To the Editor:
Immune checkpoint inhibitors (ICIs) have emerged as a promising treatment modality for patients with non–small cell lung cancer (NSCLC). However, ICI monotherapy has a relatively low response rate (approximately 20–50% in patients with NSCLC) (1), and consequently it is important to develop predictive biomarkers of response to inform clinical decisions regarding ICI use. Although PD-L1 expression on tumor cells is currently used as a predictive determinant for anti–PD-1 therapy responses, the accuracy of this test is relatively poor. Therefore, better predictive biomarkers for anti–PD-1 therapy in patients with NSCLC are needed.
Myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) play crucial immune-suppressive roles in patients with cancer (2). In addition to promoting tumor growth, the suppressive actions of MDSCs and Tregs hinder the efficacy of cancer immunotherapy. Therefore, we hypothesized that the frequency of immune-suppressive cell subsets might be correlated with the response to anti–PD-1 therapy. The fact that MDSCs and Tregs can be easily detected and affordably quantified in the peripheral blood of patients with NSCLC makes them excellent candidates for predictive biomarkers of ICIs.
To identify correlations between suppressive-cell subsets and the response to anti–PD-1 therapy, we longitudinally analyzed the frequency of immune-suppressive cells, including Tregs and MDSCs, in the blood of patients with NSCLC in an exploratory cohort (n = 34) before and after nivolumab treatment. The data were validated in an independent cohort of patients with NSCLC (n = 29). We prospectively enrolled patients with NSCLC who had failed platinum-based chemotherapy.
Patients were categorized as responders (complete response, partial response, or stable disease for more than 6 mo) or nonresponders, and blood samples were collected both before and after the first nivolumab treatment (3 mg/kg every 2 wk). The frequency of Tregs and polymorphonuclear (PMN)-MDSCs, which are the dominant population of MDSCs in patients with NSCLC, was quantified using flow cytometry. Expression of the lectin-type oxidized LDL receptor-1 (Lox-1) in PMN-MDSCs was additionally analyzed to distinguish PMN-MDSCs from neutrophils (3). At baseline, the percentage of Tregs was higher in responders than in nonresponders, but there was no significant difference in the frequency of Lox-1+ PMN-MDSCs (not shown). After the first treatment, the median percentage of Tregs was also higher in responders than in nonresponders, whereas the median percentage of Lox-1+ PMN-MDSCs was significantly lower in responders than in nonresponders (not shown). Interestingly, an inverse correlation was observed between the percentage of Tregs and Lox-1+ PMN-MDSCs (not shown).
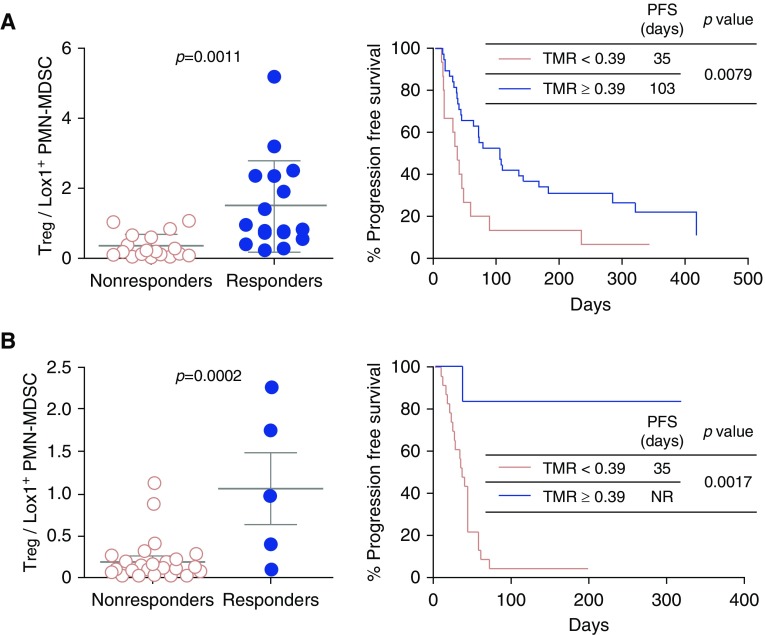
