Abstract
Point-of-care test (POCT) HbA1c assays provide rapidly available results for clinical decision-making. Accuracy and precision must be established. Venous blood samples from 300 patients were assayed for HbA1c by a laboratory technician (“laboratory assessment”) with the POCT Alere Afinion™ assay and a laboratory (Premier AffinityTM) assay. POCT results from 402 patients’ fingerstick samples assayed by nine nontechnician staff (“clinical assessment”) were compared with the laboratory assay. The laboratory assessment showed tight correlation (r2 = .977, P < .001) between the assays. Mean absolute and relative differences were 0.01 percentage points and 2.1%, respectively. CVs for the POCT and laboratory assays were <2% and <1%, respectively. The clinical assessment also showed a tight correlation between the assays (r2 = .978, P < .001), with mean absolute and relative differences of 0.2 percentage points and 3.41%, respectively. CV for the POCT assay was <2%. The POCT performed acceptably compared to the laboratory assay under realistic clinical conditions.
Keywords: glycohemoglobin, HbA1c, point of care
The glycated hemoglobin assay (glycohemoglobin, HbA1c) has become the dominant measure of chronic glycemia.1,2 With the demonstration of the causal role of chronic hyperglycemia in the pathogenesis of diabetes complications, the central role of controlling chronic glycemia in diabetes care,3,4 and its role in diagnosis,5,6 assays for glycated hemoglobin (HbA1c) have proliferated.
The National Glycohemoglobin Standardization Program (NGSP)7 has been able to align the vast majority of HbA1c assays in the U.S. and in other parts of the world with the Diabetes Control and Complications Trial (DCCT) assay.3 In addition, the NGSP has facilitated improved precision and accuracy of the commercially available HbA1c assays. The majority of the approved assays are laboratory-based; however, point-of-care test (POCT) assays performed outside of laboratories have been recognized to have advantages over central laboratory assays. First, assays performed in clinicians’ offices can provide immediate feedback to the patient and allow adjustments in treatment at the time of the office visit. Second, office-based assays are more convenient for patients as they do not require a separate visit to a central laboratory or blood drawing facility. Third, the POCT assays are more convenient for clinicians since HbA1c results can be communicated to the patient at the time of the visit and not require a separate communication to relay the results and adjust the treatment regimen accordingly. The immediate provision of HbA1c results at the time of the office visit has been shown to result in improved glycemic control.8
The main stumbling block in introducing POCT assays to clinical use has been that most assays have shown generally less precise and accurate results than the gold-standard laboratory-based assays.9-18 Most comparison studies have not been pragmatic in that they relied on laboratory professionals to perform the POCT assays, usually on whole blood samples, rather than nonlab personnel who would most likely be performing such assays.8-10,13,14-17,19-21 Therefore, the results of many comparison studies have likely maximized performance of the POCT assays and minimized the actual differences between POCT and lab-based assays that would occur in clinical practice.
In the laboratory assessment part of the current study, a laboratory technician performed a POCT assay (Alere Afinion™ AS100, Abbott Park, IL, USA) on whole venous blood samples and the results were compared to those from an NGSP-certified, DCCT-aligned laboratory assay.22 In the clinical assessment, the POCT assay was performed using fingerstick capillary samples, as indicated by the manufacturer, by nonlaboratory health care professionals in the clinic.
Materials and Methods
During the laboratory assessment, venous blood samples were taken from patients (N = 300) during their clinic visits between February 2017 and June 2017. The POCT assay was performed in the MGH Diabetes Center laboratory by a laboratory technician. The same technician measured HbA1c using the laboratory-based assay (Premier Affinity™, Trinity Biotech, Ireland). In the MGH Diabetes Center laboratory, the Premier assay has a coefficient of variation (CV) < 0.9%. The POCT assay was performed on discarded venous blood from the same sample used in the laboratory-based assay. The laboratory-based assay used daily quality control (QC) samples while the POCT performed QC monthly, as per manufacturer recommendations with a single reagent lot (#10185416).
