Abstract
Background: Thousands of biomarker tests are either available or under development for lung diseases. In many cases, adoption of these tests into clinical practice is outpacing the generation and evaluation of sufficient data to determine clinical utility and ability to improve health outcomes. There is a need for a systematically organized report that provides guidance on how to understand and evaluate use of biomarker tests for lung diseases.
Methods: We assembled a diverse group of clinicians and researchers from the American Thoracic Society and leaders from the National Heart, Lung, and Blood Institute with expertise in various aspects of precision medicine to review the current status of biomarker tests in lung diseases. Experts summarized existing biomarker tests that are available for lung cancer, pulmonary arterial hypertension, idiopathic pulmonary fibrosis, asthma, chronic obstructive pulmonary disease, sepsis, acute respiratory distress syndrome, cystic fibrosis, and other rare lung diseases. The group identified knowledge gaps that future research studies can address to efficiently translate biomarker tests into clinical practice, assess their cost-effectiveness, and ensure they apply to diverse, real-life populations.
Results: We found that the status of biomarker tests in lung diseases is highly variable depending on the disease. Nevertheless, biomarker tests in lung diseases show great promise in improving clinical care. To efficiently translate biomarkers into tests used widely in clinical practice, researchers need to address specific clinical unmet needs, secure support for biomarker discovery efforts, conduct analytical and clinical validation studies, ensure tests have clinical utility, and facilitate appropriate adoption into routine clinical practice.
Conclusions: Although progress has been made toward implementation of precision medicine for lung diseases in clinical practice in certain settings, additional studies focused on addressing specific unmet clinical needs are required to evaluate the clinical utility of biomarkers; ensure their generalizability to diverse, real-life populations; and determine their cost-effectiveness.
Keywords: precision medicine, biomarker, pulmonary
Contents
Overview
Introduction
Definitions
Biomarker
Analytical Validation
Clinical Validation
Qualification
Methods
Committee Composition
Literature Search
Framework for Biomarker Evaluation
Research Knowledge Gaps
Document Development
Framework for Biomarker Evaluation
Developing and Validating Biomarkers for Clinical Use
Status of Precision Medicine in Select Lung Diseases
Lung Cancer
Pulmonary Arterial Hypertension
Idiopathic Pulmonary Fibrosis
Asthma
Chronic Obstructive Pulmonary Disease
Sepsis
Acute Respiratory Distress Syndrome
Cystic Fibrosis
Rare Lung Diseases
Establishing Clinical Utility: Cost-Effectiveness and Applicability of Biomarkers to Diverse and Real-Life Populations
Infrastructure Needed for Widespread Adoption of Precision Medicine in Clinical Practice
Gaps in Current Research and Suggestions for Future Studies
Conclusions
Overview
Despite a dearth of objective criteria for clinical utility and ability to improve health outcomes, thousands of biomarker tests are available or under development for lung diseases. Research studies are also underway to identify novel biomarkers and seek evidence to support their translation into clinical practice. Health providers routinely use select biomarker-based tests in clinical practice, but some providers lack confidence in deciding when biomarker tests are needed. Researchers performing biomarker-related studies require greater clarity regarding the translation process.
Experts in precision medicine for a wide range of lung diseases gathered for a daylong meeting in May 2017 and wrote this systematically organized report. It provides guidance on how to understand and evaluate use of biomarker tests for lung diseases and identifies research topics that could be prioritized in the future. Common themes emerged that may help accelerate the identification and translation of clinically useful biomarker tests across multiple pulmonary diseases:
-
•
Translational studies to clinically validate biomarkers are needed, in which significance is valued over innovation.
-
•
More biomarker discovery research, in which hypothesis generation is viewed favorably, is necessary.
-
•
Efforts to integrate existing heterogeneous datasets via appropriate data-mining strategies are required.
Introduction
Precision medicine refers to understanding how a person’s biology, exposures, and lifestyle help determine approaches to prevent and treat disease (1, 2). Advances in precision medicine, including the development of biomarkers that allow the tailoring of treatments to individuals, have tremendous promise to improve population health (3, 4). Since the launch of the Precision Medicine Initiative in 2015, the NIH and other organizations have made progress toward enabling the selection of interventions and treatments on the basis of which ones will work for particular individuals (2). Box 1 provides a compelling patient story that illustrates the promise of this approach. Although focused initially on tailored therapy for cancer, precision medicine has rapidly expanded to other conditions, including lung diseases. Most notably, the NIH’s All of Us Research Program (https://allofus.nih.gov/) is recruiting and tracking more than 1 million individuals to study precision medicine broadly.
Box 1. Experience shared by Maki Inada. NIH director Dr. Francis Collins used this story during his 2016 NIH Budget Request for continued funding for precision medicine initiatives (https://www.c-span.org/video/?c4529945/collinstestimony).
During the winter of 2007, I caught a bad cold and cough that persisted for 2 months. An X-ray was ordered, and my doctors found a large, 7-cm mass in my lung. Because I was an active, healthy, 36-year-old nonsmoker, my doctors didn’t believe that this mass could be a tumor, so they sent me home with antibiotics and asked me to come back in a few weeks. During my next appointment, I felt better, although I still had a cough. Unfortunately, the new X-ray looked the same as the previous one. After further tests, I was diagnosed with non–small-cell lung adenocarcinoma. With this diagnosis, I did some research on lung cancer in Wikipedia and found that 90% of people with lung cancer are smokers, and only 15% of lung cancer patients survive 5 years. I was in total shock! However, I read further about new targeted therapies that were being developed that seemed to work well for nonsmoking Asian women. I met all three of those criteria, but next I had to find out how to get this new therapy. I met with a doctor who is a specialist in EGFR mutations in lung cancers and targeted therapies. We opted to have a surgical biopsy in order to have genetic testing done on the mutational status of my tumor, but the surgeon found tumor studs on the inside lining of my lungs and sent me away because she was not willing to operate on a stage IV patient like me. Totally devastated, I returned home to Ithaca, NY, to begin standard chemotherapy but also began taking the newly developed targeted therapy, Tarceva. During treatment, I did everything I could think of to fight my prognosis. After 3 long weeks, I learned that my tumor had an EGFR exon 19 deletion mutation. “Congratulations! Best of the bunch,” wrote my oncologist, referring to the likelihood that Tarceva would work on my tumor because of its mutation type. After 3 months and four rounds of chemotherapy plus Tarceva, my tumor shrunk in size to less than a centimeter. I was scheduled for surgery 2 weeks later and had an upper left lobectomy. I took daily Tarceva for 2 years postsurgery and then went off Tarceva to start a family. We are very lucky to have a beautiful girl! I have subsequently had three recurrences, undergone additional treatment with Tarceva, and had additional surgeries. Along the way, I acquired a common resistance mutation known as T790M, but it has been 4 years since my last surgery, and as of today, we cannot detect any signs of disease. However, if I were to have another recurrence, I am reassured in knowing that the development of other targeted therapies to inhibit resistance mutations are underway. Thanks to precision medicine, I am a 10-year nonsmoker lung cancer survivor!
Precision medicine includes the identification of biomarkers that characterize clinical heterogeneity to help diagnose diseases, aid in prognosis, and dictate optimal treatment (5). Thousands of molecular biomarkers, consisting of measures such as genetic variation, gene expression changes, and levels of proteins and metabolites, are either available or under development. For example, as of January 29, 2018, the Genetic Testing Registry contained information on nearly 14,000 tests for lung diseases (6). The National Heart, Lung, and Blood Institute (NHLBI) funds several programs in lung precision medicine, including:
-
•
Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases (CADET). Accelerates development of novel agents for diagnosis and treatment of lung diseases using strategies based on fundamental pathobiologic processes, such as mucus production.
-
•
Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS). Seeks to discover links between genes and microbes in the lung for two underrecognized lung diseases.
-
•
Precision Interventions for Severe and/or Exacerbation Prone Asthma (PrecISE) Network. Uses patient phenotypes and/or endotypes along with biomarkers in sequential adaptive clinical trials to evaluate precision interventions.
-
•
Pulmonary Vascular Disease Phenomics Program (PVDOMICS). Performs deep clinical phenotyping and blood-based omics studies to discover molecular-based subtypes of pulmonary vascular disease.
-
•
Severe Asthma Research Program (SARP). Characterizes molecular, cellular, and biological mechanisms that lead to different types of asthma via an observational longitudinal study.
-
•
Trans-Omics for Precision Medicine (TOPMed). Collects and integrates whole-genome sequencing and other omics data to understand heart-, lung-, and blood-related conditions.
Despite the promise of precision medicine and the fact that thousands of biomarker tests are available or under development for lung diseases, many have not been readily adopted in clinical practice for reasons that include lack of objective criteria for using them and uncertainty regarding their ability to broadly improve health outcomes. In addition to biomarker discovery research, efforts to translate biomarkers into clinical practice are critical to improving population health. Moreover, a strong evidence base for using genomic medicine is critical to its widespread use in clinical practice (7).
Given rapid advances in lung-related omics research and the fact that genomic medicine is on the cusp of a new era of improving population health, the time is ripe to summarize the status of lung precision medicine, with a particular focus on molecular-based biomarkers. This joint research statement between NHLBI and the American Thoracic Society (ATS) provides practical guidance regarding the assessment and use of personalized tests for lung diseases and identifies gaps in knowledge to focus future research efforts related to biomarker test development. The project on which this report is based began with a series of conference calls among ATS members and NHLBI representatives, during which key discussion points for an in-person meeting were prioritized. Each topic and lung disease area was assigned an expert, who prepared a written summary for meeting attendees to review. Subsequently, during the in-person meeting, participants agreed on a framework for biomarker evaluation, discussed the status of precision medicine in select lung diseases, and participated in breakout sessions whose major points were also discussed with the whole group.
Definitions
We used definitions that were established by the BEST (Biomarkers, Endpoints, and other Tools) Resource, a document developed by the U.S. Food and Drug Administration (FDA) and NIH to promote consistent use of biomarker terms and concepts (8).
Biomarker
BEST defines a biomarker as a “characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Molecular, histologic, radiographic, or physiologic characteristics are types of biomarkers. A biomarker is not an assessment of how an individual feels, functions, or survives.” We focused primarily on molecular biomarkers during our meeting, but we noted other types of biomarkers that were commonly used in clinical practice. Categories of biomarkers are provided in Table 1 (8).
Table 1.
Biomarker Categories
| Category | Definition |
|---|---|
| Diagnostic biomarker | Used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease |
| Monitoring biomarker | Measured serially for assessing status of a disease or medical condition or for evidence of exposure to (or effect of) a medical product or an environmental agent |
| Pharmacodynamic/response biomarker | Used to show that a biological response has occurred in an individual who has been exposed to a medical product or an environmental agent |
| Predictive biomarker | Used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent |
| Prognostic biomarker | Used to identify likelihood of a clinical event, disease recurrence, or progression in patients who have the disease or medical condition of interest |
| Safety biomarker | Measured before or after an exposure to a medical product or an environmental agent to indicate the likelihood, presence, or extent of toxicity as an adverse effect |
| Susceptibility/risk biomarker | Indicates the potential for developing a disease or medical condition in an individual who does not currently have clinically apparent disease or the medical condition |
Analytical Validation
Refers to establishing that the performance characteristics of a biomarker test are acceptable in terms of its specificity, sensitivity, accuracy, precision, and other relevant performance characteristics using a specified technical protocol, which may include specimen collection, handling, and storage procedures. Analytical validation alone does not ensure that a biomarker is clinically useful.
Clinical Validation
Refers to the process of establishing that a biomarker acceptably identifies, measures, or predicts the concept of interest. The concept is an aspect of a person’s clinical, biological, physical, or functional state that a biomarker is intended to capture or reflect.
Qualification
A conclusion based on a formal regulatory process that within a stated context of use, a medical product development tool can be relied on to have a specific interpretation and application in medical product development and regulatory review.
Methods
This project, which was approved by the ATS Program Review Subcommittee, was initiated by ATS members, who reached out to NHLBI members to participate. ATS and NHLBI members agreed that the project should be a joint effort.
Committee Composition
Four co-chairs, including two representatives from NHLBI and two ATS members, organized the ad hoc committee. Members with expertise in various aspects of precision medicine specific to pulmonary and critical care conditions were invited, as well as experts from the FDA and one patient. Participants disclosed all potential conflicts of interest, which were vetted and managed in accordance with the policies and procedures of the ATS.
Literature Search
Co-chairs performed a literature search in Medline using PubMed for biomarkers related to conditions considered. This search was supplemented by disease-specific literature searches performed by leaders of each topic. All members were allowed to supplement the literature by performing their own searches or identifying biomarkers in other ways (e.g., those used in clinical practice). The literature search conducted for this research statement was not a systematic review.
