To the Editor:
High-profile investigations have focused on alveolar regeneration after lung injury, notably expansion of the alveolar epithelial type 2 cell (AEC2) population (1–4). Investigations of AEC2 expansion, particularly mechanistic investigations using pharmacologic or genetic manipulation, require an accurate, unbiased method of quantitation to ensure valid comparison between experimental groups. AEC2 expansion is typically assessed by flow cytometry of cells recoverable from a lung digest (1–3, 5–7) or by counting cell profiles in arbitrary fields of immunostained lung sections (planimetry) (1, 2, 4, 7). AEC2 expansion is usually measured as the percentage of AEC2s that are actively cycling, as determined by incorporation of nucleoside analogs (1–7) or AEC2 number as a percentage of a larger population, for example, total lung cells (1, 2, 5); rarely is absolute AEC2 number assessed (1, 7).
These methods have not been validated and have important theoretical limitations. Flow cytometric analysis may be limited by incomplete and variable recovery of cells. Planimetric analysis may be biased by tissue inflation and shrinkage, cell loss during sectioning, the overrepresentation of larger cells in two-dimensional sections, and nonrandom sampling, which is particularly problematic for lung injury with a patchy distribution (8–10).
Evaluating AEC2 expansion as the percentage of actively cycling cells is limited by the duration of the pulse and ascertains S phase, not cell division. Expressing the number of actively cycling cells as a percentage of total AEC2s, or total AEC2 number as a percentage of a larger population, may be confounded by changes in the denominator. Epithelial regeneration requires increased absolute AEC2 numbers to replace cells lost during injury.
Stereology is an unbiased approach recommended by the American Thoracic Society for the quantitation of cell number (10) but is rarely used to assess AEC2 expansion. Here, we employed stereology as the “gold standard” method for quantifying AEC2 expansion during repair after lung injury. We measured the absolute number of AEC2s, the key parameter for alveolar regeneration, and the absolute number and percentage of actively cycling AEC2s. The results were compared with flow cytometric and planimetric analyses.
Methods
SPCCreERT2+/−;mTmG+/− mice induced with tamoxifen were treated with LPS and bromodeoxyuridine (BrdU) (6, 11).
Stereology
Lungs were inflation fixed, and systematic uniform random sampling was performed at every level using a multistage fractionator (10–12). All lung tissue except the accessory lobe was paraffin-embedded with a block sampling fraction (bsf) of 2. Blocks were exhaustively cut into 30-μm sections and stained with antibodies against green fluorescent protein (GFP) and BrdU. Cuboidal GFP+ or GFP+BrdU+ cells were counted with an optical fractionator. The section sampling fraction (ssf), height sampling fraction (hsf), and area sampling fraction (asf) were determined. The total number of GFP+ [] or GFP+BrdU+ [] cells was calculated as
where ΣQ− is the number of cells counted. Cell volume was estimated by the nucleator method (10).
Flow cytometry
Lungs were digested with dispase, elastase, and/or collagenase (1–3, 5, 6). Cells were stained with LIVE/DEAD fixable stain (Thermo Fisher) and then fixed, permeabilized, and stained for BrdU (6). Debris was excluded by forward versus side scatter gating; subsequent gates included singlets, live, GFP+, and BrdU+. The GFP+ cells are CD45−EpCAM+proSPC+ (EpCAM, epithelial cell adhesion molecule; proSPC, pro–surfactant protein C) (6; and data not shown).
where N(L) is the total number of cells in the digest.
Planimetry
The number of GFP+ or GFP+BrdU+ cells per random ×63 field of immunostained 4-μm sections was counted.
or determined by any method was multiplied by 1/recombination efficiency (11) to yield N(AEC2) or .
Results
Stereology
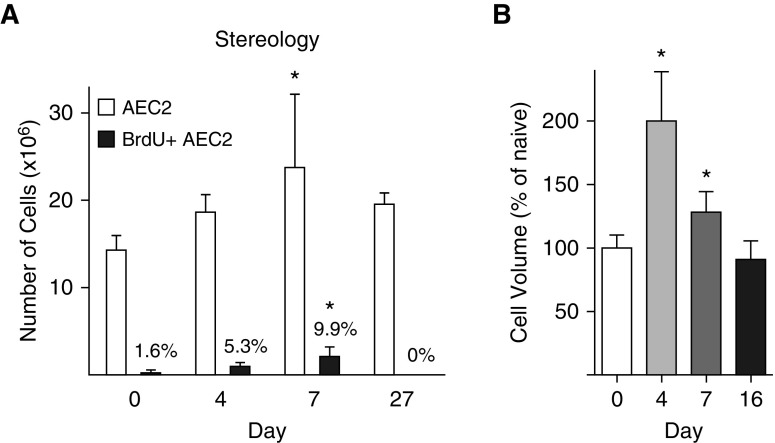
The number of AEC2s in the naive mouse lung was 14.3 ± 1.7 × 106, similar to previous estimates (13). In the LPS model, lung injury peaks on Day 3 and is followed by repair (6). During repair, AEC2 number increased by 50%, peaking on Day 7 (Figure 1A). The number and percentage of BrdU+ AEC2s also peaked on Day 7 at 2.1 ± 1.1 × 106, or 9.9%. AEC2 size increased (Figure 1B).
