Abstract
Background:
Measurement results provided by blood glucose monitoring systems (BGMS) can be affected by various influencing factors. For some BGMS using glucose oxidase (GOx)-based test strips, one of these factors is the oxygen partial pressure (pO2) of the applied blood sample. Because assessing the potential influence of pO2 when measuring capillary blood samples is not straight-forward, we performed a proof of concept study.
Method:
Influence of pO2 was investigated for two GOx-based BGMS (BGMS A and B). Measurement results of the GOx-based BGMS were compared with measurement results from a pO2-independent BGMS (BGMS C). A total of 119 samples from 60 subjects were measured, twice with BGMS C, then 6 times each with BGMS A and BGMS B or vice versa, and again twice with BGMS C. Immediately afterward, pO2 was determined. Linear regression analysis based on relative differences between results from BGMS A or BGMS B and results from BGMS C was performed to estimate the degree of pO2 influence.
Results:
The relative bias between the lowest and highest pO2 values differed by 14.3% for BGMS A, indicating a pO2 influence that might be clinically relevant, and by 9.7% for BGMS B, indicating that pO2 influence may be too small to be reliably detected because of the BGMS’ imprecision.
Conclusions:
This proof of concept study showed that with the procedures used, a potentially clinically relevant influence of pO2 in capillary blood samples on GOx-based BGMS could be detected. Further larger-scale studies are needed to verify this influence.
Keywords: blood glucose monitoring system, glucose oxidase, partial pressure of oxygen, self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is of continuing relevance for people with diabetes. As therapeutic decisions regarding insulin dosing and carbohydrate intake are often made based on measured glucose concentrations, the analytical performance of the blood glucose monitoring systems (BGMS) has an impact on the therapeutic outcome.
BGMS measurements are based on chemical reactions which, depending on the respective composition of enzymes, mediators and further components, can be influenced by various factors, for example, interfering substances present in the blood sample. Whereas glucose dehydrogenase (GDH), one of the two enzymes commonly utilized in BGMS’ reagent systems, is not affected by the partial pressure of oxygen (pO2) present in the applied blood sample, glucose oxidase (GOx) is known to be prone to oxygen interference, that is, elevated or decreased pO2 levels may cause measurement bias.1-4 The oxygen dependence of GOx-based BGMS can be minimized combining the enzyme with suitable mediators.1,3 Nevertheless part of the GOx-based BGMS available on the market is evidently influenced by pO2.5-9 ISO 15197:2013 stipulates that an interference effect exceeding 10 mg/dl or 10% at glucose concentrations <100 mg/dl or ≥100 mg/dl, respectively, shall be described in the instructions for use.10 In previous studies, we investigated the influence of different pO2 levels on BG measurements with GOx-based BGMS in laboratory settings using venous blood.8,9 Part of the tested BGMS showed considerable measurement bias at decreased or elevated pO2, irrespective of being labeled as pO2-dependent or not. The majority of BGMS labeled to be influenced by pO2 only refer to elevated oxygen levels, for example, as expected in patients undergoing oxygen therapy.7 However, the results of the studies mentioned above indicate that the effect of decreased pO2 levels might even be more relevant than the effect observed with elevated levels. Whereas high oxygen levels can lead to an underestimation of glucose concentrations, affected BGMS may overestimate glucose concentrations at decreased pO2. Low pO2 levels can be expected, for example, in elderly people or in patients with respiratory diseases but also during long distance flights or when staying at high altitude.11-18
In order to assess whether the effects observed in the laboratory studies using venous blood are relevant also for SMBG measurements, pO2 influence has to be investigated in capillary blood samples measured directly from the skin puncture site. Such studies, however, have to take into account a number of issues, like the limited volume of capillary blood that may be obtained from one skin puncture or the availability of subjects with extreme pO2. We therefore set up a proof of concept study with the objective to investigate the possible interfering effect of pO2 on GOx-based BGMS in general, irrespective of the effective magnitude of measured pO2 values.
Methods
The study was performed at the Institut für Diabetes-Technologie, Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IDT) in Germany in March 2018 in compliance with the German Medical Devices Act and with requirements of Good Clinical Practice (DIN EN ISO 14155:2012). The study was approved by the responsible Ethics Committee and exempted from approval by the German Federal Institute for Drugs and Medical Devices. The study was registered at DRKS—Deutsches Register Klinischer Studien (DRKS-ID: DRKS00014229), an approved Primary Register in the World Health Organization’s network of clinical trial registries.
