Abstract
Background:
Despite recent advances in closed-loop control of blood glucose concentration (BGC) in people with type 1 diabetes (T1D), online performance assessment and modification of artificial pancreas (AP) control systems remain a challenge as the metabolic characteristics of users change over time.
Methods:
A controller performance assessment and modification system (CPAMS) analyzes the glucose concentration variations and controller behavior, and modifies the parameters of the control system used in the multivariable AP system. Various indices are defined to quantitatively evaluate the controller performance in real time. Controller performance assessment and modification system also incorporates online learning from historical data to anticipate impending disturbances and proactively counteract their effects.
Results:
Using a multivariable simulation platform for T1D, the CPAMS is used to enhance the BGC regulation in people with T1D by means of automated insulin delivery with an adaptive learning predictive controller. Controller performance assessment and modification system increases the percentage of time in the target range (70-180) mg/dL by 52.3% without causing any hypoglycemia and hyperglycemia events.
Conclusions:
The results demonstrate a significant improvement in the multivariable AP controller performance by using CPAMS.
Keywords: artificial pancreas, biomedical system, controller performance assessment and modification
Introduction
Various automated insulin delivery systems, termed the artificial pancreas (AP), are proposed for improving glycemic regulation in people with type 1 diabetes (T1D) by utilizing dynamic glucose-insulin models and predictive controllers.1-15 Despite the proven advantages, fully automated closed-loop control of blood glucose concentration (BGC) using an AP remains challenging because of the needs for (1) reliable models to accurately describe the patient-specific time-varying glucose-insulin dynamics; (2) knowledge of insulin constraints to prevent an overdose and to improve patient safety; (3) efficient predictive control algorithms that regulate BGC in the presence of unknown disturbances like meals and physical activities; and (4) reliable and efficient controller performance assessment and modification systems (CPAMS) to assure that the AP system performs satisfactorily over time under various conditions.
Model predictive control (MPC) has gained significant popularity in various applications ranging from chemical process industries to pharmaceutical production and automated drug delivery systems.16,17 This popularity has led to an increased interest in automated controller performance assessment and modification for MPC. The information gained from the CPAMS can be used to retune or redesign the controller for performance improvement.18,19 Different AP control systems have been designed based on the MPC.9,11,20-29 Due to the complex nature of BGC dynamics in people with T1D, the performance of the MPC techniques developed by using a fixed model or fixed controller parameters for the AP system may not consistently meet the expectations. Model predictive control performance assessment is challenging because degradation in closed-loop performance can arise due to model deficiencies, poor control design parameters, or inappropriate constraints. Complex nonlinear dynamical systems such as the metabolic processes in the human body are particularly challenging to control due to the time-varying characteristics of these systems and their nonlinear behavior, presence of stochastic and unknown disturbances, and uncertain time-varying delays. An example of a system with noteworthy complexities that necessitates a safe and reliable controller integrated with a powerful CPAMS is the regulation of glucose concentrations in people with T1D. Despite advances in glucose control algorithms, the complexity of the glucose regulation problem has challenged the accurate assessment of the controller performance.30,31
In the literature, several approaches have been reported for adapting and individualizing both AP controllers and clinical open-loop therapy. The adaptation is expected to improve controller performance and provide some of the benefits that a CPAMS could provide. A data-driven AP adaptation method based on a two-phase Bayesian optimization assisted parameter learning algorithm was introduced to adapt basal and carbohydrate-ratio profiles and key feedback control parameters.32 Run-to-run approaches were proposed to update basal rate, meal bolus sizes, and the insulin to carbohydrate ratio profile.33-37 An iterative learning MPC was proposed to adapt the reference trajectory of the closed-loop controller used for glucose regulation.38-40 Individualized MPCs for AP systems were proposed based on patient-specific parameters or historical data for improved glucose regulation performance work.25,41 An adaptive learning algorithm to adjust postprandial basal and premeal bolus insulin was proposed for reducing postprandial hypoglycemia in a hybrid AP.42 Automatic adaptation of basal insulin using sensor-augmented pump therapy was developed based on a run-to-run control law.43 A learning algorithm was integrated into a MD-Logic AP system to establish an initial patient profile using open-loop data and then make periodic adjustments during closed-loop operation.44 A run-to-run control was developed for multiple-daily injection therapy where rapid- and slow-acting insulins were used based on pre- and postprandial glucose measurements.45
We developed a multivariable AP (mAP) system with a glucose concentration prediction model that is recursively updated with each new continuous glucose monitoring (CGM) information along with physiological variables reported by wearable devices. The measurable disturbances estimated from physiological variables are used both in improving glucose predictions and insulin infusion dose decisions, and in providing warnings for consumption of rescue carbs to prevent hypoglycemia.15,28 The CPAMS is developed to monitor, evaluate, and modify the mAP controller to enhance its performance and safety.31
In this work, we propose refinements to CPAMS to enhance its performance and improve glucose concentration regulation. We demonstrate that our mAP system can mitigate the effects of unknown disturbances like physical activities and meals efficiently and can control BGC in the target range (70-180) mg/dL with infrequent consumptions of reasonable amounts of rescue carbohydrates. Controller performance assessment and modification system enables these improvements by modulating the desired BGC (set-point) of the controller. Various components of the mAP are outlined below, and references are given to publications that provide the details of the algorithms. In our previous work, we have developed an adaptive MPC-based personalized multivariable, multimodule AP system capable of disturbance rejection without manual announcements for meals and exercise.28,46 To characterize the time-varying glycemic dynamics, a data-driven recursive subspace system identification technique was integrated with a physiological compartmental model to identify adaptive stable models.28,47 Assessing the performance of the recursively identified models is necessary to ensure that the model is able to provide accurate output predictions for the use in MPC algorithms. For this reason, we proposed a performance assessment technique for the recursively identified models to check certain key performance indexes (KPIs), including the risk of underpredicting undesirably high glucose levels, the risk of overpredicting dangerously low glucose levels, and the mean absolute error between predicted and measured outputs. The assessment of the clinical utility of model-based glucose predictions using Clarke error grid analysis has also been proposed.31 We have also specified the key parameters in the model to be modified through efficient ways if the poor performance of the model is observed over time based on the aforementioned KPIs.
To quantify the insulin in the bloodstream, the plasma insulin concentration (PIC) estimator was designed by using the unscented Kalman filtering technique. The PIC estimator is able to capture the variability in the temporal dynamics of patients by estimating some uncertain model parameters that have significant effects on PIC estimates. The PIC estimator is personalized by initializing the time-varying model parameters using partial least squares regression models. The PIC safety constraints were then defined to assure that a safe amount of insulin is in the body.48,49
An adaptive MPC algorithm was designed based on recursively identified state space models with dynamic adjustments of constraints and objective function weights. A feature extraction method that automatically detects the presence of meals using qualitative descriptions of CGM time-series data was also designed. The key parameters of the MPC optimization problem are modified online using the information that the feature extraction method provides about the rate and shape of variations in output measurements to improve the effectiveness of the controller against meal consumption. These adaptive controller parameters, dynamic safety constraints, and addition of exogenous physiological measurements from wearable devices empowered the control system to efficiently compute the optimal control action over diverse diurnal variations. Various KPIs were defined to evaluate the performance of the closed-loop system to modify the key controller parameters of the adaptive MPC in real time if poor performance was detected.31
Historical data can be utilized to identify behaviors and patterns of the underlying system. We showed that incorporating online learning of probable times of unknown disturbances from the amassed historical data using a recursive partial least squares model can improve the control performance by proactively mitigating the effects of impending disturbances. Hence, the controller set-point, the weights in the MPC objective functions, and the system constraints are appropriately modified in advance for anticipated periods of the disturbance effects. We have also shown that the proposed CPAMS integrated with the mAP is robust and reliable against unexpected behaviors on atypical (nonregular) days when the daily activities of the users are different from historical data used for learning and modification.31 A predictive hypoglycemia alarm module was also designed to suggest carbohydrates to be consumed to prevent predicted hypoglycemia. Using a four-step-ahead (20 minutes) prediction of glucose values and considering the presence of physical activities and the amount of active insulin in the bloodstream (PIC), this module suggests fast-acting carbohydrates if the predicted glucose value is less than a safe threshold. In case hypoglycemia is predicted, consumption of 15 to 25 g of fast-acting carbohydrates is suggested by the mAP to avoid low BGC. There is a wait period of 20 and 15 minutes between each suggested fast-acting carbohydrate during fasting time and exercise time, respectively.
