There is increasing recognition of the importance of real-world data to understand the effectiveness of treatments for type 1 diabetes (T1D). The T1D Exchange Glu online community (myglu.org) includes a diverse group of people with T1D and caregivers, and provides a platform for meaningful research to amplify the collective voice of those living with T1D.1-6
We present findings from a survey of the T1D Exchange Glu online community evaluating self-reported clinical outcomes and patient preferences impacting treatment choice in tubeless insulin pump users (Omnipod® Insulin Management System, Insulet Corp., Acton, MA, United States) compared to their previous treatment. Eligibility criteria were age ≥18 years, T1D diagnosis ≥1 year, currently using Omnipod System for 0.5 to 2 years, and previously treated with multiple daily injections (MDI) or a tubed insulin pump. Outcomes included self-reported hemoglobin A1c (HbA1c) range and total daily dose (TDD) of insulin with prior and current therapy, treatment patterns on prior therapy, treatment preferences, and factors impacting treatment choice.
Respondent characteristics (N = 147) were (mean ± SD, range): age 41.0 ± 13.8 years (18-73 years) and diabetes duration 17.8 ± 14.2 years (1-52 years). Prior treatment modality was 72% MDI and 28% insulin pump. Most respondents (71%) had been using the tubeless insulin pump system between one and two years.
HbA1c results are summarized in Figure 1. Self-reported TDD of insulin decreased significantly post-tubeless pump for prior MDI users (53 ± 37 to 42 ± 25 U/day, P < .0001, N = 95) and prior pump users (43 ± 18 to 38 ± 15 U/day, P < .001, N = 40).
Figure 1.
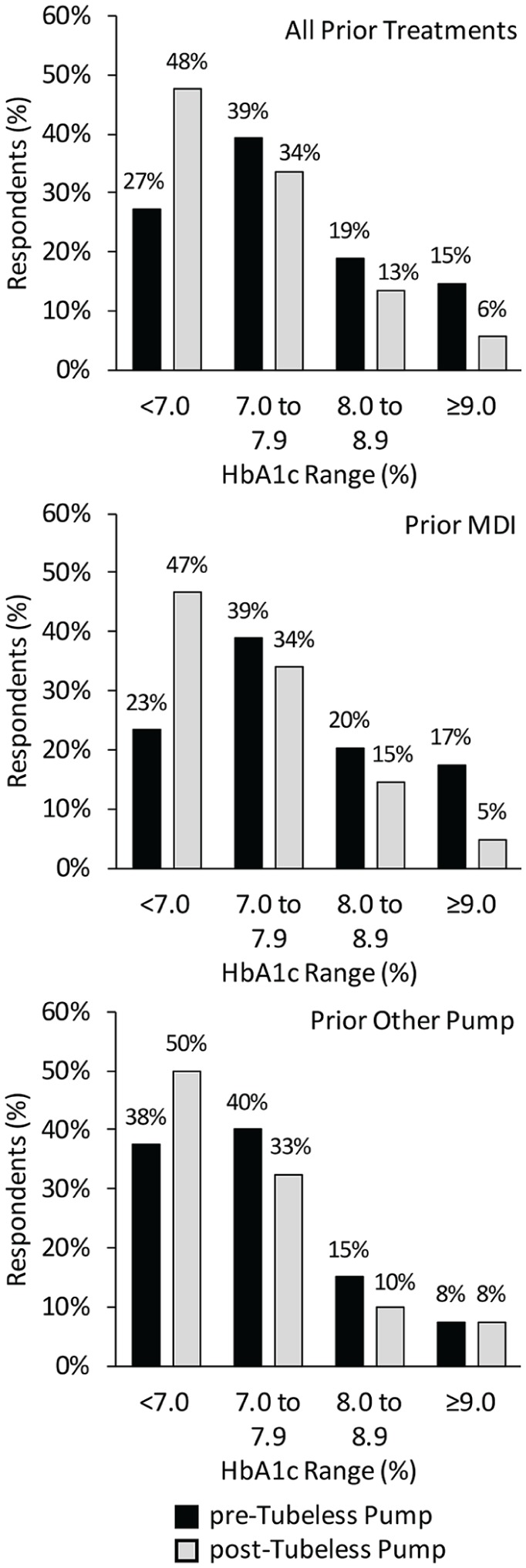
Change in self-reported HbA1c post-tubeless insulin pump therapy initiation compared to prior treatment modality.