Figure 1.
Alveolar epithelial type 2 cell (AEC2) expansion and volume measured by stereology. SPCCreERT2+/−;mTmG+/− mice were treated with LPS or saline as a control. Mice were administered bromodeoxyuridine (BrdU) 24 hours before being harvested. Lungs were inflation-fixed, cut into 3-mm slabs, divided into two blocks, and processed. One paraffin block was exhaustively cut into 30-μm sections. Sections were immunostained for green fluorescent protein and BrdU and imaged at ×63. (A) Cells were counted with an optical fractionator. The total number of AEC2s is indicated by the open bars; numbers of actively cycling (BrdU+) AEC2s are indicated by the solid bars. The percentage of total AEC2s that are BrdU+ is shown above the solid bars. (B) The volume of individual cells was measured by the nucleator method and is reported as a percentage of the naive animal. There was no difference in shrinkage in the x–y plane between naive and LPS-treated lungs (data not shown). Means and SD are shown. One-way ANOVA with Bonferroni post hoc analysis was performed. *P < 0.05 compared with naive lung.
Flow cytometry
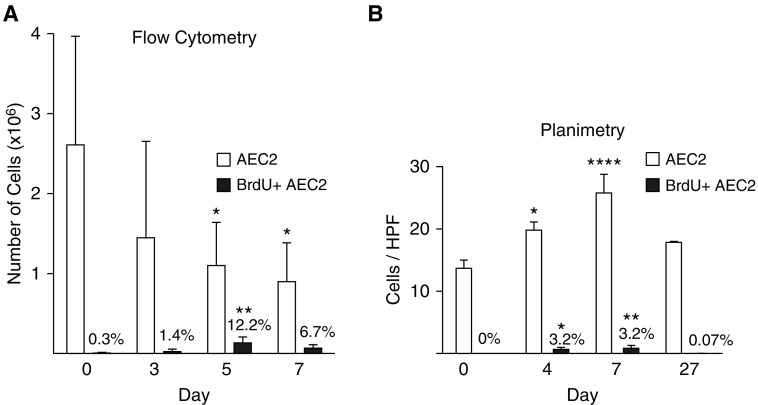
Only 2.6 ± 1.4 × 106 of the 14.3 × 106 AEC2s in the naive lung were identifiable by flow cytometry (Figure 2A). The number of AEC2s appeared to decrease during repair (Figure 2A). This decrease was attributable to fewer cells in the GFP+ gate; there were more total cells in the digest and no differences in other gates (data not shown). This pattern was also observed with unfixed, nonpermeabilized cells (data not shown). The absolute number and percentage of BrdU+ AEC2s increased, peaking on Day 5 at 0.13 ± 0.06 × 106, or 12.2%.
Figure 2.
Alveolar epithelial type 2 cell (AEC2) expansion measured by flow cytometry and planimetry. SPCCreERT2+/−;mTmG+/− mice were treated with LPS or saline as a control. Mice were administered bromodeoxyuridine (BrdU) 24 hours before being harvested. (A) Lungs were digested, and the cell suspension was stained with a LIVE/DEAD stain, then fixed and permeabilized, and stained with BrdU. Debris was gated out, followed by gates on singlets, live, green fluorescent protein (GFP), and BrdU. Flow cytometry dot plots demonstrating that GFP+ cells are equivalent to proSPC+ cells are shown in Reference 6. (B) Lungs were fixed, and sections were stained for GFP and BrdU. The total number of GFP+ and GFP+BrdU+ cells per ×63 high-power field was counted. (A and B) Total AEC2s are indicated by the open bars; actively cycling (BrdU+) AEC2s are indicated by the solid bars. The percentage of total AEC2s that are BrdU+ is shown above the solid bars. Means and SD are shown. One-way ANOVA with Bonferroni post hoc analysis was performed. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with naive lung. HPF = high-power field; proSPC = pro–surfactant protein C.
