Abstract
Rationale: Primary treatment of obstructive sleep apnea can be accompanied by a persistence of excessive sleepiness despite adherence. Furthermore, effectiveness of sleep apnea treatment is limited by poor adherence. Currently available pharmacologic options for the treatment of sleepiness in this population are limited.
Objectives: To evaluate the efficacy and safety of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor with robust wake-promoting effects, for the treatment of excessive sleepiness in participants with obstructive sleep apnea with current or prior sleep apnea treatment.
Methods: This was a double-blind, randomized, placebo-controlled, parallel-group, 12-week trial comparing solriamfetol, 37.5, 75, 150, and 300 mg, with placebo.
Measurements and Main Results: Of 476 randomized participants, 459 were included in the prespecified efficacy analyses. Coprimary endpoints (Maintenance of Wakefulness Test sleep latency and Epworth Sleepiness Scale score) were met at all solriamfetol doses (P < 0.05), with dose-dependent effects observed at Week 1 maintained over the study duration. All doses except 37.5 mg resulted in higher percentages of participants reporting improvement on Patient Global Impression of Change (key secondary endpoint; P < 0.05). Adverse events were reported in 47.9% of placebo- and 67.9% of solriamfetol-treated participants; five participants experienced serious adverse events (two [1.7%] placebo, three [0.8%] solriamfetol); none were deemed related to study drug. The most common adverse events with solriamfetol were headache (10.1%), nausea (7.9%), decreased appetite (7.6%), anxiety (7.0%), and nasopharyngitis (5.1%).
Conclusions: Solriamfetol significantly increased wakefulness and reduced sleepiness in participants with obstructive sleep apnea and excessive sleepiness; most adverse events were mild or moderate in severity.
Clinical trial registered with www.clinicaltrials.gov (NCT02348606) and www.eudract.ema.europa.eu (EudraCT 2014-005514-31).
Keywords: obstructive sleep apnea, solriamfetol, JZP-110, excessive sleepiness, TONES 3
At a Glance Commentary
Scientific Knowledge on the Subject
Excessive sleepiness associated with obstructive sleep apnea persists in some patients despite use of or attempts to use a primary obstructive sleep apnea therapy. If unaddressed, excessive sleepiness could contribute to decreased cognitive functioning, work productivity, quality of life, and increased risk for occupational and motor vehicle accidents.
What This Study Adds to the Field
This 12-week, phase III clinical trial showed that solriamfetol, 75, 150, and 300 mg, resulted in objective improvements, relative to placebo, in wakefulness on the Maintenance of Wakefulness Test; subjective improvements in sleepiness on the Epworth Sleepiness Scale; and patient- and clinician-rated improvements in global assessments of change. The safety and tolerability profile was consistent with prior studies of solriamfetol in narcolepsy. These results suggest that solriamfetol may be a potential therapeutic option for the treatment of impaired wakefulness and excessive sleepiness in individuals with obstructive sleep apnea.
Excessive sleepiness (ES) is a common complaint of individuals with obstructive sleep apnea (OSA) (1). Disruptions in sleep (e.g., awakenings) and intermittent hypoxia caused by OSA can lead to ES (2). Animal models of sleep apnea suggest that ES may also be produced by long-term intermittent hypoxia and sleep fragmentation leading to injury to wake-promoting neurons (3, 4). The consequences of ES include decreased cognitive functioning, work productivity, and quality of life, and increased risk for occupational and motor vehicle accidents (5–8). Although effective primary OSA treatment, such as positive airway pressure (PAP), generally reduces hypoxic events and sleep fragmentation (9–12), ES is estimated to persist in 12–65% of individuals who use PAP (13–16). In addition, despite recommendations for consistent PAP monitoring and the availability of adherence tracking systems (17), adherence to primary OSA therapy often varies, thereby reducing its effectiveness (18, 19). Even when such factors as treatment adherence, medications, comorbid illness, and inadequate sleep duration are controlled, ES is reported in up to 6–18% of individuals treated for OSA (13, 16, 20). Thus, ES is a persistent symptom in many patients despite OSA therapy.
Modafinil and armodafinil are approved by the U.S. Food and Drug Administration (FDA) to improve wakefulness in adults with OSA (21, 22). However, the wake-promoting effects of modafinil and armodafinil have been shown to wane throughout the day (23, 24), requiring twice daily dosing in some patients. In addition, the marketing authorization of modafinil for the treatment of OSA in Europe was withdrawn by the European Medicines Agency because of an unfavorable benefit/risk profile (25).
Solriamfetol (SUNOSI, formerly JZP-110 and ADX-N05) is an FDA-approved dopamine and norepinephrine reuptake inhibitor indicated to improve wakefulness in adult patients with excessive daytime sleepiness associated with narcolepsy or OSA. Solriamfetol has lower binding affinity to dopamine and norepinephrine transporters than traditional stimulants, and lacks the monoamine-releasing effects of amphetamines at therapeutic doses (26). This study examined the efficacy and safety of solriamfetol in the management of ES in individuals with OSA. Some of the results of the study have been previously reported in abstracts (27–29).
Methods
Study Design and Oversight
This was a clinical trial from the Treatment of OSA and Narcolepsy Excessive Sleepiness (TONES) Phase III program, the TONES 3 study. This phase III, placebo-controlled, parallel-group trial evaluated the efficacy and safety of 12 weeks of solriamfetol in adults with OSA and ES. The study was conducted at 59 clinical investigative sites in the United States, Canada, France, Germany, and the Netherlands between May 19, 2015, and December 23, 2016. The study was approved by institutional review boards or ethics committees at each site and performed in accordance with the Declaration of Helsinki; all participants provided written informed consent (www.clinicaltrials.gov identifier NCT02348606).
Participants
Participants were adults 18–75 years old, with OSA diagnosed according to the International Classification of Sleep Disorders-3 criteria (2) and with current or prior use of a primary OSA therapy including PAP, mandibular advancement device, or surgical intervention. Participants without current primary OSA therapy use or a history of a surgical intervention to treat the underlying obstruction were required to have tried to use a primary OSA therapy for at least 1 month with at least one documented adjustment to the therapy (e.g., change in PAP pressure, change in mask, change in modality). Additional inclusion criteria were baseline Epworth Sleepiness Scale (ESS) score greater than or equal to 10 (30); baseline sleep latency less than 30 minutes for the average of the first four of a five-trial, 40-minute Maintenance of Wakefulness Test (MWT) (31); and usual nightly sleep time greater than or equal to 6 hours. Patients were excluded if they did not have a usual nightly total sleep time of at least 6 hours or if they had a usual bedtime later than 1:00 a.m.; an occupation requiring nighttime shift work or variable shift work; use of any over-the-counter or prescription medications that could affect the evaluation of ES; current or past (within the past 2 yr) diagnosis of a moderate or severe substance use disorder according to DSM-5 criteria; nicotine dependence that has an effect on sleep (e.g., a subject who routinely awakens at night to smoke); or any other clinically relevant medical, behavioral, or psychiatric disorder other than OSA that is associated with ES.
