To the Editor:
Cystic fibrosis (CF) lung disease starts early in life and progresses throughout infancy and the preschool years (1, 2). Therapeutic strategies initiated early in life have the potential to reverse this process and maintain lung health. Ivacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator that increases the channel’s opening probability. Remarkable and sustained lung function improvements have been demonstrated in patients aged 6 years and older with gating CFTR mutations such as G551D on at least one allele (3, 4). A recent open-label trial provided evidence for safety and nutritional benefits in children aged 2–5 years, but lung function was not systematically assessed within this study (5). The lung clearance index (LCI) measured by multiple-breath washout has been shown to be a sensitive measure to capture lung function abnormalities in patients with CF and is feasible for use in preschool children because it requires minimal cooperation. LCI can also be used to detect treatment effects in interventional trials (6, 7). As part of GOAL (G551D Observational Study) (NCT 01521338), a multicenter longitudinal observational study to explore biomarkers as well as clinical and physiological characteristics of patients with CF with gating mutations initiated on ivacaftor therapy (8), we report the results of LCI in preschool patients assessed before and after ivacaftor treatment initiation.
Multiple-breath washout measurements based on nitrogen as the tracer gas using the EXHALYZER D (Eco Medics) were performed in preschool children with CF aged 3 to 5 years. Measurements were performed in accordance with recently published standards for preschool children (9) and modifications of the equipment to reduce dead space as described previously (2). Baseline was defined as the predose measurement immediately preceding the start of treatment; all other medications were kept constant throughout the study period. Changes in LCI from baseline to 1 month and from baseline to 6 months after the start of ivacaftor treatment were analyzed using nonparametric Wilcoxon signed-rank tests. Relative changes in LCI compared with baseline were evaluated using one-sample Kolmogorov-Smirnov tests.
This analysis includes five subjects with CF enrolled in the GOAL study in the United States and an additional four preschool-aged patients with CF started on ivacaftor at the Hospital for Sick Children in Toronto (SickKids). Baseline characteristics of the study population are displayed in Table 1. Median sweat chloride concentration at baseline was 103 mEq/L. All nine patients carried one copy of the G551D mutation, with seven patients being compound heterozygous for F508del. The average LCI at baseline was 10.6 (interquartile range [IQR], 8.9 to 11.2); seven of nine patients had LCI values above the upper limit of normal, reflecting increased ventilation inhomogeneity (2) (Figure 1A).
Table 1.
Demographic Characteristics of the Study Population at the Baseline Visit
| Characteristic | GOAL (n = 5) | SickKids (n = 4) | GOAL + SickKids (n = 9) |
|---|---|---|---|
| Age, yr | 4.2 (4.0 to 5.1) | 4.8 (3.0 to 5.8) | 4.2 (3.9 to 5.2) |
| Female, n (%) | 3 (60%) | 2 (50%) | 5 (56%) |
| Genetics, first allele, n (%) | |||
| G551D | 5 (100%) | 4 (100%) | 9 (100%) |
| Genetics, second allele, n (%) | |||
| F508del | 3 (75%) | 4 (80%) | 7 (78%) |
| 1717-1G→A | 1 (11%) | 1 (20%) | 0 (0%) |
| R1066C | 1 (11%) | 0 (0%) | 1 (25%) |
| Height z-score | 0.52 (0.47 to 1.06) | −0.49 (−1.08 to 0.14) | 0.34 (−0.92 to 0.52) |
| Weight z-score | 0.55 (−0.13 to 0.88) | −0.19 (−0.80 to −0.02) | −0.09 (−0.30 to 0.55) |
| BMI z-score | −0.12 (−0.73 to 0.62) | −0.28 (−0.55 to 0.39) | −0.12 (−0.55 to 0.62) |
| LCI | 10.6 (8.9 to 11.2) | 8.9 (7.6 to 11.6) | 9.8 (8.2 to 11.2) |
| Sweat chloride | 105 (103 to 107) | 98 (94 to 103) | 103 (96 to 105) |
| Pancreatic insufficiency, n (%) | 5 (100%) | 4 (100%) | 9 (100%) |
| Infections, n (%) | |||
| Staphylococcus aureus | 2 (50%)* | 1 (25%) | 3 (38%)* |
| Haemophilus influenzae | 2 (50%)* | 0 (0%) | 2 (25%)* |
| Pseudomonas aeruginosa | 0 (0%)* | 0 (0%) | 0 (0%)* |
| Concomitant medications, n (%) | |||
| Dornase alfa | 2 (40%) | 1 (25%) | 3 (33%) |
| Hypertonic saline | 2 (40%) | 0 (0%) | 2 (22%) |
| Oral antibiotics | 0 (0%) | 1 (25%) | 1 (11%) |
Definition of abbreviations: BMI = body mass index; GOAL = G551D Observational Study; LCI = lung clearance index; SickKids = Hospital for Sick Children in Toronto.
