Abstract
Recent studies have demonstrated that obstructive sleep apnea (OSA) is associated with the development and evolution of nonalcoholic fatty liver disease (NAFLD), independent of obesity or other shared risk factors. Like OSA, NAFLD is a prevalent disorder associated with major adverse health outcomes: Patients with NAFLD may develop cirrhosis, liver failure, and hepatocellular carcinoma. One major finding that has emerged from these studies is that the OSA–NAFLD association is related to the degree of nocturnal hypoxemia in OSA. Animal models have therefore largely focused on intermittent hypoxia, a key manifestation of OSA, to shed light on the mechanisms by which OSA may give rise to the complex metabolic disturbances that are seen in NAFLD. Intermittent hypoxia leads to tissue hypoxia and can result in oxidative stress, mitochondrial dysfunction, inflammation, and overactivation of the sympathetic nervous system, among many other maladaptive effects. In such models, intermittent hypoxia has been shown to cause insulin resistance, dysfunction of key steps in hepatic lipid metabolism, atherosclerosis, and hepatic steatosis and fibrosis, each of which is pertinent to the development and/or progression of NAFLD. However, many intriguing questions remain unanswered: Principally, how aggressively should the clinician screen for NAFLD in patients with OSA, and vice versa? In this review, we attempt to apply the best evidence from animal and human studies to highlight the relationship between these two disorders and to advocate for further trials aimed at defining these relationships more precisely.
Keywords: metabolic syndrome, sleep disordered breathing, insulin resistance, dyslipidemia, intermittent hypoxia
Obstructive sleep apnea (OSA) is a highly prevalent disorder that has been associated with multiple adverse health outcomes; among these are a variety of metabolic disorders or illnesses under the umbrella term of “metabolic syndrome,” such as hypertension, glucose dysregulation and insulin resistance, atherosclerosis, and alterations in lipid metabolism (1). It may therefore be unsurprising that OSA also is associated with nonalcoholic fatty liver disease (NAFLD) (2).
NAFLD is common in the general population, affecting up to 75% of obese individuals and 1 in 4 people worldwide (3, 4). It can be broadly subdivided into two categories: 1) nonalcoholic fatty liver (NAFL), which is characterized by hepatic steatosis without important inflammation, ballooning, or fibrosis, and which is believed to be the nonprogressive form of NAFLD, and 2) nonalcoholic steatohepatitis (NASH), which is typically characterized by hepatic steatosis, lobular inflammation, and ballooning with or without perisinusoidal fibrosis and which is believed to be the progressive form of NAFLD. Some patients with NASH subsequently develop cirrhosis, hepatocellular carcinoma, need for liver transplant, or liver-related death; indeed, NASH is the fastest growing cause of cirrhosis and hepatocellular carcinoma (5). The pathogenesis of NAFLD and the means by which some patients will progress to NASH are not completely understood, but many suggest a “multihit” model whereby some with hepatic steatosis are subsequently exposed to a variety of insults that may trigger the development of NASH (6). There are currently no U.S. Food and Drug Administration–approved agents to treat NAFLD, although several promising drugs may be on the horizon. Weight reduction is universally recommended, however, because successful and sustained weight loss may improve not only hepatic steatosis but also liver fibrosis and liver inflammation (7).
Starting in the mid-2000s, a series of reports was published that associated OSA severity with the development and progression of NAFLD. Since then, this association has been fairly well established with more than 20 studies in a variety of patient populations, both pediatric and adult. In this review, we describe the putative relationship between OSA and metabolic dysfunction in NAFLD, and we delineate both clinical and animal studies that have provided insight into this link. We end with a series of unanswered questions in the field, for which we advocate further investigation.
Sleep-disordered Breathing and Hypoxia
Animal Models of OSA
OSA is characterized by recurrent closure of the upper airway during sleep. In turn, these events have several physiological effects that are believed to be hallmarks of OSA and that may lead to further adverse outcomes (8, 9):
-
•
Intrathoracic pressure swings: Negative intrathoracic pressure during inspiration may be greatly accentuated in the presence of apneas, because the patient attempts to inspire against a collapsed oropharynx.
-
•
Sleep fragmentation: With many disordered breathing events, there is transient electroencephalographic evidence of arousals from sleep. Recurrent arousals are believed to be the major contributor to daytime sleepiness in OSA.
-
•
Hypercapnia: With each disordered breathing event, there may be an elevation in PaCO2. CO2 monitoring in OSA is not routinely performed but can generally be accomplished via transcutaneous or end-tidal CO2 detectors. This CO2 elevation may be more profound in patients with underlying lung diseases such as chronic obstructive pulmonary disease or interstitial lung disease.
-
•
Intermittent hypoxia (IH): The pattern of nocturnal hypoxia may vary considerably among patients with OSA. Some may have a very high apnea–hypopnea index (AHI) with relatively mild desaturations, and others could have few, prolonged events resulting in marked hypoxemia.
Few animals naturally exhibit OSA, so instead this illness historically has been modeled by way of mechanical obstruction of the upper airway, such as by intermittent occlusion of an endotracheal tube or tracheostomy. However, a more targeted approach, affecting one of the above-described physiological effects of OSA, is useful and has provided considerable insight into the mechanisms that link OSA to a variety of adverse outcomes (10). For instance, animals exposed to IH have elevated blood pressure (11) and develop sympathetic overactivation (12), atherosclerosis (13), and glucose and lipid dysregulation (14–16). IH may be modeled with or without intermittent hypercapnia, although data are mixed with respect to the relative physiological effects of IH versus intermittent hypercapnia in the development of disease (17–20). Sleep fragmentation can be modeled by way of a mechanical apparatus to disrupt sleep (8); this model has been shown to induce insulin resistance and increased food intake (21), tumorigenesis (22), adipose tissue inflammation (23), elevated systemic cytokine concentrations (24), and endothelial dysfunction (25). The remainder of this review is focused on the link between IH and metabolic dysfunction in NAFLD.
Unique Aspects of IH
Since reliable IH systems were first developed in the early 1990s, multiple studies have demonstrated clear differences in the physiological impact of IH versus sustained hypoxia (SH) (26–28). Chronic IH and SH have very different effects on the hypoxic ventilatory response (ventilatory sensitivity to acute hypoxia), depending on the paradigm of IH that is used (29, 30). Also relevant to the pathogenesis of OSA, IH appears to have a more profound effect than SH on weakening upper airway dilator muscle responsiveness (31). Chronic IH and SH result in dissimilar gene expression profiles in rat lungs (32), and IH but not SH appears to induce hypertension in a variety of rodent models, perhaps via sympathetic activation (33). IH and SH produce differences in cardiovascular function (34) and in various metabolic markers, including body weight, insulin resistance, and changes in the relative content of white versus brown adipose tissue (35). In rodent models, IH and SH also cause epigenetic differences (28). Although these data are still nascent, epigenetic changes may account for a major portion of the phenotypic differences between IH and SH. Thus, IH as encountered in OSA is a unique phenomenon that is associated with distinct, often maladaptive, outcomes.