Study Population
Sixty subjects were included in the evaluation (28 men, 32 women; mean age, 61.6 [range 23-82] years; 15 people with diabetes type 1, 29 with diabetes type 2, 16 people without diabetes). In order to cover a reasonably wide pO2 range, subjects with respiratory diseases or elderly people were preferred because of suspected lower capillary pO2 levels. All participants signed informed consent forms prior to the study procedures. A physician reviewed the subjects’ anamnesis and medication and checked for interfering substances indicated in the BGMS’ instructions for use. The subjects’ hematocrit values (35.0-51.0%) were verified to be within the ranges given for each BGMS.
Blood Glucose Monitoring Systems
In this study, influence of pO2 on measurement results was investigated for two GOx-based BGMS (BGMS A and B) that had shown marked pO2 influence in a previous study9 with one reagent system lot each. These two BGMS are only labeled to be sensitive to increased blood oxygen content (eg, during oxygen therapy). A GDH-based BGMS (BMGS C) was included in the investigation as pO2-independent comparison BGMS.
The BGMS were purchased from pharmacies. All systems were stored, used and maintained as stipulated in the respective manufacturer’s instructions. Control measurements were performed on each study day to ensure the proper function of each test meter and the integrity of each test strip vial used on that day.
Study Procedures
The evaluation was performed by trained study personnel in a laboratory setting in which the room temperature and humidity were checked to be within the range indicated in the manufacturer’s labelling (temperature: 21.0-23.9°C, humidity: 33.6-49.6%).
All measurements were performed on capillary blood obtained from the subject’s finger tips by skin puncture.
Measurement Procedure for Each Subject
Study personnel punctured a finger from the subject’s left hand and performed two measurements using two meters of the GDH-based comparison BGMS C, followed by six measurements with the GOx-based BGMS A (using six meters), six measurements with the GOx-based BGMS B (using six meters) and two measurements again with two meters of BGMS C. Immediately after measurements with the BGMS, one sample was taken for the determination of pO2. The pO2 was measured using a blood gas analyzer (OPTI™ CCA-TS Analyzer, OPTI Medical Systems, Inc, Roswell, GA, USA).
Subsequently two samples were collected for glucose measurements with a hexokinase-based laboratory analyzer (Cobas Integra® 400 plus, Roche Instrument Center, Rotkreuz, Switzerland).
Samples for all blood glucose (BG) measurements with the BGMS and for the pO2 measurements had to be obtained from a single skin puncture.
For each subject, this measurement procedure was repeated using a finger from the right hand. The sequence of the GOx-based BGMS was changed between left and right hands of each subject and from subject to subject.
Data Analysis
As the results of left and right hands of one subject did not show systematic differences, data sets from both hands were included in the evaluation as independent data sets if data from both hands were found to be valid. Thus, in total 119 data sets were included in the evaluation. Fifty-nine subjects provided data sets from both hands. One subject provided only one valid data set; data from the left hand had to be excluded because more than one finger prick was required for the measurements with the BGMS and blood gas analyzer.
Data analysis was performed in mg/dl. For BGMS displaying results in mmol/l, values were converted (1 mmol/l = 18.02 mg/dl).
The included samples had glucose concentrations between 84.6 and 340.1 mg/dl, measured pO2 values covered a range of 52.0-85.0 mmHg (Table 1).
Table 1.
BG Concentrations (Determined With Laboratory Analyzer) and pO2 Values Measured in 119 Included Data Sets.
| Parameter | Mean | SD | Minimum | 1st quartile | Median | 3rd quartile | Maximum |
|---|---|---|---|---|---|---|---|
| pO2 (mmHg) | 67.1 | 7.5 | 52.0 | 61.5 | 67.0 | 72.5 | 85.0 |
| Glucose (mg/dl) | 139.2 | 48.6 | 84.6 | 105.8 | 127.7 | 156.4 | 340.1 |
For the GOx-based BGMS A and B, mean values of the 6 measurements were calculated, whereas for the comparison BGMS C mean values were calculated from 4 measurements (2 measurements performed before and after the measurements with BGMS A and B). Relative biases were determined between mean values obtained with BGMS A and C and between mean values obtained with B and C to estimate pO2 influence in BGMS A and BGMS B, respectively. As a linear relationship was observed between these biases and the measured pO2 levels, linear regression equations were applied in order to estimate the extent of pO2 influence calculating the absolute value of the difference in bias between the samples with the most extreme pO2 values.
