taiwanese children, as well as other studies in non-Caucasian populations,13, 14, 15 highlight the importance of validating GLI-2012 prediction equations for spirometry in non-Caucasian populations before implementation to clinical practice. An update of GLI reference values by inclusion of more data of non-Caucasian decent is therefore recommended.
Most previous studies in Asian populations including Taiwan have employed linear regression models to generate prediction equations for spirometry,27, 28, 29, 30, 31 although it has been long known that the assumption of linearity does not necessarily hold for the relationships between age, body size and spirometric indices particularly in childhood and adolescence. Our findings lend further support that spirometric indices increase non-linearly with increasing age, height and weight in the age range of 5 to 18 years. The GAMLSS method provides suitable solution to the aforementioned long-standing problem by improving modeling that took into account the non-linear relationships between age, body size, and spirometric indices.32 In the current study, we applied the GAMLSS method to take age, height, and weight into account simultaneously. Our findings demonstrate that GAMLSS models provide a better fit to the spirometric data of healthy Taiwanese children than the conventional linear regression models.
Our results accounting for age, height, and weight as potential predictors in the prediction models of spirometric indices were comparable to previously reported prediction models in child populations.6,29,33,34 For example, Jiang et al. proposed prediction equations for spirometric reference values using a sample of Chinese children and suggested that age, height, and weight showed moderate-to-strong correlations in both boys and girls, but found that height was the most crucial predictor in their prediction equations.33 In this study, we found height and weight, but not age, were important predictors in the final prediction models for FVC and FEV1 in Taiwanese children. For FEF25-75 and PEF, age, height, and weight are important predictors in the final prediction models. Although the impact of weight on lung function was not fully appreciated, some studies29,33, 34, 35, 36 had reported that weight contributed to the prediction equations, probably because weight was related to body composition and therefore may influence lung function. The inclusion of weight in final prediction models in the current study further supports that weight might explain some variability of lung function in children, which is worth consideration in future studies developing reference values for spirometry in children.
As recommended by the American Thoracic Society and the European Respiratory Society, pulmonary function is affected by several factors including age, sex, height, weight, and ethnicity. There is a need to compare pulmonary function results of individuals with various characteristics to appropriate predicted values and LLN.7,37 We derived prediction equations for spirometry and corresponding LLN in our healthy Taiwanese childr population. We herein provide a calculator with which users can input the age, sex, height, and weight of interest, and directly obtain the corresponding predictive values, z-scores and LLN, respectively, for each spirometric index from the output. Findings from this study, specifically, reference values for spirometry as well as the calculator, would facilitate accurate interpretation of pulmonary function tests in Taiwanese children populations.
This study has several strengths. First, this study was conducted in a representative sample of healthy schoolchildren recruited from the community with a high participation rate of 90.4%. Second, the reference values for spirometry were established by a thorough analysis using both linear regression and GAMLSS method, in a large sample size of healthy children with a wide age range of 5 to 18 years. Third, considering the secular trends of pulmonary function features, it is important to update reference values periodically for the classifications of normal and abnormal reflecting the realities of the contemporary populations.7 The prediction equations derived in this study were based on the contemporary representative study cohort of Taiwanese children. To the best of our knowledge, reference values for spirometry in Taiwanese children covering a wide age range of 5 to 18 years are unavailable before the current study, although one to two decades ago, few studies reported linear regression equations for spirometry in Taiwanese children younger than 12 years of age.27, 28, 29 Compared to previous prediction equations using Chinese or Asian populations from different geographic regions, the prediction equations derived based on large sample of healthy children using advanced statistical algorithm in this study were more applicable to the child population in Taiwan.
On the other hand, some limitations should be noted. First, cross-sectional nature of the current study limits our ability to interpret longitudinal changes of pulmonary function. The longitudinal follow-up was now underway in the study participants, which may help to clarify secular trends of pulmonary function in future studies. Second, similar to most previous studies, some potential confounding factors (i.e., socioeconomic factors, diet, lifestyle, and environmental exposures) that might affect pulmonary function were not included in prediction models. Third, Quanjer et al. has suggested that at least 300 local healthy controls (150 males and 150 females) would be necessary to validate references to avoid spurious differences due to sampling error.38 Although our sample size of 757 healthy children met the requirement,38 sampling bias may still be a concern. Fourth, the extrapolation of our established prediction equations to Asian children living in other countries still needs further validation.