Treatments
The study was conducted in a fully double-blind manner. Participants were randomized (1:1:2:2:2) to 12 weeks of once-daily oral solriamfetol 37.5, 75, 150, or 300 mg, or placebo. The study drug or placebo was taken on an empty stomach within 1 hour of awakening. Participants randomized to the 150- and 300-mg doses received 75 and 150 mg, respectively, on Days 1–3, with the full dose commencing on Day 4. All study drugs were prepared in identical opaque gelatin capsules to ensure adequate double-blinding, and all study personnel were blinded to the study treatments.
Randomization was stratified by adherence or nonadherence to primary OSA therapy, with adherence defined as use greater than or equal to 4 hours per night on greater than or equal to 70% of nights for devices from which hourly usage data could be extracted, use greater than or equal to 70% of nights by daily diary for devices for which usage data could not be retrieved, or history of a surgical intervention for OSA. Nonadherence was defined as usage of a primary therapy at a level that did not meet the previously mentioned criteria or a history of a surgical intervention for OSA that was deemed by the investigator to no longer be effective at treating the obstruction. An automated system was used to randomly assign participants to treatment type. The investigator accessed an Interactive Voice or Web Response System to randomly assign participants to treatment. The master randomization code was sequestered by the quality department at Jazz Pharmaceuticals and the code was not broken or released until all study data had been collected and accepted for analysis.
Outcomes
The coprimary efficacy endpoints were change from baseline to Week 12 in mean sleep latency derived from the first four trials of a five-trial, 40-minute MWT, and change from baseline in ESS score. Change from baseline to Week 12 in sleep latency for each of the five individual MWT trials was tested as a prespecified secondary endpoint for doses that were positive on both coprimary efficacy endpoints.
The MWT provided objective assessment of the ability to remain awake (31). The ESS provided a validated measure for assessing participant-reported sleepiness; scores less than or equal to 10 are considered within the normative range (30, 32). In addition to baseline, the MWT was performed at Weeks 1, 4, and 12, whereas the ESS was administered at Weeks 1, 4, 8, and 12. All MWT evaluations were performed subsequent to an overnight stay at the study site for nocturnal polysomnography. Exploratory endpoints for polysomnography included total sleep time, number of awakenings, and wake after sleep onset at Week 12.
The key secondary endpoint was the percentage of participants at Week 12 reporting any improvement on the Patient Global Impression of Change (PGI-C) (33), assessed on a seven-point scale (1 = very much improved to 7 = very much worse); improvement was defined as ratings of “very much,” “much,” or “minimally” improved. The percentage of participants at Week 12 with any improvement as reported by the clinician on the Clinical Global Impression of Change (CGI-C) (33) was a secondary endpoint, assessed on the same scale as the PGI-C.
Other secondary endpoints, not reported here, were measures of function (10-item Functional Outcomes of Sleep Questionnaire [34]), productivity (Work Productivity and Activity Impairment Questionnaire: Specific Health Problems [35]), and health-related quality of life (36-Item Short Form Health Survey version 2 [36] and the five-dimension, five-level EuroQol [37]). Change in primary OSA therapy use was a prespecified exploratory endpoint.
Safety and tolerability were assessed based on adverse events, vital signs, electrocardiography, and laboratory tests. On days of MWT assessment, blood pressure and heart rate measurements were collected at seven time points. Risk of suicidality was evaluated at each study visit using the Columbia-Suicide Severity Rating Scale (38).
Statistical Analysis
Approximately 440 participants were planned for enrollment with approximately 55 participants in each of the solriamfetol 37.5- and 75-mg groups, and approximately 110 participants in each of the placebo and solriamfetol 150- and 300-mg groups. A sample size of 99 participants per group (placebo, 150 mg, and 300 mg) was estimated to provide at least 90% power to detect a difference between placebo and each of the 150- and 300-mg groups in the change from baseline to Week 12 of 5 minutes in the mean sleep latency on the MWT and 3.5 points on the ESS. This calculation was informed by a previous 12-week study of solriamfetol in patients with narcolepsy (39) and used common SD for the change from baseline of 10 minutes on the MWT and six points on the ESS, and a two-sided significance level of 0.05 using a Student’s t test. The two lower dose arms were not powered for statistical significance but were included to adequately characterize the minimal effective dose.
Coprimary endpoints were evaluated using a mixed-effect repeated measures model with fixed effects for treatment, time, treatment-by-time interaction, stratification factor (adherent or nonadherent to OSA therapy), and baseline value of the efficacy endpoint; results are presented as least squares (LS) mean change from baseline (SE). The PGI-C and CGI-C were evaluated using a chi-square test. A fixed hierarchical testing procedure was used to correct for multiplicity, starting with the highest solriamfetol dose for the coprimary endpoints and the key secondary endpoint, with testing proceeding to each subsequent lower dose if statistical significance was met. Doses significant for both coprimary endpoints were further tested to characterize duration of the MWT effect across the day, with identification of the first trial with a significant difference from placebo, then testing subsequent trials until nonsignificance or trial 5.
Post hoc analyses were performed to estimate effect sizes of the change from baseline to Week 12 for MWT sleep latency and ESS score based on LS mean divided by SD (Cohen’s d). Additional post hoc analyses were conducted for the modified intent-to-treat population (mITT) using a last-observation-carried-forward approach to determine the percentage of participants with normative ESS scores and with MWT sleep latency greater than or equal to 20 minutes at Week 12. The 20-minute cutoff on the MWT was based on a value of 19.4 minutes that has been reported as the lower limit of normal (40), which has also been incorporated into the American Academy of Sleep Medicine practice parameters for use of the MWT (31).
Efficacy analyses were based on the prespecified mITT, defined as all participants who were randomized, received greater than or equal to one dose of study drug, and had baseline and greater than or equal to one postbaseline evaluations of the MWT or ESS.
All analyses were performed using SAS version 9.3 or above (SAS Institute).
Results
Participant Population
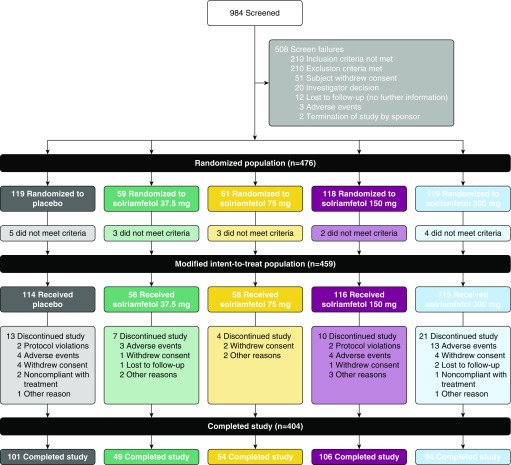
The study was conducted between May 19, 2015, when the first participant was screened, and December 23, 2016, when the last participant completed the study. Of the 474 participants who were randomized and took at least one dose of study drug, representing the safety population, 404 (85.2%) completed the study (Figure 1). Baseline demographic and clinical characteristics of the safety population were similar across treatments (Table 1). A history of a surgical intervention for OSA was reported in 17.6% and 13.5% of participants on placebo and solriamfetol, respectively. At baseline, primary OSA therapy was used by 69.7% of participants on placebo and 73.5% of participants on solriamfetol. Of these patients, 91.6% of participants on placebo and 92.7% on solriamfetol were using PAP; 2.4% on placebo and 1.1% on solriamfetol were using another type of device as a primary OSA therapy; and in 6.0% of participants on placebo and 6.1% on solriamfetol, the type of primary OSA therapy was not specified. Adverse events were the most common reason overall for withdrawal (5.1%). Those who successfully completed at least one follow-up visit (mITT population) comprised 459 participants (Figure 1).