In the clinical assessment, fingerstick blood samples were collected from 402 patients by nine nontechnician members of the MGH Diabetes Center. The POCT assay was performed by the nine staff members, including a medical assistant, a nurse, four nurse practitioners, and three physicians, during clinic visits between January and October 2018. Each examiner performed approximately 50 POCT assays per manufacturer’s directions, using his/her own individual device throughout the study. The nine staff were taught how to perform the assay by a lab technician. Venous blood samples were collected from the patients during the clinic visit and the laboratory-based assay was performed within 24 hours.
HbA1c results from each study were compiled and analyzed using linear regression and Bland-Altman analyses (Excel 2016, Microsoft, Redmond, WA, USA). To assess the precision of the POCT assays during the laboratory assessment, CVs were calculated using repeated assays (n = 5) performed for low (5 to 6.9%), midrange (7 to 8.9%), and high (≥9%) HbA1c patient samples. During the clinical assessment, the precision of the POCT assay was analyzed using 4 to 5 repeated assays performed by two staff members, on low, midrange, and high HbA1c patient fingerstick samples. Interoperator performance using the POCT assay was also compared, simultaneously examining the slope and intercept of the regression lines. Accuracy of the POCT was compared among the physicians group, the nurse group, and the medical assistant, to examine whether the POCT assay could be implemented into a practice setting and the level of personnel required.
The study was supported by a grant and donated supplies from the manufacturer. The manufacturer had no role in the design, conduct, analysis of results, or preparation of the manuscript. Only the laboratory-based HbA1c assay results were used in clinical decision-making. The study was approved by the MGH Human Research Committee and informed consent was obtained from all participants in the clinical assessment.
Results
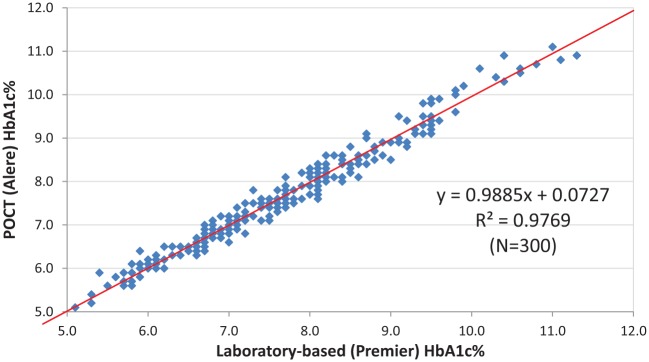
The laboratory assessment showed that the POCT assay had CVs < 2%, based on repeated assays, for all HbA1c ranges (Table 1). The laboratory-based assay had a CV < 1% for all ranges. The laboratory assessment comparison of 300 patient samples revealed a correlation of r2 = .977, P < .001 (Figure 1a). The absolute mean difference, derived from the Bland-Altman analysis, was 0.01 HbA1c percentage points (indicating a nonbiased distribution of differences) (Figure 1b) and the relative mean difference was 2.1%. In total, 72% of the assays were within ±0.2 and 97% were within ±0.4 of the laboratory assay.
Table 1.
Precision (Coefficients of Variation) of Laboratory and POCT Assays Based on Repeated Assays (n = 4-5).
| Laboratory assaya
technician performed |
POCTb
technician performed |
Clinician POCTb | |
|---|---|---|---|
| Low (5 to 6.9%) | <0.10% | 1.00% | 1.39% |
| Medium (7 to 8.9%) | <0.10% | 1.18% | 1.42% |
| High (9 and above) | 0.90% | 0.78% | 1.54% |
Premier Affinity laboratory assay.
Alere Afinion POCT assay.
Figure 1a.
Laboratory assessment. Linear regression between the Premier™ laboratory assay and the Alere Afinion assay, both performed on venous blood samples by a laboratory technician.
Figure 1b.