In this work, we enhance the CPAMS to further analyze the closed-loop behavior of the mAP and adjust the controller parameters and set-points depending on the current state of the subject, the disturbances to the metabolic system, and the performance of the controller. Our focus is to show that the proposed mAP and the CPAMS can keep the BGC in the target range (70-180) mg/dL over longer periods and mitigate the effects disturbances such as physical activity and meal efficiently without causing any hypoglycemia events and without any manual information. As the controller basal set-point value for regulating BGC has a significant effect on the glycemic results, a subject preferring to avoid consuming rescue carbohydrates during exercise may increase the basal set-point value (for example, from 110 to 140 mg/dL) in order to decrease the likelihood of hypoglycemia treatments while keeping the BGC in the target range during most of the exercise and recovery period.
A multivariable simulation software platform of virtual subjects with T1D50 is used to illustrate the performance of the mAP with the CPAMS. This multivariable glucose-insulin-physiological variable simulator (mGIPsim) is based on simulation of the glucose-insulin dynamics of virtual patients with T1D by extending Hovorka’s model. Nonlinear models are developed to describe glucose concentration variations based on user-defined scenarios for meal consumption, insulin administration, and physical activities. They compute glucose concentration values and physiological variables, such as heart rate, skin temperature, accelerometer, and energy expenditure, that are indicative of physical activities affecting glucose concentration dynamics as outputs from the simulator. Continuous glucose monitoring time series data, fingerstick BGC measurements, insulin infusion information, meal and exercise scenarios, and physiological variable measurements collected during clinical experiments were used to extend the glucose-insulin dynamics model, develop models for physiological variable predictions, and create the simulator’s virtual subjects.
Methods
In this work, CPAMS is applied to the regulation of glucose concentrations in people with T1D using automated insulin delivery with an adaptive learning MPC. We focus on automatically assessing the performance of different modules of the mAP system using various performance indices. Here, we describe briefly different components of the mAP control system and explain their key parameters. We propose a daily-based performance assessment to guarantee that the whole mAP system is functioning properly. The purpose is to increase the percentage of time in the target range (70-180 mg/dL) without causing any hypoglycemia and hyperglycemia. The proposed controller can keep the BGC in the target range with a minimum number of consumed rescue carbohydrates suggested by the hypoglycemia detection and carbohydrate suggestion module. The controller is able to compute an optimum amount of insulin under various daily conditions. Consuming less rescue carbohydrates during the day reduces the interruption times during exercise and anxiety due to hypoglycemia.
Plasma-Insulin-Cognizant Adaptive MPC
The mAP controller is an adaptive MPC algorithm cognizant of PIC for computing the optimal insulin infusion rate. It also utilizes the glycemic and PIC risk indexes that manipulate the penalty weighting matrices in the objective function. The MPC computes the optimal insulin infusion over a finite horizon using the identified time-varying glycemic models by solving at each sampling instant () the following quadratic programming problem:
| (1) |
with the objective function
where and define the glycemic model, is the disturbance term, is the model output (estimated CGM), is the reference glucose set-point target in the controller, and are the lower and upper bounds of infused insulin, respectively, and are the minimum and maximum constraints for the PIC value, respectively,is the desired value of PIC at each sampling time, and are the adaptive weights used in the MPC objective function, and is the patient-specific basal insulin rate. is a parameter that is zero during fasting period and takes on a positive value when a meal is detected using a feature extraction method. This method quantifies the rate and shape of changes in the CGM values to make the controller more aggressive during meal periods according to
| (2) |
A second-order polynomial function is fitted to the most recent three glucose concentration data at each sampling time by using ordinary least squares. Then, the derivatives of the polynomial are obtained and the first- and second-order derivatives, denoted by and , are evaluated to assign a value to parameter . The threshold is used for the meal detection, and and are patient-specific parameters personalized for each patient using the CPAMS over time.
The upper, lower, and desired values of the PIC are a linear function of the CGM values. The initial value of is 1 and then it is individualized for each subject by CPAMS daily. The states of the model are included as the two states of a state space model obtained through the recursive subspace identification and the insulin compartmental model of Hovorka’s glucose-insulin dynamic model as The time-varying positive semidefinite weighting matrix is defined as where denotes a nominal weight and is the predicted CGM. The time-varying positive semidefinite weighting matrix is defined as where denotes a nominal weight and is the PIC estimate. The and functions are defined in the Adaptive Glycemic Risk and Plasma Insulin Risk Indices section.
Adaptive Learning Controller Set-Point Determination and PIC Bounds Definition
An adaptive learning technique is proposed to modify the controller set-point based on historical information during meal and exercise periods. Historical data, including the CGM measurements and physiological variables, are used to deduce the probable times of meals consumption and physical activities. This valuable information on daily behaviors and habits can be used in the mAP system to anticipate and proactively mitigate the effects of disturbances. Therefore, the performance of the control system can be improved if the controller set-point is appropriately modified in advance for the anticipated periods of the disturbance effects. The nominal value of the reference glucose set-point target in the controller is Using historical data, different values can be assigned to the controller set-point around specific times that disturbances are expected to affect the BGC. We developed a feature extraction method on glucose concentration data for meal detection, and another machine learning algorithm for the physiological variables to detect the presence of physical activity. At the end of each day, the time windows detected for meal disturbances are assigned a lower set-point value of to make the controller more aggressive around those periods. Similarly, during exercise times, a higher set-point value of makes the controller more conservative to reduce insulin infusion in response to possible physical activities. The controller set-point values for the anticipated meal and exercise disturbance time windows of the new day are calculated by averaging the assigned set-point values to each specific time in historical data. However, as the time of meal intake or exercise may change for the new day and be different from historical data, the assigned set-point values to each specific time would be modified accordingly in real time if the expected event does not occur and irregular events are observed.
The adaptive set-point definition is also used to modify the PIC bounds including the and 31 At each CGM sampling instant, safe and reasonable set-point values are determined over the prediction/control horizon of the MPC using historical information. For example, for the periods that the chance of exercise is high, the PIC bounds are lowered in advance to improve the safety of the user by reducing the active insulin in the body. Consider as the current sampling time, and as the time index over prediction/control horizon of the MPC. The slope () and intercept of are considered to be a function of the set-point as
| (3) |
According to the definitions in Equation (3), PIC bounds change beforehand based on the historical data over the prediction/control horizon to better mitigate the effects of the unknown disturbances.
Adaptive Glycemic Risk and Plasma Insulin Risk Indices
An adaptive glycemic risk index (GRI) determines the weighting matrix for penalizing the deviation of the model output from the set-point.31 At each sampling time, the GRI is defined based on the set-point value as
| (4) |
where The GRI is considered to be a function of the controller set-point to efficiently penalize the deviations from the controller set-point and better define the aggressiveness/conservativeness of the controller.
A plasma insulin risk index is defined to manipulate the weighting matrix for penalizing the amount of input actuation (aggressiveness of insulin dosing) depending on the estimated PIC, thus suppressing the infusion rate if sufficient insulin is present in the bloodstream.31 At each sampling time, the GRI is defined as
| (5) |
where is the patient-specific basal PIC value.