Figure 1.
Participant disposition (Consolidated Standards of Reporting Trials diagram). Fifteen participants in the randomized population did not have baseline or one or more postbaseline evaluations of Maintenance of Wakefulness Test sleep latency or Epworth Sleepiness Scale score, and two did not receive solriamfetol. These participants did not meet the prespecified criteria for inclusion in the modified intent-to-treat population.
Table 1.
Demographic and Clinical Characteristics of the Safety Population at Baseline
| Variable | Placebo (n = 119) | Solriamfetol |
|||
|---|---|---|---|---|---|
| 37.5 mg (n = 58) | 75 mg (n = 62) | 150 mg (n = 117) | 300 mg (n = 118) | ||
| Age, yr, mean (SD) | 54.1 (11.4) | 57.1 (10.2) | 54.4 (11.5) | 52.7 (10.6) | 53.2 (10.6) |
| Sex, male, n (%) | 77 (64.7) | 39 (67.2) | 35 (56.5) | 72 (61.5) | 74 (62.7) |
| Race, n (%) | |||||
| White | 87 (73.1) | 45 (77.6) | 46 (74.2) | 93 (79.5) | 90 (76.3) |
| Black/African-American | 26 (21.8) | 10 (17.2) | 14 (22.6) | 18 (15.4) | 21 (17.8) |
| Asian | 4 (3.4) | 3 (5.2) | 1 (1.6) | 3 (2.6) | 6 (5.1) |
| Other | 2 (1.6) | 0 | 1 (1.6) | 3 (2.6) | 1 (0.8) |
| BMI, kg/m2, mean (SD) | 33.1 (5.2) | 34.1 (5.3) | 33.4 (5.7) | 33.3 (4.8) | 32.9 (5.6) |
| MWT sleep latency, min, mean (SD) | 12.4 (7.2) | 13.6 (8.1) | 13.1 (7.2) | 12.5 (7.2) | 12.0 (7.3) |
| ESS score, mean (SD) | 15.6 (3.3) | 15.1 (3.5) | 14.8 (3.5) | 15.1 (3.4) | 15.2 (3.1) |
| CGI-S, n (%) | |||||
| 1 = Normal, not at all ill | 0 | 0 | 0 | 0 | 0 |
| 2 = Borderline ill | 3 (2.5) | 1 (1.7) | 1 (1.6) | 2 (1.7) | 1 (0.8) |
| 3 = Mildly ill | 8 (6.7) | 5 (8.6) | 4 (6.5) | 7 (6.0) | 10 (8.5) |
| 4 = Moderately ill | 48 (40.3) | 28 (48.3) | 31 (50.0) | 53 (45.3) | 44 (37.3) |
| 5 = Markedly ill | 39 (32.8) | 14 (24.1) | 15 (24.2) | 41 (35.0) | 44 (37.3) |
| 6 = Severely ill | 15 (12.6) | 9 (15.5) | 7 (11.3) | 14 (12.0) | 17 (14.4) |
| 7 = Among the most extremely ill | 4 (3.4) | 1 (1.7) | 3 (4.8) | 0 | 2 (1.7) |
| Missing | 2 (1.7) | 0 | 1 (1.6) | 0 | 0 |
| Primary OSA therapy adherence, n (%) | |||||
| Adherent | 83 (69.7) | 40 (69.0) | 45 (72.6) | 80 (68.4) | 86 (72.9) |
| Nonadherent | 36 (30.3) | 18 (31.0) | 17 (27.4) | 37 (31.6) | 32 (27.1) |
Definition of abbreviations: BMI = body mass index; CGI-S = Clinical Global Impression of Severity; ESS = Epworth Sleepiness Scale; MWT = Maintenance of Wakefulness Test; OSA = obstructive sleep apnea.
Efficacy
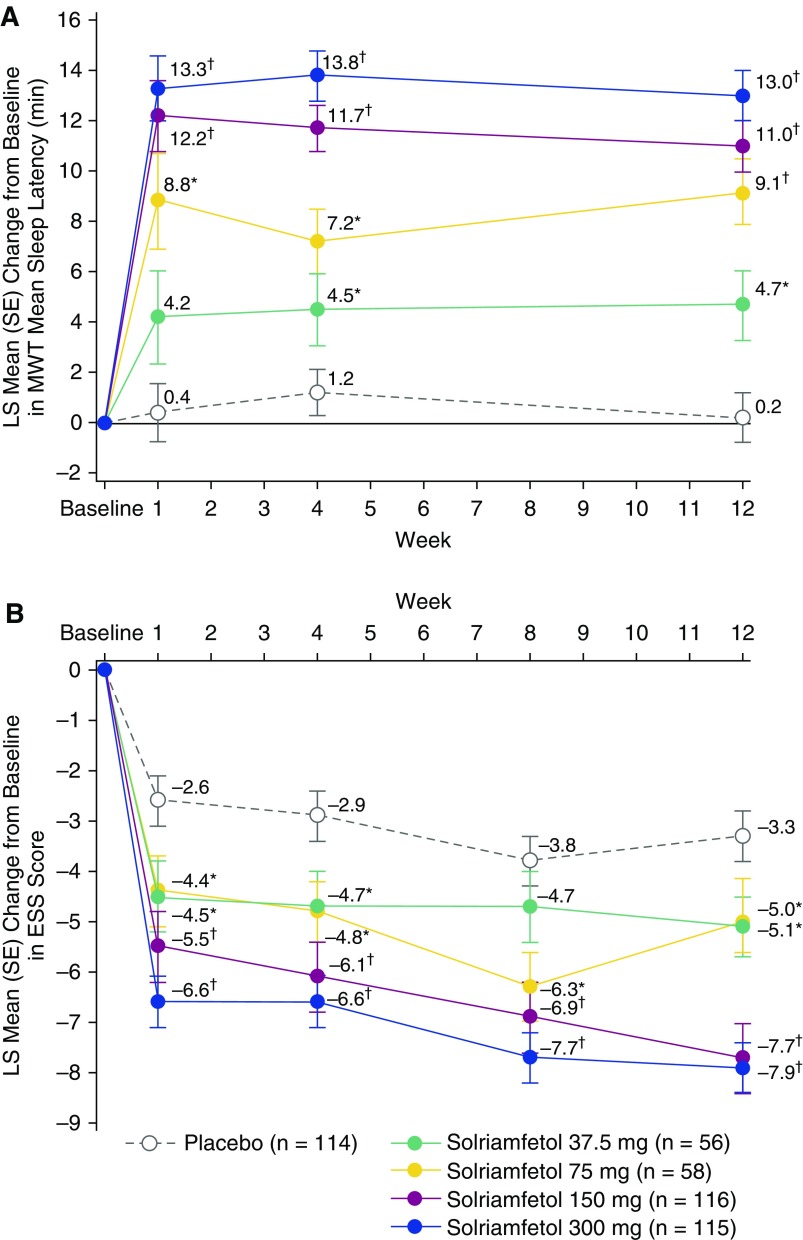
Mean treatment compliance with study drug was high, with 97.2% of scheduled doses taken. The coprimary endpoints of change from baseline at Week 12 in MWT and ESS were met at all solriamfetol doses, and the key secondary endpoint of PGI-C was met at all doses except the 37.5-mg dose (Table 2, Figures 2A and 2B). As shown in Figure 2A, solriamfetol resulted in dose-dependent increases in MWT sleep latency at Week 1, with LS mean changes from baseline that ranged from 4.2 to 13.3 minutes for the 37.5- and 300-mg doses, respectively, and that were greater than placebo (0.4 min). These increases were maintained across the 12 weeks of the study, and all solriamfetol doses resulted in improvements relative to placebo at Weeks 4 and 12 (P < 0.05). At Week 12, effect sizes (Cohen’s d) were 0.4, 0.9, 1.1, and 1.2 for solriamfetol 37.5, 75, 150, and 300 mg, respectively. The LS mean change from baseline exceeded 10 minutes at all time points with solriamfetol 150 mg (11.0–12.2 min) and 300 mg (13.0–13.8 min), whereas placebo ranged from 0.2 to 1.2 minutes.