Conclusion
This study addresses the unmet need in up-to-date reference values for spirometry using GAMLSS statistical modeling method in healthy Taiwanese children aged 5 to 18 years. Our study provides evidence that the GLI-2012 prediction equations for South East Asians are not properly matched to spirometric data in a contemporary Taiwanese child population. Our results, together with several recent studies unable to validate the GLI-2012 prediction equations in non-Caucasian populations, clearly indicate the urgent need for an update of GLI reference values by inclusion of more data of non-Caucasian decent.
Potential competing interests
The authors report no competing interests.
Ethics statement
The Institutional Review Boards of Chang Gung Memorial Hospital approved the study protocol (100–3214A3). The parents or guardians of each participant gave the written informed consent. The procedures followed in this study were in accordance with the ethical standards of The Institutional Review Boards of Chang Gung Memorial Hospital and with the Helsinki Declaration of 1975, as revised in 1983.
Author contributions
S-M. Chang and H-J. Tsai performed data analysis, interpreted the results, and drafted the manuscript. J-Y. Tzeng provided intellectual input, assisted in data analysis and interpretation, and participated in drafting and critically revising the manuscript. K-W. Yeh, L-C. Chen, S-H. Lai, S-L. Liao, M-C. Hua, M-H. Tsai contributed to participant recruitment, cohort maintenance, and data collection. J-L. Huang coordinated the cohort, raised funding for the study and staff working on the project and provided thoughtful input in interpretation of the results. T-C. Yao conceptualized, designed, and supervised the study, raised funding for the study, performed data analysis, interpreted results, and drafted the manuscript. All authors contributed to the interpretation and discussion of the results, and read and approved the final manuscript.
Submission declaration
We confirm that this manuscript has not been submitted or is not simultaneously being submitted elsewhere, and that no portion of the data has been or will be published in proceedings or transactions of meetings or symposium volumes.
Acknowledgements
Members of the PATCH study group are: Jing-Long Huang (Study Coordinator), Tsung-Chieh Yao, Ming-Han Tsai, Sui-Ling Liao, Man-Chin Hua, Shen-Hao Lai, Kuo-Wei Yeh, and Li-Chen Chen (Principal Investigators). The authors thank the study participants, their parents, their teachers and the school nurses, as well as the schools involved, for their active participation in the study. The authors also thank the Department of Education, Keelung City Government, Keelung City, Taiwan for administrative support for the study; and thank Dr. Xin Liu for providing statistical consultation. This work was supported by the Ministry of Science and Technology of Taiwan (grants MOST 103-2314-B-182-030, MOST 104-2314-B-182-046-MY2 and MOST 106-2314-B-182-051-MY3) and by Chang Gung Medical Foundation (grants CMRPG4B0031~4B0033, CMRPG3E1201~3E1205, CORPG3F0081~3F0083, CMRPG3F1711~3F1713, CORPG3F0361 and CMRPG3J0121).
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100074.
Contributor Information
Jing-Long Huang, Email: long@adm.cgmh.org.tw.
