Summary
Disorders of human skin manifest themselves with patterns of lesions ranging from simple scattered spots to complex rings and spirals. These patterns are an essential characteristic of skin disease, yet the mechanisms through which they arise remain unknown. Here we show that all known patterns of psoriasis, a common inflammatory skin disease, can be explained in terms of reaction-diffusion. We constructed a computational model based on the known interactions between the main pathogenic cytokines: interleukins IL-17 and IL-23, and tumor necrosis factor TNF-α. Simulations revealed that the parameter space of the model contained all classes of psoriatic lesion patterns. They also faithfully reproduced the growth and evolution of the plaques and the response to treatment by cytokine targeting. Thus the pathogenesis of inflammatory diseases, such as psoriasis, may be readily understood in the framework of the stimulatory and inhibitory interactions between a few diffusing mediators.
Subject Areas: Dermatology, Biological Sciences, in silico biology
Graphical Abstract
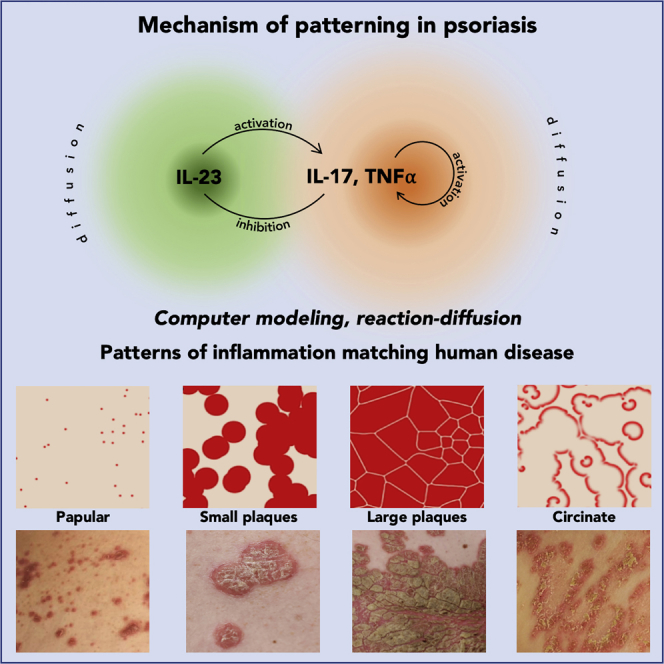
Highlights
-
•
Skin lesions in psoriasis are captured in reaction-diffusion models (RDMs)
-
•
Activator-depleted substrate RDM reflects interactions of pathogenic cytokines
-
•
Evolution of psoriatic lesions is reproduced using parameters from the Gray-Scott space
Dermatology; Biological Sciences; in silico biology
Introduction
Most skin diseases manifest themselves with reproducible patterns of skin lesions, which are conventionally described in terms of lesion morphology (e.g., macules, papules, plaques) and distribution on the skin surface (Nast et al., 2016). The biological basis of pattern formation is only understood in a few special cases. For instance, the segmental pattern of herpes zoster reflects dermatomal viral reactivation through sensory nerves, and the linear pattern in Blaschko lines represents genetic mosaicism. In most cases, however, the mechanisms by which pathological processes in the skin generate reproducible patterns remain virtually unknown (Nast et al., 2016).
The majority of skin diseases are inflammatory, which explains why the lesions are often red, elevated, and scaly (resulting from, respectively, vasodilation and hyperemia, inflammatory infiltrate and edema, and pathologically increased epidermal keratinization secondary to inflammation). The skin has a large surface (average 1.5 m2–2.0 m2) compared with its thickness (0.5 mm–4 mm; surface-to-volume ratio of approximately 650m2/m3) (Leider, 1949) and is therefore ideally suited to study the mechanisms of the spatial propagation of inflammatory processes in a tissue. Psoriasis, a chronic, autoimmune inflammatory skin disease affecting 2%–3% of the population in Western countries (Parisi et al., 2013) provides a particularly useful model. The lesions are sharply demarcated, scaly, and distributed symmetrically on the body (Christophers, 2001, Griffiths and Barker, 2007, Nestle et al., 2009). The plaques evolve from pinpoint papules by centrifugal growth, which explains the oval contour of mature lesions (Farber et al., 1985, Soltani and Van Scott, 1972). Individual plaques may merge producing polycyclic contours (Christophers, 2001, Farber et al., 1985). In some instances the plaques have the appearance of rings (referred to as annular, arciform, or circinate patterns) (Christophers, 2001, Nast et al., 2016), which is the predominant morphological feature in approximately 5% patients (Morris et al., 2001). The mechanisms responsible for these patterns are not readily explainable in terms of the lateral propagation of inflammation, in which one would expect a gradual attenuation of inflammation due to the dilution of proinflammatory agents that diffuse in the skin. In contrast, in psoriatic lesions the intensity of inflammation is preserved throughout the whole plaque and sharply suppressed at its margin over the distance of a few millimeters. We show that the phenotypic features of psoriasis can be explained in terms of interactions between key pathogenic cytokines consistent with a reaction-diffusion model. This model captures all cardinal phenotypic features of psoriasis and may provide a wider framework to understand the patterning and maintenance of inflammation in other skin diseases.