Downstream Effects of IH in OSA
OSA causes IH by recurrent collapse of the upper airway during sleep, as measured by peripheral oxyhemoglobin saturation. It may seem intuitive that arterial desaturation would result in intermittent tissue hypoxia as well. However, no study has examined the tissue-specific effects of recurrent airway closure in humans. A few studies have shown that liver enzymes may be acutely elevated in OSA and are lowered with continuous positive airway pressure (CPAP) (36), and at least one study has shown that serum creatine phosphokinase similarly may be elevated in OSA and reduced by CPAP (37). One explanation of these findings is that OSA might induce mild tissue ischemia resulting from recurrent hypoxemia, but in any case, these studies only provide inferential data about the tissue-specific effects of OSA. Because of the relative lack of insight human studies provide in this context, we must look at experimental data in animal models for clues about the tissue-specific hypoxic effects of OSA. Reinke and colleagues looked at tissue effects of IH and SH in a murine model, exposing animals to one of four profiles: SH (10% FiO2), “mild” IH (12 cycles/h), “severe” IH (60 cycles/h), and room air (38). Experiments were performed in both lean and obese mice, and tissue oxygen tension was measured in anesthetized mice under each condition by use of an oxygen microelectrode. The oxygen tension in each exposure type was reduced in obese versus lean mice, and superimposed IH caused a cyclic reduction in oxygen tension in liver, fat, and muscle tissue. This finding suggests that the IH of OSA likely causes recurrent hypoxia and reoxygenation not just in the circulation but at the tissue level as well.
As might be expected, these cycles of recurrent hypoxia and reoxygenation, both in experimental IH and in OSA, are associated with further molecular and cellular disruption. Reactive oxygen species, molecules containing one or more unpaired electrons, are highly unstable and may interfere with normal cell signaling and function, leading to oxidative stress. Oxidative stress may manifest as genetic mutations, alterations in protein function, or disruption of the cell membrane. Oxidative stress normally is countered by endogenous antioxidant systems. Several studies have demonstrated that patients with OSA have elevated markers of oxidative stress in various tissues (39, 40). Likewise, in experimental models, IH in rodents has been linked to oxidative stress in the brain, heart, and pancreas (41–43). Whether oxidative stress leads to further injury in OSA is unclear, however, because there are only scant data supporting the use of antioxidants in OSA (44, 45).
OSA and experimental IH both have been shown to increase output of the sympathetic nervous system (46–48). Muscle sympathetic nerve activity and catecholamine concentrations are both increased in OSA and are reduced to near-normal levels again with CPAP use. In fact, sympathetic nerve activity is increased in OSA not just during sleep but during wakefulness as well (49). Rodent studies have shown that IH increases carotid body chemoreflex, driving an excess of sympathetic tone (50, 51), and that IH also increases catecholamine release by the adrenal medulla (52, 53). These findings have important repercussions for a variety of metabolic conditions, including hypertension, NAFLD, and insulin resistance.
Mechanisms of Hypoxia-induced Metabolic Dysfunction in OSA
OSA, Hypoxia, and Obesity
Obesity is a clear precipitating factor in the development of NAFLD, and the development of OSA is undoubtedly related to obesity. Around 70% of patients with OSA are obese (54), although this statistic may represent somewhat of a referral bias because clinicians may not consider the diagnosis routinely in lean individuals. Weight reduction in obese individuals with OSA is associated with an improvement in OSA severity and reduced upper airway collapsibility (55, 56). Given this relationship, it is natural to wonder whether OSA itself is associated with weight gain. Interestingly, OSA treatment appears to be associated with a slight increase in body weight, refuting this paradigm (57), although one study has shown that CPAP may reduce visceral body fat even if overall weight is unchanged (58). There have been no studies of long-term IH exposure in humans. However, one study examined the impact on a variety of metabolic parameters of aerobic exercise in a hypoxic environment versus normoxia and found that hypoxia did not impact weight, blood pressure, fasting glucose, or hemoglobin A1c (59). It is well characterized that individuals at altitude (hypobaric hypoxia) lose weight (60); however, altitude is a model of chronic SH. By contrast, experimental chronic IH in rodent models that mimic severe OSA causes less weight gain, and perhaps weight loss, relative to control conditions. This observation is made in animals fed a normal chow diet or a variety of high-fat diets (61, 62).
Glucose Dysregulation in Hypoxia
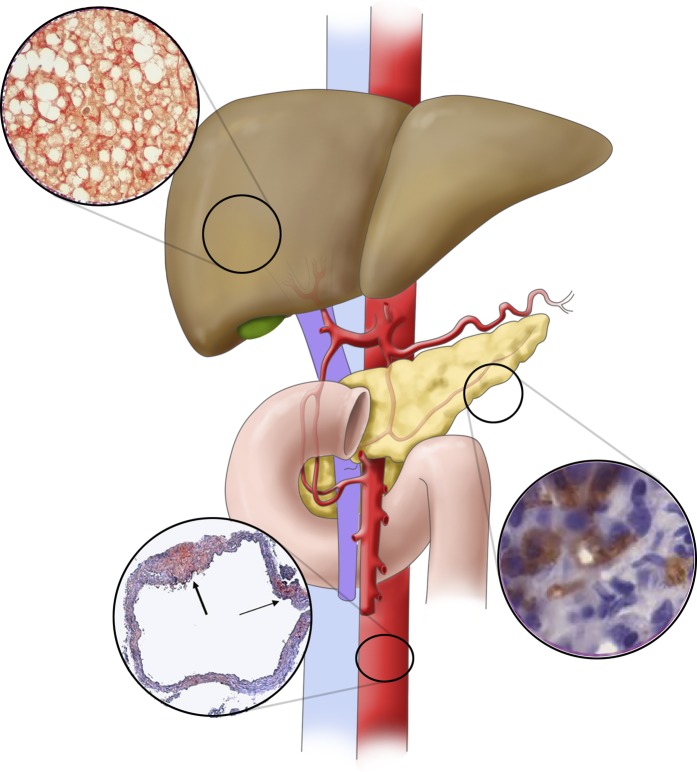
OSA has been associated with insulin resistance and glucose intolerance using a variety of clinically relevant outcomes (hemoglobin A1c, fasting glucose, homeostatic model assessment of insulin resistance, glucose tolerance testing, and the hyperinsulinemic euglycemic clamp) (63). Data are mixed about whether treating OSA with CPAP improves markers of glucose dysregulation. Other expert reviews have provided insight about such studies. There are relatively few studies in humans investigating the distinct impact of IH on glucose handling. One such study by Newhouse and colleagues placed healthy volunteers in cyclic normoxia or IH (25 s of 5% FiO2, followed by 2 min of normoxia, at a frequency of 25 events/h for 3 h). Subjects in IH had increased fasting glucose concentrations but no change in insulin sensitivity (64). These findings echo earlier findings by Louis and Punjabi, who applied a similar 8-hour protocol and found reduced insulin sensitivity and glucose effectiveness in the hypoxic arm, as measured by intravenous glucose tolerance test (65). In this study, sympathetic tone was also increased in hypoxic subjects. Experimental data in rodent models have been more consistent in their outcomes. Universally, severe chronic IH is associated with an increase in fasting glucose, marked insulin resistance, and poor glucose handling (14, 66). Recent data have implicated sympathetic nervous system function in these outcomes: One study showed that IH effects of hyperglycemia, glucose intolerance, and insulin resistance could be abrogated by adrenal medullectomy or by administration of an α-adrenergic antagonist (67). Other studies have demonstrated that IH causes pancreatic β-cell apoptosis, resulting in glucose intolerance and a reduction in insulin sensitivity (68, 69) (Figure 1).