Controller Performance Assessment and Modification During Postprandial Periods
The PIC constraints of the MPC are modified based on the parameter (Equation (2)) when a meal is detected. The two parameters and have a significant effect on the aggressiveness of the controller during meal consumption. A three-hour-long postprandial period (PP) is defined for each detected meal. At the end of each day, the detected PPs via the feature extraction method are evaluated based on some effective performance indices. The expected desired behavior of the controller during these periods is not violating the maximum acceptable change in the CGM values due to the meal and no hypoglycemia due to the insulin overdose. We define a threshold for the maximum acceptable change in the CGM values due to the meal effect as
| (6) |
where is the maximum change between two consecutive CGM data for each PP. Several factors affect the glycemic response to food, including the type, composition, and processing of the ingredients. These factors impact the rate of digestion and absorption, and subsequently, the glycemic response to the meal disturbance that must be considered in the CPAMS of the mAP. We define to be a function of the maximum change in CGM to consider the effect of different types of food on BGC. By analyzing the clinical datasets during PPs of various types of meal, we found that for of 5 and 20 the maximum acceptable change in the CGM values can be 100 and 160 respectively. So, by calculating for each PP, we can obtain threshold. At the end of each day, for each PP period, the maximum change in the CGM values due to the meal intake is compared with If the poor performance is observed, the CPAMS modifies and parameters. To calculate the maximum change in the CGM values due to the meal intake, the difference between the maximum value of CGM and the CGM value at the start of meal consumption for each PP is calculated. The average of CGM values in a past window of 30 minutes before meal detection is considered as an approximate value of CGM at the beginning of meal consumption. The 30 minutes is a sufficiently large past window to consider the delay in the effect of meal consumption on BGC. It can also take into account the effect of meal amounts, duration of meal consumption, and meal composition.
Controller performance assessment and modification system reduces the values of and parameters safely to make the controller more aggressive to better mitigate the effect of meal consumptions if the following conditions are satisfied simultaneously:
The threshold is violated.
No hypoglycemia event happens during the PPs.
No rescue carbohydrates are consumed by the user due to the predicted hypoglycemia event via the predictive hypoglycemia alarm module.
However, the CPAMS increases the values of and parameters to make the controller more conservative to avoid insulin overdosing if any of the following conditions are satisfied:
Hypoglycemia event happens during the PPs.
Rescue carbohydrates are consumed by the patient due to the predicted hypoglycemia event via the predictive hypoglycemia alarm module.
The initial values of and are considered to be 20 and 40 respectively. These parameters are modified (reduced or increased) by 2.5 and 5 respectively, if the required specified conditions are satisfied. To obtain we calculate the difference between two consecutive samples of CGM data for all CGM measurements during each PPs and then their maximum value is For example, suppose that the value of so the maximum acceptable change in the CGM values during this PP is obtained by If the observed maximum change in CGM values during this PP is larger than this value, and no hypoglycemia events were predicted by the predictive hypoglycemia alarm module, and no hypoglycemia events actually occurred during the PPs for that day, then the CPAMS reduces the values of and parameters to make the controller more aggressive to handle better the increase in the BGC due to the meal.
Controller Performance Assessment and Modification During Fasting Period
In this work, we consider three hours of PP period for each detected meal and two hours of recovery period after each detected exercise. The rest of the time on each day is considered as fasting periods. The PIC bounds of the MPC define the aggressiveness/conservativeness of the controller during different conditions. During the fasting period, the parameter is zero and the parameter is the key parameter. The initial value of is set to a safe initial value of unity. The parameter is modified for each subject by analyzing the past historical data including the CGM measurements during fasting periods. At the end of each day, the value of parameter is obtained based on the CGM values in the fasting period as
| (7) |
where the parameter is used in the GRI. At the end of each day, the value of is modified for the next day using the following equation:
| (8) |
where is the average of all values calculated using CGM data during the fasting period. For example, if the average value gets a negative value, the controller needs to be more conservative by reducing the PIC bounds for the upper, lower, and desired values.
Results
The efficacy of the proposed mAP and CPAMS is investigated by using mGIPsim.50 In addition to the CGM values, the mGIPsim generates physiological variable signals reported by noninvasive wearable devices. Aerobic exercises with treadmill and stationary bicycle are considered for testing the mAP system integrated with the CPAMS. Twenty virtual subjects are simulated for 60 days with varying times and quantities of meals consumed on each day and physical activities with different types, intensities, and durations as detailed in Tables 1 and 2. The meal and physical activity information are not entered manually to the AP system as the AP controller is designed to regulate the BGC in the presence of significant unknown disturbances such as unannounced meals and exercises. In the defined scenario, the maximum amount for the meal consumption is 70 g of carbohydrates though larger amounts can be also tested. The metabolic equivalent task (MET) values computed by the simulator from physiological signal data are used to extract information about the presence and intensity of exercise for the mAP system.