Framework for Biomarker Evaluation
Experts from the FDA provided insights into 1) the process of identifying, reviewing regulatory submissions, and accepting or qualifying biomarkers as drug development tools; and 2) approving or clearing biomarker assays, tests, and devices. The committee surveyed currently available and promising biomarkers for the conditions listed below, reviewed how specific cutoffs for analytic and clinical validity are deemed appropriate, and determined how to assess clinical utility of biomarkers.
Research Knowledge Gaps
Disease-specific experts summarized existing biomarkers and corresponding evidence specific to each condition considered. Salient knowledge gaps and common themes related to the process of biomarker discovery were identified via discussion and consensus. Suggestions for future research studies were formulated to address identified knowledge gaps.
Document Development
Co-chairs created a document outline. Disease-specific leaders sent drafts of their disease areas to co-chairs, who edited contributions into a single document. Notes taken by various group members and breakout session leaders during the in-person meeting held on May 20, 2017 formed the basis of remaining sections, which were written by co-chairs. Box 1 was provided by our patient participant. The final draft was sent to all participants for review and feedback. After multiple cycles of review and revision, all authors agreed on a final draft version.
Framework for Biomarker Evaluation
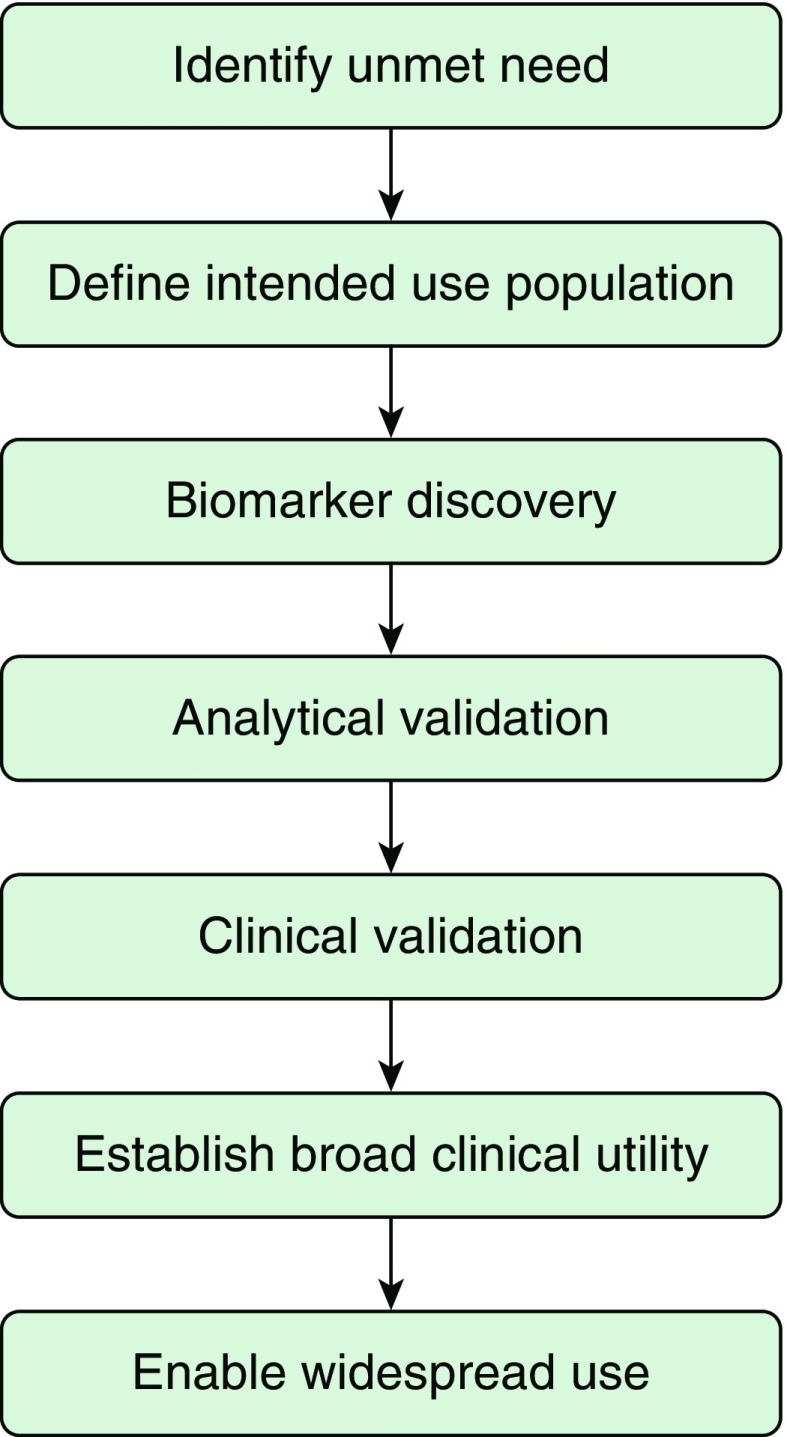
Medical products regulated by the FDA include drugs, biologics, and medical devices. Biomarker tests can be reviewed and approved by the FDA, or they can be developed within clinical laboratories under the purview of the Clinical Laboratory Improvement Act. Although we based our framework for biomarker evaluation on FDA approval processes, it also applies to laboratory-developed tests, because similar criteria must be established for any biomarker test to be clinically useful, despite potential differences in regulatory review. Figure 1 provides a summary of steps involved in identifying a biomarker and ensuring it becomes a clinically used test.
Figure 1.
Biomarker development steps.
Regulatory considerations differ whether investigators seek for biomarkers to be 1) approved biomarker tests as in vitro diagnostic devices, or 2) accepted as medical product development tools. In both cases, translating a potential biomarker into a formal biomarker test or gaining acceptance as a medical product development tool involves clearly defining the object (i.e., subject and specimen type) from which the biomarker will be measured, the assay to measure the biomarker (i.e., supplies, equipment, protocol), and interpretation method and criteria. In vitro diagnostic devices are evaluated in a patient care setting to establish a benefit-to-risk ratio for an intended use. Subsequently, their FDA approval is granted via premarket review.
Biomarkers accepted as medical product development tools, most often drug development tools, are evaluated in an investigational setting to establish their benefit-to-risk ratio under a specific context of use. Acceptance of biomarkers often occurs when biomarkers are part of the drug approval process itself, in which case drug labels, reviews, and guidances include relevant biomarker information. Other pathways include qualification of biomarkers through the Biomarker Qualification Program, which was developed to accelerate the process of biomarker integration into drug development. Alternatively, they can be identified via scientific community consensus, a lengthy process involving substantial evidence from published articles in favor of the biomarker, followed by regulatory acceptance.
The identification or use of biomarkers in drug development spans steps from basic science research to clinical trials (9). In the initial stages, biomarker candidates are selected based on understanding of molecular pathways leading to disease, or, conversely, potential biomarkers may be used to investigate molecular pathways that lead to better biomarkers, an increased understanding of drug mechanisms of action, or the identification of novel drug targets. During clinical trials, biomarkers can be used to select or stratify patients, select dose of drugs, or aid in safety and efficacy assessments. Each of these functions during clinical development may be critical for drug approval, for example, ensuring a specific prognostic biomarker aids a clinical trial design by having proper statistical power to measure efficacy in a target population. For the Biomarker Qualification Program, the evidentiary criteria framework is a process that begins with a needs assessment and context of use discussion, as these drive the level of evidence that is necessary for approval of a biomarker test (10). The context of use is a concise statement describing the biomarker, its category, and its intended use in drug development. Subsequently, evidence regarding the benefits and risk of using, or not using, a biomarker in this specific context is gathered. Qualification is granted when the evidentiary criteria, including data on assay performance, biological rationale, known associations with clinical outcomes, and reproducibility of data, support use of the biomarker in a specific context. Qualification recommendations and review documentation are made publicly available on the FDA’s Biomarker Qualification Program webpage (11).
Developing and Validating Biomarkers for Clinical Use
Ideally, biomarker development begins with a needs assessment, in which a clear question that addresses an unmet clinical need is identified (Figure 1). Although this may seem obvious, many omics studies collect samples from large numbers of patients without a clear hypothesis, partly because of the feasibility of adding on an omics component to existing biobanks and epidemiological studies. Biomarkers can be identified via broad exploratory omics projects, but the likelihood of gathering appropriate data that can later motivate translational studies to improve an unmet need is decreased.
With a clear question, biomarker discovery studies can be designed more effectively. First, an intended use population can be identified. That is, the biomarker can be studied in the individuals who will benefit from the test, increasing the likelihood that an identified biomarker will succeed at addressing an important clinical problem. The FDA and local regulatory affairs offices are often willing to provide early feedback to facilitate biomarker development, including providing advice about validation strategies and integration of tests into clinical practice. Discussions regarding the motivation and imperatives from industry partners are also helpful to seek the “widest” populations for biomarker development. A therapeutic product profile may be drafted at this stage.
Next, analytical validation of a specific test proceeds. Analytical validation includes information about the process of obtaining, storing, and transporting a sample; taking the measure via a specific assay; and interpreting data to evaluate a biomarker, including measures of sensitivity, specificity, accuracy, and precision. At this stage, measures are usually restricted to a discovery cohort and, ideally, replicated in an independent population. As a biomarker may have been selected from among many candidates, details on statistical or machine learning methods used to identify the candidate are critical, as investigators need to be mindful of overfitting when assessing performance of tests, especially those based on omics studies.
Proper analytical validation is critical to proceed with clinical validation, which establishes a biomarker test as acceptable for its intended purpose in clinical practice or as a drug development tool for a specific context of use. Clinical validation studies may be retrospective, prospective, and/or randomized clinical trials. To develop biomarkers as drug development tools intended for clinical use, the correlation to the expected outcome needs to be evaluated in a nonclinical or clinical study that includes groups with and without the biomarker. Additional evaluations in groups with and without the biomarker, and with and without treatment, may help distinguish between prognostic and predictive abilities of a biomarker. Strategies to address the salient cost limitations that arise at this stage include partnering with industry, using innovative platforms such as large biobanks, or forming a start-up company supported by an academic institution. Conflicts of interest must be managed carefully to ensure actual or perceived conflicts are avoided. After analytical and clinical validation, an overall statement about the strength of evidence for a biomarker test can be made, which may lead to FDA acceptance or approval or a Clinical Laboratory Improvement Act–certified test.
Status of Precision Medicine in Select Lung Diseases
Biomarkers are in use and under development to varying degrees for lung diseases. Rare diseases are often inherently more decipherable than common diseases, especially those related to a single gene change, thus facilitating the development of molecular pathway–driven diagnostics and therapeutics. Most complex diseases do not yet have well established omics-based biomarkers, but efforts are underway to use biomarkers to identify disease subtypes and targeted therapies. Table 2 summarizes the most prominent biomarkers for the diseases considered. The biomarkers discussed in this research statement are not intended to be comprehensive; we selected biomarkers to review for the purposes of research and not to make clinical practice recommendations.
Table 2.
Summary of Salient Pulmonary Disease Biomarkers
| Disease | Biomarker | Biomarker Category | Biological Rationale | Biomarker Function | Analytical Validity | Strength of Evidence |
|---|---|---|---|---|---|---|
| Lung cancer | PDL1 | Response | A negative costimulatory receptor expressed on activated T-cells | PDL1 expression in at least 50% of tumor cells is correlated with efficacy of pembrolizumab, an inhibitor of PD1 | Repeatable and reproducible | Found in two independent, multicenter, prospective, observational studies |
| PAH | BNP | Prognostic, predictive, monitoring, and response | Released primarily from the heart as a result of myocardial strain and used in natriuresis and vasodilation via the renin/angiotensin pathway | BNP and NT-proBNP correlate with hemodynamic parameters, disease severity, and mortality | Repeatable and reproducible | Only guideline-based biomarker for the disease |
| IPF | MMP7 | Diagnostic and prognostic | Up-regulated in the BAL fluid and serum | MMP7 overexpression in lung tissue and BAL is found in IPF | Repeatable and reproducible | Used in a multidimensional index and staging system for IPF identified retrospectively |
| IPF | PBMC gene expression profile | Diagnostic and prognostic | Decreased expression of genes in the costimulatory pathway during T-cell activation | PBMC is predictive of poor transplant-free survival | Repeatable and reproducible | Used in multiple retrospective studies |
| Asthma | FeNO | Predictive | Proinflammatory mediator predisposing to airway hyperresponsiveness | Higher FeNO levels associated with steroid responsiveness | Repeatable and reproducible | Supported by multiple RCTs |
| COPD | Fibrinogen | Prognostic | Associated with all-cause mortality and exacerbations | Fibrinogen is a measure of inflammation | Lacks sufficient sensitivity and specificity for clinical use | Qualified as a biomarker by the COPD Foundation Biomarker Qualification Consortium |
| Sepsis | Lactate | Prognostic, predictive, monitoring, and response | Signifies a shift from aerobic to anaerobic metabolism during inadequate tissue metabolism | Pyruvate is metabolized to lactate | Repeatable and reproducible | Guideline-based biomarker for the disease |
| ARDS | PaO2/FiO2 ratio | Prognostic and predictive | Defines degree of hypoxemia | Identifies disease severity | Repeatable and reproducible | Only guideline-based biomarker for the disease |
| Cystic fibrosis | Sweat chloride concentration | Diagnostic and response | Mutations of the gene encoding CFTR cause cystic fibrosis, and sweat chloride reflects the function of the CFTR protein | Elevated sweat chloride concentration >60 mM indicates CFTR dysfunction | Repeatable and reproducible | Guideline-based biomarker for the disease |
| LAM | VEGF-D | Diagnostic, prognostic, predictive, and response | Denotes active lymphangiogenesis as it is a lymphangiogenic growth factor | Higher VEGF-D levels are associated with greater rate of lung function decline | Repeatable and reproducible | Guideline-based biomarker for the disease |
| PAP | GM-CSF autoantibody level | Diagnostic and predictive | GM-CSF signaling regulates function of alveolar macrophages | Elevated GM-CSF autoantibodies are diagnostic of autoimmune PAP | Repeatable and reproducible | Demonstrated in multiple retrospective and prospective studies |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; BNP = brain natriuretic peptide; CFTR = cystic fibrosis transmembrane conductance regulator; COPD = chronic obstructive pulmonary disease; FeNO = fractional exhaled nitric oxide; GM-CSF = granulocyte–macrophage colony–stimulating factor; IPF = idiopathic pulmonary fibrosis; LAM = lymphangioleiomyomatosis; MMP7 = matrix metallopeptidase 7; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PAH = pulmonary arterial hypertension; PAP = pulmonary alveolar proteinosis; PBMC = peripheral blood mononuclear cell; PD1 = programmed cell death protein 1; PDL1 = programmed cell death ligand 1; RCT = randomized controlled trial; VEGF-D = vascular endothelial growth factor D.