To optimize the cutoff value, we evaluated the ratio of Tregs to Lox-1+ PMN-MDSCs (abbreviated as TMR). The difference between the TMRs of responders and nonresponders was significantly greater than the frequency of either Tregs or Lox-1+ PMN-MDSCs alone, with an area under the receiver operator characteristic curve of 87% (not shown). Patients with a TMR ≥ 0.39 (the cutoff value that optimized both sensitivity and specificity) had a likelihood ratio for being a responder of 3.17. The sensitivity and specificity of using this cutoff to predict response was 87.5% (95% confidence interval, 63.5–98.5%) and 72.2% (95% confidence interval, 46.5–90.3%), respectively. Moreover, patients with a TMR ≥ 0.39 had significantly longer median progression-free survival (103 vs. 35 d; P = 0.0079) than those with a TMR < 0.39 (Figure 1A). The validation cohort results confirmed that the TMR predicts the treatment outcome of nivolumab in patients with NSCLC (Figure 1B). To further evaluate the performance of the TMR as a predictive marker for anti–PD-1 therapy, we analyzed all of the available data (n = 63). The positive and negative predictive values (cutoff TMR, 0.39) were 69.2% and 91.8%, respectively.
Figure 1.
Quantification of peripheral immune-suppressive cells after the first anti–PD-1 therapy in the exploration (A) and validation (B) cohort of patients with non–small cell lung cancer. Left panel: the Treg-to-Lox-1+ polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC) ratio (TMR) was compared between nonresponders and responders. Right panel: progression-free survival (PFS) was analyzed by cutoff value 0.39 (right panels) in the exploration (A) and validation (B) cohorts. PFS was measured from the first day of anti–PD-1 therapy to tumor progression or death. Patients were censored on June 5, 2018, if alive and progression free. NR = not reached; Treg = regulatory T cells.
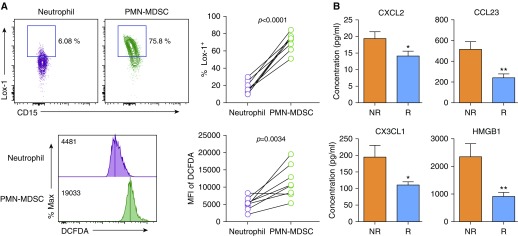
To confirm the presence of PMN-MDSCs, Lox-1 expression and reactive oxygen species (ROS) production were analyzed in PMN-MDSCs compared with neutrophils. Lox-1 was previously reported to be a PMN-MDSC–specific marker expressed on immune-suppressive PMN-MDSCs but not on neutrophils (3). High ROS production is one of the immune-suppression mechanisms in PMN-MDSCs (4). In our patients, Lox-1 was prominently expressed on PMN-MDSCs compared with neutrophils (Figure 2A). Moreover, the level of ROS production was significantly higher in PMN-MDSCs than in neutrophils (Figure 2A), indicating that the PMN-MDSCs in our study showed common features of PMN-MDSCs that can be distinguished from neutrophils.
Figure 2.
Characterization of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) in patients with non–small cell lung cancer. (A) Comparison of Lox-1 levels and reactive oxygen species production between PMN-MDSCs and neutrophils in peripheral blood of patients with non–small cell lung cancer. PMN-MDSCs were isolated from peripheral blood mononuclear cells, and neutrophils were isolated from the pellet after density gradient centrifugation of blood. Reactive oxygen species production was measured using the oxidation-sensitive dye dichlorodihydrofluorescein diacetate (DCFDA). (B) Analysis of chemokines and HMGB1 in the plasma of patients with NSCLC after the first therapy. *P < 0.05, **P < 0.01 (two-tailed Student’s t test). MFI = mean fluorescence intensity; NR = nonresponders; R = responders.