Results
Classification of Psoriasis Plaque Patterns
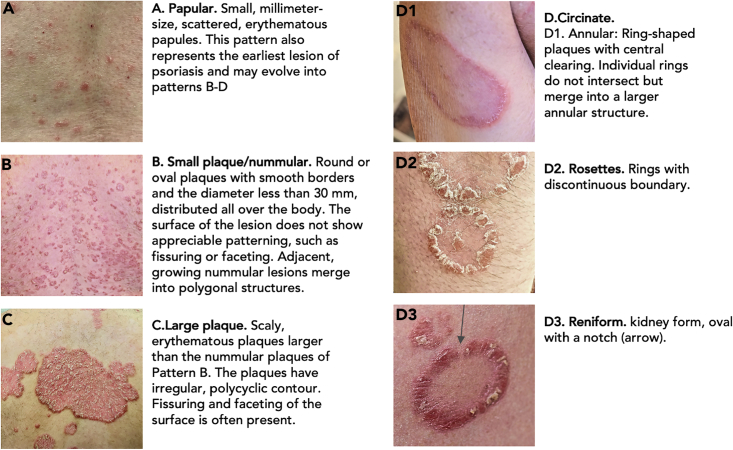
The patterns repetitively identified in the literature and in our clinical photograph repository are listed in Figure 1, with further morphological details characteristic of different patterns shown in Figure S1. As detailed in Transparent Methods, we have excluded linear psoriasis, psoriatic erythroderma, and guttate psoriasis from our classification.
Figure 1.
Patterns of Skin Lesions in Psoriasis
See also Figure S1.
A feature not explicitly discussed in the literature is the patterning of the plaque itself, manifest in the shape of the scales and/or irregularities of the plaque surface. The intensity of the inflammatory process is not homogeneous within the plaque. In the very early pinpoint papules the inflammatory infiltrate is most dense at the center, which translates into higher proliferative activity of the keratinocytes and a thicker scale centrally in the papule (Figure S1A) (Soltani and Van Scott, 1972). As the lesion grows the inflammatory infiltrate becomes more irregular, with a tendency toward higher activity at the periphery and occasional hotspots inside the plaque. A growing plaque, such as a nummular lesion, is thus often slightly thicker and scalier at the periphery than in the center. Likewise, the central portion of the plaque clears more rapidly during treatment, whereas the regression of inflammatory hotspots and the marginal region is delayed (Griffin et al., 1988). Large, mature plaques demonstrate a complex pattern of polygonal faceting rather than thickening of the margins (Figure S1D).