Figure 1.
Mechanisms by which intermittent hypoxia in obstructive sleep apnea may result in metabolic dysfunction in nonalcoholic fatty liver disease. In rodent models, intermittent hypoxia has been linked to pancreatic apoptosis (cells in inset stained for active caspase 3), resulting in insulin resistance. Intermittent hypoxia–mediated overactivation of the sympathetic nervous system is also implicated in the development of glucose dysregulation predisposing to nonalcoholic fatty liver disease. In the vasculature, intermittent hypoxia causes atherosclerosis, among many other effects on lipid metabolism. The thick arrow in the bottom inset points to atherosclerotic plaque with a necrotic core. The thin arrow points to a fatty streak. Intermittent hypoxia has been shown to directly cause hepatic steatosis and fibrosis, possibly owing to oxidative stress and mitochondrial dysfunction. Insets are adapted with permission from References 13 and 68, as well as our unpublished data.
OSA, Hypoxia, and Lipid Metabolism
Over the last 15 years, dozens of studies have attempted to determine the effect of OSA on lipid metabolism. A recent, large meta-analysis that analyzed 107 datasets encompassing over 18,000 patients showed that OSA is associated with higher triglyceride, low-density lipoprotein, and total cholesterol concentrations, as well as lower high-density lipoprotein concentrations (70), with a correlation between AHI and lower high-density lipoprotein and higher triglyceride concentrations. Accordingly, several studies have examined the effect of CPAP on lipid profiles in patients with OSA, although results have mainly been negative. The first randomized, placebo-controlled crossover trial measuring the effect of CPAP versus sham CPAP on fasting lipids showed minor differences in triglyceride and total cholesterol concentrations after 2 months of treatment (71). This study was notable for fairly rigorous methodology: Authors sampled blood for lipid profiles at seven time points during both wake and sleep and compared the area under the 24-hour concentration curve between groups as endpoints. Other studies, including a meta-analysis, have shown some modest effect of CPAP in improving lipid profiles in OSA (72). In one intriguing study, investigators examined the effect of CPAP withdrawal from CPAP-adherent subjects with severe OSA on nighttime free fatty acid, insulin, glucose, and cortisol concentrations. The authors showed that untreated OSA quickly increased nocturnal glucose and free fatty acid concentrations (73).
There are considerable data in rodent models suggesting that the hypoxic burden of OSA may be a critical factor in the development of dysfunctional lipid metabolism in OSA. Chronic IH in obese mice increases liver triglyceride content (74), upregulates pathways of hepatic liver biosynthesis (74), elevates total cholesterol and low-density lipoprotein concentrations in lean mice (16), and induces atherosclerosis (13, 75, 76) (Figure 1). The effect of IH to increase free fatty acid concentrations is reversible by pretreatment with a β-adrenergic antagonist (67), suggesting that these ill effects of hypoxia in OSA might be mitigated by pharmacologic targeting. This concept has not yet been tested in humans, however. To our knowledge, there is only one study of the isolated effect of IH on lipid concentrations in humans. Healthy subjects were exposed to normoxia or IH for 6 hours, with hypoxic gas flow sufficient to target nadir peripheral oxyhemoglobin saturations of 85%, 17 times per hour. Consistent with data obtained from subjects with OSA, free fatty acid concentrations were elevated in those exposed to IH (77). These data collectively suggest that hypoxia is likely a driver of alterations in lipid metabolism in OSA.
OSA, Hypoxia, and Hepatic Steatosis
With all the preceding data, it may come as no surprise that OSA has been independently associated with the development of NAFLD and with its progression to NASH and liver fibrosis. NAFLD is considered a hepatic manifestation of the metabolic syndrome. Therefore, glucose and lipid dysregulation are critical antecedent events in NAFLD development (78). There are now over 20 studies associating OSA with NAFLD in adult subjects (Table 1). Many early studies examined bariatric surgery cohorts because liver biopsy is widely performed at the time of surgery and because most such patients have at least NAFL. However, more recent studies have documented the link between OSA and NAFLD in those of normal weight, and even in children, and studies in obese individuals have generally been careful to examine the independent effect of OSA on NAFLD. Comparatively few studies have sought to determine the effect of CPAP on NAFLD progression, and this line of inquiry is inherently challenging for a few reasons: 1) Most patients with NAFLD do not have elevated liver aminotransferases, and diagnosis of NAFLD in the absence of liver biopsy is a rapidly evolving field, often requiring technical expertise; 2) the time scale of NAFLD progression and regression may require lengthy CPAP trials, which are difficult and costly; and 3) patients with the highest pretest probability for OSA and NAFLD are those who are morbidly obese, but this group may not represent the diversity of these illnesses.
Table 1.