Statistical Considerations
The relevance of the pO2 influence was assessed based on the following statistical considerations: Regarding other influencing factors, ISO 15197:201310 stipulates that influence quantities should be labeled in the instruction for use if the induced change in glucose measurement results exceeds 10 mg/dl or 10% (for BG concentration <100 mg/dl or ≥100 mg/dl). With respect to hematocrit, ISO 15197:201310 stipulates that hematocrit influence should be labeled if hematocrit’s effect on glucose measurement results exceeds 10 mg/dl or 10% (for BG concentration <100 mg/dl or ≥100 mg/dl) with respect to a specific midlevel hematocrit (substitute for an average hematocrit).
Therefore, if the systematic measurement difference between the most extreme pO2 values in the study exceeds 20%, at least one pO2 value can be found for which the systematic measurement differences between glucose measurements at that pO2 value and glucose measurements at both ends of the pO2 range will be at least 10% at the same time (corresponding to hematocrit criteria). In addition, the BGMS’ analytical imprecision has to be accounted for. In this study, the 97.5% quantiles for the coefficient of variation calculated from 6 (systems A and B) or 2 (system C) replicate measurements were found to be 5.69% for system A, 6.48% for system B, and 3.99% for system C. Thus, a difference in bias between the most extreme pO2 values exceeding 10%, is highly likely not to be found due to random error. In contrast, a difference in bias below 10% would indicate an effect of pO2 influence too small to be reliably detected.
Results
In order to estimate the relevance of pO2 influence for GOx-based BGMS, the absolute difference in relative bias (GOx-based BGMS versus GDH-based comparison BGMS) between the most extreme pO2 values obtained in the study was calculated using linear regression.
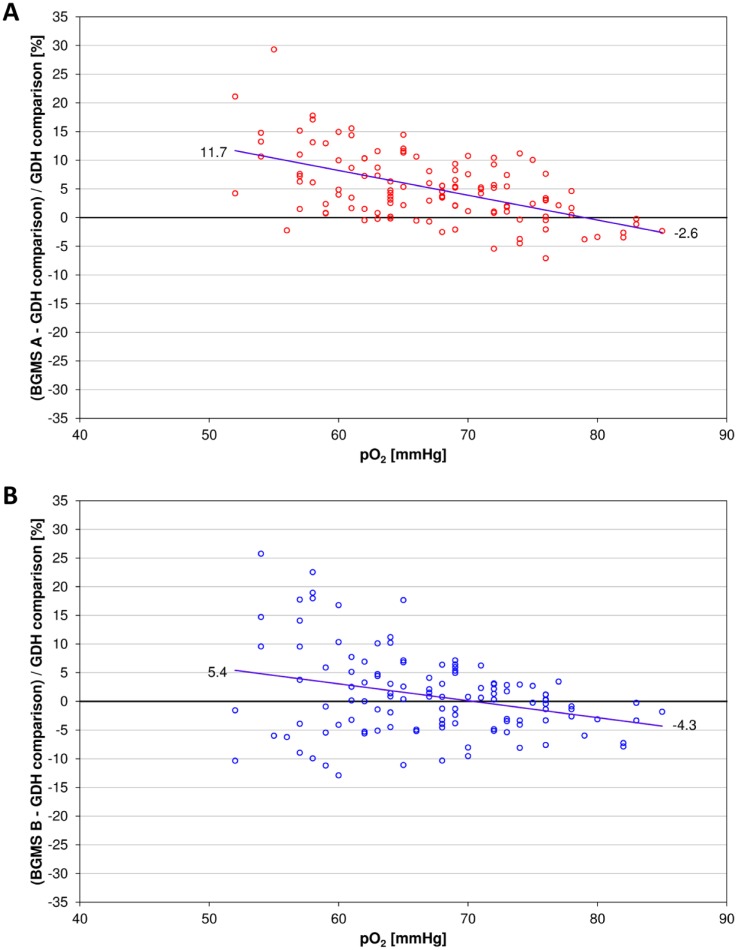
For BGMS A the relative bias at the lowest and highest pO2 value as determined from the regression equation was 11.7% and −2.6%, respectively (Figure 1A). According to the considerations described above, the resulting absolute difference in relative bias of 14.3% indicated a detectable pO2 influence that might be clinically relevant. Relative biases at the lowest and highest pO2 values calculated from the regression equation of BGMS B were 5.4% and −4.3%, respectively (Figure 1B). The difference in relative bias was 9.7%, indicating a pO2 influence, which is possibly too small to be reliably detected. Although it is close to the borderline of 10% difference in biases set for this study, the clinical relevance could not be finally assessed.