Model of Cytokine Interactions in Psoriasis
Cytokines interleukin IL-23, IL-17, and tumor necrosis factor TNF-α are central mediators in psoriatic plaque formation, as underscored by the fact that pharmacological blockade of either cytokine by monoclonal antibodies causes clinical remission in a large proportion of patients (Jabbar-Lopez et al., 2017). Interactions between the cytokines inferred from the available data are shown schematically in Figure 2A. The most important pathogenic cytokines are those of the IL-17 family, being produced primarily by TH17 lymphocytes (interaction 0) (Krueger et al., 2012). These cells require IL-23 for expansion and activation (Cosmi et al., 2008, Wilson et al., 2007, Zheng et al., 2006) and amplify the inflammatory process by inducing other proinflammatory cytokines, the most important of which is TNF-α (Boehncke and Schön, 2015). Psoriatic plaques contain both dendritic cells producing IL-23 and TH17 cells expressing the IL-23 receptor (Cosmi et al., 2008, Lee et al., 2004, Tillack et al., 2014, Wilson et al., 2007). Treatment with guselkumab, a selective therapeutic monoclonal antibody inhibiting IL-23, attenuates IL-17s in psoriatic plaques and in serum in patients with psoriasis (interaction 1) (Hawkes et al., 2018, Sofen et al., 2014, Tillack et al., 2014). This attenuation is correlated with the clinical clearing of psoriasis lesions (Sofen et al., 2014). IL-17 and TNF-α synergize with each other (Alzabin et al., 2012, Krueger et al., 2012, Xu et al., 2017): IL-17 increases the expression of TNF-α (Jovanovic et al., 1998) (interaction 2), whereas therapeutic TNF-α inhibition blocks IL-17 in responding patients (interaction 3) (Zaba et al., 2007, Zaba et al., 2009). The positive feedback of IL-17 cytokines on their own production (interactions 2 and 3) is further demonstrated by the findings that IL-17A induces IL-17C (Xu et al., 2018) and that the therapeutic inhibition of the IL-17 receptor with brodalumab reduces the expression of the IL-17 cytokine (IL-17A, C, F) (Russell et al., 2014). TNF-α downregulates IL-23 (interaction 4) either directly (Notley et al., 2008, Zakharova and Ziegler, 2005) or indirectly via the inhibition of interferons (Palucka et al., 2005, Tillack et al., 2014). Disturbance of this negative interaction is probably responsible for paradoxical induction of psoriasis in patients with rheumatoid arthritis and inflammatory bowel disease treated with TNF-α antibodies (Palucka et al., 2005, Tillack et al., 2014). This induction is readily reverted by therapeutic inhibition of the excess of IL-23 by ustekinumab, an antibody binding to the p40 chain of IL-23 (Tillack et al., 2014).
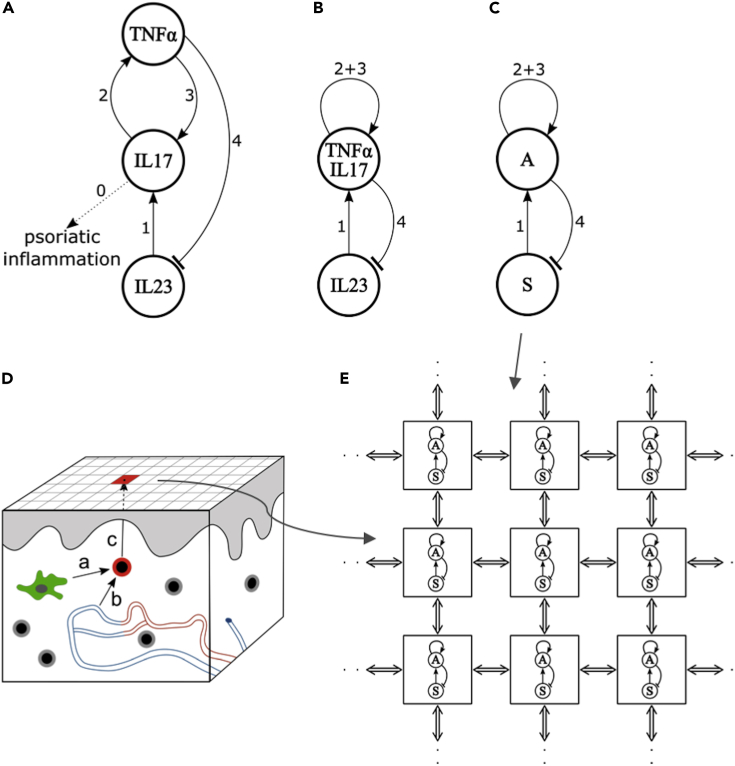
Figure 2.
Modeling Plaque Formation in Psoriasis
(A) Interactions between key cytokines involved in psoriasis plaque formation. Labels numbered 0–4 refer to the observations from which these interactions have been inferred (see Results).
(B) A simplified diagram of interactions, in which cytokines IL-17 and TNF-α are considered jointly.
(C) Diagram (B) relabeled as an activator (A) - depleted substrate (S) system.