Table 1.
Meal Scenario for 60 Days Closed-Loop Experiment Using the Multivariable Metabolic Simulator.
| Meal | Range of time | Range of amount (g) |
|---|---|---|
| Breakfast | (06:00-07:00) | (50-70) |
| Lunch | (12:00-13:00) | (50-70) |
| Dinner | (18:00-19:00) | (50-70) |
| Snack | (22:00-23:00) | (20-30) |
Table 2.
Exercise Scenario for 60 Days Closed-Loop Experiment Using the Multivariable Metabolic Simulator.
| Exercise | Range for values | ||||
|---|---|---|---|---|---|
| Time | Duration (min) | Speed | Incline | Power | |
| Treadmill | (10:00-11:00) or (16:00-17:00) | (40-60) | (4-6) | (0-2) | - |
| Bicycling | (10:00-11:00) or (16:00-17:00) | (40-60) | - | - | (70-110) |
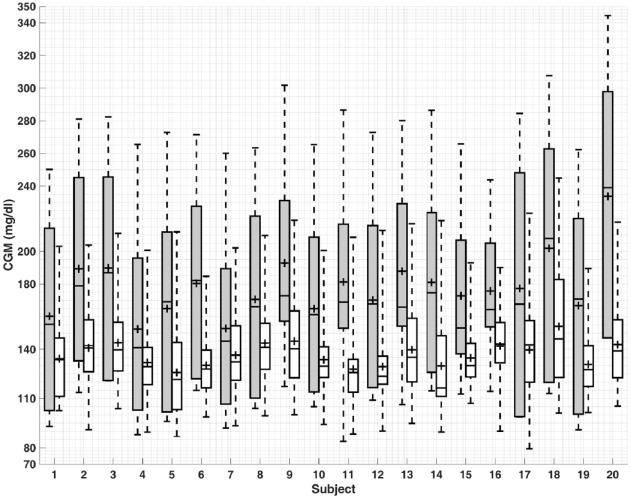
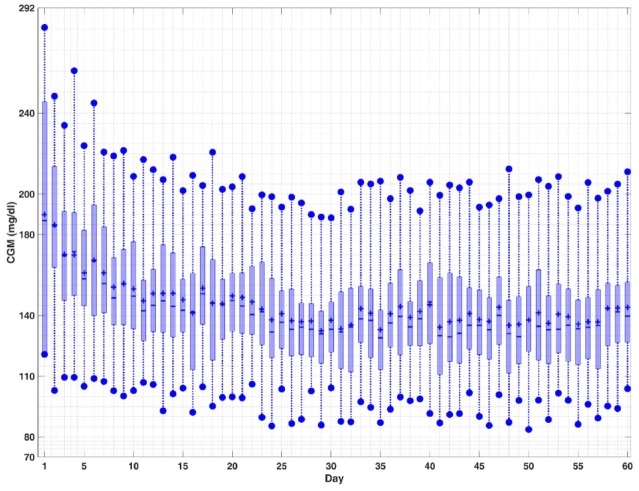
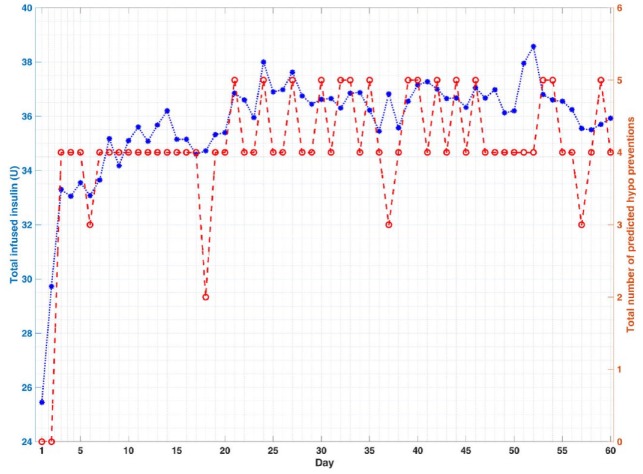
The quantitative evaluation of the closed-loop results based on the proposed algorithms is presented in Tables 3-5, where the meal and exercise specifications are different every day. The purpose of these simulations is to show that the mAP integrated with CPAMS and an adaptive learning technique is robust and reliable in learning and handling users’ habits and activities that vary over time. The average percentage of time spent in the target range (70-180) mg/dL improves significantly from 39.4% to 91.7% for all subjects over time. There are no hypoglycemia events as the BGC is never below 70 mg/dL. The minimum and maximum observed BGC values across all experiments on the last day (day 60) of the simulation are 79 and 245 mg/dL, respectively. There is also a significant improvement in the mean of CGM values from 197.1 to 137.4 mg/dL. The number of treatments for the predicted hypoglycemia events by consuming rescue carbohydrates is also low, which indicates that the whole mAP system can function satisfactorily under various conditions like exercise and meals. Overall, the results demonstrate that the mAP is able to regulate BGC effectively in the presence of significant unknown disturbances caused by the diverse timing and amounts of meals and exercise specifications while mitigating severe hypo- and hyperglycemic excursions. The closed-loop results for all subjects in Figure 1 and for a select subject during all 60 days in Figure 2 indicate that the performance of the mAP improves over time due to CPAMS and by learning from historical data. Since the meal and exercise specifications for each day are randomly selected from Tables 1 and 2, these variations affect the closed-loop behavior of the control system observed in Figure 2. In Figure 3, the controller delivers more insulin to increase the percentage of time spent in the target range. This increase in the total daily infused insulin augments the number of rescue carbohydrates to avoid potential hypoglycemia, particularly during exercise sessions when the CGM values may be low. The variations observed in the performance indices, the total daily infused insulin, and the total number of predicted hypo preventions by rescue carbohydrates for each day over time are due to different meal and exercise scenarios generated for each day. Users may specify their preferences for lower rescue carbohydrates, which cause the CPAMS and MPC to decrease insulin infusion and reduce hypoglycemia risk. This more conservative setting results in lower rescue carbohydrates, though the decreased insulin may cause slightly higher glucose values compared to a controller tuned to increase the time spent in the target range.
Table 3.
Continuous Glucose Monitoring Metrics Percentage of Time Spent in Different Ranges for Day 1, Day 60, and for All Days for Closed-Loop Control Results.
| Subject | (70-180) mg/dL | (180-250) mg/dL | (250-350) mg/dL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 60 | Overall | Day 1 | Day 60 | Overall | Day 1 | Day 60 | Overall | |
| 1 | 40.1 | 90.7 | 85.4 | 59.4 | 9.3 | 14.5 | 0.5 | 0.0 | 0.1 |
| 2 | 35.0 | 94.4 | 88.9 | 35.9 | 5.6 | 10.6 | 29.0 | 0.0 | 0.5 |
| 3 | 31.8 | 88.4 | 85.0 | 38.2 | 11.6 | 14.6 | 30.0 | 0.0 | 0.4 |
| 4 | 56.2 | 95.8 | 92.5 | 37.3 | 4.2 | 7.4 | 6.5 | 0.0 | 0.1 |
| 5 | 43.8 | 94.4 | 90.4 | 47.0 | 5.6 | 9.4 | 9.2 | 0.0 | 0.2 |
| 6 | 32.3 | 97.2 | 91.6 | 53.9 | 2.8 | 8.1 | 13.8 | 0.0 | 0.3 |
| 7 | 60.4 | 94.4 | 89.4 | 33.2 | 5.6 | 10.5 | 6.5 | 0.0 | 0.1 |
| 8 | 37.3 | 89.8 | 86.2 | 52.1 | 10.2 | 13.7 | 10.6 | 0.0 | 0.2 |
| 9 | 38.7 | 90.7 | 77.8 | 35.9 | 9.3 | 20.9 | 25.3 | 0.0 | 1.2 |
| 10 | 45.6 | 94.9 | 91.4 | 46.5 | 5.1 | 8.5 | 7.8 | 0.0 | 0.1 |
| 11 | 47.5 | 94.9 | 88.7 | 32.3 | 5.1 | 11.0 | 20.3 | 0.0 | 0.4 |
| 12 | 43.3 | 93.5 | 87.5 | 40.1 | 6.5 | 12.1 | 16.6 | 0.0 | 0.4 |
| 13 | 40.1 | 90.7 | 81.0 | 39.2 | 9.3 | 18.3 | 20.7 | 0.0 | 0.7 |
| 14 | 39.6 | 92.6 | 88.3 | 39.2 | 7.4 | 11.2 | 21.2 | 0.0 | 0.5 |
| 15 | 50.2 | 95.4 | 88.9 | 39.6 | 4.6 | 10.9 | 10.1 | 0.0 | 0.2 |
| 16 | 42.9 | 95.8 | 92.5 | 57.1 | 4.2 | 7.5 | 0.0 | 0.0 | 0.0 |
| 17 | 36.4 | 89.8 | 84.8 | 33.2 | 10.2 | 14.7 | 30.4 | 0.0 | 0.5 |
| 18 | 21.2 | 65.3 | 62.0 | 31.3 | 34.7 | 36.0 | 47.5 | 0.0 | 2.0 |
| 19 | 36.4 | 96.3 | 90.3 | 55.8 | 3.7 | 9.5 | 7.8 | 0.0 | 0.2 |
| 20 | 9.2 | 88.9 | 73.7 | 27.2 | 11.1 | 24.4 | 63.6 | 0.0 | 1.9 |
| Average | 39.4 | 91.7 | 85.8 | 41.7 | 8.3 | 13.7 | 18.9 | 0.0 | 0.5 |
Table 4.