Tsung-Chieh Yao, Email: yao@adm.cgmh.org.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Flow diagram of study sample selection. 1
References
- 1.Quanjer P.H., Borsboom G.J., Brunekreef B. Spirometric reference values for white European children and adolescents: polgar revisited. Pediatr Pulmonol. 1995;19:135–142. doi: 10.1002/ppul.1950190209. [DOI] [PubMed] [Google Scholar]
- 2.Quanjer P.H., Stanojevic S., Cole T.J. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao T.C., Tsai H.J., Chang S.W. Prediction of Allergies in Taiwanese Children Study G. Obesity disproportionately impacts lung volumes, airflow and exhaled nitric oxide in children. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174691. e0174691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korotzer B., Ong S., Hansen J.E. Ethnic differences in pulmonary function in healthy nonsmoking Asian-Americans and European-Americans. Am J Respir Crit Care Med. 2000;161:1101–1108. doi: 10.1164/ajrccm.161.4.9902063. [DOI] [PubMed] [Google Scholar]
- 5.Golshan M., Nematbakhsh M., Amra B., Crapo R.O. Spirometric reference values in a large Middle Eastern population. Eur Respir J. 2003;22:529–534. doi: 10.1183/09031936.03.00003603. [DOI] [PubMed] [Google Scholar]
- 6.Ip M.S., Karlberg E.M., Chan K.N., Karlberg J.P., Luk K.D., Leong J.C. Lung function reference values in Chinese children and adolescents in Hong Kong. II. Prediction equations for plethysmographic lung volumes. Am J Respir Crit Care Med. 2000;162:430–435. doi: 10.1164/ajrccm.162.2.9905058. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 8.Quanjer P.H., Brazzale D.J., Boros P.W., Pretto J.J. Implications of adopting the Global Lungs Initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013;42:1046–1054. doi: 10.1183/09031936.00195512. [DOI] [PubMed] [Google Scholar]
- 9.Brazzale D., Hall G., Swanney M.P. Reference values for spirometry and their use in test interpretation: a Position Statement from the Australian and New Zealand Society of Respiratory Science. Respirology. 2016;21:1201–1209. doi: 10.1111/resp.12855. [DOI] [PubMed] [Google Scholar]
- 10.Hall G.L., Thompson B.R., Stanojevic S. The Global Lung Initiative 2012 reference values reflect contemporary Australasian spirometry. Respirology. 2012;17:1150–1151. doi: 10.1111/j.1440-1843.2012.02232.x. [DOI] [PubMed] [Google Scholar]
- 11.Langhammer A., Johannessen A., Holmen T.L. Global Lung Function Initiative 2012 reference equations for spirometry in the Norwegian population. Eur Respir J. 2016;48:1602–1611. doi: 10.1183/13993003.00443-2016. [DOI] [PubMed] [Google Scholar]
- 12.Arigliani M., Canciani M.C., Mottini G. Evaluation of the global lung initiative 2012 reference values for spirometry in african children. Am J Respir Crit Care Med. 2017;195:229–236. doi: 10.1164/rccm.201604-0693OC. [DOI] [PubMed] [Google Scholar]
- 13.Abdullah N., Borhanuddin B., Shah S.A., Hassan T., Jamal R. Global Lung Initiative 2012 spirometry reference values in a large Asian cohort of Malay, Chinese and Indian ancestry. Respirology. 2018;23:1173–1179. doi: 10.1111/resp.13330. [DOI] [PubMed] [Google Scholar]
- 14.Bonner R., Lum S., Stocks J., Kirkby J., Wade A., Sonnappa S. Applicability of the global lung function spirometry equations in contemporary multiethnic children. Am J Respir Crit Care Med. 2013;188:515–516. doi: 10.1164/rccm.201212-2208LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Saad H., El Attar M.N., Hadj Mabrouk K. The recent multi-ethnic global lung initiative 2012 (GLI2012) reference values don't reflect contemporary adult's North African spirometry. Respir Med. 2013;107:2000–2008. doi: 10.1016/j.rmed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Backman H., Lindberg A., Sovijarvi A., Larsson K., Lundback B., Ronmark E. Evaluation of the global lung function initiative 2012 reference values for spirometry in a Swedish population sample. BMC Pulm Med. 2015;15:26. doi: 10.1186/s12890-015-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kainu A., Timonen K.L., Toikka J. Reference values of spirometry for Finnish adults. Clin Physiol Funct Imaging. 2016;36:346–358. doi: 10.1111/cpf.12237. [DOI] [PubMed] [Google Scholar]
