Abstract
IFN-β is reported to improve survival in patients with acute respiratory distress syndrome (ARDS), possibly by preventing sepsis-induced immunosuppression, but its therapeutic nature in ARDS pathogenesis is poorly understood. We investigated the therapeutic effects of IFN-β for postseptic ARDS to better understand its pathogenesis in mice. Postseptic ARDS was reproduced in mice by cecal ligation and puncture to induce sepsis, followed 4 days later by intratracheal instillation of Pseudomonas aeruginosa to cause pneumonia with or without subcutaneous administration of IFN-β 1 day earlier. Sepsis induced prolonged increases in alveolar TNF-α and IL-10 concentrations and innate immune reprogramming; specifically, it reduced alveolar macrophage (AM) phagocytosis and KC (CXCL1) secretion. Ex vivo AM exposure to TNF-α or IL-10 duplicated cytokine release impairment. Compared with sepsis or pneumonia alone, pneumonia after sepsis was associated with blunted alveolar KC responses and reduced neutrophil recruitment into alveoli despite increased neutrophil burden in lungs (i.e., “incomplete alveolar neutrophil recruitment”), reduced bacterial clearance, increased lung injury, and markedly increased mortality. Importantly, IFN-β reversed the TNF-α/IL-10–mediated impairment of AM cytokine secretion in vitro, restored alveolar innate immune responsiveness in vivo, improved alveolar neutrophil recruitment and bacterial clearance, and consequently reduced the odds ratio for 7-day mortality by 85% (odds ratio, 0.15; 95% confidence interval, 0.03–0.82; P = 0.045). This mouse model of sequential sepsis → pneumonia infection revealed incomplete alveolar neutrophil recruitment as a novel pathogenic mechanism for postseptic ARDS, and systemic IFN-β improved survival by restoring the impaired function of AMs, mainly by recruiting neutrophils to alveoli.
Keywords: bacterial pneumonia, innate immunity, acute lung injury, immune suppression, IFN-β
Clinical Relevance
Patients with severe peritoneal infection usually experience nosocomial infections with high mortality. Lung innate immunity, including the bactericidal capacity of alveolar macrophages, is impaired and may be associated with susceptibility to nosocomial infections. Potent immune-enhancing mediator IFN-β restores functional impairment of alveolar macrophages and may be a new therapeutic target for sepsis-related immune suppression.
Acute respiratory distress syndrome (ARDS) is characterized by pulmonary inflammation, diffuse noncardiogenic pulmonary edema, refractory hypoxemia, and acute respiratory failure (1). With an annual incidence of 86.2 per 100,000 and an in-hospital mortality rate of 38.5%, ARDS imposes a significant healthcare burden in the United States (2). With regard to pathophysiology, ARDS appears to be driven by intense pulmonary inflammation that results in capillary–alveolar leakage of protein-rich pulmonary edema fluid and depletion and/or inactivation of surfactant; in survivors, pulmonary fibrosis (3) and chronic lung impairment occur (4). ARDS is commonly triggered in critically ill patients by various extrapulmonary and/or pulmonary diseases, including sepsis and organ damage via trauma, pancreatitis, pneumonia, or aspiration-induced injury (5). Sepsis followed by ventilator-associated pneumonia in intubated patients under intensive care frequently leads to ARDS (6). Protective mechanical ventilation can improve survival (7), but specific pharmacotherapy has not been approved for clinical use in ARDS; hence, current therapy is supportive, and clinical outcomes are, in general, poor (8).
Sepsis, the most frequent cause of ARDS, has an incidence of 751,000 per year in the United States and a mortality rate of 28.6% (9, 10). Despite the vast armory of antimicrobial and antiinflammatory agents, decades of research, and the use of well-controlled study designs, most clinical trials evaluating potential therapies for sepsis have failed (11). Concurrently, understanding of sepsis has improved from its early conceptualization as excessive inflammation due to interrelated host responses occurring in distinct (albeit overlapping) stages involving the systemic inflammatory response syndrome, sepsis, and septic shock, which result in multiple-organ dysfunction syndrome and death (commonly resulting from cardiovascular collapse) (12, 13). This sequence of events is associated with progressive worsening of an imbalance between proinflammatory (e.g., TNF-α) and antiinflammatory (e.g., IL-10) mediators (14), which creates the immunosuppressed state referred to as immune paralysis (11, 15–17) and increases infection susceptibility and mortality (18); these outcomes have been documented in animal models (19) and in humans (20). Sepsis is also associated with functional disruption of multiple immune cell types, including neutrophils (21, 22), monocytes (23, 24), natural killer cells (25), dendritic cells (26), and lymphocytes (15, 27, 28); however, the question of how such abnormalities contribute to ARDS development remains unanswered.
Recently, an open-label trial of systemic IFN-β improved 28-day survival in patients with ARDS (29). IFN-β is a type I IFN family member usually produced in response to viral infections and various pathogen-associated molecules (e.g., LPS), and it exerts pleiotropic effects such as viral inhibition, tumor killing, and immune modulation (30, 31). IFN-β also stimulates expression of ecto-5′-nucleotidase (CD73) in cultured endothelial cells (32) and in human lung tissue explants (29), which is associated with increased adenosine production, improved endothelial barrier function, and reduced vascular leakage, suggesting that IFN-β may function to reduce pulmonary edema. In murine models of sepsis and endotoxin-induced shock, IFN-β improves survival, possibly by silent mating type information regulation 2 homolog 1–mediated suppression of exaggerated cytokine expression (33). Thus, IFN-β holds promise as a disease-specific therapy for ARDS, but there is insufficient knowledge about its potential therapeutic benefits.
Using an established, clinically relevant, “two-hit” sepsis model (cecal ligation and puncture [CLP]) followed by Pseudomonas aeruginosa–induced pneumonia (19, 34), we investigated the mechanism of sepsis/pneumonia-related mortality and the therapeutic effects of IFN-β administration in mice. The results revealed that antecedent sepsis affects impaired innate immunity in the lungs, especially the effector (phagocytosis) and regulatory (cytokine/chemokine secretion) functions of alveolar macrophages (AMs), and also that it reduces neutrophil migration into alveoli and causes severe acute lung injury (ALI)/ARDS and, consequently, increased mortality. Significantly, all of these abnormalities were improved by IFN-β therapy.
Methods
Sepsis-related ARDS Model
Additional methodological details are included in the data supplement. All studies were conducted using procedures approved by the Institutional Review Board at the University of Tokyo Graduate School of Medicine (Tokyo, Japan).