Studies of Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in Adult Human Subjects, Including Interventional Trials
| Study | Subjects (n), Background | NAFLD Diagnosis | NAFLD Prevalence | OSA Diagnosis | Key Findings |
|---|---|---|---|---|---|
| Tanné et al., 2005 (80) | 163 subjects with suspected OSA, mean BMI <30 kg/m2 | LFTs, biopsy in a small subset | Elevated LFTs in 20%, 13 of 18 subjects with biopsy showed steatosis | PSG | Severe OSA independently associated with elevated LFTs, insulin resistance, liver necrosis, and fibrosis |
| Jouët et al., 2007 (101) | 62 morbidly obese bariatric surgery patients, mean BMI 47.8 kg/m2 | LFTs, biopsy | LFTs increased in 47%, NASH in 34% | Polygraphy | OSA an independent risk factor for elevated LFTs but not for NASH |
| Kallwitz et al., 2007 (102) | 85 subjects undergoing bariatric surgery, mean BMI 55.0 kg/m2 | LFTs, biopsy | 99% with NAFLD, 19% with fibrosis | PSG | Subjects with OSA more likely to have elevated ALT. Trend toward increase in OSA prevalence in those with inflammatory injury and fibrosis |
| Campos et al., 2008 (103) | 200 subjects undergoing bariatric surgery, mean BMI 48.0 kg/m2 | LFTs, biopsy | 32% with NASH | PSG | Previously diagnosed OSA associated with a fourfold increased risk for the presence of NASH |
| Mishra et al., 2008 (91) | 101 subjects undergoing bariatric surgery with biopsy-proven NAFLD, BMI 52.6 kg/m2 (NASH), 47.3 kg/m2 (no NASH) | Biopsy | 78% with NASH | PSG | Subjects with NASH had lower nadir and mean O2 saturations, higher AHI, and higher ALT/AST ratio. Nadir O2 saturation was an independent predictor of NASH. Nadir and mean O2 saturations were lower in those with fibrosis |
| Polotsky et al., 2009 (92) | 90 subjects with severe obesity, mean BMI 49.0 kg/m2 | LFTs, biopsy in a subset | 70% of those with biopsy | PSG | Desaturations in >4.6% and respiratory disturbance index >15 independently associated with lobular inflammation, balloon degeneration, and fibrosis |
| Kohler et al., 2009 (104) | 94 subjects with moderate to severe OSA, mean BMI 35.0 kg/m2 | LFTs | N/A (only LFTs used as outcome measure) | Nocturnal oximetry | Both therapeutic CPAP and subtherapeutic CPAP (1 mo) reduced LFTs, but there was no between-group difference |
| Daltro et al., 2010 (105) | 40 subjects undergoing bariatric surgery, mean BMI 41.6 kg/m2 | LFTs, biopsy | NAFLD in 83%, NASH in 80% | PSG | Moderate to severe OSA associated with insulin resistance, but no relationship between AHI and NASH, or T84 and NASH |
| Ulitsky et al., 2010 (106) | 253 subjects undergoing bariatric surgery, mean BMI 48.2 kg/m2 | LFTs, biopsy | 46% with steatosis, 21% with NASH | Prior diagnosis or sleep study in chart | Trend toward OSA as an independent predictor of NASH |
| Türkay et al., 2012 (107) | 106 subjects who underwent PSG for possible OSA, BMI 29.2 kg/m2 (no NAFLD), 33.1 kg/m2 (NAFLD) | Ultrasound, no biopsy | 67% | PSG | AHI, ODI, nadir O2 saturation, T90 all independent predictors of NAFLD Duration of hypoxic events was most significant predictor of NAFLD severity |
| Aron-Wisnewsky et al., 2012 (93) | 101 subjects undergoing bariatric surgery, 91% women, mean BMI 46.9 kg/m2 | LFTs, biopsy | 8% with NASH | Nocturnal oximetry | No difference in LFTs between tertiles of ODI. Lobular inflammation, balloon degeneration, NAFLD activity score, and fibrosis were all more severe in those with highest ODI tertile. Hypoxic burden was independently predictive of liver fibrosis and NAFLD activity score |
| Sivam et al., 2012 (86) | 27 subjects with AHI ≥25, ODI ≥20, naive to CPAP, mean BMI 31.3 kg/m2 | LFTs, abdominal MRI | Undefined | PSG | No difference in intrahepatic lipid content or in any LFTs except alkaline phosphatase with CPAP vs. sham CPAP (2 mo) |
| Corey et al., 2013 (108) | 159 subjects undergoing bariatric surgery, BMI 47.5 kg/m2 (NAFLD), 45.7 kg/m2 (no NAFLD) | ALT, biopsy | 69% with NAFLD | Prior diagnosis of OSA | Absence of OSA was strongly associated with normal liver histology (OR, 5.6) |
| Minville et al., 2014 (83) | 226 referred to sleep clinic for possible OSA, mean BMI 34.5 kg/m2 | Biomarker panels (SteatoTest, NashTest, FibroTest [BioPredictive]), no biopsy | 62% | PSG | T90 independent risk factor for hepatic steatosis in multivariate analysis, and possible NASH in univariate analysis. Severity of hypoxia associated with worsened liver injury only in obese subjects |
| Pulixi et al., 2014 (109) | 159 subjects with histologic NAFLD, 80 age-, sex-, and BMI-matched control subjects, BMI <30 kg/m2 | Biopsy | 67% (predefined) | Berlin questionnaire, Epworth Sleepiness Scale score | In sleepy subjects with risk for OSA, increased prevalence of NASH and significant fibrosis |
| Lin et al., 2015 (110) | 85 subjects with NAFLD diagnosed by ultrasound, mean BMI 27.3 kg/m2 | Ultrasound, no biopsy | 100% (only subjects with NAFLD enrolled) | PSG | ODI predicted elevated ALT; average O2 saturation predicted elevated AST. OSA severity associated with increase in LFTs, cholesterol, fasting glucose |
| Agrawal et al., 2015 (111) | 100 subjects with NAFLD from liver clinic (BMI, 28.3 kg/m2), 23 OSA subjects from pulmonary clinic (BMI, 32.2 kg/m2) | Ultrasound, FibroScan (Echosens), no biopsy | 91% of subjects with OSA | PSG | Among those with OSA, AHI was an independent predictor of liver fibrosis in NAFLD |
| Petta et al., 2015 (94) | 126 subjects with elevated ALT and NAFLD, BMI 29.0 kg/m2 (no OSA), 33.5 kg/m2 (OSA) | LFTs, biopsy | 100% (only subjects with NAFLD enrolled) | STOP-Bang, polygraphy in subset of 50 | OSA prevalence higher in patients with F2–F4 fibrosis on biopsy. Liver fibrosis associated with T95 on polygraphy |
| Mesarwi et al., 2015 (95) | 35 subjects undergoing bariatric surgery, mean BMI 50.2 kg/m2 (liver fibrosis), 46.7 kg/m2 (no fibrosis) | Biopsy | 36% with hepatic fibrosis | PSG | AHI higher in those with fibrosis, and severe OSA more prevalent among those with fibrosis |
| Cakmak et al., 2015 (84) | 137 subjects who underwent PSG for suspected OSA, mean BMI 34.4 kg/m2 | Ultrasound | 83% with NAFLD | PSG | AHI and ODI higher in those with moderate to severe steatosis than in those without fatty liver. Nadir O2 saturation lower in those with NAFLD |