Lung Cancer
Lung cancer is the leading cause of cancer-related death in the world (12). Although lung cancer has been grouped into small-cell carcinoma, non–small-cell squamous-cell carcinoma, non–small-cell adenocarcinoma, and large-cell carcinoma subtypes, studies of genomic biomarkers such as those enabled by next-generation sequencing have identified more than a thousand potential signatures that accompany observed histologies (13–15). Current methods to detect lung cancer include chest imaging and biopsy, which can include bronchoscopy, transthoracic needle biopsy, and surgical lung biopsy (16). For imaging-based diagnosis, yearly chest computed tomography (CT) scans are often performed in adults, particularly those with a smoking history who are at higher risk, but many pulmonary nodules observed are of indeterminate status. Sensitivity of bronchoscopy ranges from 34% to 88%, depending on the location and size of the lesion (17). Thus, there is a need for biomarkers that guide clinical decision making.
Promising biomarkers in lung cancer include diagnostic biomarkers that can distinguish benign versus malignant lesions found on chest CT scan, monitoring biomarkers that identify individuals who are most likely to benefit from more intense screening and/or chemoprevention, and predictive and response biomarkers that can lead to use of targeted therapies. Molecular markers for early detection in lung cancer have been sought in plasma, airway epithelium, and sputum. Various potential markers have reached phase 1 and 2 studies, but there is a marked drop in the number of markers that make it past phase 3 and 4 studies. This expensive “valley of death” of biomarkers is partly due to the biased discovery populations that do not match the intended use populations for tests evaluated.
Many prognostic biomarkers are available or are being developed for lung cancer. Among these are tumor immune and microenvironment biomarkers, such as PD1 (programmed cell death protein 1), PDL1 (PD1 ligand 1), and VEGF-A (vascular endothelial growth factor-A). PDL1 is an example of a response biomarker that can influence treatment and lead to clinical benefit. PD1 is a negative costimulatory receptor that is expressed primarily on the surface of activated T cells, and when PD1 binds to one of its ligands, it can inhibit a cytotoxic T-cell response so that tumors can escape T cell–induced antitumor activity (18–24). PDL1 expression in at least 50% of tumor cells is correlated with improved efficacy of pembrolizumab, an inhibitor of programmed cell death (25). Genetic aberration biomarkers (e.g., KRAS [KRAS proto-oncogene, GTPase], ERBB2 [Erb-B2 receptor tyrosine kinase 2], BRAF [B-Raf proto-oncogene, serine/threonine kinase], and EGFR [epidermal growth factor receptor]), epithelial-to-mesenchymal transition–associated biomarkers (e.g., SNAI2 [Snail family transcriptional repressor 2], FOXC2 [Forkhead box protein C2], and TGF-β [transforming growth factor-β]), and resistance and susceptibility to treatment biomarkers (e.g., ERCC1 [ERCC excision repair 1, endonuclease noncatalytic subunit] and RRM1 [ribonucleotide reductase catalytic subunit M1]) also show promise (13).
One diagnostic biomarker that made it through the discovery-to-adoption-in-clinical-practice process was the result of carefully designed studies and substantial efforts to secure funding. The biomarker in question, which is based on a gene-expression signature observed in cytologically normal bronchial epithelial cells collected from the mainstem bronchus via bronchoscopy, has improved diagnostic performance compared with bronchoscopy alone for the detection of lung cancer among current and former smokers (26). Two independent, multicenter, prospective, observational studies, the AEGIS (Airway Epithelial Gene Expression in the Diagnosis of Lung Cancer) trials (AEGIS-1 and AEGIS-2), demonstrated that the combination of the classifier plus bronchoscopy had a sensitivity of 96% (95% confidence interval, 93–98%) in AEGIS-1 and 98% (95% confidence interval, 96–99%) in AEGIS-2 (27). Subsequently, its clinical utility was demonstrated via a registry study that showed the test improved patient health outcomes and had a positive economic benefit (i.e., avoided thoracotomies) (28). The diagnostic biomarker became a Medicare-covered test in 2016.
Knowledge gaps
Studies are needed to identify 1) noninvasive diagnostic biomarkers that are able to detect disease in its early stages (i.e., before the occurrence of irreversible lung changes) and 2) prognostic biomarkers that lead to treatment strategies based on identification of tumor subtypes.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH), a disease in which widespread obstruction of the smallest pulmonary arteries leads to pulmonary hypertension and right ventricular failure, occurs most commonly in women (threefold increased risk) and across the age spectrum (29, 30). Although therapies developed over the past two decades improve clinical function and survival for many patients, PAH remains a disease with high morbidity and mortality and no cure.
Diagnosis and early initiation of treatment in PAH is currently based on exclusion of other diseases that may cause pulmonary hypertension, including heart disease, lung disease, hypoxia, pulmonary embolism, as well as a variety of conditions, such as connective tissue diseases, portal hypertension, and HIV infection (31). Clinical testing for diagnosis includes heart and lung imaging with echocardiography, CT, nuclear medicine perfusion scanning, pulmonary arteriography, and pulmonary function testing to accurately identify other diseases that cause pulmonary hypertension. Cardiac catheterization is also required to confirm the presence of pulmonary hypertension and exclude cardiac etiologies. In the absence of other identified causes, PAH usually is sporadic (called idiopathic PAH), but 6% to 20% of cases are familial. PAH diagnosis is often delayed, sometimes many years, because the progression of underlying pulmonary vascular disease is silent until sufficiently severe to reduce cardiac output, at which time rapidly progressive symptoms or even death can happen unexpectedly. It is an enormous preventative limitation that presymptomatic pulmonary microvascular loss cannot be detected clinically.
Genetic variants, or genotypes, are biomarkers with diagnostic, prognostic, and/or predictive potential. Initially, genetic variants were identified as the mechanism explaining familial PAH. In sporadic PAH, a genetic basis may be concealed by decreased penetrance (20%), which is the basis for skipped generations. Disease-causing mutations for PAH have been identified in 10 genes (32). Most notably, BMPR2 (bone morphogenetic protein receptor type 2) mutations are responsible for the disease in 75% of familial cases and in 25% of sporadic cases. A collaborative international meta-analysis of 1,550 patients with PAH found that those with a BMPR2 mutation had onset at younger age, more severe disease, and increased risk of death compared with those without an identified mutation (33). Other genes whose variants are associated with PAH include SMAD9 (SMAD family member 9), ACVRL1 (activin A receptor–like type 1), and ENG (endoglin), which are the basis for hereditary hemorrhagic telangiectasia (34). Pulmonary venoocclusive disease, a rare pulmonary vascular disease, which is difficult to distinguish clinically from PAH, is now believed to be a phenotypic variation of another disease, pulmonary capillary hemangiomatosis. Recently, both have been shown to be caused by a homozygous mutation of EIF2AK4 (eukaryotic translation initiation factor 2α kinase 4) (35). It is especially important to recognize these conditions because some PAH therapies are not beneficial, and may carry unique risks, in pulmonary venoocclusive disease (36).
Scoring systems on the basis of clinical, hemodynamic, and exercise parameters such as the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) risk score and the French PAH registry equation are the main PAH prognostic biomarkers in clinical use (36). Composite risk scores are used because no single variable provides definitive prognostic information (36). Risk assessment must be considered in conjunction with current treatment. Over the past 2 decades, four classes (i.e., prostacyclins, endothelin receptor antagonists, phosphodiesterase inhibitors, soluble guanylate cyclase stimulators) of pharmacologic therapies have received FDA approval for PAH therapy. However, response to any specific therapy is not predictable, and these therapies may lose efficacy with time.
Brain natriuretic peptide (BNP) is a biomarker recommended for risk stratification in PAH and as a monitoring biomarker during treatment (a “normal” level of BNP is suggested as a treatment goal) (37). Acute vasodilation in the presence of nitric oxide (NO) inhalation during cardiac catheterization is the only predictive biomarker clinically used for PAH therapy, and it is associated with survival among patients with PAH on oral calcium blocker therapy (38). A whole-exome sequencing study found that vasodilator-responsive patients with PAH had an enrichment of associated variants in genes that regulate vascular smooth muscle contraction (39). A study of 25 gene expression levels in peripheral blood showed that levels of two genes could be used to stratify patients with PAH according to the subphenotype of being responsive to calcium channel blocker therapy (40). Gene expression changes have the potential to predict responses to other PAH treatments. For example, one study of 1,198 patients with PAH examining endothelin-1 pathway gene polymorphisms and treatment responses to endothelin receptor antagonists found that one variant (rs11157866) was associated with symptom improvement at 12 and 18 months (41).
Knowledge gaps
Studies to identify and validate diagnostic, prognostic, and predictive biomarkers would greatly enhance the care of patients with PAH. A special need is to distinguish patients who are unlikely to respond to medical therapy, in whom rapid priority for lung transplantation may be the best option.
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF), a condition with median survival of 3 to 5 years after diagnosis, is localized to the lung and characterized by a pattern of heterogeneous, subpleural patches of fibrotic, remodeled lung (42, 43). IPF affects 5 million people worldwide, disproportionately affects men, and is associated with cigarette smoking (44, 45). In addition, IPF incidence increases with age, is inexplicably increasing in prevalence (46, 47), and is likely underdiagnosed (43, 46, 48). Among the general population aged 50 years and older, 1.8% of individuals have reticulation, honeycombing, or other features of pulmonary fibrosis detectable by chest CT scan (48), and among asymptomatic relatives of families with two or more cases of IPF, 15% to 20% have radiographic or pathological evidence of asymptomatic or “preclinical” pulmonary fibrosis (49). Although pirfenidone (50) and nintedanib (51) have been shown to slow IPF progression, most patients with IPF are discovered in the advanced stage when little can be done to influence survival. Earlier diagnosis of IPF would enable detection of patients with a lower burden of fibrotic lung disease (52, 53) and may reveal novel molecular targets for intervention that substantially improve the prognosis of this progressive disease.
IPF is a complex, heterogeneous genetic disorder that is associated with rare and common sequence variants in many genes (e.g., MUC5B [mucin 5b, oligomeric mucus/gel-forming], TERT [telomerase reverse transcriptase], TERC [telomerase RNA component], RTEL1 [regulator of telomere elongation helicase 1], PARN [poly(A)-specific ribonuclease], SFTPC [surfactant protein C], and SFTPA2 [54–61]) and at least 11 novel loci (62, 63). Genetic risk variants play major and similar roles in the development of both familial and sporadic fibrotic idiopathic interstitial pneumonia (IIP), accounting for up to 35% of the risk of IIP (62). Although rs35705950, a promoter variant in MUC5B, is the strongest risk factor for the development of IIP, and more specifically IPF (62, 64–68), it has a low penetrance (62, 64), indicating that interactions of rs35705950 with other genetic and/or environmental factors are critical determinants of IPF risk. Besides associations of genetic variants with IPF, multiple emerging epigenetic (69–73) and transcriptional (74–77) profiles have been identified. In aggregate, these findings suggest that IPF is a heterogeneous disease and that genetic and molecular subtypes of IPF will provide essential clues to disease pathogenesis (78, 79), prognosis (80–96), treatment (50, 51, 97, 98), and survival (99), all of which remain substantial challenges in treating patients with IPF.