(D) Skin representation and simulation initialization. The skin surface is partitioned into square regions. A lesion is initiated by an activated TH17 cell (red), which is either a resident memory T cell activated by a dendritic cell (green, interaction a) or has migrated from circulation through a capillary wall (interaction b). The area of microinflammation around the activated TH17 cell is considered as a “seed” region, and its projection to the surface (arrow c) is colored in red. The epidermis, the upper layer of the skin, is shaded in gray and capillaries in the dermis are colored in red (arterioles) and blue (venules). Skin-resident memory T cells are marked in gray.
(E) Detail of skin surface representation. Each region is a two-dimensional projection of the underlying activator-depleted substrate system of proinflammatory cytokines and represents a computational cell implementing reaction system (C). These computational cells are interconnected (double arrows), allowing for the diffusion of cytokines.
Computational Model Construction
To analyze whether the molecular-level interactions depicted in Figure 2A can account for the observed plaque patterns and the response of the disease to treatment, we constructed a mathematical model. We followed the standard method of simplifying the modeled system to focus on its essence and make it more amenable to analysis (Bak, 1996, Gaines, 1977, Prusinkiewicz, 1998). This simplification reduced the size of the parameter space and thus, to the extent possible, the use of parameters for which quantitative data are currently unavailable. It has also related the problem of plaque pattern formation to a known class of reaction-diffusion systems, which provided guidance for the exploration of the parameter space, and facilitated the analysis and interpretation of the results.
We have pursued the following train of thought. The mutual promotion of cytokines IL-17 and TNF-α, represented by interactions 2 and 3 in Figure 2A, suggests that their concentrations may change in concert. Assuming this is the case, we reduced the three-substance graph in Figure 2A by representing IL-17 and TNF-α jointly. The resulting two-substance graph (Figure 2B) has the structure of an activator-depleted substrate reaction-diffusion model (Gierer and Meinhardt, 1972, Marcon et al., 2016) (Figure 2C). In this model, the substrate S with concentration s is locally converted into the activator A with concentration a according to the canonical equations (Gierer and Meinhardt, 1972, Meinhardt, 1982):
| (Equation 1) |
The term ka2s indicates that the conversion is autocatalytically promoted by the activator, with the rate controlled by parameter k. The activator concentration increases at the expense of the substrate, thus the activator downregulates the substrate. Parameters ρa0 and ρs0 are the rates of the base production of the activator and the substrate, and μa and μs control their turnover. The remaining terms, and , represent diffusion of the activator and substrate at rates controlled by parameters Da and Ds, respectively (for simplicity, diffusion is not explicitly represented in Figures 2A–2C). Consistent with Figures 2B and 2C, we identify variable a with the concentration of cytokines TNF-α and IL-17 and s with the concentration of IL-23:
In the simulations, a patch of skin surface (Figure 2D) is represented by an array of interconnected computational “cells,” each of which performs local computation according to Equation 1 (Figure 2E). The initial state in all simulations is a uniform distribution of IL-23 in the whole array, except for randomly distributed small “seed” areas with a high concentration of IL-17 and TNF-α. These areas represent IL-17-secreting cells (such as the TH17-cell) that either have been activated in situ (Figure 2D, interaction a) or have migrated from the circulation to the skin (Figure 2D, interaction b) (Krueger et al., 2012).
Exploration of the Model Parameter Space
Currently it is not feasible to measure the diffusion of cytokines in human skin, hence there are no experimental data to provide suggestions for the parameter values of the model. Consequently, we adopted a reverse strategy, where we explored the model parameter space by searching for values that would yield psoriasis patterns observed in patients (Figure 1). To guide this search, we referred to the Gray-Scott reaction-diffusion system (Gray and Scott, 1984), for which the parameter space has been thoroughly explored:
| (Equation 2) |
We observe (see also Yamamoto and Miorandi, 2010, Yamamoto et al., 2011) that Equation 2 are a special case of Equation 1, where
The parameter space and details of six patterns obtained for specific parameter values are shown in Figure 3. These patterns correspond visually to the six types of psoriasis identified in patients (Figure 1). Note that, consistent with the common assumption of the Gray-Scott reaction-diffusion model, the ratio of the diffusion rates of substrate and activator was set to Ds:Da = 2 (Pearson, 1993). This is a departure from the much larger ratios typically used in reaction-diffusion models (Diego et al., 2018, Gierer and Meinhardt, 1972, Kondo and Miura, 2010, Lengyel and Epstein, 1991, Marcon et al., 2016, Vastano et al., 1987). On biochemical grounds, this departure is justified by the commensurate small size of the three cytokines, implying comparable diffusion rates (see Table S1). The small ratio of diffusion rates does not preclude Turing instability and spontaneous pattern emergence for carefully chosen values of the remaining parameters (see Figure S2). Nevertheless, the parameter values leading to the formation of plaque patterns are compatible with the “filtering” operation mode, in which the patterns do not emerge spontaneously in a homogeneous medium and elaborate the initial pre-patterns instead (Diego et al., 2018, Lee et al., 1993, Muratov and Osipov, 2000, Pearson, 1993). This latter mode is more pertinent to the development of psoriasis plaques, which is initiated by activated TH17 cells in the skin (Figure 2D).