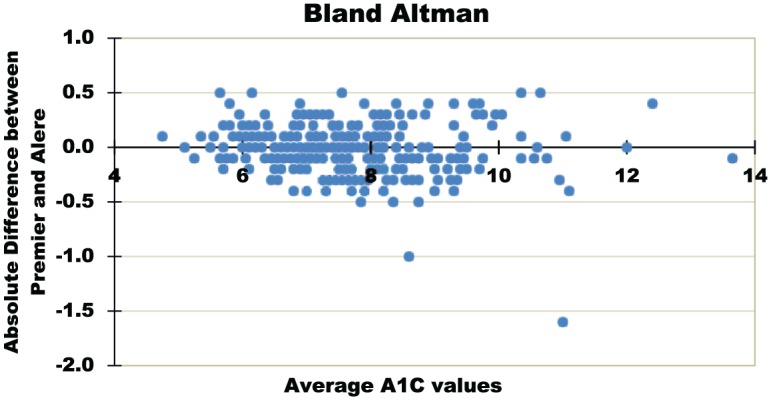
Laboratory assessment. Bland-Altman analysis of laboratory-based, Premier Affinity assay and the POCT, Alere Afinion assay, both performed on venous blood samples by a laboratory technician. N = 300.
The clinical assessment showed again that the POCT assay had acceptable precision based on repeated (n = 4-5) assays of samples divided into low, medium and high HbA1c values (Table 1). The low range (5.0% to 6.9%) CV was 1.39%, the midrange (7.0% to 8.9%) was 1.42%, and the high range (>9.0%) was 1.54%. The CV of the laboratory assay remained <0.9% during the clinical assessment.
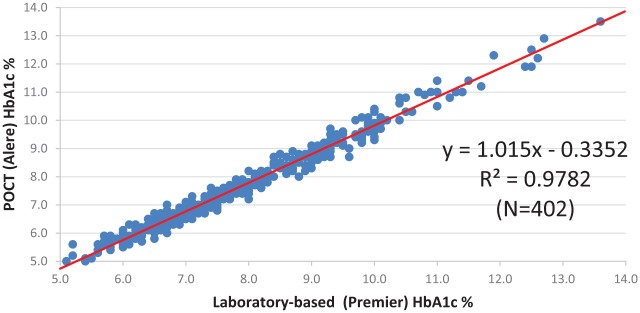
The comparison between the gold-standard laboratory method and the POCT assay used in the clinical setting revealed a correlation of r2 = .978, P < .001, mirroring the results from the laboratory assessment (Figure 2a). The mean absolute and relative differences were 0.2 percentage points and 3.41%, respectively. The Bland-Altman analysis (Figure 2b) showed that 53% of the POCT assays were within ±0.2 and 85% were within ±0.4 of the laboratory assay. There was a negative bias of 0.2, indicating that the POCT values were on average 0.2 HbA1c percentage points lower than the laboratory values.
Figure 2a.
Clinical assessment. Linear regression between the Premier™ laboratory assay and the Alere Afinion assay, with the laboratory-based assay performed on venous blood samples by a laboratory technician and the POCT assay performed on fingerstick capillary samples by nontechnician clinical staff.
Figure 2b.
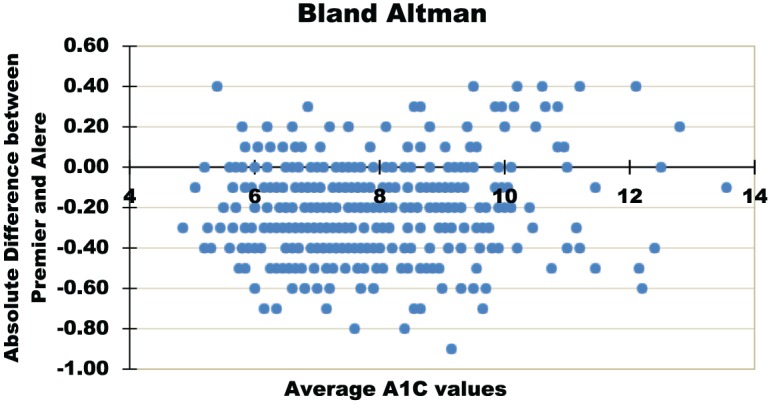
Clinical assessment. Bland-Altman analysis of laboratory-based, Premier Affinity assay and the POCT, Alere Afinion assay. The laboratory-based assay was performed on venous blood samples by a laboratory technician and the POCT assay was performed on fingerstick capillary samples by nontechnician clinical staff. N = 402.