| Qi et al., 2016 (85) | 175 nonobese subjects | Ultrasound, no biopsy | 61% | PSG | No differences in TG, LFTs, CRP, HOMA-IR with worsening OSA. Nadir O2 saturation predicted NAFLD presence |
| Trzepizur et al., 2016 (81) | 1,285 subjects without excessive alcohol intake, suspected of having OSA, mean BMI 30.7 kg/m2 | LFTs, hepatic steatosis index, FibroMeter (Echosens) NAFLD score | 73% with steatosis | PSG or home sleep testing | OSA severity and mean nocturnal O2 saturation both associated with increased risk of hepatic steatosis. Severe OSA associated with 2.5-fold higher risk of liver fibrosis but only in univariate modeling |
| Buttacavoli et al., 2016 (87) | 15 subjects with severe OSA, mean BMI 35.4 kg/m2 | Ultrasound, FibroScan | 87% with steatosis | Polygraphy | No significant change in hepatic steatosis or fibrosis after 6–12 mo of therapeutic CPAP |
| Jullian-Desayes et al., 2016 (88) | 103 subjects with moderate to severe OSA, mean BMI 28.3 kg/m2 | LFTs, FibroMax (SteatoTest, NashTest, FibroTest) | 44% with moderate to severe steatosis, 44% with fibrosis | PSG | No difference in steatosis, NASH, or liver fibrosis between those who got 6–12 wk of CPAP vs. sham CPAP |
| Trzepizur et al., 2018 (112) | 124 subjects with at least one criterion for metabolic syndrome, suspected of having OSA | FibroScan | 27% with significant fibrosis | PSG | Dose–response relationship between OSA severity and liver stiffness. Severe OSA was independently associated with significant fibrosis |
| Chen et al., 2018 (82) | 160 subjects who underwent PSG for OSA symptoms, mean BMI 28.0 kg/m2 | LFTs, ultrasound, acoustic radiation force impulse ultrasound | 64% with steatosis | PSG | LFTs and liver steatosis severity increased with increasing OSA severity. AST and ALT both reduced with 3 mo of CPAP therapy |
| Toyama et al., 2018 (113) | 61 male subjects with OSA on CPAP, abdominal adiposity, mean BMI <30 kg/m2 | Abdominal CT (hepatic steatosis) | 41% with steatosis | PSG | AHI and T90 were greater in subjects with fatty liver than in those without. Liver fat content did not change after a mean 31 mo of therapy, but among those with fatty liver at baseline, CPAP reduced liver fat content |
Definition of abbreviations: AHI = apnea–hypopnea index; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; CPAP = continuous positive airway pressure; CRP = C-reactive protein; CT = computed tomography; HOMA-IR = homeostatic model assessment of insulin resistance; LFT = liver function test (aminotransferase level unless otherwise indicated); MRI = magnetic resonance imaging; N/A = not applicable; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis; ODI = oxygen desaturation index; OR = odds ratio; OSA = obstructive sleep apnea; PSG = polysomnogram; STOP-Bang = snoring, tiredness, observed apnea, high blood pressure, body mass index, age, neck circumference, and male sex questionnaire; TG = triglycerides; Tx = percentage of total sleep time with oxyhemoglobin saturation less than x%.
The link between hypoxia and steatosis in NAFLD has been examined in several studies. Drager and colleagues (79) determined that chronic IH in obese mice causes elevations in hepatic triglyceride content and histological evidence of hepatic steatosis (Figure 1). In humans, many associative studies have examined the effect of OSA on hepatic steatosis, with results seemingly supportive of the role of OSA in modulating this early NAFLD phenotype. In fact, several markers of OSA severity have been linked to liver fat content, including AHI (80–82), time with oxyhemoglobin saturation less than 90% (83), oxyhemoglobin desaturation index (84), and mean (81) and nadir (85) nocturnal oxyhemoglobin saturation. Although some earlier studies were not adjusted for common covariates such as obesity (80), careful multivariate analysis has been performed in larger recent studies.
By contrast, results of studies investigating the impact of CPAP on hepatic steatosis have been largely negative. In a crossover study design of 2 months of CPAP versus sham CPAP in 27 subjects with moderate to severe OSA, Sivam and colleagues showed no change in intrahepatic lipid content, based on magnetic resonance imaging/spectroscopy (86). Similarly negative results were observed in other cohorts (87, 88). Though discouraging, these studies to date have been limited either by small cohorts studied or by limited time on CPAP. Large-scale studies of the impact of CPAP on a variety of markers of lipid metabolism, including hepatic steatosis, are forthcoming.
OSA, Hypoxia, and Hepatic Inflammation and Fibrosis in NAFLD
Although glucose and lipid dysregulation and hepatic steatosis are critical features of early NAFLD, well-designed studies have now convincingly demonstrated that liver fibrosis is the only pathologic feature of NAFLD that is associated with death or need for liver transplant (89, 90). As such, the most critical questions in evaluating the disease-modifying potential of OSA in NAFLD are whether OSA is associated with more severe liver fibrosis in NAFLD and whether CPAP may mitigate the progression of fibrosis. The majority of studies to date that have looked at markers of liver fibrosis as an outcome have shown that OSA is associated with increased fibrosis (Table 1), and a few studies have linked the hypoxic burden of OSA to worsened fibrosis as well (91–93). Some of these studies have used inflammatory markers (cytokine or aminotransferase concentrations) as outcomes, and several biopsy-based studies have found associations between OSA and histologic NASH. However, these studies are not definitive, and one negative study merits particular attention, given its sample size: Trzepizur and colleagues examined a cohort of nearly 1,300 subjects with suspected OSA. Those with severe OSA were found to have a 2.5-fold higher risk for liver fibrosis, but this association did not hold after multivariate adjustment (81). A few studies have examined the prevalence of OSA in patients with NAFLD and liver fibrosis (94, 95), but these studies are lacking either in sample size or in definitive OSA diagnosis. In general, results of CPAP trials that have examined liver fibrosis as an outcome in subjects with OSA and NAFLD have been negative (87, 88), but studies of surrogates of NASH progression such as aminotransferase concentrations have appeared more promising.