On Day −4, sepsis (“hit 1”) was induced in male C57BL/6JJcl mice by CLP (19, 35) in selected cohorts. Sham laparotomy was conducted for control animals. On Day −1, mouse groups received subcutaneous IFN-β (700,000 U/kg; PBL Assay Science) or saline. On Day 0, pneumonia (“hit 2”) was induced by intratracheal instillation of P. aeruginosa (ATCC27853; American Type Culture Collection) as described by Robertson and colleagues (36). For control animals, normal saline was administered intratracheally.
Sepsis, Survival, and Lung Injury
Survival was evaluated over the entire 11-day study period. Lung injury was quantified using a lung histopathology score (measured according to American Thoracic Society recommendations) (37), tissue myeloperoxidase (MPO) activity, and protein/albumin concentrations as well as cytokine concentrations in BAL fluid (BALF) at the indicated times. Bacterial burden within the lungs was evaluated with colony counts in BALF. Lung tissue expression of Ly6g/c was evaluated by immunohistochemistry with antimouse Ly6g/c rat IgG (Abcam).
Hematology and Blood Chemistry
Complete blood counts were measured using a MEK-6458 automated analyzer (Nihon Kohden). Blood chemistry was evaluated with a urea nitrogen B test kit for blood urea nitrogen and a transaminase CII test kit for hepatic enzymes (both from Wako Pure Chemical Industries).
Cytokine Concentrations
Concentrations of cytokines (IL-6, TNF-α, KC, and IL-10) in peritoneal lavage, serum, and BALF were measured by flow cytometry using a BD Cytometric Bead Array Kit (BD Biosciences) according to the manufacturer’s instructions.
Macrophage Effector and Regulatory Function
The phagocytic capacity of AMs on Day 0 was evaluated using pH-sensitive fluorescently labeled bioparticles (IncuCyte pHrodo Green E. coli Bioparticles for Phagocytosis; Essen BioScience). Fluorescent areas were monitored using a time-lapse imaging system with IncuCyte ZOOM (Essen BioScience) according to the manufacturer’s instructions. Phagocytosis of fluorescently labeled latex beads (Cayman Chemicals) was also measured in peritoneal macrophages (PMs) and AMs on Day 0 by flow cytometry. The secretion capacity of KC in AMs in culture media was measured (as detailed above) after phagocytosis. The effects of TNF-α and IL-10 on the capacity of AMs to secrete proinflammatory cytokines was measured in cultured wild-type AMs incubated with or without TNF-α or IL-10, followed by measurement of the IL-6 concentration in the media (as detailed above).
Statistical Analyses
Statistical analyses were conducted using SigmaPlot version 12.0 (Systat Software). Data are expressed as the mean ± SD. Survival between treatment groups was compared by log-rank testing, and the odds ratio (OR) was calculated. Comparisons of parametric data were made using the t test. Comparisons of nonparametric data were made using the Mann-Whitney rank-sum test. Multiple-group comparisons were done using one-way analysis of variance with post hoc analyses (Holm-Sidak or Dunn’s method) for multiple comparisons and the Mann-Whitney rank-sum test for pairwise comparisons. P < 0.05 was considered significant.
Results
Sepsis Increased Pneumonia-related Mortality in the Two-Hit Sepsis-induced ARDS Model
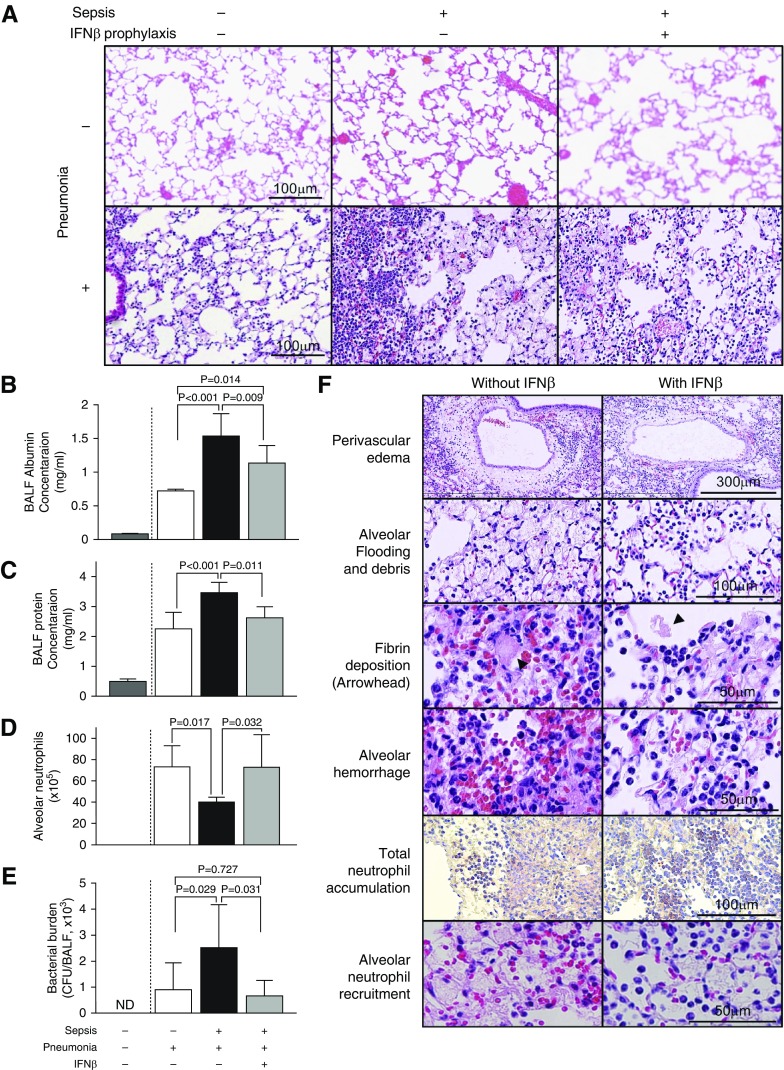
Four mouse groups were evaluated: 1) peritoneal sepsis alone with intratracheal instillation of saline, 2) pneumonia alone with sham laparotomy, 3) sepsis followed by pneumonia, and 4) sepsis followed by IFN-β prophylaxis followed by pneumonia (Figure 1). Despite administration of broad-spectrum antibiotics immediately after CLP, blood cultures were positive in more than half of mice, suggesting that bacteremia was present (data not shown). Sepsis or pneumonia alone resulted in minimal mortality (0% in cohort 1 or 14.8% in cohort 2, respectively), but sepsis followed 4 days later by pneumonia resulted in very high mortality (90.5% in cohort 3) (Figure 1). Antecedent sepsis did not result in detectable cellular infiltration or histological changes in lung parenchyma before lung infection (Figure 2A, upper panels). In contrast, compared with mice with pneumonia alone, mice with sepsis followed by pneumonia experienced markedly increased ALI as characterized by perivascular edema, alveolar flooding, fibrin deposition, hemorrhage, and neutrophil infiltration (Figure 2A, lower panels; Figure 2F; see also Figures E1–E4 in the data supplement), with increased concentrations of protein and albumin in BALF (Figures 2B and 2C) and a worse Lung Injury Score (Figure E5B). Antecedent sepsis resulted in higher total lung MPO activity (Figure E5A). In contrast to severe damage in lung parenchyma, other vital organ functions, such as the liver, the kidneys, and the coagulation system, were preserved because complete blood counts (Table 1) and serum chemistries (Table 2) were almost within normal range 18 hours after pneumonia onset. Taken together, these data support the notion that pneumonia in the context of antecedent sepsis results in higher mortality caused by increased ALI and ARDS.