To assess the projected variability of the POCT on the HbA1c target value, we examined the POCT values when the laboratory HbA1c was 7.0%. The range of values for the different clinicians ranged from 6.6% (n = 1) to 6.8% (n = 4), reflecting the negative bias of the POCT results compared to the laboratory assay.
Interoperator performance using the POCT assay was also compared, simultaneously examining the slope and intercept of the regression lines. Comparisons among the physicians group, the nurses group and the medical assistant, showed no significance differences between the physicians group and the nurses group (interaction = .121, P = .406 ) or between the nurses group and the medical assistant (interaction = .725, P = .756), but a significant difference between the physicians group and the single medical assistant (interaction = .507, P = .013).
Discussion
The laboratory assessment demonstrated that in the ideal setting with a trained laboratory technician, the POCT assay performs acceptably compared with a laboratory-based assay. Several DCCT-aligned and NGSP certified POCT assays are commercially available22 with FDA approval through CLIA waiver for use as diabetes management tools.23 When performed in ideal conditions by a trained laboratory technician, several of the approved assays have also been shown to perform acceptably when compared to a laboratory-based assay.9-14 The level of training of the staff performing POCT assays (laboratory technician or care provider) is often unclear.18,24
Our clinical assessment used POCT devices in the clinical setting for which they were designed. Historically, the precision and accuracy of POCT HbA1c assays have suffered when performed in the clinical setting by nonlaboratory staff,11 even when in prior studies the same assays performed by laboratory technicians had been shown to correlate well with laboratory-based assays. The major differences between the laboratory and clinical assessments in the current study include: (1) in the laboratory assessment, assays were performed on discarded venous samples as opposed to using fingerstick samples in the clinical study; and (2) a single laboratory technician performed the POCT assays in the laboratory as opposed to having nine nontechnician staff perform the assays during the clinical assessment, as per manufacturer’s instructions. The limitations of the clinical assessment study include the use of professional clinical staff who were expert in diabetes care, albeit not laboratory technicians. This may limit the generalizability of the current study.
Conclusion
The POCT assay performed acceptably under realistic clinical conditions, albeit with a slightly negative bias compared with the laboratory result.
Footnotes
Authors’ Note: This manuscript has not been previously presented in any format.
Abbreviations: CLIA, Clinical Laboratory Approval Amendments; CV, coefficient of variation; DCCT, Diabetes Control and Complications Trial; HbA1c, hemoglobin A1c; MGH, Massachusetts General Hospital; NGSP, National Glycohemoglobin Standardization Program; POCT, point-of-care test; QC, quality control.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded through an investigator-initiated funding request to Alere, the manufacturer of the Afinion point-of-care assay. Alere had no role in the design, conduct, analysis of data, or the preparation of this publication. Alere supplied the equipment, reagents, and other supplies necessary to perform the POCT assay. EB and FMP were supported by a T32 grant (#T32 DK007028) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
ORCID iDs: Francesca M. Perez  https://orcid.org/0000-0002-8449-9835
https://orcid.org/0000-0002-8449-9835
Erica Basque  https://orcid.org/0000-0001-8766-0789
https://orcid.org/0000-0001-8766-0789
References
- 1. Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341-346. [DOI] [PubMed] [Google Scholar]