All continuous variables are presented as medians with interquartile ranges.
One GOAL subject did not have microbiology assessments performed at baseline.
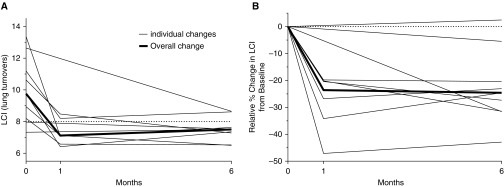
Figure 1.
(A) Absolute and (B) relative median changes per subject in lung clearance index (LCI) in preschool children treated with ivacaftor. Absolute LCI significantly improved 1 month after the first dose of ivacaftor (median [interquartile range (IQR)] LCI at 1 mo, 7.1 [6.6 to 8.5]; P = 0.02). The improvement in LCI was maintained 6 months after the first dose (median [IQR] LCI, 7.5 [7.3 to 7.6]; P = 0.03). Similarly, relative LCI significantly improved from baseline at both 1 month (median [IQR] change in LCI from baseline, −23.6% [−34.2 to −20.2]; P < 0.001) and 6 months after treatment (median [IQR] change in LCI from baseline, −24.6 [−31.4 to −20.4]; P < 0.001). The dotted lines represent an upper limit of normal of 8 in A and a change of 0% in B.
Mean sweat chloride concentrations decreased to 46 mEq/L 1 month after ivacaftor initiation (mean change [IQR], −56 [−61 to −47]). Within-patient LCI significantly improved 1 month after the first dose of ivacaftor (median LCI at 1 mo, 7.10 [6.6 to 8.5]; P = 0.02). The improvement in LCI was maintained 6 months after the first dose (median LCI, 7.3 [7.3 to 7.6]; P = 0.03). Assessing relative changes in LCI at 1 month and 6 months after ivacaftor treatment yielded similar results. LCI significantly improved from baseline at both 1 month (median change in LCI from baseline, −23.6% [IQR, −34.2 to −20.2]; P < 0.001) and 6 months post-treatment (median change in LCI from baseline, −24.6% [IQR, −31.4 to −20.4]; P < 0.001). Thus, rapid and sustained improvements in LCI were demonstrated with ivacaftor therapy that exceeded the between-test reproducibility and thus the physiologically relevant change for quarterly LCI measurements in health, which we recently demonstrated to be 15% in preschool children (10). The only two patients not showing an improvement in LCI had normal baseline values of LCI. The observed effect size was similar to what has been described in older patients with CF treated with ivacaftor (6, 11). This study also supports the concept that LCI is a suitable outcome measure to capture changes in lung function in preschool children on CFTR modulator therapy, and also that ivacaftor helps improve early airway disease even in young children.
Footnotes
Supported by the Cystic Fibrosis Foundation.
Originally Published in Press as DOI: 10.1164/rccm.201802-0243LE on April 3, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ranganathan SC, Hall GL, Sly PD, Stick SM, Douglas TA Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Early lung disease in infants and preschool children with cystic fibrosis: what have we learned and what should we do about it? Am J Respir Crit Care Med. 2017;195:1567–1575. doi: 10.1164/rccm.201606-1107CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanojevic S, Davis SD, Retsch-Bogart G, Webster H, Davis M, Johnson RC, et al. Progression of lung disease in preschool patients with cystic fibrosis. Am J Respir Crit Care Med. 2017;195:1216–1225. doi: 10.1164/rccm.201610-2158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. KIWI Study Group. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4:107–115. doi: 10.1016/S2213-2600(15)00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1:630–638. doi: 10.1016/S2213-2600(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, et al. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis: a pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med. 2013;188:456–460. doi: 10.1164/rccm.201302-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson PD, Latzin P, Ramsey KA, Stanojevic S, Aurora P, Davis SD, et al. ATS Assembly on Pediatrics. Preschool multiple-breath washout testing: an official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2018;197:e1–e19. doi: 10.1164/rccm.201801-0074ST. [DOI] [PubMed] [Google Scholar]
- 10.Oude Engberink E, Ratjen F, Davis SD, Retsch-Bogart G, Amin R, Stanojevic S. Inter-test reproducibility of the lung clearance index measured by multiple breath washout. Eur Respir J. 2017;50:1700433. doi: 10.1183/13993003.00433-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane M, Gonska T, Jensen R, Avolio J, Klingel M, Stanojevic S, et al. Lung clearance index response in patients with CF with class III CFTR mutations. Thorax. 2016;71:476–477. doi: 10.1136/thoraxjnl-2015-207894. [DOI] [PubMed] [Google Scholar]