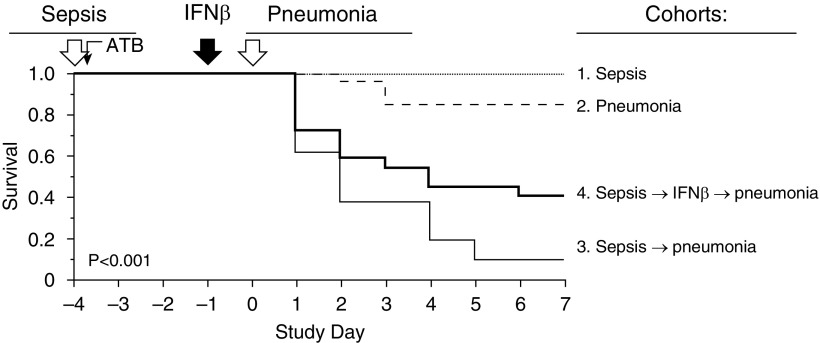
Figure 1.
Reproduction of sepsis-related acute respiratory distress syndrome and the therapeutic effects of IFN-β in mice. Sepsis was induced by cecal ligation and puncture (CLP) and pneumonia by transtracheal instillation of Pseudomonas aeruginosa (4.5 × 106 colony-forming unit [cfu]). Mice in cohort 1 (n = 8) underwent CLP and tracheal instillation of saline. Mice in cohort 2 (n = 27) underwent sham laparotomies instead of CLP followed by tracheal instillation of P. aeruginosa. Mice in cohort 3 (n = 21) underwent CLP followed by tracheal instillation of P. aeruginosa, and mice in cohort 4 (n = 22) underwent CLP followed by tracheal instillation of P. aeruginosa with systemic IFN-β prophylaxis. All mice received broad-spectrum antibiotics by subcutaneous injection within 1 hour of CLP or sham surgery. Saline (cohort 3; n = 21) and IFN-β (cohort 4; n = 22) were administered subcutaneously (volume, 200 μl). The panel shows Kaplan-Meier analysis of four cohorts of mice. On Day 7, survival in cohort 3 (9.5%) was dramatically lower than that in cohort 1 (100%) or cohort 2 (85.2%) (log-rank test, P < 0.0001). Compared with cohort 3, IFN-β–treated mouse survival (cohort 4) was improved markedly (40.9%) (log-rank test, P = 0.034). Data represent the combined results of three separate survival experiments, each with similar results. ATB = antibiotics.
Figure 2.
Pneumonia-related lung injury was exacerbated by sepsis and reduced by IFN-β administration. (A) Lung histology before (upper panels) or 18 hours after (lower panels) tracheal instillation of P. aeruginosa. Presence (+) or absence (−) of infection is indicated. Note that abnormalities or cohort differences were not detectable before pneumonia onset (upper panels), whereas afterward, lung inflammation in mice with only pneumonia was mild (bottom left), but its marked increase was accompanied by alveolar edema fluid in mice with sepsis without or with IFN-β prophylaxis (bottom middle and right panels) (n = 6–9 mice/group). Hematoxylin and eosin staining, ×20 magnification. Scale bars: 100 μm. (B) Albumin concentrations in the BAL fluid (BALF). (C) Protein concentration in BALF. (D) Enumeration of neutrophils recovered from BALF. (E) Remaining bacterial cfus in BALF. All samples were collected 18 hours after pneumonia onset in the mice indicated. (F) Histological features of acute lung injury in representative lung tissues from mice without (left, cohort 3) or with (right, cohort 4) IFN-β administration. Hematoxylin and eosin staining is shown, except for the panels labeled “Total neutrophil accumulation,” which show immunohistochemical staining of neutrophils with anti-Ly6g/c antibody. Larger images of the two lower-right panels in A and the pairs of panels labeled “Perivascular edema” and “Fibrin deposition” in F are shown in Figures E2–E4. Scale bars: 300 μm, 100 μm, and 50 μm. ND = not evaluated, not detected.
Table 1.
Effect of Systemic IFN-β on Complete Blood Counts of Mice with Septic Peritonitis Followed by Pneumonia
| Complete Blood Count* | Sepsis (+), Pneumonia (+), IFN-β (−) (n = 10) | Sepsis (+), Pneumonia (+), IFN-β (+) (n = 9) | P Value |
|---|---|---|---|
| Hemoglobin, g/dl (13.92 ± 0.50) | 12.8 (12.4–13.8) | 12.7 (11.6–13.15) | 0.23 |
| MCV, fl (52.65 ± 0.55) | 45.8 (45.2–46.2) | 45.1 (44.6–45.7) | 0.22 |
| MCH, pg (15.32 ± 0.15) | 14.4 (14.3–14.6) | 14.3 (14.3–14.6) | 0.61 |
| MCHC, g/dl (29.11 ± 0.26) | 31.4 (31.2–31.9) | 31.5 (31.3–32.4) | 0.46 |
| White blood cell count, /μl (3,520 ± 1,312) | 2,750 (2,150–3,452) | 2,230 (885–2,400) | 0.06 |
| Platelets, ×104/μl (96.35 ± 31.68) | 46.8 (37.3–52.3) | 59.9 (52.4–62.0) | 0.016 |
Definition of abbreviations: MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume.
Data are displayed as median (interquartile range). Differences among groups were compared by using the Mann-Whitney rank-sum test.
Reference values for 10-week-old C57BL/6J male mice are shown in parentheses (mean ± SD). Data were obtained from the following website: www.crj.co.jp/cms/pdf/info_common/17/8003381/control_data_6j_may2010.pdf (accessed on October 18, 2017).
Table 2.