Table 2.
Hierarchical Testing at Week 12 of Coprimary and Key Secondary Efficacy Endpoints in the Modified Intent-to-Treat Population
| Endpoint | Difference from Placebo (95% CI); P Value |
|||
|---|---|---|---|---|
| Solriamfetol, 300 mg | Solriamfetol, 150 mg | Solriamfetol, 75 mg | Solriamfetol, 37.5 mg | |
| MWT, LS mean difference | 12.8 (10.0 to 15.6); <0.0001 | 10.7 (8.1 to 13.4); <0.0001 | 8.9 (5.6 to 12.1); <0.0001 | 4.5 (1.2 to 7.9); 0.0086 |
| ESS, LS mean difference | −4.7 (−5.9 to −3.4); <0.0001 | −4.5 (−5.7 to −3.2); <0.0001 | −1.7 (−3.2 to −0.2); 0.0233 | −1.9 (−3.4 to −0.3); 0.0161 |
| PGI-C, % difference | 39.6 (28.7 to 50.4); <0.0001 | 40.5 (29.8 to 51.3); <0.0001 | 23.3 (8.6 to 38.0); 0.0035 | 6.2 (−9.7 to 22.2); 0.4447 |
Definition of abbreviations: CI = confidence interval; ESS = Epworth Sleepiness Scale; LS = least squares; MWT = Maintenance of Wakefulness Test; PGI-C = Patient Global Impression of Change.
A fixed hierarchical testing procedure was used to correct for multiplicity, starting with the highest solriamfetol dose for the coprimary endpoints and followed by the key secondary endpoint; testing proceeded in that order for each subsequent lower dose, with statistical significance claimed only for those outcomes above the break in the hierarchy.
Figure 2.
Change from baseline on the coprimary efficacy endpoints (modified intent-to-treat population). (A) Least squares (LS) mean change from baseline in Maintenance of Wakefulness Test sleep latency in minutes for all treatment groups and (B) LS mean change in Epworth Sleepiness Scale score for all treatment groups. *P < 0.05 and †P < 0.0001 versus placebo. ESS = Epworth Sleepiness Scale; MWT = Maintenance of Wakefulness Test.
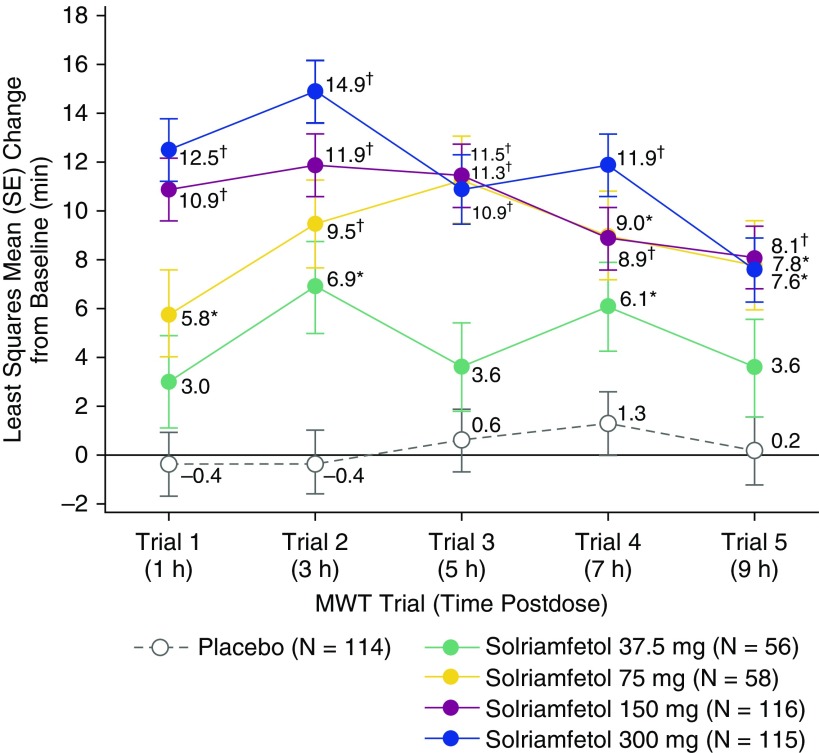
Change from baseline in sleep latency on each of the five individual MWT trials at Week 12 was significantly greater with solriamfetol 75-, 150-, and 300-mg doses compared with placebo, demonstrating efficacy of solriamfetol from 1 to 9 hours after dosing (Figure 3). The 37.5-mg dose showed a significant difference relative to placebo for trial 2 only, based on the prespecified testing sequence.
Figure 3.
Change from baseline in sleep latency for each of the five individual trials in the Maintenance of Wakefulness Test (MWT) at Week 12 (modified intent-to-treat population). Individual MWT trials, each of 40-minute duration, were performed at 2-hour intervals beginning 2 hours after awakening and 1 hour after dosing at the approximate times postdose shown in parentheses. The result at trial 4 for 37.5 mg was of nominal significance based on the prespecified testing sequence. *P < 0.05 and †P < 0.0001 versus placebo.
Solriamfetol treatment resulted in dose-dependent decreases in ESS score relative to placebo at Week 1 that remained stable over the 12-week study duration (Figure 2B). These decreases were greater than placebo for all doses at all time points except for the 37.5-mg dose at Week 8. Effect sizes at Week 12 were 0.4, 0.4, 1.0, and 1.0 for solriamfetol 37.5, 75, 150, and 300 mg, respectively. ESS scores decreased by more than seven points with the 150- and 300-mg doses at Week 12 (P < 0.0001), whereas placebo decreased by 3.3 points.