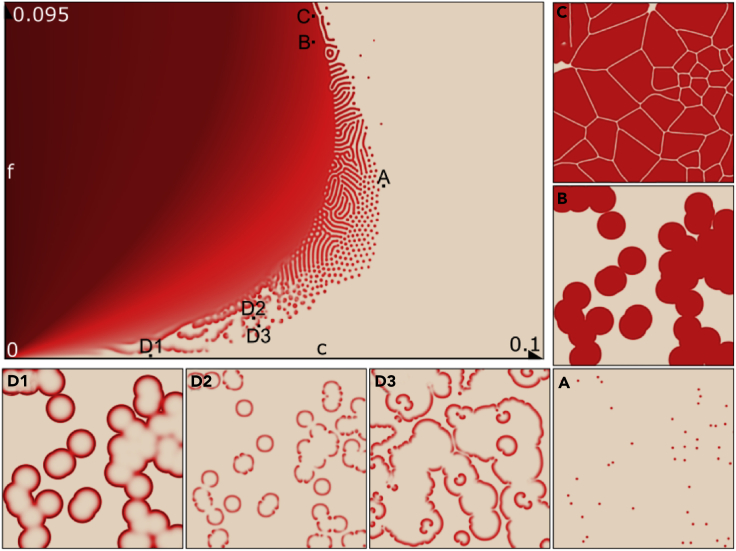
Figure 3.
Parameter Space of the Model and Selected Patterns
Top left: A comprehensive representation of the range of patterns generated using Equation 2 for different values of the synthetic parameters c and f.
(A–D) Magnified views of patterns generated using select parameter values. These labels and patterns correspond to the psoriatic skin lesions identified in Figure 1.
The Development of Lesions and Response to Treatment
The simulated development of psoriasis lesions and the response to treatment are shown in Figure 4 and in Videos S1, S2, S3, S4, S5, and S6. The development was simulated by using the forward Euler method to advance the state of the reaction-diffusion model over time, given an initial random distribution of small papules. The parameter values and initial conditions for each of these simulations are listed in Table S2, with additional information characterizing the sensitivity of simulations to the variation of (individual) parameter values collected in Table S3. Minimum values of the activator A, representing cytokines IL-17 and TNF-α, needed to initiate pattern formation are given in Table S4. The simulated patterns shown in Figure 4 have a striking resemblance to the actual patterns of psoriatic skin lesions shown in Figure 1. Next, we simulated the effect of therapy by increasing the decay rate of cytokines IL-17 and TNF-α (activator A), which mimics real-life treatment with an anti-cytokine antibody. Interestingly, the simulated lesion clearing was not simply a time reversal of the processes of plaque formation: the interior of the plaques cleared first, producing annular lesions (Figure 4, row 5). The residual lesions dispersed slowly, eventually disappearing entirely or leaving residual spots (Figure 4, row 6). These results closely resemble clinical situations, in which residual annular or papular lesions are often observed (Figure S1C).