Effect of Systemic IFN-β on Blood Chemistry of Mice with Septic Peritonitis Followed by Pneumonia
| Blood Chemistry* | Sepsis (+), Pneumonia (+), IFN-β (−) (n = 17) | Sepsis (+), Pneumonia (+), IFN-β (+) (n = 18) | P Value |
|---|---|---|---|
| BUN, mg/dl (33.67 ± 4.35) | 16.0 (10.8–19.8) | 12.0 (9.5–14.1) | 0.042 |
| AST, mg/dl (68.2 ± 24.1) | 93.0 (64.1–110.2) | 85.3 (67.7–99.8) | 0.44 |
| ALT, mg/dl (29.8 ± 7.6) | 13.5 (8.2–6.6) | 9.6 (6.5–11.3) | 0.072 |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen.
Data are presented as median (interquartile range). Differences among groups were compared by using the Mann-Whitney rank-sum test.
Reference values for 10-week-old C57BL/6J male mice are shown in parentheses (mean ± SD). Data were obtained from the following website: www.crj.co.jp/cms/pdf/info_common/17/8003381/control_data_6j_may2010.pdf (accessed on October 18, 2017).
Therapeutic Efficacy of IFN-β Prophylaxis for ARDS and Survival
In mice subjected to sepsis followed by pneumonia, a single IFN-β dose before pneumonia onset improved their overall survival dramatically compared with untreated mice (P = 0.034; log-rank test) (Figure 1). Nineteen (90%) of 21 mice in cohort 3 (minus IFN-β prophylaxis) were dead by Day 7, whereas only 13 (59%) of 22 mice in cohort 4 (plus IFN-β prophylaxis) were dead by this time. Thus, IFN-β prophylaxis was associated with an 85% reduction in 7-day mortality (OR, 0.15; 95% confidence interval, 0.03–0.82; P = 0.045) (Figure 1). IFN-β prophylaxis did not reduce total pulmonary neutrophil accumulation, as revealed by inspection of lung histology (Figure 2A) and measurements of total lung MPO activity (Figure E5A). However, IFN-β prophylaxis improved the concentrations of protein and albumin in BALF (Figures 2B and 2C), indicating its protective effect to prevent intraalveolar protein leakage. In addition, IFN-β increased the number of neutrophils recruited into the alveoli significantly (P = 0.032), as revealed by BALF analyses (Figure 2D) and lung histology (Figure 2F, bottom panels). IFN-β reduced the pulmonary bacterial burden measured 18 hours after pneumonia onset (P = 0.031) (Figure 2E). Consequently, IFN-β reduced the Lung Injury Score significantly (P = 0.006) (Figure E5B) by reducing the severity of abnormalities characteristic of ALI compared with untreated mice (Figure 2F). Collectively, these data indicated that IFN-β prophylaxis improved mouse survival and lung epithelial permeability, and also reduced histologically observable lung injury, findings that are consistent with the concept of ARDS.
Sepsis Reprogrammed the Alveolar Cytokine Response to Lung Infection
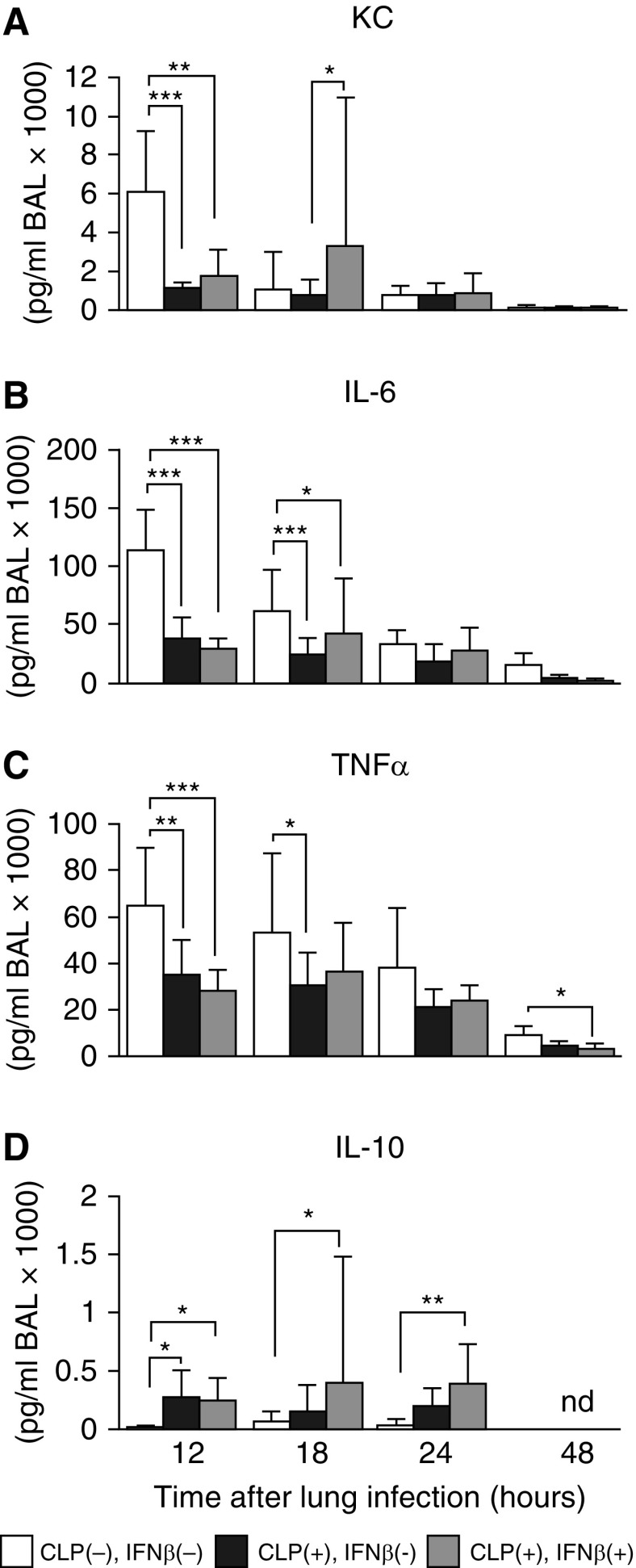
CLP-induced peritoneal sepsis altered the alveolar cytokine signaling responses to subsequent bacterial pneumonia markedly in a cytokine-specific manner. Peritoneal sepsis was associated with a significant reduction in the acute, pulmonary infection–specific increase in concentrations of KC, IL-6, and TNF-α within alveoli as measured in BALF (Figure 3A–3C). In sharp contrast, peritoneal sepsis was associated with a significant and prolonged increase in the pulmonary infection–stimulated increase in IL-10 concentrations within alveoli as measured in BALF (Figure 3D). Prophylactic IFN-β improved KC expression in lungs significantly (P = 0.048) as measured in BALF (Figure 3A) 18 hours after pneumonia onset. These results indicate that CLP altered the acute cytokine response in alveoli to lung infection, thus providing an explanation for impaired recruitment of neutrophils into the intraalveolar space. Importantly, IFN-β reversed the CLP-stimulated reprogramming of alveolar cytokine responses to lung infection (Figure 3A).