The percentage of respondents reporting HbA1c within a given range pre-tubeless insulin pump (black bars) and post-tubeless insulin pump (gray bars) is plotted for all respondents (N = 143) and stratified by prior therapy: prior multiple daily injections (N = 103) and prior other pump (N = 40). The proportion of respondents meeting the American Diabetes Association(ADA)7 treatment target of HbA1c <7% was significantly higher post-tubeless pump overall (P < .0001) and for prior multiple daily injection users (P < .0001), and was higher for prior users of other insulin pumps but did not reach significance (P = .13). Overall, there was a significant (P < .0001) shift in self-reported HbA1c range from high to lower levels associated with tubeless insulin pump use.
Treatment patterns on prior therapy included bolus injections at least three times per day for 87% of prior MDI users. Forty-four percent of prior pump users reported disconnecting their tubed pump an average of at least two times per day. The most commonly reported duration of pump disconnection was 10 to 30 minutes (65% of respondents), with 22% reporting disconnecting for >30 minutes to one hour.
The decision to initiate tubeless insulin pump therapy was made primarily by respondents (46%) or with some input from their healthcare provider (45%). Respondents’ three highest-ranked reasons for stopping prior therapy to initiate tubeless pump therapy included wanting better control of diabetes and wanting to lower HbA1c, as well as wanting more lifestyle flexibility for prior MDI users and not wanting to be connected to tubing for prior pump users. The most frequently reported benefits of the tubeless pump were lifestyle improvements (85%), improvement in glucose control (73%), lower HbA1c (60%), and ability to exercise more (50%).
This survey study of the T1D Exchange Glu online community demonstrated that tubeless insulin pump therapy was associated with an improvement in self-reported HbA1c and a significant reduction in daily insulin use over up to two years compared to prior treatment with MDI or other insulin pumps. Perceived improvement in diabetes management and features of the tubeless insulin pump that supported lifestyle flexibility were significant factors in the adoption of this technology. The results of this study underscore the importance of access and choice of diabetes management technology to positively impact treatment satisfaction and outcomes.
Acknowledgments
The authors wish to thank the T1D Exchange for their support of the study. They also thank Howard Zisser, MD, of Verily Life Sciences LLC for his contributions to the study design and Jialun He, PhD, and Kate Dryga, MS, of Insulet Corp. for analysis and editorial support.
Footnotes
Authors’ Note: Data from this manuscript were previously presented at the American Diabetes Association 77th Scientific Sessions in San Diego, CA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The study was sponsored by Insulet Corporation and conducted by the T1D Exchange, a program of Unitio, Inc. Drs Ly and Huyett are full-time employees of Insulet Corporation. Dr Layne was an employee of Insulet Corporation at the time the study was completed.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was sponsored by Insulet Corporation.
ORCID iD: Trang T. Ly  https://orcid.org/0000-0002-5995-1447
https://orcid.org/0000-0002-5995-1447
References
- 1. Pinsker JE, Schoenberg BE, Garey C, Runion A, Larez A, Kerr D. Perspectives on long-distance air travel with type 1 diabetes. Diabetes Technol Ther. 2017;19(12):744-748. [DOI] [PubMed] [Google Scholar]
- 2. Forlenza GP, Argento NB, Laffel LM. Practical considerations on the use of continuous glucose monitoring in pediatrics and older adults and nonadjunctive use. Diabetes Technol Ther. 2017;19(S3):S13-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinsker JE, Kraus A, Gianferante Det al. Techniques for exercise preparation and management in adults with type 1 diabetes. Can J Diabetes. 2016;40(6):503-508. [DOI] [PubMed] [Google Scholar]
- 4. Barnard K, Crabtree V, Adolfsson Pet al. Impact of type 1 diabetes technology on family members/significant others of people with diabetes. J Diabetes Sci Technol. 2016;10(4):824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sampson Perrin AJ, Guzzetta RC, Miller KMet al. A web-based study of the relationship of duration of insulin pump infusion set use and fasting blood glucose level in adults with type 1 diabetes. Diabetes Technol Ther. 2015;17(5):307-310. [DOI] [PubMed] [Google Scholar]
- 6. Bevan A. T1D exchange: an online community for people touched by type 1 diabetes. AADE Pract. 2017;5(4):44-46. [Google Scholar]
- 7. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42(S1):S61-S70. [DOI] [PubMed] [Google Scholar]