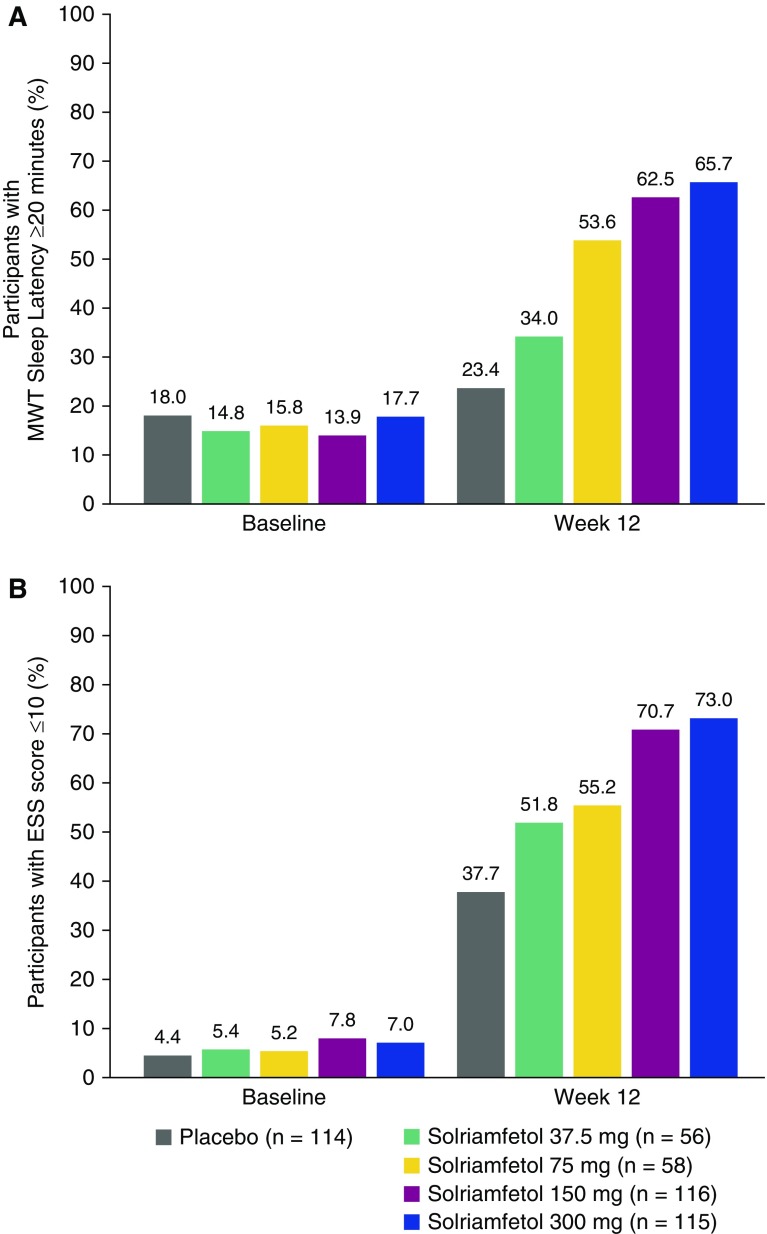
At Week 12, 23.4% of participants in the placebo group had a mean MWT sleep latency greater than or equal to 20 minutes, whereas the percentages were 34.0%, 53.6%, 62.5%, and 65.7% among participants treated with solriamfetol 37.5, 75, 150, and 300 mg, respectively (Figure 4A). This is in contrast to baseline, when the percentage of participants with MWT sleep latency greater than or equal to 20 minutes was 18.0% among those randomized to placebo, and ranged from 13.9% to 17.7% for those randomized to solriamfetol. Similarly, at Week 12, 51.8–73.0% of participants in the solriamfetol groups had ESS scores less than or equal to 10; this value was 37.7% in the placebo group (Figure 4B). These percentages contrast with baseline, when 4.4% and 5.2–7.8% of participants randomized to placebo and solriamfetol, respectively, had ESS scores less than or equal to 10.
Figure 4.
Percentage of participants (A) with Maintenance of Wakefulness Test sleep latency greater than or equal to 20 minutes and (B) with Epworth Sleepiness Scale (ESS) scores less than or equal to 10 among participants in the modified intent-to-treat population with missing data imputed using last-observation-carried-forward. Values at baseline include some participants with ESS scores of 10, because the inclusion criterion was an ESS score greater than or equal to 10. MWT = Maintenance of Wakefulness Test.
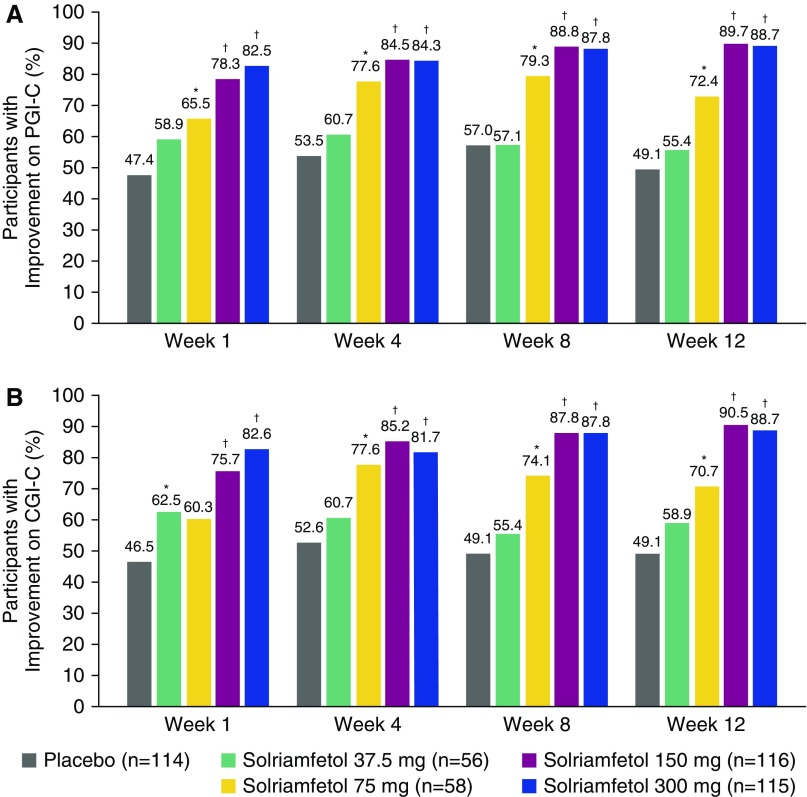
At Week 12, significantly higher percentages of participants on solriamfetol 75 mg (72.4%; P < 0.05), 150 mg (89.7%; P < 0.0001), and 300 mg (88.7%; P < 0.0001) reported overall improvement on the PGI-C relative to placebo (49.1%) (Figure 5A). These effects were dose-dependent and apparent as early as Week 1. Results were generally similar on the CGI-C (Figure 5B).
Figure 5.
Percentage of participants with improvement on the Global Impression of Change scales (modified intent-to-treat population): (A) Patient Global Impression of Change scale and (B) Clinical Global Impression of Change scale results. *P < 0.05 and †P < 0.0001 for difference between solriamfetol and placebo. CGI-C = Clinical Global Impression of Change scale; PGI-C = Patient Global Impression of Change scale.
There were no meaningful differences in response to solriamfetol between the subgroups of participants who were adherent or nonadherent to primary OSA therapy (data not shown).
Adherence with Primary OSA Therapy
All parameters for adherence with primary OSA therapy remained relatively constant during the course of the study. The mean (SD) change from baseline to Weeks 9–12 in the percentage of nights participants used a primary OSA therapy was 0.8% (12.1) for placebo (n = 69) and 1.1% (12.0) for the combined solriamfetol group (n = 218). Similarly, for participants who had electronically retrievable data, the mean (SD) change from baseline to Weeks 9–12 in the average number of hours per night subjects used their OSA device was −0.3 (0.9) and −0.3 (1.2) for placebo (n = 43) and the combined solriamfetol group (n = 133), respectively.