Planimetry
The number of AEC2s per high-power field increased by 90%, peaking on Day 7, at which point 3.2% were BrdU+ (Figure 2B).
Discussion
In comparison with stereology, flow cytometry underestimated the number of AEC2s in the naive lung. Moreover, flow cytometry falsely demonstrated a decline in AEC2 number during regeneration. The number of actively cycling AEC2s during repair was also grossly underestimated. The percentage of cycling AEC2s was higher and peaked earlier by flow cytometry than by stereology, suggesting that the AEC2s detectable in the digest may not be representative of all AEC2s: cycling cells may be preferentially recoverable. These findings raise concern that apparent reduced AEC2 expansion in diseased lungs (1) or knockout mice (1, 5, 6) may be confounded by impaired recoverability from the digest of an injured lung.
Planimetric analysis overestimated AEC2 expansion during repair, perhaps because AEC2s hypertrophy and/or because the reduced compliance of injured lungs limits inflation, thereby decreasing lung volume and increasing cell density. The percentage of actively cycling cells was underestimated, probably because nuclei are less likely than cytoplasm/membrane to appear in a section. However, counting only cells with observable nuclei overestimated the percentage of actively cycling cells (data not shown), likely because cells in S phase and their nuclei enlarge. These results are consistent with a comparison between planimetric and stereologic quantitation of airway inflammatory cells (8); the novelty of our study lies in the inclusion of flow cytometric data and the application of stereology to the field of alveolar epithelial regeneration.
Although stereology is labor-intensive and requires expertise, our data demonstrate that flow cytometric and planimetric analyses are not reliable. As our findings presumably apply to other cell types, studies of tissue regeneration should include stereologic approaches.
Footnotes
Supported by NIH grants R01HL131608 (R.L.Z.), P01HL014985 (R.M.T.), and R24HL123767 (R.M.T.); the Boettcher Foundation (R.L.Z.); the University of Colorado Denver Department of Medicine Outstanding Early Career Scholars Program (R.L.Z.); and the Villum Foundation (J.R.N.).
Author Contributions: Conception and design: R.L.Z., R.M.T., P.M.H., D.M.H., and J.R.N. Data analysis and acquisition: N.L.J., N.P., J.M., and E.F.R. Interpretation of data: R.L.Z., R.M.T., and J.R.N. Drafting or revising the manuscript: R.L.Z., E.F.R., P.M.H., R.M.T., and J.R.N. Final approval: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201709-1838LE on March 13, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, et al. Hyaluronan and TLR4 promote surfactant-protein-C–positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22:1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, et al. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell. 2017;21:120–134. e127. doi: 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, et al. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. Proc Natl Acad Sci USA. 2011;108:15990–15995. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dial CF, Tune MK, Doerschuk CM, Mock JR. Foxp3+ regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am J Respir Cell Mol Biol. 2017;57:162–173. doi: 10.1165/rcmb.2017-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClendon J, Jansing NL, Redente EF, Gandjeva A, Ito Y, Colgan SP, et al. Hypoxia-inducible factor 1α signaling promotes repair of the alveolar epithelium after acute lung injury. Am J Pathol. 2017;187:1772–1786. doi: 10.1016/j.ajpath.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paris AJ, Liu Y, Mei J, Dai N, Guo L, Spruce LA, et al. Neutrophils promote alveolar epithelial regeneration by enhancing type II pneumocyte proliferation in a model of acid-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1062–L1075. doi: 10.1152/ajplung.00327.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratu VA, Erpenbeck VJ, Fehrenbach A, Rausch T, Rittinghausen S, Krug N, et al. Cell counting in human endobronchial biopsies: disagreement of 2D versus 3D morphometry. PLoS One. 2014;9:e92510. doi: 10.1371/journal.pone.0092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll ML, Carroll NG, James AL. Do bronchial biopsies represent mast cell density in airways? A stereological study. Eur Respir J. 2006;28:612–621. doi: 10.1183/09031936.06.00037006. [DOI] [PubMed] [Google Scholar]
- 10.Hsia CC, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansing NL, McClendon J, Henson PM, Tuder RM, Hyde DM, Zemans RL. Unbiased quantitation of alveolar type II to alveolar type I cell transdifferentiation during repair after lung injury in mice. Am J Respir Cell Mol Biol. 2017;57:519–526. doi: 10.1165/rcmb.2017-0037MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knust J, Ochs M, Gundersen HJ, Nyengaard JR. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat Rec (Hoboken) 2009;292:113–122. doi: 10.1002/ar.20747. [DOI] [PubMed] [Google Scholar]
- 13.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]