Continuous Glucose Monitoring Metrics for Different Statistics for Day 1, Day 60, and Overall Days and Number of Hypo Treatments Based on Predictive Hypoglycemia Alarms on Day 60 for Closed-Loop Results.
| Subject | Mean (mg/dL) | Min (mg/dL) | Max (mg/dL) | # of Predicted hypo preventions by rescue carbs (amounts in g) on day 60 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 60 | Overall | Day 1 | Day 60 | Overall | Day 1 | Day 60 | Overall | P | E | F | |
| 1 | 181.0 | 139.9 | 148.3 | 102.0 | 103.0 | 89.0 | 250.0 | 203.0 | 261.0 | 0 | 4 (60) | 0 |
| 2 | 207.7 | 140.1 | 139.8 | 114.0 | 91.0 | 74.0 | 281.0 | 204.0 | 281.0 | 0 | 4 (60) | 1 (15) |
| 3 | 212.4 | 143.0 | 145.4 | 121.0 | 104.0 | 84.0 | 282.0 | 211.0 | 282.0 | 0 | 4 (60) | 0 |
| 4 | 171.5 | 129.6 | 136.8 | 100.0 | 90.0 | 84.0 | 265.0 | 201.0 | 265.0 | 2 (40) | 4 (80) | 0 |
| 5 | 186.7 | 121.4 | 136.7 | 102.0 | 87.0 | 78.0 | 273.0 | 212.0 | 273.0 | 2 (30) | 4 (60) | 0 |
| 6 | 200.8 | 128.4 | 140.8 | 122.0 | 99.0 | 79.0 | 271.0 | 185.0 | 271.0 | 0 | 3 (45) | 0 |
| 7 | 170.8 | 135.6 | 140.4 | 106.0 | 93.0 | 83.0 | 260.0 | 202.0 | 260.0 | 0 | 3 (45) | 1 (15) |
| 8 | 191.6 | 144.7 | 142.8 | 110.0 | 99.0 | 83.0 | 263.0 | 210.0 | 263.0 | 0 | 4 (60) | 0 |
| 9 | 204.0 | 149.7 | 153.7 | 117.0 | 100.0 | 87.0 | 302.0 | 219.0 | 302.0 | 0 | 3 (75) | 0 |
| 10 | 183.3 | 129.8 | 137.0 | 114.0 | 94.0 | 80.0 | 265.0 | 201.0 | 265.0 | 1 (15) | 4 (60) | 0 |
| 11 | 192.8 | 125.9 | 140.0 | 110.0 | 88.0 | 81.0 | 287.0 | 209.0 | 287.0 | 3 (54) | 4 (72) | 0 |
| 12 | 189.1 | 129.1 | 140.9 | 116.0 | 90.0 | 83.0 | 273.0 | 213.0 | 277.0 | 0 | 4 (60) | 1 (15) |
| 13 | 197.8 | 144.6 | 148.4 | 106.0 | 95.0 | 85.0 | 280.0 | 217.0 | 280.0 | 0 | 1 (25) | 1 (25) |
| 14 | 199.1 | 133.0 | 138.5 | 115.0 | 90.0 | 78.0 | 286.0 | 219.0 | 286.0 | 0 | 3 (60) | 2 (40) |
| 15 | 184.6 | 135.7 | 143.4 | 113.0 | 107.0 | 84.0 | 266.0 | 193.0 | 266.0 | 0 | 2 (30) | 0 |
| 16 | 182.9 | 140.6 | 138.2 | 114.0 | 90.0 | 81.0 | 244.0 | 190.0 | 244.0 | 0 | 3 (45) | 1 (15) |
| 17 | 203.0 | 138.7 | 142.9 | 99.0 | 79.0 | 72.0 | 284.0 | 223.0 | 284.0 | 0 | 5 (75) | 0 |
| 18 | 230.2 | 161.6 | 167.7 | 120.0 | 101.0 | 86.0 | 308.0 | 245.0 | 308.0 | 0 | 5 (75) | 0 |
| 19 | 190.4 | 129.3 | 141.3 | 100.0 | 101.0 | 80.0 | 262.0 | 190.0 | 262.0 | 0 | 4 (60) | 0 |
| 20 | 262.1 | 147.7 | 157.5 | 147.0 | 105.0 | 89.0 | 344.0 | 218.0 | 344.0 | 0 | 3 (45) | 0 |
| Average | 197.1 | 137.4 | 144.0 | 112.0 | 95.0 | 82.0 | 277.0 | 208.0 | 278.0 | 0.4 (7) | 3.55 (60) | 0.35 (6) |
Average represents the mean of each index over all subjects; P includes three postprandial periods for breakfast, lunch, and dinner and each postprandial period is three hours; E includes two exercise sessions (bike and treadmill), each exercise session is one hour on day 60; F includes fasting period excluding P and E periods.
Table 5.
Number of Predicted Hypo Preventions by Rescue Carbohydrates on Day 1, Day 60, and Overall.
| Subject | # of Predicted hypo preventions by rescue carbohydrates | ||
|---|---|---|---|
| Day 1 | Day 60 | Overall | |
| 1 | 0 | 4 | 225 |
| 2 | 0 | 5 | 307 |
| 3 | 0 | 4 | 242 |
| 4 | 4 | 6 | 300 |
| 5 | 2 | 6 | 299 |
| 6 | 0 | 3 | 212 |
| 7 | 3 | 4 | 212 |
| 8 | 0 | 4 | 250 |
| 9 | 1 | 3 | 192 |
| 10 | 0 | 5 | 333 |
| 11 | 2 | 7 | 331 |
| 12 | 0 | 5 | 256 |
| 13 | 0 | 2 | 136 |
| 14 | 3 | 5 | 326 |
| 15 | 1 | 2 | 152 |
| 16 | 0 | 4 | 295 |
| 17 | 0 | 5 | 328 |
| 18 | 0 | 5 | 158 |
| 19 | 0 | 4 | 222 |
| 20 | 0 | 3 | 172 |
| Average | 0.8 | 4.3 | 247.4 |
Figure 1.
Comparison of model predictive control results for the first (gray) and last (white) days of simulation for all 20 subjects. The bottom and top of the boxes are the first and third quartiles and the line inside the box is the median. The whisker’s ends represent the minimum and maximum values and + indicates mean values.
Figure 2.
Closed-loop results for a select subject. The top and bottom edges of the boxes are the first and third quartiles, and the line inside the box is the median. The whisker ends represent minimum and maximum values, and plus signs (+) indicate mean values.
Figure 3.
Closed-loop results for a select subject. The star signs (*) indicate the total daily infused insulin (U) and the circle signs (o) indicate the total number of predicted hypo preventions by rescue carbohydrates at each day.
Discussion
The rescue carbohydrates intake may not be convenient during an exercise and may lead to weight gain in the long term. However, in a real-life scenario, the person may choose to start a meal early or eat a snack prior to exercise to prevent hypoglycemia which would reduce the number of carbohydrates consumed for the sole purpose of maintaining glycemia. Many people with diabetes consume carbohydrates as part of their management plan for exercise, so the recommended 30 g per session on average for day 60 may match their standard treatment regimen. The combination of increased constraints on PIC during the identified exercise period (ie, reducing insulin administration prior to the start) and recommendation of carbohydrate consumption match the recommendations given to people with T1D.51 Some people with T1D may also prefer to define their controller basal set-point values instead of the default value of For these reasons, we present the closed-loop results for different basal set-point values to show how the performance indices defined in Tables 3 and 4 change for each case. These results illustrate that for patients who may want to consume less rescue carbohydrates, an increase in the controller basal set-point can reduce the number of hypoglycemia treatments. However, it may cause a slight increase in the percentage of time in (180-250) and (250-400) mg/dL ranges.
In Tables 6 and 7, the results for four different cases are presented: case 1: case 2: case 3: and case 4: The results show that modifying the controller basal-set-point value could be an option to reduce rescue carbohydrates consumptions. In Table 8, the P-values between CGM values of day 1 and 60 have been calculated for different cases to show the statistical significance of the differences. The next step in our studies is to test offline the proposed techniques in clinical experiments to further evaluate the insulin suggestions. After the successful performance of this step, the proposed mAP will be utilized in clinical experiments to automate insulin delivery in real time.
Table 6.