Figure 1.
Relative biases between BGMS A (A) and B (B) and GDH-based BGMS C plotted versus pO2. The regression line is represented as blue line. The mean bias is indicated at the lowest and highest pO2 values, respectively.
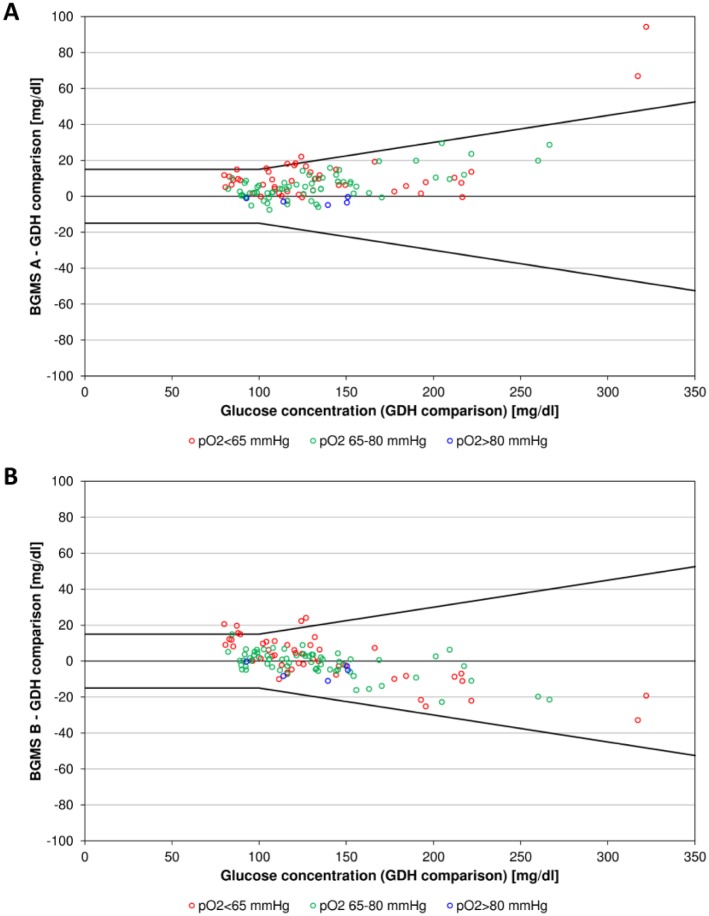
In order to visualize a possible effect of varying glucose concentrations on pO2 influences, samples were categorized by 3 ranges of different pO2 levels (<65 mmHg, 65-80 mmHg, >80 mmHg) and represented with different colors in difference plots (Figure 2). For both GOx-based BGMS low glucose concentration samples seem to show stronger pO2 influence in terms of a larger bias. However, more data are required, especially at low and high glucose concentrations, to suitably assess this possible dependence of the pO2 influence from glucose concentrations.
Figure 2.
Difference plots showing biases between mean glucose concentrations measured with GOx-based BGMS A (A) and B (B) and pO2-independent comparison BGMS C plotted versus glucose concentrations measured with BGMS C. Data points are colored depending on the category in which the sample’s pO2 value fell (<65 mmHg, 65-80 mmHg, >80 mmHg). The black lines indicating differences from the comparison measurements of ±15 mg/dl at glucose concentrations <100 mg/dl and ±15% at glucose concentrations ≥100 mg/dl are included for visual guidance.
Discussion
In this proof of concept study, we investigated the possible influence of pO2 in capillary blood samples on measurements with GOx-based BGMS. Two BGMS were selected for the study that had shown marked pO2 influence in a previous study using venous blood.9
The pO2 influence was estimated comparing the differences in relative bias between the two GOx-based BGMS and a pO2-independent comparison BGMS at the lowest and highest pO2 levels observed during the study. The resulting differences were 14.3% for BGMS A and 9.7% for BGMS B. According to the statistical considerations made for the study, the result for BGMS A indicates a detectable pO2 influence which might be clinically relevant. In BGMS B, pO2 influence is close to the borderline of 10% difference in biases set for this study and is therefore possibly too small to be reliably detected.
Dependence of pO2 influence from glucose concentrations may be suspected, because low glucose concentration samples seemingly showed slightly larger pO2 influence for both GOx-based BGMS in this study.