Biomarkers are playing an emerging role in IPF diagnosis, treatment, and early detection. In addition to demographics and physiology (100), serum and lavage concentrations of MMP7 (matrix metallopeptidase 7) (83, 96, 101) and a 52-gene peripheral blood expression signature have proven to be the most reproducible prognostic biomarkers in IPF (102, 103). Although further validation is needed, serum concentrations of surfactant proteins A and D (87, 104, 105), ICAM (intercellular adhesion molecule 1) (101), MUC1 (mucin 1, cell surface associated) (105–107), CH13L1 (chitinase 3–like 1) (84), CXCL13 (C-X-C motif chemokine ligand 13) (108), POSTN (periostin) (109), anti-HSP70 (110), CCL18 (C-C motif chemokine ligand 18) (111), and collagen degradation products (112) are also associated with IPF progression. Sequence variants in MUC5B (113), TOLLIP (Toll-interacting protein) (67), and TLR3 (Toll-like receptor 3) (114), as well as aging biomarkers (peripheral blood mononuclear cell [PBMC] telomere length [115] and free mitochondrial DNA) have also been associated with survival in IPF.
Although several biomarkers, including the MUC5B promoter variant (48, 116), serum surfactant protein D, MMP7 (49), and serum galectin-3 (117), have been associated with early forms of interstitial lung disease, it is not yet clear which, if any, of these peripheral blood biomarkers will prove useful for predicting the presence of earlier forms of pulmonary fibrosis in diverse, unselected patient groups. Thus, additional diagnostic biomarkers are needed to improve the detection of early forms of pulmonary fibrosis.
Knowledge gaps
Studies are needed to identify risk and diagnostic biomarkers for IPF, as well as drug response biomarkers for currently available therapies, such as pirfenidone and nintedanib.
Asthma
Asthma is a chronic disease of the airways characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and underlying inflammation (118). Its diagnosis relies on medical history, physical examination, and pulmonary function tests (118). Consideration of alternative diagnoses is important and may include bronchoprovocation with methacholine, histamine, cold air, or exercise challenge; chest radiography; and allergy testing (118). Asthma places a significant economic burden on the United States, with a total cost of $81.9 billion in 2013 (119). Management of asthma and choice of appropriate medications is currently based on established clinical guidelines that do not include use of biomarkers (118).
Studies have clustered patients with asthma on the basis of clinical characteristics, with the goal of identifying disease endotypes that lead to tailored therapies. One such study identified transcriptional differences between clinically defined asthma and nonasthma groups and further characterized subgroups of patients with asthma via hierarchical clustering and topological data analysis (120). Although the clinical utility of stratifying patients on the basis of such markers has not been demonstrated, the findings do show that gene expression of circulating cells does not follow current clinical classifications of asthma severity (120).
The gold standard for determining lower airway inflammation type involves invasive bronchial biopsies; therefore, identifying noninvasive biomarkers would enhance patient care. One potential predictive biomarker for asthma is periostin, an important regulator of inflammatory cell infiltration and activation (121). Periostin appears to protect mice from allergic airway inflammation and accelerates allergen-induced eosinophil recruitment in the lung (122, 123). Furthermore, periostin appears to be involved in eosinophil recruitment, airway remodeling, and development of a Th2 phenotype and contributes to increased expression of inflammatory mediators (124, 125). Data demonstrating that periostin can be used for clinical decision making in patients with asthma are currently lacking. The fraction of exhaled NO (FeNO) is another potential noninvasive predictive and response biomarker. FeNO levels may represent epithelial activity on the basis of nitric oxide synthase that indicates eosinophilic airway inflammation and may be predictive of corticosteroid responsiveness. Meta-analyses thus far have not found that choosing therapies on the basis of FeNO levels resulted in improved clinical outcomes.
Novel biologics directed at the 10% to 15% of patients with asthma with persistent severe eosinophilic asthma have been developed recently, including mepolizumab and reslizumab, which are anti–IL-5; dupilumab, which is anti–IL-4; and benralizumab, which is anti–IL-5 receptor. These biologics are expensive, costing $30,000 to $40,000 per year, and there is no reliable way of identifying which patients respond to them. Thus, response biomarkers for these treatments are needed. Of the biomarkers that could be useful for eosinophilic asthma (i.e., eosinophil count in sputum or peripheral blood, FeNO, periostin), none have high accuracy, reproducibility, sensitivity, or specificity. Because their measures are correlated, using sputum eosinophil count as a response biomarker is currently the most reasonable choice, because of its low cost and simple assay.
Knowledge gaps
Studies are needed to identify prognostic and response asthma biomarkers, particularly for expensive drugs such as asthma biologics. In addition, there is a need to leverage existing data from large real-life populations of individuals with asthma to accelerate biomarker discovery.
Chronic Obstructive Pulmonary Disease
In the United States, chronic obstructive pulmonary disease (COPD) was the second leading cause of disability-adjusted life years lost and the fourth leading cause of death in 2010 (126). Over the next 15 years and owing largely to the aging population, the number of patients with COPD is expected to double (127). Aside from smoking cessation, there are no disease-modifying therapies (128). Instead, current therapies are targeted at improving patient symptoms and reducing exacerbations, defined clinically by acute worsening of patient symptoms beyond the day-to-day variation (128). However, these therapies are imprecise. The number needed to treat (NNT) for long-acting bronchodilators, mainstays of COPD management, to prevent a single exacerbation over 1 year is 16. That is, the average number of patients with COPD who need to be treated with long-acting bronchodilators to prevent one exacerbation over a year is 16. A common second-line therapy for COPD is a combination of inhaled corticosteroids (ICS) and a long-acting β2-agonist, which is prescribed to patients who experience exacerbations frequently (i.e., two or more exacerbations or a hospitalization within the previous year) (128). The NNT for ICS/long-acting β2-agonist to prevent a single exacerbation over 1 year is 20 (129), and to prevent one hospitalization is greater than 30 (129). Disconcertingly, the long-term use of ICS has been associated with increased rates of pneumonia, with an NNT to induce harm of 20 (130).
COPD is diagnosed based on patient symptoms, persistent airflow limitation that is not fully reversible, and a history of exposure to cigarette smoke or significant biomass (128). Therapeutic choices are largely guided by severity of patient symptoms, as no biomarkers or objective measurements are available. Not surprisingly, there is tremendous variation in the rate of exacerbation among patients and across different healthcare systems (131). Promising biomarkers are in development. In 2015, as a result of COPD Foundation Biomarker Qualification Consortium work, the FDA qualified plasma fibrinogen as a prognostic biomarker for all-cause mortality and exacerbations in patients with COPD (132). Plasma fibrinogen lacks sufficient resolution (i.e., sensitivity or specificity) to be useful clinically, but it is anticipated that use of this biomarker to enrich clinical trials for patients with COPD at moderate to high risk of mortality or exacerbation will increase the rate of success for identifying novel therapeutics. Other prognostic blood biomarkers that have been evaluated include C-reactive protein (which is associated with exacerbations and mortality), leukocytosis (which is associated with exacerbation and mortality), IL-6 (which is associated with mortality), and adiponectin (which is associated with FEV1 decline). Lung-specific proteins that demonstrate promise as prognostic biomarkers include surfactant protein D, which is associated with exacerbation and mortality, and SCGB1A1 (secretoglobin family 1A member 1) and CCL18, which are associated with FEV1 decline (133). Although all of these proteins show statistically significant associations with one or more important COPD outcomes, the associations have low effect sizes, making them unsuitable for clinical use (134).
Peripheral eosinophil counts have been recently touted as a response biomarker to guide ICS use in COPD (135), after the observation that ICS appear to have larger beneficial effects in patients with COPD with higher peripheral eosinophil count (136). However, there is currently no consensus on the threshold value at which ICS should be used in patients with COPD. Some have advocated an eosinophil threshold of 2% (135, 137), whereas others have used a 300/μl cutoff (138). Other concerns in the use of peripheral eosinophil counts as a biomarker for ICS use include the lack of stability of peripheral eosinophil counts within individuals over time and the relatively weak correlation between airway eosinophil and peripheral eosinophil counts (139). Nonetheless, given the widespread availability and low cost of eosinophil count measures, using them as biomarkers deserves additional study.
Knowledge gaps
Studies are needed to identify prognostic biomarkers that enable discovery of novel therapeutics, as it is very difficult clinically to separate patients with COPD according to disease severity and according to “active” versus “burnt-out” disease.
Sepsis
Sepsis is a syndrome characterized by a dysregulated inflammatory response to microbial infection that can lead to organ damage and death. A consensus definition for sepsis offered in 1992 defines it as a concern for infection and the presence of two or more systemic inflammatory response syndrome (SIRS) criteria (temperature >38°C or <36°C; heart rate >90 beats/min; respiratory rate >20 breaths/min or PaCO2 <32 mm Hg; white blood cell count >12,000/μl, <4,000/μl, or >10% immature bands) (140). This definition was extremely broad, however, as up to 50% of hospitalized patients meet these criteria at some point during their stay (141). In 2016, a more specific sepsis definition termed “Sepsis 3” was proposed: suspected infection and a change in Sequential Organ Failure Assessment score of at least 2 (142). Sepsis using this definition is associated with in-hospital mortality rate greater than 10% (143). Debate about whether sepsis should be defined narrowly (Sepsis 3, high-mortality cohort) versus prior definitions with SIRS (less specific but potentially present earlier) continues. The clinical performance of most sepsis biomarkers was evaluated using the old definition, and their diagnostic and prognostic utility may change in the Sepsis 3 era.
Plasma lactate is the most established biomarker in sepsis, with higher levels indicating more severe disease. According to Sepsis 3, septic shock is clinically identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater and serum lactate level greater than 2 mmol/L in the absence of hypovolemia (143). Although this definition is associated with hospital mortality rates greater than 40%, even a modest elevation of lactate (2.1–4.0 mmol/L) has been associated with increased risk of death in normotensive patients with sepsis (144, 145). Lactate clearance is also associated with mortality. Patients with sepsis who fail to decrease lactate by at least 10% in the first 6 hours of treatment have at least twice the likelihood of death as those whose lactate declines by at least 10% (146). Randomized controlled trials that use lactate clearance as an endpoint in sepsis have been inconclusive, but, nonetheless, measurement of 6-hour lactate clearance was included in the 2012 Surviving Sepsis Guidelines and is incorporated in the Sepsis Centers for Medicare & Medicaid Services Core (SEP-1) Measure (147).
Lactate is by no means the only biomarker in sepsis. A 2010 review identified 178 candidate biomarkers in 3,370 references (148). The Acute Physiology and Chronic Health Evaluation (APACHE) 3 score includes numerous routine clinical labs (e.g., creatinine, pH, and sodium) that are each individually highly associated with ICU mortality.
Biomarkers that distinguish bacterial from viral sepsis or sterile SIRS, thus allowing more targeted antimicrobial use, are also highly needed. Among these is procalcitonin, another biomarker that is often used in clinical practice and has been widely studied. Procalcitonin is highly associated with both sepsis and sepsis mortality, although its diagnostic performance in a meta-analysis was found to be modest (sensitivity and specificity of 71% to identify sepsis among critically ill patients), and, hence, its clinical utility for diagnosis is limited (149). On the other hand, because procalcitonin is a marker of bacterial infection, some algorithms that incorporate serial procalcitonin measurement have been shown to be useful for avoiding prolonged courses of antibiotics (150). A recent whole blood gene expression study identified a gene signature that can separate sterile inflammation from infection and viral from bacterial infection with sensitivity of 94% for bacterial infection (151).
Knowledge gaps
Studies are needed to identify and validate biomarkers that differentiate bacterial, fungal, and viral sepsis from sterile inflammation, and thus enable targeted and narrow treatment in sepsis care.
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is a syndrome characterized by acute onset of respiratory failure, hypoxia (PaO2/FiO2 ratio < 300), and bilateral infiltrates not fully explained by heart failure. It occurs in critically ill patients with diverse risk factors, including direct lung injury (e.g., aspiration, pneumonia) and indirect lung injury (e.g., sepsis, pancreatitis, burns). ARDS carries substantial morbidity and mortality and is estimated to occur in 10% of patients who are admitted to ICUs and 23% of patients who receive mechanical ventilation (152). Treatment of ARDS involves a low-pressure, low–tidal volume ventilation strategy, a practice that has contributed to markedly falling mortality in ARDS over time (153–155).