Safety
There were no deaths. The incidence of adverse events was higher with solriamfetol (67.9%) than with placebo (47.9%) and the incidence of adverse events and discontinuations caused by adverse events was generally dose dependent (Table 3). Events leading to study discontinuation in greater than or equal to three participants who received solriamfetol were anxiety (four participants), feeling jittery (four participants), nausea (three participants), dizziness (three participants), and chest discomfort (three participants). In most participants, adverse events were of mild or moderate severity in the placebo (93.0%) and solriamfetol (94.6%) groups. Seven serious adverse events were reported in five participants, two (1.7%) on placebo (goiter in one participant; motor vehicle accident, back pain, and sciatica in another participant), and three (0.8%) on solriamfetol (one participant each with bile duct obstruction and streptococcal endocarditis in solriamfetol 37.5-mg group and hyperglycemia in solriamfetol 150-mg group); none of these was considered by the investigator to be related to study medication. The most frequently reported adverse events with solriamfetol, defined as occurring in greater than or equal to 5% of participants in any treatment group, included headache (10.1%), nausea (7.9%), decreased appetite (7.6%), anxiety (7.0%), and nasopharyngitis (5.1%) (Table 3); most of these events were dose dependent. Insomnia was reported in two participants receiving placebo (1.7%), and in one (1.7%), 0 (0%), three (2.6%), and 11 (9.3%) participants receiving solriamfetol 37.5, 75, 150, and 300 mg, respectively. All insomnia events were mild or moderate in severity, but led to study drug interruption in one participant and withdrawal from the study in another participant, both of whom were receiving solriamfetol 300 mg. There were no statistically significant or clinically meaningful changes in polysomnography parameters of total sleep time, number of awakenings, or wake after sleep onset at Week 12 in the mITT population (see Table E1 in the online supplement).
Table 3.
Adverse Events
| Adverse event | Placebo (n = 119) | Solriamfetol |
||||
|---|---|---|---|---|---|---|
| 37.5 mg (n = 58) | 75 mg (n = 62) | 150 mg (n = 117) | 300 mg (n = 118) | All Doses (n = 355) | ||
| Any adverse event, n (%) | 57 (47.9) | 37 (63.8) | 30 (48.4) | 83 (70.9) | 91 (77.1) | 241 (67.9) |
| Serious adverse event, n (%) | 2 (1.7) | 2 (3.4) | 0 | 1 (0.9) | 0 | 3 (0.8) |
| Adverse event leading to study drug discontinuation, n (%) | 4 (3.4) | 3 (5.2) | 2 (3.2) | 5 (4.3) | 16 (13.6) | 26 (7.3) |
| Most common adverse events,*n (%) | ||||||
| Headache | 10 (8.4) | 4 (6.9) | 5 (8.1) | 10 (8.5) | 17 (14.4) | 36 (10.1) |
| Nausea | 7 (5.9) | 3 (5.2) | 3 (4.8) | 10 (8.5) | 12 (10.2) | 28 (7.9) |
| Decreased appetite | 1 (0.8) | 1 (1.7) | 3 (4.8) | 9 (7.7) | 14 (11.9) | 27 (7.6) |
| Anxiety | 0 | 1 (1.7) | 2 (3.2) | 6 (5.1) | 16 (13.6) | 25 (7.0) |
| Nasopharyngitis | 8 (6.7) | 2 (3.4) | 1 (1.6) | 7 (6.0) | 8 (6.8) | 18 (5.1) |
| Diarrhea | 1 (0.8) | 1 (1.7) | 3 (4.8) | 5 (4.3) | 8 (6.8) | 17 (4.8) |
| Dry mouth | 2 (1.7) | 1 (1.7) | 1 (1.6) | 5 (4.3) | 9 (7.6) | 16 (4.5) |
| Insomnia | 2 (1.7) | 1 (1.7) | 0 | 3 (2.6) | 11 (9.3) | 15 (4.2) |
| Feeling jittery | 0 | 3 (5.2) | 3 (4.8) | 1 (0.9) | 7 (5.9) | 14 (3.9) |
| Sinusitis | 3 (2.5) | 1 (1.7) | 4 (6.5) | 0 | 3 (2.5) | 8 (2.3) |
| Irritability | 0 | 3 (5.2) | 0 | 4 (3.4) | 1 (0.8) | 8 (2.3) |
| Pruritus | 0 | 3 (5.2) | 0 | 1 (0.9) | 0 | 4 (1.1) |
Most common adverse events were those reported in ≥5% in any treatment group.
Two participants in the placebo group (1.7%) and one in the solriamfetol 300-mg group (0.9%) reported suicidal ideation on the Columbia-Suicide Severity Rating Scale. There were no reports of suicidal behavior.
At Week 12, vital signs taken at seven time points during the day from predose to 9 hours postdose (Table 4) showed small mean (95% confidence interval [CI]) increases from baseline in blood pressure, with the highest at the 300-mg dose of solriamfetol (2.5 [95% CI, 0.4–4.6] and 1.5 [0.3–2.7] mm Hg for systolic and diastolic, respectively) relative to minimal changes with placebo (−0.2 [95% CI, −1.7 to 1.4] mm Hg systolic; 0.0 [95% CI, −0.9 to 1.0] mm Hg diastolic). Small dose-dependent mean effects were observed on heart rate with solriamfetol 150 and 300 mg (increases of 2.2 [95% CI, 1.0–3.4] and 2.9 [95% CI, 1.7–4.1] beats per minute, respectively, relative to 0.1 [95% CI, −0.9 to 1.1] beats per minute with placebo). No apparent effects of solriamfetol on blood pressure or heart rate were observed on predose vital sign measures at Week 12.
Table 4.
Change from Baseline at Week 12 in Vital Signs Averaged across Predose to 9 Hours Postdose among Participants with Nonmissing Values
| Vital Sign | Placebo (n = 99) | Solriamfetol, 37.5 mg (n = 49) | Solriamfetol, 75 mg (n = 53) | Solriamfetol, 150 mg (n = 103) | Solriamfetol, 300 mg (n = 91) |
|---|---|---|---|---|---|
| Heart rate, bpm | 0.1 (−0.9 to 1.1) | 0.7 (−1.3 to 2.7) | 0.8 (−0.8 to 2.3) | 2.2 (1.0 to 3.4) | 2.9 (1.7 to 4.1) |
| Blood pressure, mm Hg | |||||
| Systolic | −0.2 (−1.7 to 1.4) | 1.8 (−0.6 to 4.1) | 0.5 (−1.8 to 2.8) | 0.7 (−0.8 to 2.1) | 2.5 (0.4 to 4.6) |
| Diastolic | 0.0 (−0.9 to 1.0) | 0.6 (−0.7 to 2.0) | −0.2 (−2.0 to 1.5) | 0.5 (−0.5 to 1.6) | 1.5 (0.3 to 2.7) |
Definition of abbreviation: bpm = beats per minute.
Data are mean (95% confidence interval).
Laboratory evaluations did not indicate effects of clinical relevance related to solriamfetol, and no clinically significant arrhythmias or morphology changes were observed by electrocardiography.