Interestingly, despite mixed results in humans, the available data do suggest that rodents exposed to IH develop liver fibrosis, oxidative damage, and hepatic inflammatory injury, and when exposed to IH plus another hepatic insult (e.g., acetaminophen), they may also exhibit marked hepatocellular inflammation and necrosis (96–98). Modeling hypoxia in OSA and NAFLD is problematic, however, because IH generally causes weight reduction in mice with diet-induced obesity. Moon and colleagues have described a possible link between hypoxia-inducible factor 1 and liver fibrosis (99), and our group has shown that deletion of hypoxia-inducible factor 1α in hepatocytes reduces hepatic expression of lipogenic genes and protects against the development of liver fibrosis in a mouse model of NAFLD (100). It therefore seems biologically plausible that the IH of OSA may accentuate liver injury in NAFLD, leading to a shift in the phenotype toward NASH and liver fibrosis. Additional studies are clearly needed to elucidate further the nature of this relationship.
Future Directions
Open Questions for Clinical Research
As clinicians, our primary task is to determine ways to diagnose and treat illness, and this goal can occasionally be lost in the jungle of available trial and bench research data. Practical questions remain for the clinician:
-
1.
Should patients with NAFLD routinely be screened for OSA, and vice versa? To answer this question, we need an accurate estimate of the prevalence of NAFL, NASH, and advanced fibrosis in patients with OSA, as well as of the prevalence of OSA in patients with NAFLD. Prospective studies are needed to fill this gap and to assess the impact of early interventions.
-
2.
There are few hard indications to treat patients with mild OSA in the absence of sleep-related symptoms, so if a patient has NAFLD and mild OSA, should this nudge the clinician toward recommending CPAP?
-
3.
There are no currently available U.S. Food and Drug Administration–approved drugs for NAFLD. Weight loss and dietary discretion are mainstays of therapy. If a patient with NAFLD is found to have OSA, should this prompt more aggressive treatment of NAFLD (e.g., early referral for bariatric surgery)?
-
4.
Why have results of CPAP trials in subjects with OSA and NAFLD been largely negative? How can we design better trials to evaluate response to OSA treatment? Should OSA treatment aside from CPAP be considered? What outcomes would be most appropriate in trial design? How can we identify high-risk patients to enroll in clinical trials?
It is our view that patients with NAFLD, particularly those who are obese and even those without specific sleep-related symptoms, should be screened for OSA, because a large percentage of those with OSA are relatively asymptomatic. However, at this time, we do not recommend that those with mild OSA be treated in the absence of other sleep-related symptoms or major comorbidities. We advocate aggressive attempts at weight reduction in patients with NAFLD as with any other manifestation of metabolic syndrome. Trial design will likely require considerable multicenter efforts to recruit sufficient subjects to demonstrate the effect of OSA therapy on NAFLD progression, and in light of poor CPAP adherence inherent to most clinical trials in OSA, we advocate that trials be more reflective of clinical practice and that alternative therapies, such as an oral appliance, might be considered in CPAP-nonadherent subjects.
Open Questions for Laboratory-based Research
Research in animal models has the potential to elucidate mechanisms that might tie together OSA and NAFLD, and considerable progress is being made. Ongoing issues for bench researchers include the following:
-
1.
In light of the often disparate results afforded by laboratory research and clinical trials, is IH an acceptable model of OSA? In focusing on IH, are we neglecting other aspects of OSA that might themselves worsen NAFLD progression, such as sympathetic overactivation, mitochondrial dysfunction, and the balance of the microbiome?
-
2.
Several studies have now demonstrated that low-intensity IH (low frequency or higher-nadir oxygen saturation as measured by pulse oximetry target) may actually be beneficial in a variety of outcomes. Might this be the case in NAFLD? Similarly, if hypoxic burden is clinically relevant for NAFLD progression, what feature of IH is most critical (e.g., nadir saturation, time with saturation <85% or <90%, oxyhemoglobin desaturation index, slope of the desaturation)?
-
3.
What model of NAFLD should be used? Several models recapitulate the histologic and genomic effects of human NAFLD, but are there advantages of diet-based approaches over other models?
Conclusions
NAFLD and OSA are exceedingly common in the general population, and OSA is associated with the development of key injurious stimuli that give rise to NAFLD as well as the progression of NAFL to NASH and liver fibrosis. This association may be due to the hypoxic burden of OSA, although OSA is an enormously heterogeneous disease state that results in a variety of physiological changes aside from IH. There is experimental evidence linking IH with a variety of key features of NAFLD, including glucose and lipid dysregulation, and hepatic inflammation, oxidative stress, and fibrosis. Further studies are needed to investigate this relationship, and several key questions remain for the clinician and trialist alike. High-quality evidence is required to provide data on screening for OSA in NAFLD and vice versa so that an optimal approach to the management of OSA and NAFLD can be realized.
Acknowledgments
Acknowledgment
The authors thank L.C.W. for illustrations and A.S.M. for helpful discussions.
Footnotes
Author Contributions: Conception and initial draft: O.A.M.; critical revisions: R.L. and A.M.