Figure 3.
Sepsis interfered with the normal alveolar cytokine responses to pneumonia, which were partially restored by systemic IFN-β in mice. (A–D) Cytokine concentrations in BALF collected at the times indicated after pneumonia onset in mice without CLP (white bars) or with CLP (black bars), and with CLP accompanied by IFN-β prophylaxis (gray bars). Data are the mean ± SD of 10–12 (12 h and 18 h) or 4–6 (24 and 48 h) mice/group. *P < 0.05; **P < 0.01; ***P < 0.001. KC = keratinocyte chemoattractant; nd = not detected.
Sepsis Initiates a Temporospatial Program of Innate Immune Cytokine Signaling
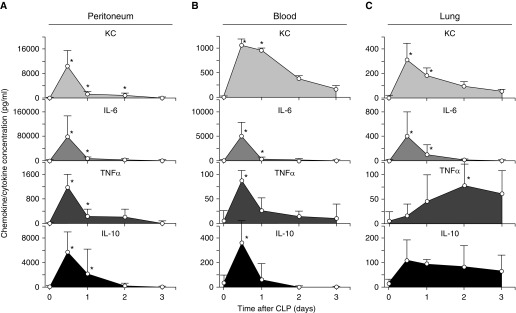
CLP caused time-, location-, and cytokine-specific changes in KC, IL-6, TNF-α, and IL-10 concentrations in the peritoneum, blood, and lungs (Figure 4). The peritoneum and blood showed similar cytokine profiles, peaking at 12 hours and declining thereafter quickly, although the magnitude was greater in the peritoneum (Figures 4A and 4B). In the alveolar space, in which KC and IL-6 concentrations showed similar rapid increases and declined thereafter, TNF-α and IL-10 concentrations increased slowly and persisted at high levels for up to 3 days (Figure 4C). Thus, CLP-induced peritoneal sepsis initiated a complex “wave” of cytokine signaling that emanated from its peritoneal epicenter, penetrated the alveolar space, and persisted for at least 3 days. TNF-α and IL-10 concentrations differed in their time-course profiles in the other body compartments and remained high 3–4 days after CLP.
Figure 4.
Effect of sepsis on cytokine responses in each body compartment. (A–C) Increased cytokine concentrations in the peritoneum, blood, and lungs initiated by CLP-mediated sepsis. Symbols represent the mean ± SD cytokine concentrations measured using a BD Cytometric Bead Array in (A) peritoneal lavage fluid, (B) serum, and (C) BALF obtained from mice (n = 5 per point) at the times indicated. Data were adjusted to take into account specimen-specific dilution factors (peritoneal lavage fluid, serum, and BALF are 13, 1, and 30, respectively). *P < 0.05.
Sepsis Reprograms the Effector and Regulatory Functions of AMs
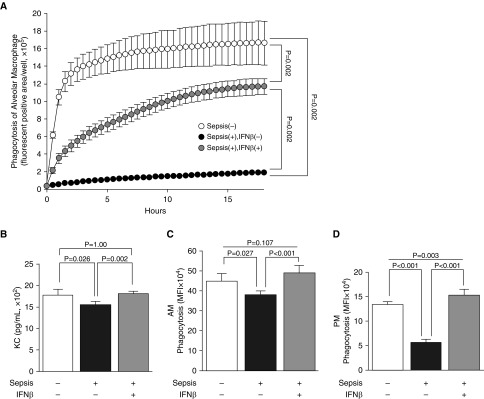
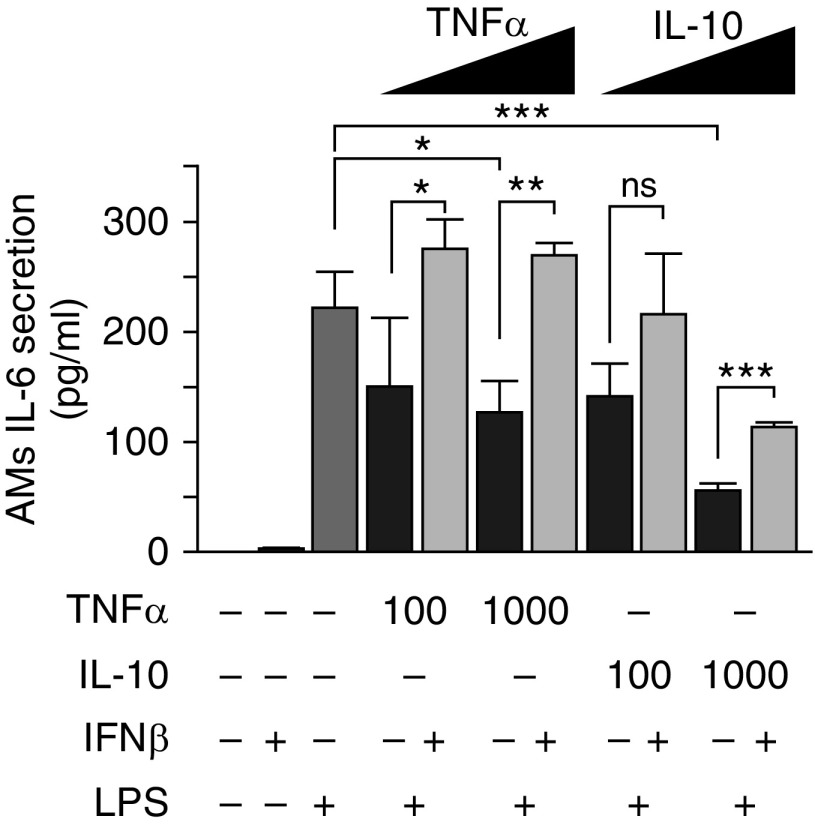
AMs clear pathogens directly through their role as tissue-resident (primary) phagocytes and indirectly by recruiting (secondary) phagocytes (neutrophils) onto the alveolar surface. Hence, we evaluated the effects of sepsis on AM numbers, phagocytic capacity, and chemokine/cytokine secretion. AM numbers increased slightly 4 days after CLP compared with those of unmanipulated controls (1.5 ± 0.3 × 105 vs. 0.9 ± 0.3 × 105; n = 4/group; P < 0.01) (Figure E6). AM numbers were not influenced by IFN-β before or 18 hours after pneumonia onset (P = 0.09 or P = 0.125, respectively) (Figure E6). By contrast, the bactericidal capacity of AMs 4 days after CLP to phagocytose fluorescently labeled Escherichia coli decreased after CLP but was restored after IFN-β treatment (Figure 5A). Similarly, the capacity of AMs to secrete KC decreased after CLP but normalized after IFN-β treatment (Figure 5B). Phagocytosis of fluorescently labeled latex beads in AMs showed similar patterns but with different magnitudes (Figure 5C). To ascertain if these effects were limited to AMs or were generalized, PMs were also evaluated 4 days after CLP. Compared with controls, the phagocytic capacity of PMs was reduced markedly by CLP and, importantly, normalized by IFN-β treatment (Figure 5D). To further explore these abnormalities and determine their relationship with the prolonged increase in TNF-α and/or IL-10 concentrations in the lung caused by CLP (Figure 4C), normal AMs were exposed to each cytokine in vitro, after which IL-6 secretion in response to LPS was measured. The capacity of AMs to secrete IL-6 was reduced significantly (P = 0.031) by exposure to TNF-α, whereas IFN-β treatment restored it completely (Figure 6). Similarly, IL-10 exposure resulted in a concentration-dependent reduction in IL-6 secretion that was reversed completely by IFN-β treatments at low concentrations and partially at high concentrations (Figure 6). These data suggest that CLP-induced cytokine exposure impaired the innate immune effector and regulatory functions of macrophages locally in the peritoneum and heterotopically in alveoli. Therefore, antecedent sepsis did not change AM numbers markedly but reduced phagocytic clearance and chemokine secretion at hit 2 (pneumonia). This finding was reproduced in vitro. IFN-β improved phagocytic clearance and chemokine secretion at hit 2 (pneumonia) in vivo and reversed the cytokine-induced impairment in cytokine release.