To elucidate the mechanism involved in the response to nivolumab, we analyzed 40 cytokines and chemokines in plasma from patients after the first nivolumab treatment by multiplex immunoassay. The levels of three chemokines (CXCL2, CCL23, and CX3CL1) and HMGB1 were significantly higher in nonresponders than in responders (Figure 2B). CXCL2, CCL23, and CX3CL1 were previously reported to promote the recruitment of MDSCs (5–7), and HMGB1 is also involved in MDSC differentiation (8).
Accumulation of MDSCs is linked to a poor prognosis in patients with NSCLC (9), but their specific role in anti–PD-1 therapy has not been studied. Our data demonstrate that factors involved in MDSC proliferation and recruitment are markedly higher in nonresponders than in responders after anti–PD-1 therapy, which might impair the efficacy of anti–PD-1 therapy. Lox-1+ PMN-MDSC numbers increased with anti–PD-1 therapy in nonresponders, suggesting that Lox-1+ PMN-MDSCs are a specific subset of MDSCs with immunosuppressive function in patients with NSCLC.
Interestingly, the frequency of peripheral Tregs was significantly higher in responders than in nonresponders. The presence of Tregs in tumors has been associated with poor survival, but a recent report demonstrated that a higher frequency of circulating Tregs is associated with a favorable response to anti–PD-1 therapy (10). In addition, we observed that tumor Treg numbers were inversely correlated with peripheral Treg numbers in the mouse tumor model (not shown). These data support our observation of high Treg numbers in peripheral blood associated with a favorable response to anti–PD-1 therapy.
In conclusion, the TMR in the blood after the first nivolumab treatment predicts the early response in patients with NSCLC and may provide clinicians with vital information concerning whether to continue or stop further anti–PD-1 therapy.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Soonmyung Paik for valuable advice regarding their research.
Footnotes
Supported by grants from the Basic Science Research Program through the National Research Foundation of the Republic of Korea (2015R1C1A1A01054596, 2012R1A5A2A44671346, 2017R1D1A1B03029874, 2017R1A5A1014560, 2018M3A9H3024850, 2017M3A9E9072669, and 2017M3A9E8029717) and the Research Resettlement Fund for the New Faculty of Seoul National University.
Author Contributions: Conception and design: H.R.K, B.C.C, S.-Y.S., S.-J.H., and J.-I.Y. Collection and assembly of data: H.R.K. and S.-M.P. Data analysis and interpretation: H.R.K., S.-M.P., S.-U.S., I.J., H.I.Y., D.I.G., B.C.C., S.-Y.S., S.-J.H., and J.-I.Y. Manuscript writing: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201808-1502LE on October 19, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aaf8943. pii: aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan HH, Jiang J, Pang Y, Achyut BR, Lizardo M, Liang X, et al. CCL9 induced by TGFβ signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer Res. 2015;75:5283–5298. doi: 10.1158/0008-5472.CAN-15-2282-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36:2095–2104. doi: 10.1038/onc.2016.367. [DOI] [PubMed] [Google Scholar]
- 7.Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. OncoImmunology. 2015;5:e1062208. doi: 10.1080/2162402X.2015.1062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Sun J, Rong R, Li L, Shang W, Song D, et al. HMGB1 promotes myeloid-derived suppressor cells and renal cell carcinoma immune escape. Oncotarget. 2017;8:63290–63298. doi: 10.18632/oncotarget.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamauchi Y, Safi S, Blattner C, Rathinasamy A, Umansky L, Juenger S, et al. Circulating and tumor myeloid-derived suppressor cells in resectable non-small cell lung cancer. Am J Respir Crit Care Med. 2018;198:777–787. doi: 10.1164/rccm.201708-1707OC. [DOI] [PubMed] [Google Scholar]
- 10.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.