Originally Published in Press as DOI: 10.1164/rccm.201806-1109TR on November 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 2.Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124–1135. doi: 10.1016/j.metabol.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 8.Chopra S, Polotsky VY, Jun JC. Sleep apnea research in animals: past, present, and future. Am J Respir Cell Mol Biol. 2016;54:299–305. doi: 10.1165/rcmb.2015-0218TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis EM, O’Donnell CP. Rodent models of sleep apnea. Respir Physiol Neurobiol. 2013;188:355–361. doi: 10.1016/j.resp.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 12.Tamisier R, Pépin JL, Rémy J, Baguet JP, Taylor JA, Weiss JW, et al. 14 Nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 13.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24:843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol (1985) 2007;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher EC, Bao G, Miller CC., III Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol (1985) 1995;78:1516–1521. doi: 10.1152/jappl.1995.78.4.1516. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell CP, Schwartz AR, Smith PL, Robotham JL, Fitzgerald RS, Shirahata M. Reflex stimulation of renal sympathetic nerve activity and blood pressure in response to apnea. Am J Respir Crit Care Med. 1996;154:1763–1770. doi: 10.1164/ajrccm.154.6.8970368. [DOI] [PubMed] [Google Scholar]
- 19.Xue J, Zhou D, Poulsen O, Imamura T, Hsiao YH, Smith TH, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine-oxide. Am J Respir Cell Mol Biol. 2017;57:581–588. doi: 10.1165/rcmb.2017-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton CE, Jernigan NL, Kanagy NL, Walker BR, Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J Appl Physiol (1985) 2011;111:980–988. doi: 10.1152/japplphysiol.01286.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baud MO, Magistretti PJ, Petit JM. Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J Sleep Res. 2013;22:3–12. doi: 10.1111/j.1365-2869.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 22.Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang SX, Khalyfa A, Wang Y, Carreras A, Hakim F, Neel BA, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes. 2014;38:619–624. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushal N, Ramesh V, Gozal D. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One. 2012;7:e45610. doi: 10.1371/journal.pone.0045610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep (Basel) 2014;37:1817–1824. doi: 10.5665/sleep.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundar KM, Prchal JT. The cornerstone of the aberrant pathophysiology of obstructive sleep apnea: tissue responses to chronic sustained versus intermittent hypoxia. Am J Respir Cell Mol Biol. 2017;56:419–420. doi: 10.1165/rcmb.2017-0028ED. [DOI] [PubMed] [Google Scholar]
- 27.Kumar GK, Klein JB. Analysis of expression and posttranslational modification of proteins during hypoxia. J Appl Physiol (1985) 2004;96:1178–1186, discussion 1170–1172. doi: 10.1152/japplphysiol.00818.2003. [DOI] [PubMed] [Google Scholar]
- 28.Nanduri J, Semenza GL, Prabhakar NR. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1096–L1100. doi: 10.1152/ajplung.00325.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves SR, Gozal E, Guo SZ, Sachleben LR, Jr, Brittian KR, Lipton AJ, et al. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol (1985) 2003;95:1767–1774. doi: 10.1152/japplphysiol.00759.2002. [DOI] [PubMed] [Google Scholar]
- 30.Mayer CA, Ao J, Di Fiore JM, Martin RJ, MacFarlane PM. Impaired hypoxic ventilatory response following neonatal sustained and subsequent chronic intermittent hypoxia in rats. Respir Physiol Neurobiol. 2013;187:167–175. doi: 10.1016/j.resp.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Skelly JR, Rowan SC, Jones JF, O’Halloran KD. Upper airway dilator muscle weakness following intermittent and sustained hypoxia in the rat: effects of a superoxide scavenger. Physiol Res. 2013;62:187–196. doi: 10.33549/physiolres.932405. [DOI] [PubMed] [Google Scholar]
- 32.Wu W, Dave NB, Yu G, Strollo PJ, Kovkarova-Naumovski E, Ryter SW, et al. Network analysis of temporal effects of intermittent and sustained hypoxia on rat lungs. Physiol Genomics. 2008;36:24–34. doi: 10.1152/physiolgenomics.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, et al. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension. 2003;42:1130–1136. doi: 10.1161/01.HYP.0000101691.12358.26. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez TA, Jourdan-Le Saux C, Joy A, Zhang J, Dai Q, Mifflin S, et al. Chronic and intermittent hypoxia differentially regulate left ventricular inflammatory and extracellular matrix responses. Hypertens Res. 2012;35:811–818. doi: 10.1038/hr.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, et al. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. Am J Respir Cell Mol Biol. 2017;56:477–487. doi: 10.1165/rcmb.2016-0243OC. [DOI] [PubMed] [Google Scholar]
- 36.Chen LD, Lin L, Zhang LJ, Zeng HX, Wu QY, Hu MF, et al. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: a meta-analysis. Clin Respir J. 2018;12:373–381. doi: 10.1111/crj.12554. [DOI] [PubMed] [Google Scholar]
- 37.Lentini S, Manka R, Scholtyssek S, Stoffel-Wagner B, Lüderitz B, Tasci S. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest. 2006;129:88–94. doi: 10.1378/chest.129.1.88. [DOI] [PubMed] [Google Scholar]
- 38.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol (1985) 2011;111:881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. 2005;172:915–920. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- 42.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 43.Polak J, Shimoda LA, Drager LF, Undem C, McHugh H, Polotsky VY, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:1483–1490, 1490A–1490B. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 45.Wu K, Su X, Li G, Zhang N. Antioxidant carbocysteine treatment in obstructive sleep apnea syndrome: a randomized clinical trial. PLoS One. 2016;11:e0148519. doi: 10.1371/journal.pone.0148519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol. 2010;174:156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–893. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 49.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol (1985) 2004;96:1236–1242, discussion 1196. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 51.Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J. 2012;39:1492–1500. doi: 10.1183/09031936.00141511. [DOI] [PubMed] [Google Scholar]
- 52.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol (1985) 2012;113:1304–1310. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 55.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–855. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 57.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–264. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- 58.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 59.Gatterer H, Haacke S, Burtscher M, Faulhaber M, Melmer A, Ebenbichler C, et al. Normobaric intermittent hypoxia over 8 months does not reduce body weight and metabolic risk factors – a randomized, single blind, placebo-controlled study in normobaric hypoxia and normobaric sham hypoxia. Obes Facts. 2015;8:200–209. doi: 10.1159/000431157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, et al. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring) 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- 61.Alvarez-Martins I, Remédio L, Matias I, Diogo LN, Monteiro EC, Dias S. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Arch. 2016;468:919–932. doi: 10.1007/s00424-016-1797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol. 2012;303:R700–R709. doi: 10.1152/ajpregu.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 64.Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, et al. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep. 2017;5:e13106. doi: 10.14814/phy2.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol (1985) 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, et al. Intermittent hypoxia-induced glucose intolerance is abolished by α-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab. 2014;307:E1073–E1083. doi: 10.1152/ajpendo.00373.