Even in populations without acute serious diseases, capillary blood samples can cover a broad range of pO2 values.19 In addition, certain groups of patients may commonly show more extreme pO2 levels. Elevated pO2 may occur, for example, in patients undergoing oxygen therapy, whereas decreased pO2 values can be expected, for example, in patients with respiratory diseases, in elderly people or also at high altitude and during long distance flights.7,11-18 Considering that chronic obstructive pulmonary disease (COPD) is described as being associated with type 2 diabetes and considering that the still increasing prevalence of diabetes mainly affects elderly people, BG measurements of a significant number of patients using SMBG might be affected by measurement deviations caused by decreased pO2 when using oxygen-sensitive BGMS.20,21 When measuring blood samples with low pO2, these BGMS tend to overestimate BG concentration and therefore patients might be at risk of not detecting hypoglycemia, especially if pO2 influence was more pronounced at low glucose levels, as suspected from results obtained in this study.
This investigation was designed as a proof of concept study with a limited number of subjects to assess whether the methodical approach was suitable for investigation of pO2 influence on BGMS. Although this proof of concept study indicated that pO2 influence might be relevant for oxygen sensitive GOx-based BGMS, the study entails several limitations that have to be taken into account when interpreting this study’s results. On the one hand the subjects’ BG concentrations did neither adequately cover the BGMS’ measuring ranges nor the low / hypoglycemic range which could be particularly relevant with affected BGMS overestimating BG at decreased pO2.8,9 As the study population consisted of subjects without severe acute or chronic diseases (besides diabetes), the range of pO2 values obtained during the study was probably tighter than the range typically found among users of BGMS.
Another limitation that should be kept in mind is that the study setting only allows the investigation of a potential influence of pO2 in general. The relevant pO2 value in the blood sample for a patient’s SMBG measurement cannot be assessed due to methodical reasons, as the blood volume required for pO2 determination in this study was substantially larger than the volume needed for SMBG (≥60 µl compared to usually <1 µl). The pO2 was obtained after the measurements with the BGMS to minimize the blood volume removed before the individual BGMS measurements, and, therefore, to perform BGMS measurements in conditions as similar as possible to patient SMBG measurements. In addition, wiping blood off the fingertip between measurements with different meters of a BGMS was avoided (whenever possible). For the same reason, samples measured with the GDH-based BGMS immediately before and after the measurements with the GOx-based BGMS were used for comparison measurements to enable accounting for glucose changes, and the samples for the laboratory analyzer, which require a comparably large blood volume, were taken at the end of the measurement series. Therefore, as the study focused on the relevance of low pO2 values, the pO2 used in this study is expected to be a conservative estimate for the pO2 relevant for each of the BGMS measurements included in the evaluation.
Finally, potential interfering effects of decreased or increased pO2 levels do not relate to all GOx-based BGMS, but are limited to the subgroup of oxygen-sensitive systems. In addition, it has to be mentioned that numerous other factors (eg, interfering drugs, hematocrit, ambient temperature) can affect SMBG measurements, irrespective of the BGMS being based on GOx or GDH.
Besides indicating a possible relevance of pO2 influence in SMBG measurements, this study confirmed adequacy of the applied procedures for an assessment of pO2 influences on BGMS using capillary blood samples in a straightforward setting. However, taking into account the possible clinical impact of pO2 influence on SMBG, a further investigation of the indicated relevance of pO2 influences on oxygen-sensitive BGMS, including the objective of dependence of pO2 influence from glucose concentrations, should be considered. For this purpose, larger scale studies are needed to generate valid conclusions. These evaluations should be performed on a sufficiently large number of subjects and focus on representative distributions of glucose concentration and pO2 values. Because of the suspected relevance of decreased pO2 levels, preferably including subjects with respiratory diseases, for example, COPD should be considered. In case the relevance of the suspected pO2 influence on oxygen-sensitive GOx-based BGMS will be verified, further studies might also address the impact of varying pO2 on continuous glucose monitoring (CGM) systems if their sensors are based on GOx.22
Conclusion
The results of this proof of concept study indicate a detectable influence of pO2 on oxygen-sensitive GOx-based BGMS. As this interference effect might be clinically relevant, additional studies should be performed in order to further characterize the pO2 influence. Relevant information regarding pO2 influence, as well as information about all other possibly interfering factors, should be included in the respective BGMS’ instructions for use, to enable patients and health care providers to choose adequate BGMS for specific health conditions.