Discussion
In this study of adult participants with OSA and ES with current or prior use of a primary OSA therapy, management with solriamfetol increased the ability to maintain wakefulness and reduced subjective sleepiness, with global improvements reported by participants and clinicians. These effects were generally dose-dependent, were seen by Week 1 after treatment initiation, and were maintained throughout the 12-week assessment period, except that the 37.5-mg dose had no effect on the PGI-C and was not durable across the day on the MWT.
The magnitude of the improvement at Week 12 was substantial, as demonstrated by large effect sizes on the MWT and ESS at doses of 150 and 300 mg and the high percentages of participants who achieved normative ESS values and mean MWT sleep latency within the lower limit of normal. Furthermore, the increase in MWT sleep latency was maintained throughout the 9-hour period following morning dosing for the 75-, 150-, and 300-mg doses. The magnitude of change is similar to that observed in studies of solriamfetol in narcolepsy (39, 41, 42). PGI-C and CGI-C data showed similarly robust results.
A higher percentage of participants (7.3%) receiving solriamfetol withdrew because of adverse events compared with placebo (3.4%). The most common adverse events with solriamfetol (headache, nausea, decreased appetite, anxiety, and nasopharyngitis) were consistent with wake-promoting agents used in the treatment of ES in OSA (43, 44). Insomnia events were similar in the placebo and solriamfetol groups for doses 37.5–150 mg and occurred more frequently with the solriamfetol 300-mg dose (9.3% of participants).
Participants in this study included adults with an International Classification of Sleep Disorders-3 OSA diagnosis and current or prior use of a primary OSA therapy, along with baseline ESS scores greater than or equal to 10 and baseline MWT latencies less than 30 minutes. Participants who were nonadherent to OSA therapy were included to study a population more representative of OSA patients in the clinical setting (19). This population differs from pivotal trials for modafinil and armodafinil for the treatment of ES in OSA, in which all participants were adherent with PAP therapy, and in which ESS greater than or equal to 10 was the only entry criterion for persistent ES (24, 45, 46). Meta-analyses of the modafinil and armodafinil studies in OSA showed decreases of two to three points on the ESS with treatment compared with placebo (43, 44), which is consistent with a reported minimal clinically important difference of two to three points on the ESS (47). In this study, decreases of greater than four points on the ESS were observed at doses of 150- and 300-mg solriamfetol; however, there are currently no head-to-head studies of solriamfetol and modafinil/armodafinil, and MWT outcomes from this study, in particular, cannot be directly compared with the modafinil/armodafinil pivotal trials because of differences in maximal test duration (20 or 30 min compared with the 40 min in this study). Adherence or nonadherence to a primary OSA therapy, which was a stratification factor in the randomization and was included as a fixed effect in the statistical model, was not significant for either MWT or ESS, suggesting baseline adherence was not a predictor of outcome. In addition, there were no effects of solriamfetol on primary OSA therapy usage.
Clinical guidelines and treatment algorithms for the management and long-term care of OSA suggest that PAP should be discussed as a first-line treatment option in adults with OSA, and that suboptimal adherence with PAP, short sleep duration, pressure leaks, inadequate pressure, or another sleep disorder should be ruled out when there is persistent ES (48). This study required that participants have a minimum of 6 hours of usual nightly total sleep and included patients with OSA who had suboptimal adherence with a primary OSA therapy to study a population more representative of patients with OSA in the clinical setting; however, the importance of obtaining adequate sleep and treating the underlying obstruction in patients with OSA should not be minimized or ignored, because short sleep duration (≤6 h) and untreated OSA are well-established risk factors for cardiovascular and cerebrovascular morbidity and mortality (49–54) that is not addressed by solriamfetol.
The main limitation of this study was the 12-week duration, which limits the ability to assess longer-term outcomes related to safety and efficacy, including any potential long-term cardiovascular consequences. Long-term safety and maintenance of efficacy in patients from this and other studies are being evaluated in a separate 1-year extension study. The lack of an active comparator also limits the ability to draw conclusions between these results and other therapies. In addition, patients with OSA who had refused to try a primary therapy were excluded from this study, so these data should not be extrapolated to patients who have refused to initiate a primary OSA therapy.
In conclusion, in this 12-week, phase III clinical trial, solriamfetol, 75, 150, and 300 mg, resulted in objective improvements in wakefulness, subjective improvements in sleepiness, and global improvements as evaluated by participants and clinicians. The safety and tolerability profile was consistent with prior studies of solriamfetol in individuals with narcolepsy, and similar to other wake-promoting agents used in the treatment of ES in OSA.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the participants, all the study investigators (listed in the online supplement), study staff, and nursing team for their participation in this research. The authors also thank Joan Li for her role as a Jazz Pharmaceuticals medical monitor in this research. Data were analyzed by INC Research (now Syneos Health) with oversight and compensation by the sponsor. Manuscript drafts, after the first manuscript draft written/inputted by the authors, were prepared with the assistance of The Curry Rockefeller Group, LLC, who was paid by the sponsor. All relevant data are provided within the manuscript and supporting files.
Footnotes
This study was funded by Jazz Pharmaceuticals, which licenses the product (worldwide except for certain Asian countries) from SK Biopharmaceuticals, which discovered solriamfetol.