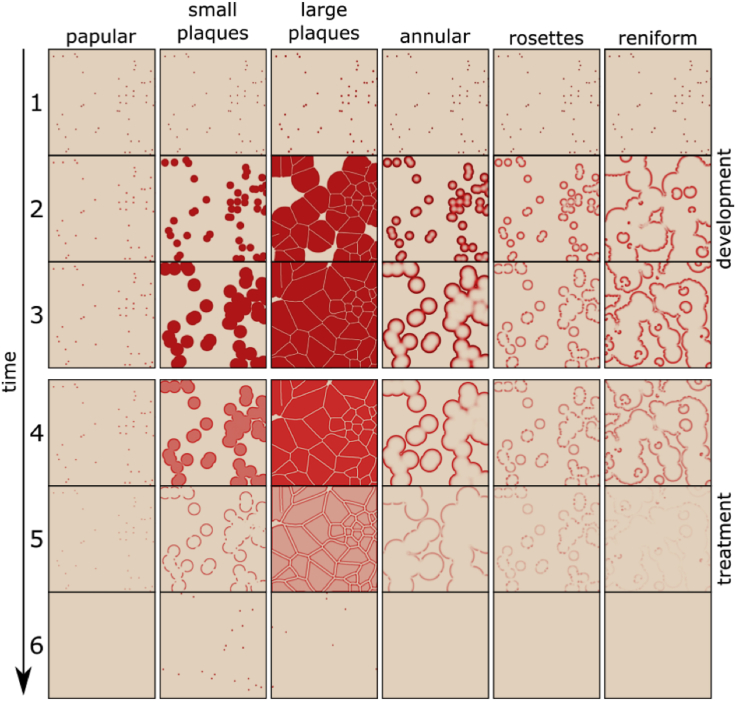
Figure 4.
The Simulated Progression of Different Types of Psoriatic Lesions
Rows 1–3: Development of the lesions. The earliest stage of a papule (Row 1) consists of randomly distributed small seed areas. Later forms of the disease (Rows 2 and 3) correspond to patterns identified in Figures 1 and 3. Rows 4–6: The effect of treatment simulated by increasing the decay rate of IL-17 and TNF-α. Note that the treatment does not result in a simple reversal of the original pattern development, but produces residual lesions with more activity at the margin of the plaques (Row 5). In some instances, residual papules persist (Row 6).
Finally, to verify that the modeling results do not critically depend on the reduction of the three-substance system in Figure 2A to the two-substance system in Figure 2B, we have constructed a simulation model corresponding directly to Figure 2A (see Supplemental Equations). Guided in part by parameter values found for the two-substance model (Tables S2 and S3), we found values for which the three-substance model produces qualitatively the same plaque patterns (Table S5). This result validates the simplification underlying the two-substance model.
Discussion
Since the foundation of dermatology as a medical specialty in the beginning of the 19th century, morphological patterns provided a useful and robust criterion for the diagnosis and classification of skin diseases. However, the mechanisms by which skin diseases produce diverse patterns remained unknown. We have shown that all major morphological types of the common skin disease psoriasis (papular, small plaque, large plaque, and different forms of circinate patterns) can be generated by a reaction-diffusion model with different parameter values. The model is based on the currently known up- and down-regulating interactions between three proinflammatory cytokines: TNF-α, IL-23, and IL-17. These interactions are not direct chemical reactions, but are mediated by immunologically active cells stimulating or inhibiting the release and proliferation of intermediary cytokines. The model has a spatiotemporal character, explaining the emergence of patterns during disease development and their disappearance during subsequent treatment. Reaction-diffusion thus provides a promising framework for studying mechanisms underlying the progress and treatment of psoriasis. As detailed data regarding the interaction and diffusion of cytokines involved in psoriasis become available, more elaborate models may be constructed to re-create the actual biological processes in the skin with increased accuracy. Recent advances in the theoretical understanding of reaction-diffusion (Diego et al., 2018) suggest that the resulting models may also become more robust to parameter changes, currently limited to narrow ranges.
Inflammatory patterns related to psoriasis are found in other diseases as well. For example, annular lesions are seen in erythema multiforme, dermatophytosis, and erythema annulare centrifugum; reniform patterns in erythema gyratum repens, urticaria, and lupus erythematosus; and rosettes in granuloma annulare. We thus hypothesize that reaction-diffusion models can be applied further to explain the patterns of other inflammatory skin diseases and suggest their treatment by selective cytokine inhibition. Eventually, reaction-diffusion models could provide a framework for understanding the pathogenesis and pharmacologic intervention of a broad spectrum of skin diseases.
Limitations of the Study
The model is based on qualitative results describing the interaction of cytokines in the skin, but its predictions have not yet been confirmed by measurements of cytokine concentrations and propagation rates in the skin. Such measurements are currently difficult for a combination of technical and ethical reasons and thus are left as a topic for further research, which we hope our work will motivate.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Mikolaj Cieslak for insightful discussions and comments, and Robert Munafo for advice on Gray-Scott reaction-diffusion models and their parameter space. This work was supported by the Natural Sciences and Engineering Research Council of Canada Discovery Grants 2014-05325 and 2019-06279 to P.P., and unrestricted research grants from the Department of Medicine, University of Alberta, and Department of Dermatology, Bispebjerg Hospital, University of Copenhagen to R.G.