Continuous Glucose Monitoring Metrics Including the Percentage of Time Spent in Different Ranges for Closed-Loop Results of Four Different Basal Set-Point Values at Day 60.
| Subject | (70-180) mg/dL | (180-250) mg/dL | (250-300) mg/dL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | |
| 1 | 90.7 | 89.4 | 87.0 | 69.4 | 9.3 | 10.6 | 13.0 | 30.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 | 94.4 | 93.5 | 79.2 | 72.2 | 5.6 | 6.5 | 20.8 | 27.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| 3 | 88.4 | 79.2 | 71.8 | 65.7 | 11.6 | 20.8 | 28.2 | 34.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| 4 | 95.8 | 94.0 | 89.8 | 71.3 | 4.2 | 6.0 | 10.2 | 28.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 5 | 94.4 | 93.1 | 76.9 | 70.8 | 5.6 | 6.9 | 23.1 | 27.8 | 0.0 | 0.0 | 0.0 | 1.4 |
| 6 | 97.2 | 94.9 | 93.5 | 76.4 | 2.8 | 5.1 | 6.5 | 23.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 7 | 94.4 | 88.0 | 79.2 | 73.1 | 5.6 | 12.0 | 20.8 | 26.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 8 | 89.8 | 75.5 | 65.7 | 67.1 | 10.2 | 24.5 | 34.3 | 32.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| 9 | 90.7 | 81.9 | 71.8 | 56.0 | 9.3 | 18.1 | 28.2 | 40.3 | 0.0 | 0.0 | 0.0 | 3.7 |
| 10 | 94.9 | 93.1 | 76.4 | 72.7 | 5.1 | 6.9 | 23.6 | 27.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| 11 | 94.9 | 90.3 | 73.1 | 83.8 | 5.1 | 9.7 | 26.9 | 16.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 12 | 93.5 | 92.6 | 86.6 | 65.3 | 6.5 | 7.4 | 13.4 | 34.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 13 | 90.7 | 92.1 | 62.5 | 67.6 | 9.3 | 7.9 | 37.5 | 32.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 14 | 92.6 | 86.1 | 76.4 | 71.3 | 7.4 | 13.9 | 23.6 | 26.9 | 0.0 | 0.0 | 0.0 | 1.9 |
| 15 | 95.4 | 92.6 | 79.6 | 76.4 | 4.6 | 7.4 | 20.4 | 23.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 16 | 95.8 | 92.6 | 78.7 | 73.6 | 4.2 | 7.4 | 21.3 | 26.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 17 | 89.8 | 72.7 | 72.7 | 61.6 | 10.2 | 27.3 | 27.3 | 37.0 | 0.0 | 0.0 | 0.0 | 1.4 |
| 18 | 65.3 | 60.2 | 53.7 | 47.2 | 34.7 | 37.5 | 39.8 | 44.9 | 0.0 | 2.3 | 6.5 | 7.9 |
| 19 | 96.3 | 95.4 | 91.2 | 70.8 | 3.7 | 4.6 | 8.8 | 29.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 20 | 88.9 | 75.5 | 66.2 | 63.9 | 11.1 | 24.5 | 33.8 | 33.8 | 0.0 | 0.0 | 0.0 | 2.3 |
| Average | 91.7 | 86.6 | 76.6 | 68.8 | 8.3 | 13.3 | 23.1 | 30.3 | 0.0 | 0.1 | 0.3 | 0.9 |
Table 7.
Continuous Glucose Monitoring Metrics Including Different Statistics for Day 60 and Total Number of Predicted Hypo Preventions by Rescue Carbohydrates for All 60 Days via Predictive Hypoglycemia Alarm Module for Closed-Loop Results of Four Different Basal Set-Point Values.
| Subject | Mean (mg/dL) at day 60 | Min (mg/dL) at day 60 | Max (mg/dL) at day 60 | Total # of predicted hypo preventions by rescue carbohydrates for all 60 days | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | |
| 1 | 139.9 | 148.5 | 154.3 | 163.6 | 103.0 | 98.0 | 104.0 | 106.0 | 203.0 | 210.0 | 214.0 | 224.0 | 225 | 197 | 164 | 145 |
| 2 | 140.1 | 146.1 | 148.3 | 157.4 | 91.0 | 91.0 | 95.0 | 102.0 | 204.0 | 214.0 | 225.0 | 237.0 | 307 | 274 | 253 | 238 |
| 3 | 143.0 | 151.4 | 159.7 | 167.0 | 104.0 | 104.0 | 108.0 | 107.0 | 211.0 | 221.0 | 227.0 | 237.0 | 242 | 222 | 189 | 163 |
| 4 | 129.6 | 134.3 | 147.3 | 154.5 | 90.0 | 86.0 | 83.0 | 101.0 | 201.0 | 213.0 | 216.0 | 240.0 | 300 | 290 | 277 | 245 |
| 5 | 121.4 | 131.4 | 152.3 | 160.4 | 87.0 | 85.0 | 99.0 | 98.0 | 212.0 | 219.0 | 239.0 | 253.0 | 299 | 266 | 243 | 234 |
| 6 | 128.4 | 139.5 | 146.7 | 145.5 | 99.0 | 108.0 | 108.0 | 94.0 | 185.0 | 194.0 | 203.0 | 213.0 | 212 | 189 | 168 | 150 |
| 7 | 135.6 | 140.4 | 144.7 | 152.9 | 93.0 | 106.0 | 104.0 | 101.0 | 202.0 | 218.0 | 227.0 | 244.0 | 212 | 198 | 194 | 180 |
| 8 | 144.7 | 155.3 | 164.5 | 164.4 | 99.0 | 105.0 | 109.0 | 106.0 | 210.0 | 225.0 | 235.0 | 245.0 | 250 | 236 | 206 | 167 |
| 9 | 149.7 | 160.8 | 166.0 | 178.0 | 100.0 | 105.0 | 117.0 | 115.0 | 219.0 | 245.0 | 237.0 | 257.0 | 192 | 172 | 140 | 125 |
| 10 | 129.8 | 137.4 | 145.8 | 155.6 | 94.0 | 86.0 | 94.0 | 102.0 | 201.0 | 207.0 | 218.0 | 228.0 | 333 | 288 | 234 | 223 |
| 11 | 125.9 | 135.5 | 144.9 | 145.4 | 88.0 | 83.0 | 91.0 | 94.0 | 209.0 | 221.0 | 227.0 | 237.0 | 331 | 305 | 277 | 252 |
| 12 | 129.1 | 138.2 | 148.8 | 161.0 | 90.0 | 91.0 | 96.0 | 94.0 | 213.0 | 218.0 | 225.0 | 241.0 | 256 | 229 | 219 | 183 |
| 13 | 144.6 | 153.7 | 171.1 | 166.6 | 95.0 | 104.0 | 115.0 | 113.0 | 217.0 | 208.0 | 234.0 | 235.0 | 136 | 100 | 87 | 59 |
| 14 | 133.0 | 135.5 | 149.7 | 158.0 | 90.0 | 93.0 | 96.0 | 100.0 | 219.0 | 224.0 | 241.0 | 254.0 | 326 | 308 | 274 | 258 |
| 15 | 135.7 | 147.8 | 141.1 | 147.8 | 107.0 | 114.0 | 85.0 | 93.0 | 193.0 | 205.0 | 215.0 | 221.0 | 152 | 133 | 125 | 127 |
| 16 | 140.6 | 141.4 | 153.1 | 156.9 | 90.0 | 101.0 | 107.0 | 108.0 | 190.0 | 200.0 | 210.0 | 224.0 | 295 | 272 | 237 | 203 |
| 17 | 138.7 | 154.6 | 154.8 | 164.5 | 79.0 | 97.0 | 98.0 | 96.0 | 223.0 | 235.0 | 240.0 | 252.0 | 328 | 304 | 292 | 269 |
| 18 | 161.6 | 169.6 | 174.1 | 179.8 | 101.0 | 105.0 | 102.0 | 101.0 | 245.0 | 252.0 | 263.0 | 269.0 | 158 | 141 | 108 | 83 |
| 19 | 129.3 | 139.5 | 150.6 | 149.6 | 101.0 | 100.0 | 108.0 | 97.0 | 190.0 | 194.0 | 208.0 | 212.0 | 222 | 193 | 191 | 157 |
| 20 | 147.7 | 157.8 | 167.1 | 168.9 | 105.0 | 110.0 | 112.0 | 109.0 | 218.0 | 228.0 | 241.0 | 253.0 | 172 | 158 | 116 | 93 |
| Average | 137.4 | 145.9 | 154.2 | 159.9 | 95.0 | 98.6 | 101.6 | 101.9 | 208.0 | 217.6 | 227.3 | 238.8 | 247 | 224 | 200 | 178 |
Average represents the mean of each index over all subjects.