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherwani SI, Aldana C, Usmani S, Adin C, Kotha S, Khan M, et al. Intermittent hypoxia exacerbates pancreatic β-cell dysfunction in a mouse model of diabetes mellitus. Sleep (Basel) 2013;36:1849–1858. doi: 10.5665/sleep.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang Y, Zhang Q, Tan J, Li L, An X, Lei P. Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and protective effects of antioxidant intervention. Nutr Diabetes. 2014;4:e131. doi: 10.1038/nutd.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10:475–489. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184:355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 72.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234:446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 73.Chopra S, Rathore A, Younas H, Pham LV, Gu C, Beselman A, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102:3172–3181. doi: 10.1210/jc.2017-00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol (1985) 2005;99:1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 75.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song D, Fang G, Greenberg H, Liu SF. Chronic intermittent hypoxia exposure-induced atherosclerosis: a brief review. Immunol Res. 2015;63:121–130. doi: 10.1007/s12026-015-8703-8. [DOI] [PubMed] [Google Scholar]
- 77.Mahat B, Chassé É, Mauger JF, Imbeault P. Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J Transl Med. 2016;14:212. doi: 10.1186/s12967-016-0965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 79.Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783–790. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–1296. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 81.Trzepizur W, Boursier J, Mansour Y, Le Vaillant M, Chollet S, Pigeanne T, et al. Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group. Association between severity of obstructive sleep apnea and blood markers of liver injury. Clin Gastroenterol Hepatol. 2016;14:1657–1661. doi: 10.1016/j.cgh.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 82.Chen LD, Zhang LJ, Lin XJ, Qi JC, Li H, Wu Z, et al. Association between continuous positive airway pressure and serum aminotransferases in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2018;275:587–594. doi: 10.1007/s00405-017-4840-0. [DOI] [PubMed] [Google Scholar]
- 83.Minville C, Hilleret MN, Tamisier R, Aron-Wisnewsky J, Clement K, Trocme C, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145:525–533. doi: 10.1378/chest.13-0938. [DOI] [PubMed] [Google Scholar]
- 84.Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, et al. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon. 2015;15:e32655. doi: 10.5812/hepatmon.32655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qi JC, Huang JC, Lin QC, Zhao JM, Lin X, Chen LD, et al. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath. 2016;20:529–535. doi: 10.1007/s11325-015-1232-9. [DOI] [PubMed] [Google Scholar]
- 86.Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40:913–918. doi: 10.1183/09031936.00177011. [DOI] [PubMed] [Google Scholar]
- 87.Buttacavoli M, Gruttad’Auria CI, Olivo M, Virdone R, Castrogiovanni A, Mazzuca E, et al. Liver steatosis and fibrosis in OSA patients after long-term CPAP treatment: a preliminary ultrasound study. Ultrasound Med Biol. 2016;42:104–109. doi: 10.1016/j.ultrasmedbio.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 88.Jullian-Desayes I, Tamisier R, Zarski JP, Aron-Wisnewsky J, Launois-Rollinat SH, Trocme C, et al. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: data from randomized trials. Respirology. 2016;21:378–385. doi: 10.1111/resp.12672. [DOI] [PubMed] [Google Scholar]
- 89.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e9, quiz e39–e40. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mishra P, Nugent C, Afendy A, Bai C, Bhatia P, Afendy M, et al. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28:1080–1086. doi: 10.1111/j.1478-3231.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 92.Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aron-Wisnewsky J, Minville C, Tordjman J, Lévy P, Bouillot JL, Basdevant A, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–233. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 94.Petta S, Marrone O, Torres D, Buttacavoli M, Cammà C, Di Marco V, et al. Obstructive sleep apnea is associated with liver damage and atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2015;10:e0142210. doi: 10.1371/journal.pone.0142210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep (Basel) 2015;38:1583–1591. doi: 10.5665/sleep.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007–1013. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 97.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–G877. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 98.Savransky V, Reinke C, Jun J, Bevans-Fonti S, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia and acetaminophen induce synergistic liver injury in mice. Exp Physiol. 2009;94:228–239. doi: 10.1113/expphysiol.2008.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1α-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582–G592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mesarwi OA, Shin MK, Bevans-Fonti S, Schlesinger C, Shaw J, Polotsky VY. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11:e0168572. doi: 10.1371/journal.pone.0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jouët P, Sabaté JM, Maillard D, Msika S, Mechler C, Ledoux S, et al. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17:478–485. doi: 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 102.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41:918–921. doi: 10.1097/01.mcg.0000225692.62121.55. [DOI] [PubMed] [Google Scholar]
- 103.Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916–1923. doi: 10.1002/hep.22241. [DOI] [PubMed] [Google Scholar]
- 104.Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78:141–146. doi: 10.1159/000170785. [DOI] [PubMed] [Google Scholar]
- 105.Daltro C, Cotrim HP, Alves E, de Freitas LA, Araújo L, Boente L, et al. Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence? Obes Surg. 2010;20:1536–1543. doi: 10.1007/s11695-010-0212-1. [DOI] [PubMed] [Google Scholar]
- 106.Ulitsky A, Ananthakrishnan AN, Komorowski R, Wallace J, Surapaneni SN, Franco J, et al. A noninvasive clinical scoring model predicts risk of nonalcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2010;20:685–691. doi: 10.1007/s11695-010-0118-y. [DOI] [PubMed] [Google Scholar]
- 107.Türkay C, Ozol D, Kasapoğlu B, Kirbas I, Yıldırım Z, Yiğitoğlu R. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care. 2012;57:244–249. doi: 10.4187/respcare.01184. [DOI] [PubMed] [Google Scholar]
- 108.Corey KE, Misdraji J, Zheng H, Malecki KM, Kneeman J, Gelrud L, et al. The absence of obstructive sleep apnea may protect against non-alcoholic fatty liver in patients undergoing bariatric surgery. PLoS One. 2013;8:e62504. doi: 10.1371/journal.pone.0062504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pulixi EA, Tobaldini E, Battezzati PM, D’Ingianna P, Borroni V, Fracanzani AL, et al. Risk of obstructive sleep apnea with daytime sleepiness is associated with liver damage in non-morbidly obese patients with nonalcoholic fatty liver disease. PLoS One. 2014;9:e96349. doi: 10.1371/journal.pone.0096349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin QC, Chen LD, Chen GP, Zhao JM, Chen X, Huang JF, et al. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath. 2015;19:273–280. doi: 10.1007/s11325-014-1008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agrawal S, Duseja A, Aggarwal A, Das A, Mehta M, Dhiman RK, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9:283–291. doi: 10.1007/s12072-015-9615-3. [DOI] [PubMed] [Google Scholar]
- 112.Trzepizur W, Boursier J, Le Vaillant M, Ducluzeau PH, Dubois S, Henni S, et al. on the behalf of the METABOL group. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. 2018 doi: 10.1183/13993003.00601-2018. DOI: 10.1183/13993003.00601-2018. [DOI] [PubMed] [Google Scholar]
- 113.Toyama Y, Murase K, Azuma M, Hamada S, Tachikawa R, Tanizawa K, et al. Impact of long-term continuous positive airway pressure on liver fat in male obstructive sleep apnea patients with fatty liver. Sleep Biol Rhythms. 2018;16:117–124. [Google Scholar]