The most common ARDS biomarker is the PaO2/FiO2 ratio, serving as a diagnostic, prognostic, and predictive biomarker. In 2012, a group of ARDS experts convened to define ARDS and ARDS severity thresholds and found that more complex models could not out-perform PaO2/FiO2 ratio thresholds as a predictor of mortality in ARDS (156). Thus, in the 2012 “Berlin Criteria,” ARDS severity was defined using the PaO2/FiO2 ratio, with severe less than 100, moderate 100 to 200, and mild 200 to 300 categories. PaO2/FiO2 is not only diagnostic and prognostic but also a predictive biomarker, as patients with lower PaO2/FiO2 ratios benefit from adjunctive ventilator treatment. Specifically, in patients with PaO2/FiO2 ratio less than 150, neuromuscular blockade showed a strong trend toward improved mortality (157). Furthermore, prone positioning, which had been ineffective in studies that included all patients with ARDS, achieved a striking mortality benefit when applied early to the subset of patients with severe disease (155).
Promising plasma-based biomarkers in ARDS have been identified via targeted proteomics studies, representing biological pathways such as markers of inflammation, coagulation, and epithelial and endothelial dysfunction (158). Although biomarkers have shown associations in independent cohorts, none have been validated for clinical use. Attempts to incorporate multiple inflammatory, epithelial, and endothelial plasma biomarkers that were individually associated with ARDS mortality into a model that includes the APACHE score found that limited prognostic value was added by the biomarkers (159). Recent ARDS biomarker work has focused on endotyping disease—that is, “splitting” the syndrome into multiple endotypes, rather than “lumping” all patients into one. For example, latent class modeling has been used to identify two distinct endotypes of ARDS, including a hyperinflammatory subset characterized by high levels of IL-8 and tumor necrosis factor-α and low levels of bicarbonate. Patients with this hyperinflammatory endotype in three independent trials had a much higher risk of death and, importantly, a differential response to both high positive end-expiratory pressure and conservative fluid treatment strategies (160, 161).
Knowledge gaps
Studies are needed to identify biomarkers associated with ARDS-specific mortality (as opposed to general ICU mortality) and development of biomarkers that validate ARDS subtypes with distinct prognosis and pathophysiology. Such biomarkers are critical for designing targeted and adequately powered ARDS clinical trials.
Cystic Fibrosis
Cystic fibrosis, the most common lethal genetic disorder among white individuals, affecting more than 30,000 people in the United States and more than 70,000 people worldwide (162), is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein. CFTR dysfunction leads to mucus obstruction, infection, and inflammation that produce obstructive lung disease, airway remodeling, bronchiectasis, and ultimately respiratory failure. Untreated cystic fibrosis causes death in infancy. The median age of survival of patients with cystic fibrosis has risen to 47.7 years, thanks to advances in symptomatic therapies and, potentially, the more recent introduction of CFTR modulators, treatments that target specific CFTR-causing mutations. Indeed, more than half of patients with cystic fibrosis are now adults, a trend that has been increasing for several decades (163).
Diagnostic biomarker tests for cystic fibrosis are well established, and some can serve as prognostic biomarkers. Patients with cystic fibrosis are typically identified within weeks of birth through newborn screening, which is followed with confirmatory testing at cystic fibrosis care centers. Newborn screening includes testing of immunoreactive trypsinogen in bloodspots with or without screening for known CFTR mutations. Diagnosis is based on signs and symptoms of disease, or family history plus evidence of CFTR dysfunction (i.e., elevated sweat chloride concentration > 60 mM) and/or the detection of two well-characterized CFTR variants associated with disease. Immunoreactive trypsinogen/DNA-based cystic fibrosis newborn screening algorithms have 97.8% sensitivity and 99.7% specificity to diagnose or exclude cystic fibrosis (164). The sweat chloride concentration test demonstrates excellent sensitivity and specificity to diagnose cystic fibrosis, and it can effectively discriminate between subjects without CFTR mutations, heterozygous carriers, and patients with two disease-causing mutations (165). Patients with one or more mutation who retain partial CFTR function frequently have pancreatic sufficiency, higher lung function, and lower sweat chloride values, which are associated with later diagnosis, better growth, and increased longevity (163). Subjects with indeterminate genetic testing and intermediate sweat chloride concentration values (i.e., 30–60 mM) are classified as having CFTR-related metabolic syndrome or CFTR-related disorder if testing is performed outside of newborn screening. A minority of these individuals go on to meet cystic fibrosis diagnostic criteria, but their clinical course is typically milder than that of patients with classic cystic fibrosis (164).
The development of effective therapies for cystic fibrosis has been linked directly to the development of relevant disease and pharmacodynamic biomarkers. These vary depending on what aspects of cystic fibrosis are targeted (e.g., obstructive lung disease, lung infection, pancreatic insufficiency). For pulmonary therapies, pharmacodynamic and safety biomarkers with regulatory approval that have emerged as reliable surrogate endpoints (i.e., substitutes for clinical efficacy measures) include FEV1% predicted, risk of pulmonary exacerbations, and the respiratory symptom domain of the Cystic Fibrosis Quality of Life Questionnaire–Revised. Among these, FEV1% predicted generally has the greatest precision and power to detect therapeutic effect, although it does not have an established minimal clinically important difference that is universally applied to pulmonary therapies. Furthermore, some therapeutic targets have little effect on FEV1% predicted (e.g., antiinflammatories).
Multiple-breath washout and the lung clearance index have emerged as sensitive pharmacodynamic biomarkers of airway obstruction for young patients with cystic fibrosis and patients with less-advanced disease (166, 167). Sensitive pulmonary function tests such as these are critical for the assessment of new therapies in cystic fibrosis, as improved lung function of study participants (either due to advances in baseline therapies or evaluation in younger patients) limits the ability to detect drug efficacy via older, established endpoints (e.g., FEV1, pulmonary exacerbation risk). Multiple-breath washout/lung clearance index demonstrates excellent reliability, with mean coefficient of variation within one session in children reported between 3% and 7%, and excellent validity (discriminating between patients with cystic fibrosis and control subjects without cystic fibrosis in 22 of 23 studies) (168). It has increased sensitivity compared with FEV1% predicted to detect biologic activity of at least four different pulmonary therapies in young patients with cystic fibrosis with preserved lung function (169–172). How this test will be used in clinical care as a disease-monitoring biomarker is currently unclear, but it is attractive for some cystic fibrosis populations, as it is effort independent, does not require forced expiratory maneuvers, and is sensitive in the setting of mild disease.
Sweat chloride concentration is an invaluable diagnostic and pharmacodynamic biomarker in cystic fibrosis. Its value is highly dependent on CFTR expression and activity, and sweat is not affected by disease state compared with other tissues where CFTR is expressed (173). As a pharmacodynamic biomarker, it has been adapted for use in multicenter clinical trials of CFTR modulators and has repeatedly demonstrated the capacity to detect biologic activity and associate with benefit as measured by other established surrogate endpoints. It offers low within-patient variance (19.3–19.8 mmol/L in placebo-treated control patients with cystic fibrosis and minimal-function CFTR mutations) and has thresholds associated with clinical status to diagnose cystic fibrosis and CFTR disorders (e.g., pancreatic-sufficient cystic fibrosis, subjects with intermediate sweat chloride concentration values, and subjects without cystic fibrosis) (163, 173). As a biomarker to monitor individual CFTR modulator response, it has not demonstrated direct associations with established pulmonary surrogate endpoints, such as FEV1% predicted, on a patient-specific basis (174). There are many likely reasons for this, including variability of baseline lung function, disease state, and secondary pulmonary therapies of patients with cystic fibrosis that independently impact FEV1. Nevertheless, data from clinical trials analyzed in aggregate demonstrate a clear direct relationship between change in sweat chloride concentration and change in FEV1% predicted (175).
There are numerous additional biomarkers used in cystic fibrosis for clinical care and research, some of which are summarized in Table 3 (176, 177). Cystic fibrosis care is currently undergoing a transformation as a result of the development of mutation-specific CFTR modulators coupled with early diagnosis.
Table 3.
Prominent Cystic Fibrosis Biomarkers
| Modality | Examples | Biomarker type | Comments |
|---|---|---|---|
| CFTR function | Nasal potential difference | Diagnostic and response | Nasal potential difference and intestinal current measurement are secondary tests of CFTR function that can be used to 1) diagnose cystic fibrosis and 2) detect CFTR bioactivity of drugs that improve CFTR function |
| Intestinal current measurement | |||
| Imaging | Chest X-ray | Prognostic, monitoring, and response | Chest X-ray sensitivity limits its use |
| Chest CT | CT radiation concerns limit regular use | ||
| Chest MRI | MRI tools emerging | ||
| Hyperpolarized gas | |||
| Perfusion | |||
| Sputum and/or serum inflammatory biomarkers | Sputum (e.g., NE, IL-1β, IL-6, IL-8, HMGB-1, total cell count, and % PMNs), serum (hsCRP, calprotectin, SAA, and GCSF) | Prognostic, predictive, and response | NE in BAL fluid predicts bronchiectasis in young patients with cystic fibrosis |
| Sputum access limited to those with established disease | |||
| High variability and low sensitivity limit use | |||
| Respiratory tract microbial analysis | Detection of pathogens (e.g., Pseudomonas aeruginosa, MRSA, and Burkholderia sp.) | Diagnostic, prognostic, monitoring, and response | Routine part of cystic fibrosis care to monitor disease and guide treatment |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; CT = computed tomography; GCSF = granulocyte colony–stimulating factor; HMGB-1 = high-mobility group box 1 protein; hsCRP = high sensitivity C-reactive protein; MRI = magnetic resonance imaging; MRSA = methicillin-resistant Staphyloccus aureus; NE = neutrophil elastase; PMN = polymorphonuclear leukocytes; SAA = serum amyloid A.
Knowledge gaps
Studies are needed to assess how existing or new biomarkers can increase therapeutic precision for mutation-specific CFTR modulators, the use of which requires new pharmacodynamic endpoints and disease-monitoring tools, and to identify prognostic biomarkers for disease exacerbations and rapid lung function decline that might benefit all patients and lead to novel mutation-agnostic therapies.
Rare Lung Diseases
Rare diseases are defined in the United States as conditions that affect fewer than 200,000 people. There are more than 6,800 rare diseases, which collectively affect an estimated 25 million Americans and represent a substantial burden of morbidity, mortality, health care utilization, and caregiver costs. Although the number of rare lung diseases has not been formally defined, there are approximately several hundred entities depending on the extent of delineation of subgroups, with growing subtypes as additional genetic etiologies are increasingly identified. Some rare diseases (i.e., cystic fibrosis, IPF), are discussed in other sections of this document. Here, biomarkers are described for lymphangioleiomyomatosis (LAM), pulmonary alveolar proteinosis (PAP), and Hermansky-Pudlak syndrome (HPS). Common challenges in biomarker development for rare lung diseases include limited patient cohorts for validation studies and the lack of resources and incentives to facilitate transfer of assays out of the research domain into certified clinical laboratories.
LAM
LAM is a rare neoplasm that affects women almost exclusively and results in progressive cystic lung disease and respiratory failure. Although chest CT findings may suggest the diagnosis of LAM, additional criteria are required for confident diagnosis, such as either tuberous sclerosis complex or renal angiomyolipoma. However, the majority of patients with LAM lack these additional features, and therefore lung biopsy is often performed for diagnostic certainty. Serum VEGF-D (vascular endothelial growth factor D), a key growth factor in tumor metastasis, is the most successful biomarker used in clinical care of patients with LAM. After the observation that serum levels of VEGF-D were elevated in patients with LAM compared with healthy control subjects (178), VEGF-D levels in patients with LAM were compared with those of individuals with other causes of cystic lung disease, and it was found that VEGF-D could be a diagnostic biomarker (179). The 2016 ATS/Japanese Respiratory Society Clinical Practice Guideline, which provides evidence-based recommendations for the diagnosis and treatment of patients with LAM, formally evaluated the role of VEGF-D as a potential noninvasive diagnostic test for LAM (180). On the basis of a systematic review of seven studies, serum VEGF-D testing had a low false-positive rate and a high false-negative rate, and thus, its use may eliminate the need for an invasive lung biopsy in patients who present with cystic lung disease that lacks confirmatory features of LAM. Those with positive tests could be diagnosed with confidence, whereas those with a negative test would proceed with usual diagnostic testing (i.e., lung biopsy).
There are also data to suggest that serum VEGF-D may have roles as a prognostic, predictive, and pharmacodynamics biomarker. The MILES (Multicenter International LAM Efficacy of Sirolimus) trial was a double-blind trial of sirolimus versus placebo in women with moderate to severe pulmonary impairment due to LAM (181). In this trial, a higher baseline VEGF-D level was associated with both better lung function response in the sirolimus group and more rapid lung function decline in the placebo group. Serum VEGF-D level greater than 800 pg/ml was also associated with a faster rate of decline of FEV1 compared with patients with a serum VEGF-D less than 800 pg/ml (182). The utility of VEGF-D as a biomarker has also been demonstrated in other clinical trials, but there are no data indicating that extent of VEGF-D reduction correlates with clinically meaningful outcome measures.
Knowledge gaps
Longitudinal studies are needed to refine the use of VEGF-D in treatment and disease monitoring, as well as studies to identify novel diagnostic biomarkers.