Table 8.
P-values of Statistical Differences Between Day 1 and 60 Continuous Glucose Monitoring Values for Closed-Loop Results of Four Different Basal Set-Point Values.
| Subject | P-values of statistical differences between day 1 and 60 CGM values | |||
|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |
| 1 | 4.7×10^−25 | 4.4×10^−26 | 3.4×10^−34 | 1.5×10^−30 |
| 2 | 2.7×10^−47 | 8.9×10^−50 | 1.1×10^−53 | 1.9×10^−52 |
| 3 | 1.5×10^−53 | 2.4×10^−51 | 6.6×10^−48 | 4.3×10^−43 |
| 4 | 1.2×10^−27 | 1.7×10^−23 | 5.0×10^−22 | 5.9×10^−13 |
| 5 | 6.3×10^−50 | 2.9×10^−39 | 4.8×10^−29 | 8.6×10^−22 |
| 6 | 1.8×10^−73 | 3.2×10^−71 | 6.0×10^−75 | 2.3×10^−73 |
| 7 | 1.7×10^−21 | 2.2×10^−18 | 3.1×10^−25 | 9.6×10^−30 |
| 8 | 1.4×10^−30 | 8.6×10^−29 | 2.2×10^−28 | 1.1×10^−33 |
| 9 | 9.0×10^−35 | 1.9×10^−32 | 1.4×10^−22 | 8.4×10^−23 |
| 10 | 2.5×10^−43 | 5.8×10^−54 | 3.7×10^−54 | 2.0×10^−52 |
| 11 | 1.0×10^−50 | 4.1×10^−48 | 3.5×10^−60 | 1.2×10^−73 |
| 12 | 3.6×10^−45 | 9.8×10^−43 | 2.6×10^−53 | 2.2×10^−47 |
| 13 | 2.9×10^−33 | 1.2×10^−48 | 2.1×10^−23 | 1.7×10^−57 |
| 14 | 1.1×10^−47 | 1.3×10^−41 | 6.9×10^−33 | 7.5×10^−19 |
| 15 | 8.8×10^−42 | 2.8×10^−46 | 1.2×10^−62 | 9.6×10^−72 |
| 16 | 9.9×10^−36 | 4.7×10^−59 | 1.2×10^−59 | 1.9×10^−65 |
| 17 | 2.6×10^−33 | 3.3×10^−29 | 7.0×10^−34 | 4.3×10^−28 |
| 18 | 1.5×10^−40 | 2.7×10^−40 | 2.1×10^−33 | 2.0×10^−32 |
| 19 | 1.3×10^−51 | 4.8×10^−48 | 1.9×10^−47 | 7.5×10^−49 |
| 20 | 1.3×10^−93 | 2.0×10^−89 | 4.7×10^−83 | 2.0×10^−81 |
| Average | 8.5×10^−23 | 1.1×10^−19 | 3.3×10^−23 | 2.9×10^−14 |
Abbreviation: CGM, continuous glucose monitoring.
The MET, or simply metabolic equivalent, is a physiological measure expressing the energy cost of physical activity (PA) and is defined as the ratio of metabolic rate (and therefore the rate of energy consumption) during a specific PA to a reference metabolic rate. MET is used as a means of expressing the intensity and energy expenditure of activities in a way that is comparable among people. We have developed a real-time MET estimation algorithm by using noninvasive measurements of physiological variables. In this approach, the MET values are computed by using heart rate, galvanic skin response, skin temperature, blood volume pulse, and accelerometer information streaming in real time from a wristband.52
The controller set-point for the current time is defined based on the condition of the patient at that sampling time and historical data are used to define the controller set-point for future values over the prediction/control horizon. The controller set-point values for the anticipated meal and exercise disturbance time windows of the new day are calculated by averaging the assigned set-point values for the specific time in the historical data which corresponds with the prediction time. In these results, all historical data are used. For example, if historical data shows that a patient consumes a meal around 12:00 pm but the presence of meal is not detected at 12:00 today, the set-point for 12:00 would be the default value (eg, 110 mg/dL) even though the controller has used a set-point of 80 mg/dL for that specific time in the previous sampling times. We observed that this modification increases the amount of PIC without increasing the risk of hypoglycemia. Considering a set-point of 80 mg/dL for the future sampling time based on historical data does not imply that the real CGM values will be driven to that value by controller. Rather, it means that the controller would act more aggressively if a meal consumption is detected. However, we anticipate implementing machine learning techniques in future work to implement a moving window for historical data. It may also be suitable to provide the option of modifying the controller set-point based historical data as a user choice. If a user has a well-organized life with regular exercise and meal habits, she/he can use this option to provide better glycemic control in a fully automated manner without meal and exercise announcements.
Conclusion
In this work, a CPAMS is proposed for our mAP that uses an adaptive learning MPC. The mAP efficiency is evaluated online by means of different key KPIs. CPAMS can learn the user’s habits and activities over time by utilizing historical data such as CGM data and physiological variables. The proposed adaptive learning mAP system integrated with the CPAMS is evaluated by simulation studies. Different amounts and times for meal consumption and various exercise sessions were used on different days to challenge the mAP system and assess its performance. The results illustrate that a significant improvement can be achieved in controller performance by using CPAMS without causing any hypoglycemia or hyperglycemia episodes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIDDK DP3 DK101075-01 and DP3 DK101077-01, and Juvenile Diabetes Research Foundation grant A18-0036-001 made possible through collaboration between the JDRF and The Leona M. and Harry B. Helmsley Charitable Trust.