- 2. Sacks DB, Arnold M, Bakris GL, et al. Executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:793-798. [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:978-986. [DOI] [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonyureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 5. Nathan DM, Balkau B, Bonora E, et al. for the International Expert Committee on the Diagnosis of Diabetes. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S13-S27. [DOI] [PubMed] [Google Scholar]
- 7. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE; NGSP Steering Committee. National Glycohemoglobin Standardization Program: a five-year progress report. Clin Chem. 2001;47:1985. [PubMed] [Google Scholar]
- 8. Cagliero E, Levina E, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22:1785-1789. [DOI] [PubMed] [Google Scholar]
- 9. Torregrosa ME, Molina J, Argente CR, Ena J. Accuracy of three hemoglobin A1c point-of-care systems for glucose monitoring in patients with diabetes mellitus. Endocrinol Nutr. 2015;62:478. [DOI] [PubMed] [Google Scholar]
- 10. Affret A, Griz LH, Cesse EÂ, Specht Y, Carvalho EM, Fontbonne A. Assessment of a glycated hemoglobin point-of-care analyzer (A1CNow+) in comparison with an immunoturbidimetric method: a diagnostic accuracy study. Sao Paulo Med J. 2015;133:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leca V, Ibrahim Z, Maraninchi M, et al. Point-of-care measurements of HbA1c: simplicity does not mean laxity of controls. Diabetes Care. 2012;35:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strauss SM, Rosedale M, Pesce MA, et al. Point-of-care HbA1c testing with the A1cnow test kit in general practice dental clinics: a pilot study involving its accuracy and practical issues in its use. Point Care. 2014;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manley SE, Hikin LJ, Round RA, et al. Comparison of IFCC-calibrated HbA(1c) from laboratory and point of care testing systems. Diab Res Clin Pract. 2014;105:364. [DOI] [PubMed] [Google Scholar]
- 14. Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem. 2014;60:1062-1072. [DOI] [PubMed] [Google Scholar]
- 15. Paknikar S, Sarmah R, Sivaganeshan L, et al. Long-term performance of point-of-care hemoglobin A1c assays. J Diabetes Sci Technol. 2016;10:1308-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou R, Gao Z-Q, Tong Q, et al. Improvement in the quality of HbA1c determination by using commutable specimens with IFCC-values. Lab Med. 2017;48:146-153. [DOI] [PubMed] [Google Scholar]
- 17. Lee K, Jun SH, Han M, et al. Performance evaluation of SD A1cCare as a HbA1c analyzer for point-of-care testing. Clin Biochem. 2015;48:625. [DOI] [PubMed] [Google Scholar]
- 18. Solvik UO, Roraas T, Christensen NG, Sandberg S. Diagnosing diabetes mellitus: performance of hemoglobin A1c point-of-care instruments in general practice offices. Clin Chem. 2013;59:1790-1801. [DOI] [PubMed] [Google Scholar]
- 19. Abai NS, Nyirenda M, Reddy T, Ramjee G. Good correlation between the Afinion AS100 analyser and the ABX Pentra 400 analyser for the measurement of glycosylated heamoglobin and lipid levels in older adults in Durban, South Africa. S Afr Med J 2017;108:50-55. [DOI] [PubMed] [Google Scholar]
- 20. Jain A, Rao N, Sharifi M, et al. Evaluation of point of care Afinion AS100 analyser in a community setting. Ann Clin Biochem 2017;54:331-341. [DOI] [PubMed] [Google Scholar]
- 21. Sobolesky PM, Smith BE, Saenger AK, et al. Multicenter assessment of hemoglobin A1c point-of-care device for diagnosis of diabetes mellitus. Clin Biochem. 2018;61:18-22.16, 19-21. [DOI] [PubMed] [Google Scholar]
- 22. National Glycohemoglobin Standardization Program. List of NGSP Certified Methods. Available at: http://www.ngsp.org/certified.asp. Accessed July 2017.
- 23. US Food and Drug Administration. FDA Executive Summary Clinical Chemistry and Clinical Toxicology Devices Panel. Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ClinicalChemistryandClinicalToxicologyDevicesPanel/UCM511599.pdf. Accessed July 2017.
- 24. Manthei DM, Wesener N, Twarkowski D, Giacherio DA, Schoeder LF. Retrospective accuracy study of point-of-care hemoglobin A1c in field conditions. Clin Chem. 2017;63:780-783. [DOI] [PubMed] [Google Scholar]