PAP
PAP is a rare syndrome characterized by the accumulation of surfactant in alveolar macrophages and alveolar spaces resulting in respiratory compromise due to hypoxemia. PAP occurs because of a number of disparate but intersecting pathogenic mechanisms and is not a single disease. Common symptoms include cough, dyspnea on exertion, fatigue, and weight loss. PAP syndrome is identified based on a compatible history, typical radiologic findings, BAL cytology and/or lung biopsy findings, and compatible biomarkers (183).
Granulocyte-macrophage colony-stimulating factor (GM-CSF) plays a vital role in the catabolism of surfactant and other alveolar macrophage functions critical for surfactant homeostasis, alveolar stability, lung function, and innate host defense. Disruption of GM-CSF signaling causes PAP syndrome in many patients. Autoimmune PAP is caused by the disruption of GM-CSF signaling by anti–GM-CSF antibodies (GMAb) and provides rationale for GMAb as a diagnostic biomarker. In fact, GMAb may have the strongest scientific rationale of any biomarker in pulmonary medicine, including the demonstration that transfer of GM-CSF autoantibodies confers PAP disease (184, 185). Elevated serum GM-CSF is used as a diagnostic biomarker for autoimmune PAP in patients with typical clinical and radiologic findings. The test is noninvasive and has high precision, and the sensitivity and specificity approach 100% at a cutoff serum GMAb level of 5 μg/ml (186). In addition, because autoimmune PAP responds better to whole-lung lavage and GM-CSF therapy than other causes of PAP, GM-CSF autoantibodies also serve as a predictive biomarker. Although widely accepted as a key diagnostic test, GM-CSF autoantibody testing is currently available only on a research basis.
A number of additional biomarkers are useful for identification of other causes of PAP syndrome and can be used in an algorithmic manner (183). For example, patients with negative serum GM-CSF autoantibodies and elevated levels of serum GM-CSF without apparent underlying diseases known to be associated with PAP need further evaluation for hereditary PAP by analyzing GM-CSF receptor gene (CSF2RA or CSF2RB) mutations. Whole-blood GM-CSF signaling tests, such as the GM-CSF–induced STAT5 (signal transduction and activator of transcription 5) phosphorylation index test, may support the diagnosis of primary PAP.
Knowledge gaps
Studies are needed to identify and validate diagnostic biomarkers focused on PAP syndrome that is not due to autoimmune causes.
HPS
HPS is a recessive disorder that is associated with oculocutaneous albinism and bleeding diatheses due to platelet dysfunction. HPS gene products are ubiquitously expressed, and recessive mutations result in defects in hetero-oligomeric intracellular protein trafficking complexes. There are currently 10 genetic loci associated with HPS in humans, and pulmonary fibrosis has been associated with some genes (i.e., HPS1 [HPS1, biogenesis of lysosomal organelles complex 3 subunit 1], AP3B1 (adaptor-related protein complex 3 subunit β1), and HPS4 [HPS4, biogenesis of lysosomal organelles complex 3 subunit 2]) but not others. In the affected subtypes, pulmonary fibrosis has been reported to develop in all patients typically in the third to fourth decades of life, with most patients succumbing within 3 to 10 years of diagnosis in the absence of lung transplantation. Although HPS can be diagnosed based on clinical features and evaluation of platelet-dense granules by electron microscopy, the subtypes are clinically indistinguishable. Therefore, genetic testing serves as both a diagnostic and a prognostic biomarker in HPS. Advances in genetic sequencing technologies have dramatically improved affordability, accessibility, and scope of genetic analyses, although access and cost remain limitations in clinical practice. HPS mutations are identified in most, but not all, patients with the clinical syndrome of HPS, suggesting incomplete sensitivity of testing methods and the likelihood of additional causal genes.
Knowledge gaps
Studies are needed to create affordable and easily interpretable genetic tests on the basis of known HPS mutations, and continued studies are needed to identify biomarkers in those patients who do not have a known mutation.
Establishing Clinical Utility: Cost-Effectiveness and Applicability of Biomarkers to Diverse and Real-Life Populations
Clinical utility of a validated biomarker test must be established before it is widely adopted (187, 188). That is, evidence that the test improves patient health outcomes in the real world and has a positive economic benefit must be obtained. As costs of biomarker tests decline, performing such testing in large populations is possible, which should facilitate translation of biomarkers into clinical practice (189). Although clinical trials are necessary to explore the short-term benefits and risks of testing for specific conditions (190, 191), the clinical utility of biomarker tests, particularly among asymptomatic individuals, in terms of long-term impact on morbidity, mortality, quality of life, and diagnosis and treatment costs remains uncertain (189, 192). Furthermore, the benefits and costs of integrating biomarker testing into routine clinical care have not been systematically assessed (193). Some considerations during validation and early use maximize the likelihood that a biomarker test will be clinically useful. First, capturing appropriate data for those using biomarkers outside of originally studied settings is important to, for example, identify biomarker–medication interactions with already approved therapeutics. Second, considering the generalizability of the biomarker test beyond the originally studied populations, and including diverse, real-life populations during validation, will help ensure biomarker tests are broadly useful.
Relatively few genomics studies have been conducted in individuals with diverse ancestries (194). As of 2009, only 4% of genome-wide association studies were conducted in individuals not of European descent (195), and although by 2016 this figure rose to 20%, most of the studies in individuals who were not European were limited to individuals of Asian ancestry (194). Reasons that few studies have been completed of individuals of African and Latin American ancestry and indigenous people include that 1) researchers have avoided the increased difficulty of identifying true from spurious associations when ancestral differences among cases and controls may differ, which happens more often in admixed populations; 2) logistical issues have led researchers to make use of large datasets established by large medical centers, some of which are not accessible to minority individuals; and 3) minority individuals elect not to participate in research studies for cultural or historical reasons (194, 196). Despite potential challenges, including diverse populations in precision medicine research is imperative to ensure biomarker tests advance health for all (196, 197).
Resource allocation may improve if biomarker tests lead to earlier and better targeting of treatments and thereby reduce healthcare costs. However, the extent of this improvement is unknown, as studies of biomarker testing cost-effectiveness that assess their relative value in terms of health benefits and monetary costs are lacking (198). Patient health outcomes are influenced by intersecting factors at the health system, provider, and patient levels that affect a chain of events, including who gets tested, screened, or treated; when; and which specific technology is received (199). Access to biomarker tests and related services often depends on cost and coverage of services by health plans, and differential access to biomarker tests may increase health care–related disparities (200–206).
Infrastructure Needed for Widespread Adoption of Precision Medicine in Clinical Practice
Beyond establishing clinical utility, there are additional barriers to implementation of precision medicine that must be addressed before use of biomarkers can become routine in clinical practice. First, clarity regarding test reimbursement is needed. Few U.S. insurance providers have developed clear policies regarding reimbursement of molecular studies, and fewer than 50% of ordered tests are eventually covered (202, 207, 208). In countries with centralized payer systems, heterogeneity exists regarding criteria used for evaluating clinical effectiveness of orphan drugs and personalized medicine diagnostics; the bar for coverage approval is considerably higher for diagnostic testing than for orphan drugs, despite the greater costs associated with the latter (209). Economic concerns influence use of genomic tests and medications coupled to them, even when patients are knowledgeable regarding genomic testing and results and are receptive to undergoing testing (210–220).
Second, providers lack adequate training in genomics and confidence in deciding when biomarker tests are needed (213, 221–223). This is partly because the majority of today’s physicians graduated medical school before the introduction of most biomarkers. Approaches to overcome the lack of knowledge among active pulmonary healthcare providers include developing effective electronic health record alerts and decision support tools, training genetic counselors who specialize in pulmonary diseases, and developing pulmonary-specific programs in continuing medical education in genetics and genomics. ATS has made strides in this regard, including hosting relevant postgraduate courses and through participation in the Inter-Society Coordinating Committee for Practitioner Education in Genomics (224).
Third, rapid dissemination of clinically actionable information to health providers is critical for widespread adoption of precision medicine. For example, there are no centralized databases supported to guarantee that genetic information concerning pathogenicity of sequence variants is reliably curated in a sustained manner, and in the absence of universally adopted guidelines, interpretation of clinically actionable variants often differs across clinical sequencing laboratories (225). The Clinical Genome Resource (ClinGen) is attempting to address this deficiency through the establishment of standards for variant interpretation and a publicly accessible informatics infrastructure to efficiently exchange expertly curated information (226). Although ClinGen has established eight Clinical Domain Working Groups covering 31 diseases, expert panels in pulmonary disease have yet to be convened.
Gaps in Current Research and Suggestions for Future Studies
An impediment to precision medicine shared across most diseases considered was the lack of effective translation of promising biomarkers into widely used clinical tests. Common themes emerged for future studies to address this gap and help accelerate the identification of clinically useful biomarker tests:
-
•
There are limited options for validating biomarkers in prospective trials because of the high costs associated with this endeavor. Despite this difficulty, additional translational studies to clinically validate biomarkers are needed, in which significance is valued over innovation.
-
•
More biomarker discovery research, in which hypothesis generation is viewed favorably, is necessary. This includes gathering cohorts for specimen collection or leveraging existing large datasets and databases so that specific clinical unmet needs can be addressed.
-
•
Efforts to integrate relevant existing datasets are required. This includes careful merging of heterogeneous data types within cohorts as well as merging of data across cohorts to facilitate biomarker discovery research via appropriate data-mining strategies.
Conclusions
Despite notable advances in lung precision medicine, with thousands of biomarker tests either available or under development for several lung diseases, few biomarkers are in widespread clinical use. This ATS/NHLBI Research Statement summarizes the process of developing biomarkers, reviews current biomarkers for various pulmonary diseases, outlines barriers that must be overcome, and suggests research priorities to achieve the promises of lung precision medicine.
Acknowledgments
This statement was prepared by an ad hoc subcommittee of the ATS Assembly on Behavioral Science and Health Services Research, and the Section on Genetics and Genomics.
Members of the subcommittee are as follows:
Ann Chen Wu, M.D., M.P.H.1 (Co-Chair)
James P. Kiley, Ph.D.2 (Co-Chair)
Patricia J. Noel, Ph.D.2 (Co-Chair)
Blanca E. Himes, Ph.D.3 (Co-Chair)
Shashi Amur, Ph.D.4
Esteban G. Burchard, M.D., M.P.H.5
John P. Clancy, M.D.6
Joshua Galanter, M.D., M.A.S.7
Maki Inada, Ph.D.8
Tiffanie K. Jones, M.D., M.P.H.9
Jonathan A. Kropski, M.D.10
James E. Loyd, M.D.10
Lawrence M. Nogee, M.D.11
Benjamin A. Raby, M.D.12,13
Angela J. Rogers, M.D.14
David A. Schwartz, M.D.15,16
Don D. Sin, M.D., M.P.H.17,18
Avrum Spira, M.D., M.Sc.19
Scott T. Weiss, M.D., M.S.12,20
Lisa R. Young, M.D.10,21
1Precision Medicine Translational Research Center, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts; 2Division of Lung Diseases at the NHLBI at the National Institutes of Health, Bethesda, Maryland; 3Department of Biostatistics, Epidemiology and Informatics, and 9Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 4Office of Translational Sciences, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland; 5Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California; 6Division of Pulmonary Medicine, Cincinnati Children’s Hospital, Cincinnati, Ohio; 7Genentech Inc., South San Francisco, California; 8Department of Biology, School of Humanities and Sciences, Ithaca College, Ithaca, New York; 10Division of Allergy, Pulmonary and Critical Care Medicine, Department of Medicine, and 21Division of Pulmonary Medicine, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee; 11The Johns Hopkins Hospital, Johns Hopkins University School of Medicine, Baltimore, Maryland; 12Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts; 13Division of Respiratory Disease, Department of Pediatrics, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts; 14Department of Pulmonary and Critical Care Medicine, Stanford University, Stanford, California; 15Department of Medicine and 16Department of Immunology, School of Medicine, University of Colorado, Aurora, Colorado; 17Centre for Heart Lung Innovation at St. Paul’s Hospital, Vancouver, British Columbia, Canada; 18Department of Medicine at the University of British Columbia, Vancouver, British Columbia, Canada; 19Pulmonary, Allergy, Sleep & Critical Care Medicine, Department of Medicine, School of Medicine, Boston University, Boston, Massachusetts; and 20Partners HealthCare Center for Personalized Genetic Medicine, Partners HealthCare Biobank, Boston, Massachusetts.
Footnotes
This research statement was approved by the American Thoracic Society October 2018 and the National Heart, Lung, and Blood Institute September 2018
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.201810-1895ST.
You may print one copy of this document at no charge. However, if you require more than one copy, you must place a reprint order. Domestic reprint orders: amy.schriver@sheridan.com; international reprint orders: louisa.mott@springer.com.