Author Contributions
P.P. and R.G. designed research; L.R. and P.P. created the mathematical model and performed computer simulations; L.R., P.P. and R.G. wrote the paper.
Declaration of Interests
L.R., P.P., and R.G. have no conflict to declare.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.008.
Contributor Information
Przemyslaw Prusinkiewicz, Email: pwp@ucalgary.ca.
Robert Gniadecki, Email: r.gniadecki@ualberta.ca.
Supplemental Information
References
- Alzabin S., Abraham S.M., Taher T.E., Palfreeman A., Hull D., McNamee K., Jawad A., Pathan E., Kinderlerer A., Taylor P.C. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann. Rheum. Dis. 2012;71:1741–1748. doi: 10.1136/annrheumdis-2011-201024. [DOI] [PubMed] [Google Scholar]
- Bak P. Springer; 1996. How Nature Works. The Science of Self-Organized Criticality. [Google Scholar]
- Boehncke W.-H., Schön M.P. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- Christophers E. Psoriasis–epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001;26:314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- Cosmi L., De Palma R., Santarlasci V., Maggi L., Capone M., Frosali F., Rodolico G., Querci V., Abbate G., Angeli R. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego X., Marcon L., Müller P., Sharpe J. Key features of turing systems are determined purely by network topology. Phys. Rev. X. 2018;8 [Google Scholar]
- Farber E.M., Nall L., Strefling A. Psoriasis: a disease of the total skin. J. Am. Acad. Dermatol. 1985;12:150–156. doi: 10.1016/s0190-9622(85)70019-9. [DOI] [PubMed] [Google Scholar]
- Gaines B.R. System identification, approximation and complexity. Int. J. Gen. Syst. 1977;3:145–174. [Google Scholar]
- Gierer A., Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Gray P., Scott S.K. Autocatalytic reactions in the isothermal, continuous stirred tank reactor. Chem. Eng. Sci. 1984;39:1087–1097. [Google Scholar]
- Griffin T.D., Lattanand A., VanScott E.J. Clinical and histologic heterogeneity of psoriatic plaques. Therapeutic relevance. Arch. Dermatol. 1988;124:216–220. [PubMed] [Google Scholar]
- Griffiths C.E., Barker J.N. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- Hawkes J.E., Yan B.Y., Chan T.C., Krueger J.G. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J. Immunol. 2018;201:1605–1613. doi: 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar-Lopez Z.K., Yiu Z.Z.N., Ward V., Exton L.S., Mohd Mustapa M.F., Samarasekera E., Burden A.D., Murphy R., Owen C.M., Parslew R. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J. Invest. Dermatol. 2017;137:1646–1654. doi: 10.1016/j.jid.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Kondo S., Miura T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science. 2010;329:1616–1620. doi: 10.1126/science.1179047. [DOI] [PubMed] [Google Scholar]
- Krueger J.G., Fretzin S., Suárez-Fariñas M., Haslett P.A., Phipps K.M., Cameron G.S., McColm J., Katcherian A., Cueto I., White T. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012;130:145–154.e9. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.J., McCormick W.D., Ouyang Q., Swinney H.L. Pattern formation by interacting chemical fronts. Science. 1993;261:192–194. doi: 10.1126/science.261.5118.192. [DOI] [PubMed] [Google Scholar]
- Lee E., Trepicchio W.L., Oestreicher J.L., Pittman D., Wang F., Chamian F., Dhodapkar M., Krueger J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leider M. On the weight of the skin. J. Invest. Dermatol. 1949;12:187–191. [PubMed] [Google Scholar]
- Lengyel I., Epstein I.R. Modeling of turing structures in the chlorite–iodide–malonic Acid–starch reaction system. Science. 1991;251:650–652. doi: 10.1126/science.251.4994.650. [DOI] [PubMed] [Google Scholar]
- Marcon L., Diego X., Sharpe J., Müller P. High-throughput mathematical analysis identifies Turing networks for patterning with equally diffusing signals. Elife. 2016;5 doi: 10.7554/eLife.14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. Academic Press; 1982. Models of Biological Pattern Formation. [Google Scholar]
- Morris A., Rogers M., Fischer G., Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr. Dermatol. 2001;18:188–198. doi: 10.1046/j.1525-1470.2001.018003188.x. [DOI] [PubMed] [Google Scholar]