Figure 5.
The innate immune functions of macrophages are reduced after sepsis and restored by IFN-β. (A) Phagocytic capacity of alveolar macrophages (AMs) collected on Day 0 from the mice indicated (n = 6 per group) measured by the uptake of pH-sensitive fluorescent dye–labeled bioparticles (Escherichia coli) (IncuCyte pHrodo Green E. coli Bioparticles for Phagocytosis; Essen BioScience) using time-lapse fluorescence imaging (IncuCyte Zoom; Essen BioScience). Time-course increase in fluorescent areas indicates capacity to phagocytose and exposure of the content to low pH in the phagosome. P values indicate differences between each group at 18 hours of incubation. (B) KC secretion of AMs stimulated with E. coli bioparticles (pHrodo) (n = 6 mice per group). (C) Phagocytic capacity of AMs (n = 5 per group) measured by uptake of fluorescently labeled beads using flow cytometry. (D) Phagocytic capacity of peritoneal macrophages (PMs) collected by peritoneal lavage from mice and evaluated as described in C (n = 5 mice/group). MFI = mean fluorescent intensity.
Figure 6.
Reproduction of the TNF-α– and IL-10–mediated AM innate immune hyporesponsiveness and correction by IFN-β in vitro. AMs collected by BAL from unmanipulated mice were cultured with TNF-α or IL-10 at the concentrations indicated (pg/ml) with or without IFN-β (1,000 U/ml), and stimulated with LPS as indicated. IL-6 released into the culture medium was measured by ELISA as described in the Methods section. *P < 0.05, **P < 0.01, ***P < 0.001. ns = not significant.
Discussion
We used a murine two-hit infection model (34) to reproduce sepsis-related ARDS and study both the pathogenesis of ARDS and the therapeutic effects of IFN-β. CLP-mediated peritoneal sepsis (hit 1) followed 4 days later by respiratory tract infection with P. aeruginosa (hit 2) resulted in markedly increased mortality compared with either insult alone. Mortality was attributed to ARDS resulting from increased ALI when the lung infection occurred during a period of sepsis-induced alveolar innate immune hyporesponsiveness, which comprised effects on AMs, including reduced phagocytic capacity by macrophages and reduced infection-stimulated expression of neutrophil chemoattractants by AMs. Importantly, sepsis-mediated immune reprogramming was associated with reduced neutrophil recruitment into alveoli, despite increased neutrophil recruitment into the pulmonary interstitium, referred to as incomplete alveolar neutrophil recruitment. IFN-β reversed the suppressive effects of prior sepsis on the immune functions of AMs, improved neutrophil recruitment from the pulmonary interstitium into the intraalveolar space, improved bacterial clearance in the lung, reduced sepsis-related ARDS, and improved mouse survival.
The conclusion that the CLP-induced sepsis → pneumonia infection model employed in the present study recapitulated postseptic pneumonia-related ALI and ARDS faithfully is supported by four main observations. First, CLP initiated a “systemic wave” of cytokine reprogramming resulting in an alveolar (and peritoneal) innate immune hyporesponsiveness corresponding to systemic inflammatory response syndrome and the compensatory antiinflammatory response syndrome, which are complex, interconnected pro- and antiinflammatory processes initiated by severe infection that lead to immune paralysis (14). Second, the Lung Injury Score (37) increased 18 hours after pneumonia onset after sepsis compared with nonsepsis control animals and resulted in reproduction of the specific histopathological abnormalities seen in patients with sepsis-related ARDS (5). Third, parenchymal lung inflammation, edema, and endothelial permeability in the lung increased when pneumonia followed sepsis compared with nonsepsis control animals. This finding is consistent with the concept that exaggerated neutrophil lung inflammation and pulmonary edema are central to the pathogenesis of ARDS (5). Finally, compared with results in nonsepsis control animals, mortality was sixfold higher when pneumonia followed sepsis, a finding consistent with reports of impaired lung defenses after sepsis in humans (38) and mice (39). It will be interesting to determine if the two-hit model approach results in ARDS when other initial insults are deployed (e.g., cerulein-induced pancreatitis, ischemia–reperfusion injury, or peritoneal/systemic administration of endotoxins).