Author Disclosures: J.G. is an employee of Genentech. L.M.N. received royalties from UpToDate for authoring chapters on Genetic Surfactant Dysfunction Disorder. B.A.R. has stocks, stock options, or other equity interests in CureSpark; and served as editor for UpToDate; his spouse has been a blind reader for clinical trial data analysis by Paraxel. D.A.S. served on an advisory committee for NuMedii; received research support from, holds intellectual property, stock, stock options, or other ownership in, and is an employee of Eleven P15; holds intellectual property on patent 8673565 for methods and compositions for risk prediction, diagnosis, prognosis, and treatment of pulmonary disorders; holds intellectual property on patent US 2016/0060701 A1 for methods for predicting risk of interstitial pneumonia; and has two patents pending on the composition and methods of repeating or preventing fibrotic diseases and biomarkers for the diagnosis and treatment of fibrotic diseases. D.D.S. served on an advisory committee for Boehringer Ingelheim; as a consultant for Sanofi and Regeneron Pharmaceuticals; and as a speaker for AstraZeneca and Novartis; and received research support from AstraZeneca, Boehringer Ingelheim, and Merck. A.S. is an employee of Johnson and Johnson; is a founder of Metera Pharmaceuticals Inc.; and served as a consultant for and has stocks, stock options, or other equity interests in Veracyte. L.R.Y. served on an advisory committee for Boehringer Ingelheim; and holds intellectual property on a patent for the use of VEGF-D as a diagnostic test in lymphangioleiomyomatosis. A.C.W., J.P.K., P.J.N., B.E.H., S.A., E.G.B., J.P.C., M.I., T.K.J., J.A.K., J.E.L., A.J.R., and S.T.W. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Behavioral Science and Health Services Research, and the Section on Genetics and Genomics
References
- 1.Nimmesgern E, Benediktsson I, Norstedt I. Personalized medicine in Europe. Clin Transl Sci. 2017;10:61–63. doi: 10.1111/cts.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson SJ, Rehm HL. Building the foundation for genomics in precision medicine. Nature. 2015;526:336–342. doi: 10.1038/nature15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013;5:189sr4. doi: 10.1126/scitranslmed.3005785. [DOI] [PubMed] [Google Scholar]
- 5.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46. doi: 10.1002/cpt.136. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. Genetic testing registry[accessed 2018 Nov 5]. Available from: https://www.ncbi.nlm.nih.gov/gtr/
- 7.Khoury MJ. No shortcuts on the long road to evidence-based genomic medicine. JAMA. 2017;318:27–28. doi: 10.1001/jama.2017.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA-NIH Biomarker Working GroupBEST (Biomarkers, EndpointS, and other Tools) resource. Silver Spring, MD: U.S. Food and Drug Administration. 2016[accessed 2018 Nov 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338448/ [PubMed]
- 9.Institute of Medicine. Evolution of translational omics: lessons learned and the path forward. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 10.Leptak C, Menetski JP, Wagner JA, Aubrecht J, Brady L, Brumfield M, et al. What evidence do we need for biomarker qualification? Sci Transl Med. 2017;9:eaal4599. doi: 10.1126/scitranslmed.aal4599. [DOI] [PubMed] [Google Scholar]
- 11.FDA List of qualified biomarkers 2018[accessed 2018 Nov 5]. Available from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535383.htm
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 13.Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nat Rev Cancer. 2016;16:525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Cancer Genome Atlas Research Network. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma Nature 2014511543–550.[Published erratum appears in Nature 514:262.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst A, Silvestri GA, Johnstone D American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693–1717. doi: 10.1378/chest.123.5.1693. [DOI] [PubMed] [Google Scholar]
- 17.Rivera MP, Mehta AC, Wahidi MM.Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer 3rd edAmerican College of Chest Physicians evidence-based clinical practice guidelinesChest 2013143e142S–e165S. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 24.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 26.Whitney DH, Elashoff MR, Porta-Smith K, Gower AC, Vachani A, Ferguson JS, et al. Derivation of a bronchial genomic classifier for lung cancer in a prospective study of patients undergoing diagnostic bronchoscopy. BMC Med Genomics. 2015;8:18. doi: 10.1186/s12920-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. AEGIS Study Team. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373:243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, et al. Clinical utility of a bronchial genomic classifier in patients with suspected lung cancer. Chest. 2016;150:210–218. doi: 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 29.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1) Suppl:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 31.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Rivas G, Jerjes-Sánchez C, Rodriguez D, Garcia-Pelaez J, Trevino V. A systematic review of genetic mutations in pulmonary arterial hypertension. BMC Med Genet. 2017;18:82. doi: 10.1186/s12881-017-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadinnapola C, Bleda M, Haimel M, Screaton N, Swift A, Dorfmüller P, et al. NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation. 2017;136:2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:390–398. doi: 10.1183/16000617.0077-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan AC, Dusek AC, McMahon TJ. Treatment-related biomarkers in pulmonary hypertension. Am J Respir Cell Mol Biol. 2015;52:663–673. doi: 10.1165/rcmb.2014-0438TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1:250–258. doi: 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemnes AR, Trammell AW, Archer SL, Rich S, Yu C, Nian H, et al. Peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation. 2015;131:401–409, discussion 409. doi: 10.1161/CIRCULATIONAHA.114.013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benza RL, Gomberg-Maitland M, Demarco T, Frost AE, Torbicki A, Langleben D, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1345–1354. doi: 10.1164/rccm.201501-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King T, Costabel U, Cordier J, DoPico G, du Bois R, Lynch D, et al. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 43.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 46.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 47.Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11:1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 48.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191:417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 51.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 52.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 53.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 55.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 57.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med. 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 60.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191:646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47:512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fingerlin TE, Zhang W, Yang IV, Ainsworth HC, Russell PH, Blumhagen RZ, et al. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 2016;17:74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364:1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68:436–441. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 67.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borie R, Crestani B, Dieude P, Nunes H, Allanore Y, Kannengiesser C, et al. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One. 2013;8:e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, et al. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:525–535. doi: 10.1164/rccm.201201-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Rabinovich EI, Kapetanaki MG, Steinfeld I, Gibson KF, Pandit KV, Yu G, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e33770. doi: 10.1371/journal.pone.0033770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang IV, Pedersen BS, Rabinovich E, Hennessy CE, Davidson EJ, Murphy E, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173:188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaminski N. Microarray analysis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3) Suppl:S32–S36. [PubMed] [Google Scholar]
- 77.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68:1114–1121. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cha SI, Ryerson CJ, Lee JS, Kukreja J, Barry SS, Jones KD, et al. Cleaved cytokeratin-18 is a mechanistically informative biomarker in idiopathic pulmonary fibrosis. Respir Res. 2012;13:105. doi: 10.1186/1465-9921-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res. 2012;159:218–227. doi: 10.1016/j.trsl.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 83.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korthagen NM, van Moorsel CH, Barlo NP, Ruven HJ, Kruit A, Heron M, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105:106–113. doi: 10.1016/j.rmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishii H, Mukae H, Kadota J, Kaida H, Nagata T, Abe K, et al. High serum concentrations of surfactant protein A in usual interstitial pneumonia compared with non-specific interstitial pneumonia. Thorax. 2003;58:52–57. doi: 10.1136/thorax.58.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greene KE, King TE, Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 88.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, et al. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med. 2010;134:1136–1142. doi: 10.5858/2009-0144-OA.1. [DOI] [PubMed] [Google Scholar]
- 89.Vuorinen K, Myllärniemi M, Lammi L, Piirilä P, Rytilä P, Salmenkivi K, et al. Elevated matrilysin levels in bronchoalveolar lavage fluid do not distinguish idiopathic pulmonary fibrosis from other interstitial lung diseases. APMIS. 2007;115:969–975. doi: 10.1111/j.1600-0463.2007.apm_697.x. [DOI] [PubMed] [Google Scholar]
- 90.Huh JW, Kim DS, Oh YM, Shim TS, Lim CM, Lee SD, et al. Is metalloproteinase-7 specific for idiopathic pulmonary fibrosis? Chest. 2008;133:1101–1106. doi: 10.1378/chest.07-2116. [DOI] [PubMed] [Google Scholar]
- 91.Prasse A, Pechkovsky DV, Toews GB, Schäfer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–1693. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 92.Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:382–385. doi: 10.1164/rccm.2103112. [DOI] [PubMed] [Google Scholar]
- 93.Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med. 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 94.Furuhashi K, Suda T, Nakamura Y, Inui N, Hashimoto D, Miwa S, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med. 2010;104:1204–1210. doi: 10.1016/j.rmed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 95.Kadota J, Mizunoe S, Mito K, Mukae H, Yoshioka S, Kawakami K, et al. High plasma concentrations of osteopontin in patients with interstitial pneumonia. Respir Med. 2005;99:111–117. doi: 10.1016/j.rmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 96.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loomis-King H, Flaherty KR, Moore BB. Pathogenesis, current treatments and future directions for idiopathic pulmonary fibrosis. Curr Opin Pharmacol. 2013;13:377–385. doi: 10.1016/j.coph.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adamali HI, Maher TM. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel Ther. 2012;6:261–272. doi: 10.2147/DDDT.S29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 100.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 101.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herazo-Maya JD, Sun J, Molyneaux PL, Li Q, Villalba JA, Tzouvelekis A, et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med. 2017;5:857–868. doi: 10.1016/S2213-2600(17)30349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takahashi H, Fujishima T, Koba H, Murakami S, Kurokawa K, Shibuya Y, et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162:1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 105.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3–L7. doi: 10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 107.Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med. 2006;260:429–434. doi: 10.1111/j.1365-2796.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 108.Vuga LJ, Tedrow JR, Pandit KV, Tan J, Kass DJ, Xue J, et al. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:966–974. doi: 10.1164/rccm.201309-1592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tajiri M, Okamoto M, Fujimoto K, Johkoh T, Ono J, Tominaga M, et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir Investig. 2015;53:73–81. doi: 10.1016/j.resinv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187:768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 112.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 113.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG, et al. The Toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:1442–1450. doi: 10.1164/rccm.201304-0760OC. [DOI] [PubMed] [Google Scholar]
- 115.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2:557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ho JE, Gao W, Levy D, Santhanakrishnan R, Araki T, Rosas IO, et al. Galectin-3 is associated with restrictive lung disease and interstitial lung abnormalities. Am J Respir Crit Care Med. 2016;194:77–83. doi: 10.1164/rccm.201509-1753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.National Asthma Education and Prevention Program. Third Expert Panel on the Diagnosis and Management of Asthma. Expert panel report 3: guidelines for the diagnosis and management of asthma - full report 2007. Bethesda, MD: National Heart, Lung and Blood Institute; 2007 [Google Scholar]
- 119.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 120.Bigler J, Boedigheimer M, Schofield JPR, Skipp PJ, Corfield J, Rowe A, et al. U-BIOPRED Study Group‖A severe asthma disease signature from gene expression profiling of peripheral blood from U-BIOPRED cohorts Am J Respir Crit Care Med 20171951311–1320. [DOI] [PubMed] [Google Scholar]
- 121.Li W, Gao P, Zhi Y, Xu W, Wu Y, Yin J, et al. Periostin: its role in asthma and its potential as a diagnostic or therapeutic target. Respir Res. 2015;16:57. doi: 10.1186/s12931-015-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–4966. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bentley JK, Chen Q, Hong JY, Popova AP, Lei J, Moore BB, et al. Periostin is required for maximal airways inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2014;134:1433–1442. doi: 10.1016/j.jaci.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao P, Simpson JL, Zhang J, Gibson PG. Galectin-3: its role in asthma and potential as an anti-inflammatory target. Respir Res. 2013;14:136. doi: 10.1186/1465-9921-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. U.S. Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khakban A, Sin DD, FitzGerald JM, McManus BM, Ng R, Hollander Z, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years: a population-based perspective. Am J Respir Crit Care Med. 2017;195:287–291. doi: 10.1164/rccm.201606-1162PP. [DOI] [PubMed] [Google Scholar]