- Muratov C.B., Osipov V.V. Static spike autosolitons in the Gray-Scott model. J. Phys. A Math. Gen. 2000;33:8893–8916. [Google Scholar]
- Nast A., Griffiths C.E.M., Hay R., Sterry W., Bolognia J.L. The 2016 International League of Dermatological Societies’ revised glossary for the description of cutaneous lesions. Br. J. Dermatol. 2016;174:1351–1358. doi: 10.1111/bjd.14419. [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Kaplan D.H., Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Notley C.A., Inglis J.J., Alzabin S., McCann F.E., McNamee K.E., Williams R.O. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J. Exp. Med. 2008;205:2491–2497. doi: 10.1084/jem.20072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka A.K., Blanck J.-P., Bennett L., Pascual V., Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc. Natl. Acad. Sci. U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi R., Symmons D.P.M., Griffiths C.E.M., Ashcroft D.M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Invest. Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- Pearson J.E. Complex patterns in a simple system. Science. 1993;261:189–192. doi: 10.1126/science.261.5118.189. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P. In search of the right abstraction: the synergy between art, science, and information technology in the modeling of natural phenomena. In: Sommerer C., Mignonneau L., editors. Art @ Science. Springer; 1998. pp. 60–68. [Google Scholar]
- Russell C.B., Rand H., Bigler J., Kerkof K., Timour M., Bautista E., Krueger J.G., Salinger D.H., Welcher A.A., Martin D.A. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J. Immunol. 2014;192:3828–3836. doi: 10.4049/jimmunol.1301737. [DOI] [PubMed] [Google Scholar]
- Sofen H., Smith S., Matheson R.T., Leonardi C.L., Calderon C., Brodmerkel C., Li K., Campbell K., Marciniak S.J., Jr., Wasfi Y. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J. Allergy Clin. Immunol. 2014;133:1032–1040. doi: 10.1016/j.jaci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Soltani K., Van Scott E.J. Patterns and sequence of tissue changes in incipient and evolving lesions of psoriasis. Arch. Dermatol. 1972;106:484–490. [PubMed] [Google Scholar]
- Tillack C., Ehmann L.M., Friedrich M., Laubender R.P., Papay P., Vogelsang H., Stallhofer J., Beigel F., Bedynek A., Wetzke M. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–577. doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]
- Vastano J.A., Pearson J.E., Horsthemke W., Swinney H.L. Chemical pattern formation with equal diffusion coefficients. Phys. Lett. A. 1987;124:320–324. [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Xu T., Ying T., Wang L., Zhang X.D., Wang Y., Kang L., Huang T., Cheng L., Wang L., Zhao Q. A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A. Oncotarget. 2017;8:81860–81872. doi: 10.18632/oncotarget.19899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Lu H., Lee Y.-H., Wu Y., Liu K., Shi Y., An H., Zhang J., Wang X., Lai Y. An interleukin-25-mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787–798.e4. doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Yamamoto L., Miorandi D. Lecture Notes in Computer Science. 2010. Evaluating the robustness of activator-inhibitor models for cluster head computation; pp. 143–154. [Google Scholar]
- Yamamoto L., Miorandi D., Collet P., Banzhaf W. Recovery properties of distributed cluster head election using reaction–diffusion. Swarm Intelligence. 2011;5:225–255. [Google Scholar]
- Zaba L.C., Cardinale I., Gilleaudeau P., Sullivan-Whalen M., Suárez-Fariñas M., Fuentes-Duculan J., Novitskaya I., Khatcherian A., Bluth M.J., Lowes M.A. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba L.C., Suárez-Fariñas M., Fuentes-Duculan J., Nograles K.E., Guttman-Yassky E., Cardinale I., Lowes M.A., Krueger J.G. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J. Allergy Clin. Immunol. 2009;124:1022–1110.e1–e395. doi: 10.1016/j.jaci.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova M., Ziegler H.K. Paradoxical anti-inflammatory actions of TNF-alpha: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J. Immunol. 2005;175:5024–5033. doi: 10.4049/jimmunol.175.8.5024. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2006;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.