Acknowledgments
The authors would like to thank the subjects who participated in the study as well as Manuela Link, MD, Eva Zschornack, MD, Martina Tesar, Natalie Neuburger, Tuba Alkan, and other IDT staff who contributed to the conduct of the study. The study was registered at DRKS—Deutsches Register Klinischer Studien (DRKS-ID: DRKS00014229).
Footnotes
Abbreviations: BG, blood glucose; BGMS, blood glucose monitoring system; CGM, continuous glucose monitoring; COPD, chronic obstructive pulmonary disease; GDH, glucose dehydrogenase; GOx, glucose oxidase; ISO, International Organization for Standardization; pO2, partial pressure of oxygen; SD, standard deviation; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB, SP, NJ, CL, and CH are employees of the IDT. RH is employee of Roche Diabetes Care GmbH, Mannheim, Germany. GF is general manager of the IDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Bayer, Dexcom, LifeScan, Menarini Diagnostics, Metronom Health, Novo Nordisk, Roche, Sanofi, Sensile and Ypsomed.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The performance of this investigator-initiated study was supported by Roche Diabetes Care GmbH with a grant.
ORCID iDs: Stefan Pleus  https://orcid.org/0000-0003-4629-7754
https://orcid.org/0000-0003-4629-7754
Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
References
- 1. Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10:10-26. [Google Scholar]
- 2. Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814-825. [DOI] [PubMed] [Google Scholar]
- 3. Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel). 2010;10:4558-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaubey A, Malhotra BD. Mediated biosensors. Biosens Bioelectron. 2002;17:441-456. [DOI] [PubMed] [Google Scholar]
- 5. Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2:349-362. [DOI] [PubMed] [Google Scholar]
- 6. Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124:257-266. [DOI] [PubMed] [Google Scholar]
- 7. Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29:1062-1070. [DOI] [PubMed] [Google Scholar]
- 8. Baumstark A, Schmid C, Pleus S, Haug C, Freckmann G. Influence of partial pressure of oxygen in blood samples on measurement performance in glucose-oxidase-based systems for self-monitoring of blood glucose. J Diabetes Sci Technol. 2013;7:1513-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmid C, Baumstark A, Pleus S, Haug C, Tesar M, Freckmann G. Impact of partial pressure of oxygen in blood samples on the performance of systems for self-monitoring of blood glucose. Diabetes Technol Ther. 2014;16:156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International Organization for Standardization. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2013. [Google Scholar]
- 11. Blom H, Mulder M, Verweij W. Arterial oxygen tension and saturation in hospital patients: effect of age and activity. BMJ. 1988;297:720-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia RF, Borderias CL, Casanova MC, et al. Air travel and respiratory diseases. Arch Bronconeumol. 2007;43:101-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Connor TM, Barry PJ, Jahangir A, Finn C, Buckley BM, El-Gammal A. Comparison of arterial and venous blood gases and the effects of analysis delay and air contamination on arterial samples in patients with chronic obstructive pulmonary disease and healthy controls. Respiration. 2011;81:18-25. [DOI] [PubMed] [Google Scholar]
- 14. Coker RK, Partridge MR. What happens to patients with respiratory disease when they fly? Thorax. 2004;59:919-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seccombe LM, Kelly PT, Wong CK, Rogers PG, Lim S, Peters MJ. Effect of simulated commercial flight on oxygenation in patients with interstitial lung disease and chronic obstructive pulmonary disease. Thorax. 2004;59:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mays EE. An arterial blood gas diagram for clinical use. Chest. 1973;63:793-800. [DOI] [PubMed] [Google Scholar]
- 17. Ak A, Ogun CO, Bayir A, Kayis SA, Koylu R. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2006;210:285-290. [DOI] [PubMed] [Google Scholar]
- 18. deMol P, Krabbe HG, de Vries ST, et al. Accuracy of handheld blood glucose meters at high altitude. PLoS One. 2010;5(11):e15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. Partial pressure of oxygen in capillary blood samples from the fingertip. J Diabetes Sci Technol. 2013;7:1648-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27:2478-2484. [DOI] [PubMed] [Google Scholar]
- 21. Mirrakhimov AE. Chronic obstructive pulmonary disease and glucose metabolism: a bitter sweet symphony. Cardiovasc Diabetol. 2012;11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cappon G, Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G. Wearable continuous glucose monitoring sensors: a revolution in diabetes treatment. Electronics. 2017;6:1-16. [Google Scholar]