ORCID iD: Ali Cinar  https://orcid.org/0000-0002-1607-9943
https://orcid.org/0000-0002-1607-9943
References
- 1. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cameron F, Ly TT, Forlenza GP, et al. Inpatient clinical trial of a fully closed-loop artificial pancreas using only CGM and accelerometer data for insulin dosing. Diabetes Technol Ther. 2016;18:A23-A24. [Google Scholar]
- 4. Jacobs PG, El Youssef J, Reddy R, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18(11):1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramkissoon CM, Bertachi A, Beneyto A, Bondia J, Vehi J. Detection and control of unannounced exercise in the artificial pancreas without additional physiological Signals. IEEE J Biomed Heal Inf. 2019. [DOI] [PubMed] [Google Scholar]
- 6. Beneyto A, Bertachi A, Bondia J, Vehi J. A new blood glucose control scheme for unannounced exercise in type 1 diabetic subjects. IEEE Trans Control Syst Technol. 2018. [Google Scholar]
- 7. Turksoy K, Hajizadeh I, Littlejohn E, Cinar A. Multivariate statistical monitoring of sensor faults of a multivariable artificial pancreas. IFAC-PapersOnLine. 2017;50(1):10998-11004. [Google Scholar]
- 8. Ly TT, Weinzimer SA, Maahs DM, et al. Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes. 2017;18(5):348-355. [DOI] [PubMed] [Google Scholar]
- 9. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sánchez-Peña R, Colmegna P, Grosembacher L, et al. Artificial pancreas: first clinical trials in Argentina. IFAC-PapersOnLine. 2017;50(1):7731-7736. [Google Scholar]
- 11. Cameron FM, Ly TT, Buckingham BA, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527-532. doi: 10.1089/dia.2017.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinsker JE, Lee JB, Dassau E, et al. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39(7):1135-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forlenza GP, Deshpande S, Ly TT, et al. Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care. 2017;40(8):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia CE, Prett DM, Morari M. Model predictive control: theory and practice–A survey. Automatica. 1989;25(3):335-348. [Google Scholar]
- 17. Rawlings JB, Mayne DQ. Model Predictive Control: Theory and Design. Madison, WI: Nob Hill Publishing; 2009. [Google Scholar]
- 18. Schäfer J, Cinar A. Multivariable MPC system performance assessment, monitoring, and diagnosis. J Process Control. 2004;14(2):113-129. [Google Scholar]
- 19. Huang B, Shah SL. Performance Assessment of Control Loops: Theory and Applications. New York: Springer Science & Business Media; 2012. [Google Scholar]
- 20. Incremona GP, Messori M, Toffanin C, Cobelli C, Magni L. Model predictive control with integral action for artificial pancreas. Control Eng Pr. 2018;77:86-94. [Google Scholar]
- 21. Shi D, Dassau E, Doyle FJ., III Adaptive zone model predictive control of artificial pancreas based on glucose-and velocity-dependent control penalties. IEEE Trans Biomed Eng. 2018;66(4):1045-1054. doi: 10.1109/TBME.2018.2866392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905. [DOI] [PubMed] [Google Scholar]
- 23. Gondhalekar R, Dassau E, Doyle FJ. Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat type 1 diabetes. Automatica. 2016;71:237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakrabarty A, Zavitsanou S, Doyle FJ, Dassau E. Event-triggered model predictive control for embedded artificial pancreas systems. IEEE Trans Biomed Eng. 2018;65(3):575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Messori M, Kropff J, Del Favero S, et al. Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol Ther. 2017;19(10):560-571. [Google Scholar]
- 26. Boiroux D, Duun-Henriksen AK, Schmidt S, et al. Overnight glucose control in people with type 1 diabetes. Biomed Signal Process Control. 2018;39:503-512. [Google Scholar]
- 27. El Fathi A, Smaoui MR, Gingras V, Boulet B, Haidar A. The artificial pancreas and meal control: an overview of postprandial glucose regulation in type 1 diabetes. IEEE Control Syst Mag. 2018;38(1):67-85. [Google Scholar]
- 28. Hajizadeh I, Rashid M, Cinar A. Plasma-insulin-cognizant adaptive model predictive control for artificial pancreas systems. J Process Control. 2019;77:97-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laguna Sanz AJ, Doyle FJ, III, Dassau E. An enhanced model predictive control for the artificial pancreas using a confidence index based on residual analysis of past predictions. J Diabetes Sci Technol. 2017;11(3):537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng J, Hajizadeh I, Yu X, et al. Multi-level supervision and modification of artificial pancreas control system. Comput Chem Eng. 2018;112:57-69. doi: 10.1016/J.COMPCHEMENG.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hajizadeh I, Samadi S, Sevil M, Rashid M, Cinar A. Performance assessment and modification of an adaptive model predictive control for automated insulin delivery by a multivariable artificial pancreas. Ind Eng Chem Res. 2019;58(26);11506-11520. doi: 10.1021/acs.iecr.8b06202. [DOI] [Google Scholar]
- 32. Shi D, Dassau E, Doyle FJ., III Multivariate learning framework for long-term adaptation in the artificial pancreas. Bioeng Transl Med. 2019;4(1):61-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palerm CC, Zisser H, Jovanovič L, Doyle FJ., III A run-to-run framework for prandial insulin dosing: handling real-life uncertainty. Int J Robust Nonlin. 2007;17(13):1194-1213. [Google Scholar]
- 34. Palerm CC, Zisser H, Jovanovič L, Doyle FJ., III A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes. J Process Control. 2008;18(3-4):258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toffanin C, Visentin R, Messori M, Di Palma F, Magni L, Cobelli C. Toward a run-to-run adaptive artificial pancreas: in silico results. IEEE Trans Biomed Eng. 2017;65(3):479–488. [DOI] [PubMed] [Google Scholar]
- 36. Toffanin C, Messori M, Cobelli C, Magni L. Automatic adaptation of basal therapy for type 1 diabetic patients: a run-to-run approach. Biomed Signal Process Control. 2017;31:539-549. [Google Scholar]
- 37. Magni L, Forgione M, Toffanin C, et al. Run-to-run tuning of model predictive control for type 1 diabetes subjects: in silico trial. J Diabetes Sci Technol. 2009;3(5):1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Gao F, Doyle FJ., III Survey on iterative learning control, repetitive control, and run-to-run control. J Process Control. 2009;19(10):1589-600. [Google Scholar]
- 39. Wang Y, Dassau E, Doyle FJ., III Closed-loop control of artificial pancreatic $β$-cell in type 1 diabetes mellitus using model predictive iterative learning control. IEEE Trans Biomed Eng. 2009;57(2):211-219. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Zhang J, Zeng F, et al. “‘Learning’” can improve the blood glucose control performance for type 1 diabetes mellitus. Diabetes Technol Ther. 2017;19(1):41-48. [DOI] [PubMed] [Google Scholar]
- 41. Messori M, Incremona GP, Cobelli C, Magni L. Individualized model predictive control for the artificial pancreas: in silico evaluation of closed-loop glucose control. IEEE Control Syst Mag. 2018;38(1):86-104. [Google Scholar]
- 42. Resalat N, El Youssef J, Reddy R, Castle J, Jacobs PG. Adaptive tuning of basal and bolus insulin to reduce postprandial hypoglycemia in a hybrid artificial pancreas. J Process Control. 2019;80:247-254. [Google Scholar]
- 43. Herrero P, Bondia J, Giménez M, Oliver N, Georgiou P. Automatic adaptation of basal insulin using sensor-augmented pump therapy. J Diabetes Sci Technol. 2018;12(2):282-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller S, Nimri R, Atlas E, Grunberg EA, Phillip M. Automatic learning algorithm for the MD-logic artificial pancreas system. Diabetes Technol Ther. 2011;13(10):983-990. [DOI] [PubMed] [Google Scholar]
- 45. Campos-Cornejo F, Campos-Delgado DU, Espinoza-Trejo D, et al. An advisory protocol for rapid-and slow-acting insulin therapy based on a run-to-run methodology. Diabetes Technol Ther. 2010;12(7):555-565. [DOI] [PubMed] [Google Scholar]
- 46. Hajizadeh I, Rashid M, Samadi S, et al. Multivariable AP with adaptive control. In: Sánchez-Peña RS, Cherñavvsky DR, eds. The Artificial Pancreas. US: Academic Press; 2019: 59–77. [Google Scholar]
- 47. Hajizadeh I, Rashid M, Turksoy K, et al. Incorporating unannounced meals and exercise in adaptive learning of personalized models for multivariable artificial pancreas systems. J Diabetes Sci Technol. 2018;12(5):953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hajizadeh I, Rashid M, Turksoy K, et al. Plasma insulin estimation in people with type 1 diabetes mellitus. Ind Eng Chem Res. 2017;56(35):9846-9857. [Google Scholar]
- 49. Hajizadeh I, Rashid M, Samadi S, et al. Adaptive and personalized plasma insulin concentration estimation for artificial pancreas systems. J Diabetes Sci Technol. 2018;12(3):639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Samadi S, Rashid M, Sevil M, et al. Multivariable simulation platform for type 1 diabetes mellitus. The 18th Annual Diabetes Technology Meeting; North Bethesda, Maryland; Novermber 8-10, 2018. [Google Scholar]
- 51. Perkins BA, Riddell MC. Type 1 diabetes and exercise: using the insulin pump to maximum advantage. Can J Diabetes. 2006;30(1):72-79. [Google Scholar]
- 52. Sevil M, Rashid M, Maloney Z, et al. Real-time estimation of energy expenditure using noninvasive wearable sensors for multivariable artificial pancreas system. 5th IEEE Conference on Biomedical and Health Informatics, At Treasure Island Hotel; 2018; Las Vegas, NV. [Google Scholar]