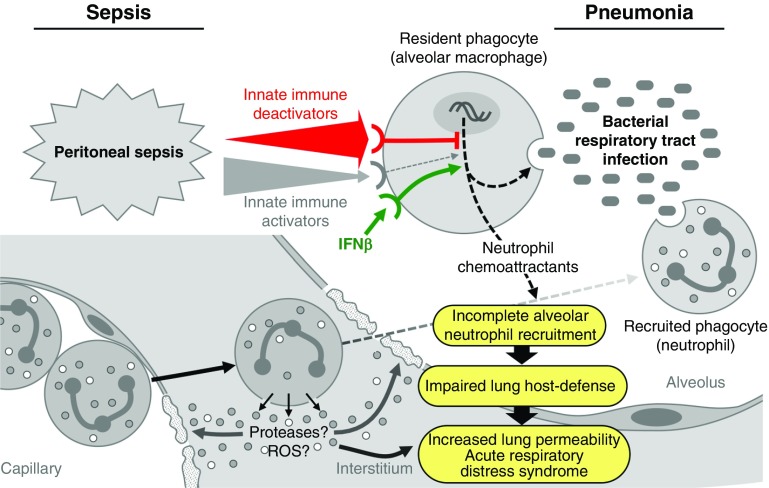
Our observation of incomplete neutrophil recruitment into alveoli during postsepsis lung infection is consistent with the work of Delano and colleagues (40), and we identified a mechanism that could explain the paradox of increased neutrophilic inflammation during innate immune hyporesponsiveness and ARDS development (Figure 7). P. aeruginosa is cleared from the respiratory surface initially by primary (resident) phagocytes (AMs) and progressively thereafter by secondary phagocytes (circulating neutrophils), which are recruited into alveoli by KC (and other chemokines) released by AMs (41). Sepsis reduced the phagocytic capacity of alveolar (and peritoneal) macrophages as well as Pseudomonas-stimulated KC secretion by AMs. Sepsis also reduced alveolar KC concentrations and neutrophil recruitment into alveoli (both measured in BALF) simultaneously with increases in neutrophilic lung inflammation/MPO activity and Lung Injury Score. Thus, neutrophil localization in the pulmonary vasculature and interstitium, rather than within alveoli, shifted the balance of host responses from rapid bacterial clearance to ALI, leading to ARDS.
Figure 7.
Proposed mechanism for sepsis-related lung injury, acute respiratory distress syndrome (ARDS), and the therapeutic efficacy of IFN-β. Initially, bacteria entering alveoli interact with alveolar macrophages (primary phagocytes), which initiate primary microbial clearance and release chemokines and cytokines to recruit/activate neutrophils (secondary phagocytes) and initiate secondary microbial clearance and other immune responses rapidly. Sepsis initiates a systemic wave of innate immune reprogramming comprising rapid-onset, transient activation and delayed, prolonged deactivation. This resulted in prolonged alveolar hyporesponsiveness, including reductions in the effector (phagocytosis) and regulatory (chemokine secretion) functions of alveolar macrophages. This reprogramming serves as an “immunological lens” by focusing and amplifying the attack locally and reducing effects distally, and, over time, it leads to the resolution and restoration of homeostasis. When a respiratory tract infection occurs during the period of sepsis-induced hyporesponsiveness, the concomitant reduction in infection-stimulated alveolar neutrophil chemoattractant concentrations leads to frustrated or incomplete alveolar neutrophil recruitment. Consequently, fewer neutrophils are recruited into alveoli, but increased numbers are recruited to the pulmonary vasculature and interstitium. This alters the kinetics of secondary microbial clearance, thereby shifting the balance from rapid microbial clearance to increased acute lung injury and ARDS development. IFN-β functions therapeutically in this context by reversing sepsis-mediated innate immune reprogramming to enhance rapid microbial clearance and, consequently, reduce acute lung injury and ARDS development. See the main text for further details. ROS = reactive oxygen species.
Numerous reports have documented that sepsis mediates immune suppression via host factors such as IL-10 (42) and TNF-α (43) and pathogen-associated molecular pattern molecules such as LPS (23) and peptidoglycans (44), all of which increase after CLP in rodents (34, 45, 46). The importance of AMs in neutrophil recruitment into alveoli is supported by a report showing that depletion of AMs before Pseudomonas lung infection reduced the early increase in alveolar KC and MIP-2 concentrations, delayed neutrophil recruitment into alveoli, reduced bacterial clearance, increased lung injury, and increased mouse mortality (41). The importance of KC is supported by a report showing that bacterial lung infection in KC-deficient mice resulted in reduced recruitment of neutrophils into alveoli, reduced bacterial clearance, and increased mortality compared with control animals (47), whereas another study showed that CLP reduced concentrations of KC receptors on neutrophils, thereby impeding neutrophil recruitment (48). Taken together, these observations support a mechanism in which sepsis → pneumonia–related ARDS results from alterations in the temporospatial timing and quality of the innate immune responses to lung infection (i.e., incomplete alveolar neutrophil recruitment) rather than from a simple increase in the magnitude of the inflammatory response.
Several observations regarding the therapeutic efficacy of IFN-β in the two-hit model have translational implications for the potential development of IFN-β as therapy for ARDS and for its mechanism of action. In a study by Bellingan and colleagues (29), administration of IFN-β to patients with established ARDS (mostly sepsis related) for 6 consecutive days was associated with an 81% reduction in 28-day mortality. In contrast, in our murine study, administration of IFN-β only once before lung infection was associated with an 85% reduction in the OR for 7-day mortality, suggesting that IFN-β may be a therapeutically effective prophylaxis against ARDS development in high-risk patients. IFN-β also normalized the effector and regulatory functions of AMs (i.e., phagocytosis and KC secretion), restored alveolar KC concentration, and normalized alveolar neutrophil recruitment, suggesting that its therapeutic mechanism of action may involve reversal of sepsis-mediated innate immune hyporesponsiveness. This mechanism of action is supported by a report indicating that IFN-β is essential for expression of proinflammatory mediators in macrophages and restores LPS responsiveness to immunosuppressed IFN-β–deficient macrophages (49). The importance of type I IFN signaling in recruiting neutrophils into the infected peritoneum is also supported by the work of Kelly-Scumpia and colleagues (50). Further support comes from the study by Bellingan and coworkers, who identified treatment-related changes in immune/inflammatory biomarkers, including myxovirus resistance protein 1, β2-microglobulin, CD73, IL-8, and IL-6 (29). IFN-β has been shown to induce CD73 expression on pulmonary vascular endothelial cells and to reduce pulmonary vascular leakage after intestinal ischemia–reperfusion injury in mice (32), so its therapeutic effects in our model may be explained by improved vascular barrier function. IFN-β treatment reduced protein and albumin concentrations in BALF significantly, although the wet weight/dry weight ratio of the lungs did not change (data not shown). Hence, our observation supports the notion that IFN-β helps to prevent intraalveolar protein leakage and may have contributed to the dramatic improvement in mouse survival. These results support the feasibility and clinical application of IFN-β therapy for sepsis-mediated ARDS in humans.
Study limitations meant that we did not evaluate the functional difference between the neutrophils in the lung interstitium and endoalveolar neutrophils to estimate the contribution of interstitial neutrophils to lung injury after pneumonia onset. Besides, we could not establish a dose–response relationship or evaluate the timing, frequency, or route of IFN-β administration to optimize the therapeutic benefit. Nor were we able to define the temporal envelope of innate immune reprogramming and vulnerability to infection. TNF-α and IL-10 were implicated as mediators of sepsis-mediated AM reprogramming, but our results did not exclude the contribution of other factors (e.g., endotoxins). There may other potential direct effects of IFN-β on other cell types such as neutrophils, although in the present study, systemic IFN-β did not alter the neutrophil phenotype or proportions in the lungs and peripheral blood (data not shown). A randomized clinical trial (CORDIS [Community Research and Development Information Service] number 305853) is underway to confirm the clinical benefit of IFN-β therapy for ARDS. It would be very interesting to know if IFN-β is of benefit for ARDS associated with other initial insults, as discussed above.