Author Contributions: Concept and design of the study, D.C., L.P.C., H.W., Y.L., J.B., and K.P.S. Acquisition of data, P.K.S., R.R., G.K.Z., M.G., A.M., and K.P.S. Analysis of data, P.K.S., R.R., G.K.Z., M.G., D.C., L.P.C., H.W., Y.L., J.B., A.M., and K.P.S. Drafting of manuscript, P.K.S., R.R., G.K.Z., M.G., D.C., L.P.C., H.W., Y.L., J.B., A.M., and K.P.S. Revising manuscript critically for important intellectual content, P.K.S., R.R., G.K.Z., M.G., D.C., L.P.C., H.W., Y.L., J.B., A.M., and K.P.S. Approval of final manuscript, P.K.S., R.R., G.K.Z., M.G., D.C., L.P.C., H.W., Y.L., J.B., A.M., and K.P.S. The study protocol was developed by Jazz Pharmaceuticals with input from K.P.S. Data were collected by the study investigators, who had confidentiality agreements with the sponsor. Analyses were conducted by the contract research organization under the supervision of the sponsor. All the authors take responsibility for the accuracy and completeness of the data and analyses reported, and for the fidelity of the studies to the protocols. The first manuscript draft was written by the first and last authors (P.K.S. and K.P.S.), with input from all the authors. All authors made the decision to submit the manuscript for publication and approved the manuscript for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201806-1100OC on December 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Adam Blackman, Charles George, Colin Shapiro, Colin Shapiro, Heike Benes, Ingo Fietze, Geert Mayer, Peter Young, Gert Jan Lammers, Mansoor Ahmed, Akinyemi Ajayi, James Andry, Roy Artal, Bijan Bastani, Richard Bogan, Bruce Corser, Christopher Drake, Helene Emsellem, Milton Erman, Neil Feldman, Nancy Foldvary, Mark Gotfried, John Hudson, Lois Krahn, Daniel Lorch, Atul Malhotra, James Maynard, Emmanuel Mignot, Michael Neeb, Joseph Ojile, Alvin Thomas Perkins, Russell Rosenberg, Asim Roy, Pradeep Sahota, R. Bart Sangal, Andrew Schreiber, Paula Schweitzer, Ralph Steele, Thomas Stern, Thomas Stern, Sarah Stolz, Kingman Strohl, Todd Swick, Todd J. Swick, Stephen G. Thein, Robert Thomas, Michael Thorpy, Terri Weaver, Kerri Wilks, David Winslow, Paul Wylie, Gary Zammit, and Phyllis Zee
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. The International Classification of Sleep Disorders (ICSD-3) 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, et al. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci. 2007;27:10060–10071. doi: 10.1523/JNEUROSCI.0857-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 6.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 7.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 8.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164:2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 9.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 10.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 11.McDaid C, Durée KH, Griffin SC, Weatherly HL, Stradling JR, Davies RJ, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–436. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan CE. Nasal positive airway pressure and sleep apnea: reflections on an experimental method that became a therapy. Am J Respir Crit Care Med. 2018;198:581–587. doi: 10.1164/rccm.201709-1921PP. [DOI] [PubMed] [Google Scholar]
- 13.Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, et al. Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22:389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 14.Koutsourelakis I, Perraki E, Economou NT, Dimitrokalli P, Vagiakis E, Roussos C, et al. Predictors of residual sleepiness in adequately treated obstructive sleep apnoea patients. Eur Respir J. 2009;34:687–693. doi: 10.1183/09031936.00124708. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pépin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Lévy P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33:1062–1067. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 17.Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, et al. ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borker PV, Patel SR. Managing sleep apnea in those who fail continuous positive airway pressure: dealing with the invisible epidemic. Am J Respir Crit Care Med. 2018;198:837–838. doi: 10.1164/rccm.201805-1001ED. [DOI] [PubMed] [Google Scholar]
- 20.Verbruggen AE, Dieltjens M, Wouters K, De Volder I, Van de Heyning PH, Braem MJ, et al. Prevalence of residual excessive sleepiness during effective oral appliance therapy for sleep-disordered breathing. Sleep Med. 2014;15:269–272. doi: 10.1016/j.sleep.2013.11.781. [DOI] [PubMed] [Google Scholar]
- 21.North Wales, PA: Teva Pharmaceuticals USA; 2015. Provigil (modafinil) tablets [package insert] [Google Scholar]
- 22.Frazer, PA: Cephalon; 2017. Nuvigil (armodafinil) tablets [package insert] [Google Scholar]
- 23.Schwartz JR, Feldman NT, Bogan RK, Nelson MT, Hughes RJ. Dosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy. Clin Neuropharmacol. 2003;26:252–257. doi: 10.1097/00002826-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Roth T, White D, Schmidt-Nowara W, Wesnes KA, Niebler G, Arora S, et al. Effects of armodafinil in the treatment of residual excessive sleepiness associated with obstructive sleep apnea/hypopnea syndrome: a 12-week, multicenter, double-blind, randomized, placebo-controlled study in nCPAP-adherent adults. Clin Ther. 2006;28:689–706. doi: 10.1016/j.clinthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency (EMA) Modafinil: summary of product characteristics and package leaflet. [accessed 2015 May 27]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Modafinil_31/WC500096080.pdf.
- 26.Baladi MG, Forster MJ, Gatch MB, Mailman RB, Hyman DL, Carter LP, et al. Characterization of the neurochemical and behavioral effects of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2018;366:367–376. doi: 10.1124/jpet.118.248120. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Li J, et al. A phase 3, randomized, placebo-controlled, double-blind, 12-week, multicenter study of the efficacy and safety of JZP-110 for the treatment of excessive sleepiness in patients with obstructive sleep apnea [abstract] Sleep. 2017;40:A237. [Google Scholar]
- 28.Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Li J, et al. Results of a randomized, placebo-controlled, double-blind, 12-week, multicenter study of solriamfetol (JZP-110) for the treatment of excessive sleepiness in patients with obstructive sleep apnea [abstract] Sleep Med. 2017;40:e298. [Google Scholar]
- 29.Strohl KP, Zammit GK, Gotfried M, Chen D, Li J, Carter LP, et al. Results of a randomized, placebo-controlled, double-blind, 12-week, multicenter study of JZP-110 for the treatment of excessive sleepiness in patients with obstructive sleep apnea [abstract] Chest. 2017;152:A1062–A1063. [Google Scholar]
- 30.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 31.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 32.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–849. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 33.Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville, MD: National Institute of Mental Health; 1976. U.S. Department of Health, Education, and Welfare Publication No. (ADM) 76-338. [Google Scholar]
- 34.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly MC. Work productivity and activity impairment questionnaire: specific health problem V2.0 (WPAI:SHP) [accessed 2018 May 17]. Available from: http://www.reillyassociates.net/WPAI_SHP.html. [PubMed]
- 36.Ware JE, Jr, Kosinski M, Bjorner JB, Turner-Bowker D, Maruish ME. User’s manual for the SF-36v2 health survey. 2nd ed. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 37.The EuroQol Group. EQ-5D-5L user guide. 2013 [accessed 2017 Apr 27]. Available from: http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-5L_UserGuide_2015.pdf.
- 38.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruoff C, Swick TJ, Doekel R, Emsellem HA, Feldman NT, Rosenberg R, et al. Effect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b study. Sleep. 2016;39:1379–1387. doi: 10.5665/sleep.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doghramji K, Mitler MM, Sangal RB, Shapiro C, Taylor S, Walsleben J, et al. A normative study of the maintenance of wakefulness test (MWT) Electroencephalogr Clin Neurophysiol. 1997;103:554–562. doi: 10.1016/s0013-4694(97)00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogan RK, Feldman N, Emsellem HA, Rosenberg R, Lu Y, Bream G, et al. Effect of oral JZP-110 (ADX-N05) treatment on wakefulness and sleepiness in adults with narcolepsy. Sleep Med. 2015;16:1102–1108. doi: 10.1016/j.sleep.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Thorpy MJ, Shapiro C, Mayer G, Corser BC, Emsellem H, Plazzi G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85:359–370. doi: 10.1002/ana.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sukhal S, Khalid M, Tulaimat A. Effect of wakefulness-promoting agents on sleepiness in patients with sleep apnea treated with CPAP: a meta-analysis. J Clin Sleep Med. 2015;11:1179–1186. doi: 10.5664/jcsm.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuan YC, Wu D, Huang KW, Chi NF, Hu CJ, Chung CC, et al. Effects of modafinil and armodafinil in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Clin Ther. 2016;38:874–888. doi: 10.1016/j.clinthera.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Pack AI, Black JE, Schwartz JR, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:1675–1681. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 46.Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–471. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 47.Patel S, Kon SSC, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197:961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 49.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 50.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 53.Pergola BL, Moonie S, Pharr J, Bungum T, Anderson JL. Sleep duration associated with cardiovascular conditions among adult Nevadans. Sleep Med. 2017;34:209–216. doi: 10.1016/j.sleep.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Ford ES. Habitual sleep duration and predicted 10-year cardiovascular risk using the pooled cohort risk equations among US adults. J Am Heart Assoc. 2014;3:e001454. doi: 10.1161/JAHA.114.001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.