- 18.Fasola S., La Grutta S., Cibella F., Cilluffo G., Viegi G. Global lung function initiative 2012 reference values for spirometry in south Italian children. Respir Med. 2017;131:11–17. doi: 10.1016/j.rmed.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 19.Cole T.J., Stanojevic S., Stocks J., Coates A.L., Hankinson J.L., Wade A.M. Age- and size-related reference ranges: a case study of spirometry through childhood and adulthood. Stat Med. 2009;28:880–898. doi: 10.1002/sim.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigby R.A., Stasinopoulos D.M. Generalized additive models for location, scale and shape (with discussion) Appl Statist. 2005;54:504–544. [Google Scholar]
- 21.Yao T.C., Ou L.S., Yeh K.W., Lee W.I., Chen L.C., Huang J.L. Associations of age, gender, and BMI with prevalence of allergic diseases in children: PATCH study. J Asthma. 2011;48:503–510. doi: 10.3109/02770903.2011.576743. [DOI] [PubMed] [Google Scholar]
- 22.Yao T.C., Lee W.I., Ou L.S., Chen L.C., Yeh K.W., Huang J.L. Reference values of exhaled nitric oxide in healthy Asian children aged 5 to 18 years. Eur Respir J. 2012;39:378–384. doi: 10.1183/09031936.00013911. [DOI] [PubMed] [Google Scholar]
- 23.Yao T.C., Tsai H.J., Tu Y.L. Multiplexed immunoglobulin E sensitization in relation to exhaled nitric oxide in a population sample of children. Allergy. 2014;69:678–682. doi: 10.1111/all.12378. [DOI] [PubMed] [Google Scholar]
- 24.Yao T.C., Chang S.W., Hua M.C. Tobacco smoke exposure and multiplexed immunoglobulin E sensitization in children: a population-based study. Allergy. 2016;71:90–98. doi: 10.1111/all.12775. [DOI] [PubMed] [Google Scholar]
- 25.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Cole T.J., Green P.J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 27.Pan W.H., Chen J.Y., Haung S.L. Reference spirometric values in healthy Chinese neversmokers in two townships of Taiwan. Chin J Physiol. 1997;40:165–174. [PubMed] [Google Scholar]
- 28.Tsai M.C., Jeng M.J., Chang H.L. Spirometric reference equations for healthy children aged 6 to 11 years in Taiwan. J Chin Med Assoc. 2010;73:21–28. doi: 10.1016/s1726-4901(10)70017-4. [DOI] [PubMed] [Google Scholar]
- 29.Jeng M.J., Chang H.L., Tsai M.C. Spirometric pulmonary function parameters of healthy Chinese children aged 3-6 years in Taiwan. Pediatr Pulmonol. 2009;44:676–682. doi: 10.1002/ppul.21038. [DOI] [PubMed] [Google Scholar]
- 30.Ip M.S., Karlberg E.M., Karlberg J.P., Luk K.D., Leong J.C. Lung function reference values in Chinese children and adolescents in Hong Kong. I. Spirometric values and comparison with other populations. Am J Respir Crit Care Med. 2000;162:424–429. doi: 10.1164/ajrccm.162.2.9905057. [DOI] [PubMed] [Google Scholar]
- 31.Stanojevic S., Wade A., Lum S., Stocks J. Reference equations for pulmonary function tests in preschool children: a review. Pediatr Pulmonol. 2007;42:962–972. doi: 10.1002/ppul.20691. [DOI] [PubMed] [Google Scholar]
- 32.Stanojevic S., Wade A., Stocks J. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M., Gao Y., Zhong N.S., Chen W.Q., Guan W.J., Zheng J.P. Spirometric reference values for healthy Han children aged 5-15 years in Guangzhou, southern China. Pediatr Pulmonol. 2015;50:1009–1016. doi: 10.1002/ppul.23099. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y.N., Wang J., Dong G.H. Predictive equations using regression analysis of pulmonary function for healthy children in Northeast China. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063875. e63875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doctor T.H., Trivedi S.S., Chudasama R.K. Pulmonary function test in healthy school children of 8 to 14 years age in south Gujarat region, India. Lung India. 2010;27:145–148. doi: 10.4103/0970-2113.68317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chhabra S.K., Vijayan V.K., Rahman M., Mittal V., Singh P.D. Regression equations for spirometry in children aged 6 to 17 years in Delhi region. Indian J Chest Dis Allied Sci. 2012;54:59–63. [PubMed] [Google Scholar]
- 37.Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. American Thoracic Society. [DOI] [PubMed] [Google Scholar]
- 38.Quanjer P.H., Stocks J., Cole T.J., Hall G.L., Stanojevic S., Global Lungs I. Influence of secular trends and sample size on reference equations for lung function tests. Eur Respir J. 2011;37:658–664. doi: 10.1183/09031936.00110010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.