In conclusion, using a clinically relevant two-hit sepsis-related ARDS mouse model, we found that antecedent sepsis affects impaired innate immunity in the lungs, especially the effector and regulatory functions of AMs, which resulted in incomplete neutrophil trafficking into the alveoli and, consequently, worse survival. Prophylactic systemic IFN-β restored impaired macrophage functions and improved the survival of CLP-injured mice.
Acknowledgments
Acknowledgment
The authors thank Yoko Suzuki for excellent technical support and Professors Yasuyuki Seto, Department of Stomach and Esophageal Surgery, and Kyoji Moriya, Division of Infection Control and Prevention Service, The University of Tokyo Hospital, for their valuable comments on the manuscript. The authors also thank Arshad Makhdum, Ph.D., of the Edanz Group (www.edanzediting.com/ac) for editing a draft of the manuscript.
Footnotes
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology-Japan; the Japan Society for the Promotion of Science (A232490720001 [Y. Yamada, N.Y., S.N., K.U.], B24390364 [Y. Yamada, K.U.], C26462747 [S.N., K.U., K.D., N.Y.], 15H06176 and 17K17043 [T.H.]); the Japan Ministry of Health, Labor and Welfare (H24-Nanchitou [Nanchi]-Ippan-035, H24-Rinkensui-Ippan-003 [K.U.]); and the National Heart, Lung, and Blood Institute (HL085453 [B.C.T.]).
Author Contributions: T.H., K.U., B.C.T., Y. Yamamura, and Y. Yamada: planned the experiments; T.H., H.T., K.U., Y.K., T.T., S.M., and K.N.: conducted experiments; T.H., H.T., K.U., B.C.T., Y.K., T.S., K.D., E.N., S.N., N.Y., N.M., K.C., and Y. Yamada: interpreted the results; and T.H., K.U., B.C.T., and Y. Yamada: drafted the manuscript for important intellectual content. All authors contributed to the final manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0261OC on January 24, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005;33:1549–1556. doi: 10.1097/01.ccm.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederman MS, Fein AM. Sepsis syndrome, the adult respiratory distress syndrome, and nosocomial pneumonia: a common clinical sequence. Clin Chest Med. 1990;11:633–656. [PubMed] [Google Scholar]
- 7.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 8.Diaz JV, Brower R, Calfee CS, Matthay MA. Therapeutic strategies for severe acute lung injury. Crit Care Med. 2010;38:1644–1650. doi: 10.1097/CCM.0b013e3181e795ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payen D, Monneret G, Hotchkiss R. Immunotherapy - a potential new way forward in the treatment of sepsis. Crit Care. 2013;17:118. doi: 10.1186/cc12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torgersen C, Moser P, Luckner G, Mayr V, Jochberger S, Hasibeder WR, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–1847. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 19.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 20.Merlino JI, Yowler CJ, Malangoni MA. Nosocomial infections adversely affect the outcomes of patients with serious intraabdominal infections. Surg Infect (Larchmt) 2004;5:21–27. doi: 10.1089/109629604773860273. [DOI] [PubMed] [Google Scholar]
- 21.Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–712. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 22.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 23.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza-Fonseca-Guimaraes F, Parlato M, Fitting C, Cavaillon JM, Adib-Conquy M. NK cell tolerance to TLR agonists mediated by regulatory T cells after polymicrobial sepsis. J Immunol. 2012;188:5850–5858. doi: 10.4049/jimmunol.1103616. [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 27.Andreu-Ballester JC, Tormo-Calandín C, Garcia-Ballesteros C, Pérez-Griera J, Amigó V, Almela-Quilis A, et al. Association of γδ T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol. 2013;20:738–746. doi: 10.1128/CVI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, et al. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 29.Bellingan G, Maksimow M, Howell DC, Stotz M, Beale R, Beatty M, et al. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med. 2014;2:98–107. doi: 10.1016/S2213-2600(13)70259-5. [DOI] [PubMed] [Google Scholar]
- 30.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 31.Mahieu T, Libert C. Should we inhibit type I interferons in sepsis? Infect Immun. 2007;75:22–29. doi: 10.1128/IAI.00829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiss J, Yegutkin GG, Koskinen K, Savunen T, Jalkanen S, Salmi M. IFN-β protects from vascular leakage via up-regulation of CD73. Eur J Immunol. 2007;37:3334–3338. doi: 10.1002/eji.200737793. [DOI] [PubMed] [Google Scholar]
- 33.Yoo CH, Yeom JH, Heo JJ, Song EK, Lee SI, Han MK. Interferon β protects against lethal endotoxic and septic shock through SIRT1 upregulation. Sci Rep. 2014;4:4220. doi: 10.1038/srep04220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, et al. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res. 2008;150:278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frevert CW, Martin TR. Sepsis and the lung host response. Semin Respir Crit Care Med. 2004;25:85–93. doi: 10.1055/s-2004-822308. [DOI] [PubMed] [Google Scholar]
- 39.Hoogerwerf JJ, Leendertse M, Wieland CW, de Vos AF, de Boer JD, Florquin S, et al. Loss of suppression of tumorigenicity 2 (ST2) gene reverses sepsis-induced inhibition of lung host defense in mice. Am J Respir Crit Care Med. 2011;183:932–940. doi: 10.1164/rccm.201006-0934OC. [DOI] [PubMed] [Google Scholar]
- 40.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, et al. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A, Marchesi F. IL-10 and macrophages orchestrate gut homeostasis. Immunity. 2014;40:637–639. doi: 10.1016/j.immuni.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, et al. TNF-α is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama K, Okugawa S, Yanagimoto S, Kitazawa T, Tsukada K, Kawada M, et al. Involvement of IRAK-M in peptidoglycan-induced tolerance in macrophages. J Biol Chem. 2004;279:6629–6634. doi: 10.1074/jbc.M308620200. [DOI] [PubMed] [Google Scholar]
- 45.Hellman J, Roberts JD, Jr, Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277:14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 46.Otero-Antón E, González-Quintela A, López-Soto A, López-Ben S, Llovo J, Pérez LF. Cecal ligation and puncture as a model of sepsis in the rat: influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor α levels. Eur Surg Res. 2001;33:77–79. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 47.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B4 augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188:3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CM, et al. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am J Respir Crit Care Med. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- 49.Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-β to the murine macrophage response to the Toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- 50.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]