- 128.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 129.Suissa S. Number needed to treat: enigmatic results for exacerbations in COPD. Eur Respir J. 2015;45:875–878. doi: 10.1183/09031936.00216514. [DOI] [PubMed] [Google Scholar]
- 130.Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax. 2013;68:540–543. doi: 10.1136/thoraxjnl-2012-202709. [DOI] [PubMed] [Google Scholar]
- 131.Roberts CM, Lopez-Campos JL, Pozo-Rodriguez F, Hartl S European COPD Audit team. European hospital adherence to GOLD recommendations for chronic obstructive pulmonary disease (COPD) exacerbation admissions. Thorax. 2013;68:1169–1171. doi: 10.1136/thoraxjnl-2013-203465. [DOI] [PubMed] [Google Scholar]
- 132.Miller BE, Tal-Singer R, Rennard SI, Furtwaengler A, Leidy N, Lowings M, et al. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease: perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016;193:607–613. doi: 10.1164/rccm.201509-1722PP. [DOI] [PubMed] [Google Scholar]
- 133.Sin DD, Hollander Z, DeMarco ML, McManus BM, Ng RT From Discovery to Clinical Implementation. Biomarker development for chronic obstructive pulmonary disease: from discovery to clinical implementation. Am J Respir Crit Care Med. 2015;192:1162–1170. doi: 10.1164/rccm.201505-0871PP. [DOI] [PubMed] [Google Scholar]
- 134.Hollander Z, DeMarco ML, Sadatsafavi M, McManus BM, Ng RT, Sin DD. Biomarker development in COPD: moving from P values to products to impact patient care. Chest. 2017;151:455–467. doi: 10.1016/j.chest.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 135.Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47:1374–1382. doi: 10.1183/13993003.01370-2015. [DOI] [PubMed] [Google Scholar]
- 136.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 137.Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2016;71:118–125. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–398. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 139.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 140.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 141.Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192:958–964. doi: 10.1164/rccm.201502-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 145.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 146.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 147.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 150.Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 151.Sweeney TE, Wong HR, Khatri P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci Transl Med. 2016;8:346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 153.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 154.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guérin C, Reignier J, Richard JC. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;369:980–981. doi: 10.1056/NEJMc1308895. [DOI] [PubMed] [Google Scholar]
- 156.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 157.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 158.Levitt JE, Rogers AJ. Proteomic study of acute respiratory distress syndrome: current knowledge and implications for drug development. Expert Rev Proteomics. 2016;13:457–469. doi: 10.1586/14789450.2016.1172481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. NHLBI ARDS Clinical Trials Network. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. ARDS Network. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spielberg DR, Clancy JP. Cystic fibrosis and its management through established and emerging therapies. Annu Rev Genomics Hum Genet. 2016;17:155–175. doi: 10.1146/annurev-genom-090314-050024. [DOI] [PubMed] [Google Scholar]
- 163.Cystic Fibrosis Foundation Patient Registry2016 Annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2016 [Google Scholar]
- 164.Brewington J, Clancy JP. Diagnostic testing in cystic fibrosis. Clin Chest Med. 2016;37:31–46. doi: 10.1016/j.ccm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 165.Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis. Pediatrics. 1996;97:524–528. [PubMed] [Google Scholar]
- 166.Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. J Cyst Fibros. 2016;15:416–423. doi: 10.1016/j.jcf.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 167.Horsley A, Wild JM. Ventilation heterogeneity and the benefits and challenges of multiple breath washout testing in patients with cystic fibrosis. Paediatr Respir Rev. 2015;16:15–18. doi: 10.1016/j.prrv.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 168.Kent L, Reix P, Innes JA, Zielen S, Le Bourgeois M, Braggion C, et al. European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) Standardisation Committee. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13:123–138. doi: 10.1016/j.jcf.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 169.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, et al. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37:806–812. doi: 10.1183/09031936.00072510. [DOI] [PubMed] [Google Scholar]
- 170.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, et al. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65:379–383. doi: 10.1136/thx.2009.125831. [DOI] [PubMed] [Google Scholar]
- 171.Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. VX14-809-109 Investigator Group. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5:557–567. doi: 10.1016/S2213-2600(17)30215-1. [DOI] [PubMed] [Google Scholar]
- 172.Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1:630–638. doi: 10.1016/S2213-2600(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 173.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143:14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 175.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16:41–44. doi: 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 176.Muhlebach MS, Clancy JP, Heltshe SL, Ziady A, Kelley T, Accurso F, et al. Biomarkers for cystic fibrosis drug development. J Cyst Fibros. 2016;15:714–723. doi: 10.1016/j.jcf.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Szczesniak R, Turkovic L, Andrinopoulou ER, Tiddens HAWM. Chest imaging in cystic fibrosis studies: What counts, and can be counted? J Cyst Fibros. 2017;16:175–185. doi: 10.1016/j.jcf.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 179.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med. 2016;194:748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Radzikowska E, Jaguś P, Sobiecka M, Chorostowska-Wynimko J, Wiatr E, Kuś J, et al. Correlation of serum vascular endothelial growth factor-D concentration with clinical presentation and course of lymphangioleiomyomatosis. Respir Med. 2015;109:1469–1475. doi: 10.1016/j.rmed.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 183.Suzuki T, Trapnell BC. Pulmonary alveolar proteinosis syndrome. Clin Chest Med. 2016;37:431–440. doi: 10.1016/j.ccm.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, et al. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med. 2010;182:49–61. doi: 10.1164/rccm.201001-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, et al. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med. 2009;361:2679–2681. doi: 10.1056/NEJMc0904077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, et al. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods. 2014;402:57–70. doi: 10.1016/j.jim.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Francescatto L, Katsanis N. Newborn screening and the era of medical genomics. Semin Perinatol. 2015;39:617–622. doi: 10.1053/j.semperi.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Green RC, Rehm HL, Kohane IS.Clinical genome sequencing Ginsberg GS, Willard HF.Genomic and personalized medicine2nd edSan Diego: Academic Press; 2013102–122. [Google Scholar]
- 189.Kotze MJ, Lückhoff HK, Peeters AV, Baatjes K, Schoeman M, van der Merwe L, et al. Genomic medicine and risk prediction across the disease spectrum. Crit Rev Clin Lab Sci. 2015;52:120–137. doi: 10.3109/10408363.2014.997930. [DOI] [PubMed] [Google Scholar]
- 190.Mestan KK, Ilkhanoff L, Mouli S, Lin S. Genomic sequencing in clinical trials. J Transl Med. 2011;9:222. doi: 10.1186/1479-5876-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vassy JL, Lautenbach DM, McLaughlin HM, Kong SW, Christensen KD, Krier J, et al. MedSeq Project. The MedSeq Project: a randomized trial of integrating whole genome sequencing into clinical medicine. Trials. 2014;15:85. doi: 10.1186/1745-6215-15-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lazaridis KN, McAllister TM, Babovic-Vuksanovic D, Beck SA, Borad MJ, Bryce AH, et al. Implementing individualized medicine into the medical practice. Am J Med Genet C Semin Med Genet. 2014;166C:15–23. doi: 10.1002/ajmg.c.31387. [DOI] [PubMed] [Google Scholar]
- 193.Berg JS, Powell CM. Potential uses and inherent challenges of using genome-scale sequencing to augment current newborn screening. Cold Spring Harb Perspect Med. 2015;5:a023150. doi: 10.1101/cshperspect.a023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489–494. doi: 10.1016/j.tig.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 196.Bustamante CD, Burchard EG, De la Vega FM. Genomics for the world. Nature. 2011;475:163–165. doi: 10.1038/475163a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, et al. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. 2016;375:655–665. doi: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Phillips KA, Veenstra DL, Ramsey SD, Van Bebber SL, Sakowski J. Genetic testing and pharmacogenomics: issues for determining the impact to healthcare delivery and costs. Am J Manag Care. 2004;10:425–432. [PubMed] [Google Scholar]
- 199.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 200.Gibons A. Study results: employer-based coverage of genetic counseling services. Benefits Q. 2004;20:48–68. [PubMed] [Google Scholar]
- 201.Meckley LM, Neumann PJ. Personalized medicine: factors influencing reimbursement. Health Policy. 2010;94:91–100. doi: 10.1016/j.healthpol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 202.Hresko A, Haga SB. Insurance coverage policies for personalized medicine. J Pers Med. 2012;2:201–216. doi: 10.3390/jpm2040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cohen J, Wilson A, Manzolillo K. Clinical and economic challenges facing pharmacogenomics. Pharmacogenomics J. 2013;13:378–388. doi: 10.1038/tpj.2011.63. [DOI] [PubMed] [Google Scholar]
- 204.Graf MD, Needham DF, Teed N, Brown T. Genetic testing insurance coverage trends: a review of publicly available policies from the largest US payers. Per Med. 2013;10:235–243. doi: 10.2217/pme.13.9. [DOI] [PubMed] [Google Scholar]
- 205.Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann Intern Med. 1998;129:412–416. doi: 10.7326/0003-4819-129-5-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 206.Hargraves JL, Hadley J. The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Serv Res. 2003;38:809–829. doi: 10.1111/1475-6773.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu CY, Loomer S, Ceccarelli R, Mazor KM, Sabin J, Clayton EW, et al. Insurance coverage policies for pharmacogenomic and multi-gene testing for cancer. J Pers Med. 2018;8:E19. doi: 10.3390/jpm8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Trosman JR, Van Bebber SL, Phillips KA. Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract. 2010;6:238–242. doi: 10.1200/JOP.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Degtiar I. A review of international coverage and pricing strategies for personalized medicine and orphan drugs. Health Policy. 2017;121:1240–1248. doi: 10.1016/j.healthpol.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 210.Issa AM, Tufail W, Hutchinson J, Tenorio J, Baliga MP. Assessing patient readiness for the clinical adoption of personalized medicine. Public Health Genomics. 2009;12:163–169. doi: 10.1159/000189629. [DOI] [PubMed] [Google Scholar]
- 211.O’Daniel J, Lucas J, Deverka P, Ermentrout D, Silvey G, Lobach DF, et al. Factors influencing uptake of pharmacogenetic testing in a diverse patient population. Public Health Genomics. 2010;13:48–54. doi: 10.1159/000217795. [DOI] [PubMed] [Google Scholar]
- 212.Weldon CB, Trosman JR, Gradishar WJ, Benson AB, III, Schink JC. Barriers to the use of personalized medicine in breast cancer. J Oncol Pract. 2012;8:e24–e31. doi: 10.1200/JOP.2011.000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rogausch A, Prause D, Schallenberg A, Brockmöller J, Himmel W. Patients’ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics. 2006;7:49–59. doi: 10.2217/14622416.7.1.49. [DOI] [PubMed] [Google Scholar]
- 214.Issa AM, Hutchinson JF, Tufail W, Fletcher E, Ajike R, Tenorio J. Provision of personalized genomic diagnostic technologies for breast and colorectal cancer: an analysis of patient needs, expectations and priorities. Per Med. 2011;8:401–411. doi: 10.2217/pme.11.39. [DOI] [PubMed] [Google Scholar]
- 215.Haga SB, O’Daniel JM, Tindall GM, Lipkus IR, Agans R. Survey of US public attitudes toward pharmacogenetic testing. Pharmacogenomics J. 2012;12:197–204. doi: 10.1038/tpj.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.DeFrank JT, Salz T, Reeder-Hayes K, Brewer NT. Who gets genomic testing for breast cancer recurrence risk? Public Health Genomics. 2013;16:215–222. doi: 10.1159/000353518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Armstrong K, Putt M, Halbert CH, Grande D, Schwartz JS, Liao K, et al. The influence of health care policies and health care system distrust on willingness to undergo genetic testing. Med Care. 2012;50:381–387. doi: 10.1097/MLR.0b013e31824d748b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lipkus IM, Vadaparampil ST, Jacobsen PB, Miree CA. Knowledge about genomic recurrence risk testing among breast cancer survivors. J Cancer Educ. 2011;26:664–669. doi: 10.1007/s13187-011-0248-5. [DOI] [PubMed] [Google Scholar]
- 219.Tzeng JP, Mayer D, Richman AR, Lipkus I, Han PK, Valle CG, et al. Women’s experiences with genomic testing for breast cancer recurrence risk. Cancer. 2010;116:1992–2000. doi: 10.1002/cncr.24990. [DOI] [PubMed] [Google Scholar]
- 220.Lillie SE, Brewer NT, O’Neill SC, Morrill EF, Dees EC, Carey LA, et al. Retention and use of breast cancer recurrence risk information from genomic tests: the role of health literacy. Cancer Epidemiol Biomarkers Prev. 2007;16:249–255. doi: 10.1158/1055-9965.EPI-06-0525. [DOI] [PubMed] [Google Scholar]
- 221.Wu AC, Mazor KM, Ceccarelli R, Loomer S, Lu CY. Access to guideline-recommended pharmacogenomic tests for cancer treatments: experience of providers and patients. J Pers Med. 2017;7:E17. doi: 10.3390/jpm7040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miller FA, Krueger P, Christensen RJ, Ahern C, Carter RF, Kamel-Reid S. Postal survey of physicians and laboratories: practices and perceptions of molecular oncology testing. BMC Health Serv Res. 2009;9:131. doi: 10.1186/1472-6963-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomarkers. 2013;17:219–225. doi: 10.1089/gtmb.2012.0165. [DOI] [PubMed] [Google Scholar]
- 224.National Human Genome Research Institute. Inter-society Coordinating Committee for Practitioner Education in Genomics (ISCC) 2018. [accessed 2018 Nov 5]. Available from: https://www.genome.gov/27554614/intersociety-coordinating-committee-for-practitioner-education-in-genomics-iscc/
- 225.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–D985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, et al. ClinGen. ClinGen--the Clinical Genome Resource. N Engl J Med. 2015;372:2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]