The release of extracellular vesicles (EVs) by fungi is a fundamental cellular process. EVs carry several biomolecules, including pigments, proteins, enzymes, lipids, nucleic acids, and carbohydrates, and are involved in physiological and pathological processes. EVs may play a pivotal role in the establishment of fungal infections, as they can interact with the host immune system to elicit multiple outcomes.
KEYWORDS: drug targets, extracellular vesicles, fungal infections, immune response
ABSTRACT
The release of extracellular vesicles (EVs) by fungi is a fundamental cellular process. EVs carry several biomolecules, including pigments, proteins, enzymes, lipids, nucleic acids, and carbohydrates, and are involved in physiological and pathological processes. EVs may play a pivotal role in the establishment of fungal infections, as they can interact with the host immune system to elicit multiple outcomes. It has been observed that, depending on the fungal pathogen, EVs can exacerbate or attenuate fungal infections. The study of the interaction between fungal EVs and the host immune system and understanding of the mechanisms that regulate those interactions might be useful for the development of new adjuvants as well as the improvement of protective immune responses against infectious or noninfectious diseases. In this review, we describe the immunomodulatory properties of EVs produced by pathogenic fungi and discuss their potential as adjuvants for prophylactic or therapeutic strategies.
INTRODUCTION
Extracellular vesicles (EVs) are spherical structures delimited by a bilayered membrane that are produced by prokaryotic and eukaryotic cells (1, 2). EVs transport various biomolecules, such as proteins, lipids, nucleic acids, and carbohydrates, and they are involved in several aspects of physiology and pathogenicity (2–4). EVs are classified as apoptotic bodies, ectosomes, or exosomes, depending upon their cellular origin and size (5). Apoptotic bodies are the largest (50 to 5,000 nm in size) and are derived from apoptotic cells. Ectosomes, also called microvesicles, are generated by outward budding from the plasma membrane, followed by pinching off and release to the extracellular space, resulting in EVs ranging from 100 to 1,000 nm in size. Exosomes are the smallest EVs (30 to 150 nm), and these structures originate from endosomal compartments (5–7). In this review, we adopt EVs as a general term for all of the above-mentioned vesicles.
In fungi, the first visual evidence of the production of EVs was produced in 1972 in Aspergillus nidulans (8). Microscopic evidence of fungal EVs was reproduced in 1973 in Cryptococcus neoformans (9), 1990 in Candida albicans (10), and 1998 in Saccharomyces cerevisiae (11), but the first characterization of extracellular membranous structures as fungal EVs dates to 2007 in the C. neoformans model (12). So far, the production of EVs has been observed in a number of fungal species (13–19). The composition of fungal EVs can vary, depending on the availability of nutrition and the immunological activity of host cells, and they typically contain proteins, RNA, lipids, complex carbohydrates, and pigments (20, 21). Due to the heterogeneity in their content, fungal EVs are able to participate in a number of physiological processes, including biofilm formation, the transport of virulence factors, and modulation of the host immune response (22, 23).
Deep mycoses, such as cryptococcosis, candidiasis, and aspergillosis, are responsible for approximately 1,270,000 annual global cases, and the mortality rates from these mycoses are comparable to those from malaria (24, 25). The drugs currently approved for treating human mycoses usually have low efficacy and high toxicity, and the widespread use of these medications is selecting for resistant strains (26–29). Given the high incidence of fungal diseases worldwide and their therapeutic limitations, it is important to study the biology of pathogenic fungi in an attempt to develop new immune interventions (30). In this review, we discuss the immunomodulatory potential of fungal EVs. Additionally, we highlight strategies where fungal EVs could be used as therapeutic targets and/or as components of therapeutic and prophylactic strategies.
THE INTERACTION OF FUNGAL EVs WITH THE IMMUNE SYSTEM
Most of the data resulting from the immunomodulatory effects of EVs are derived from studies involving Gram-negative bacteria (31). Macrophages that internalize Neisseria gonorrhoeae EVs undergo apoptosis due to the presence of the porin PorB within the vesicles, resulting in altered mitochondrial permeability and cytochrome c release (32). EVs derived from Escherichia coli can also cause apoptosis in human intestinal epithelial cells due to interleukin-8 (IL-8) production, possibly mediated by the intracellular receptor NOD-1 (33, 34). Similar effects were observed in Gram-positive bacteria, where the listeriolysin O within EVs produced by Listeria monocytogenes decreased the viability of J774 macrophages (35). Streptococcus pneumoniae EVs also induce inflamatory cytokine production and cell maturation (36). Additionally, EVs derived from Streptococcus pneumoniae interact with complement components that cannot directly interact with the bacteria, thus avoiding phagocytosis (36). Fungal EVs also possess immunogenic properties (37). The proteins, RNA, lipids, carbohydrates, and pigments in fungal EVs are recognized by pattern recognition receptors (PRRs) expressed on leukocytes and activate immune responses (38). These collective findings show that EVs of fungi might positively or negatively modulate the activation of innate immunity.
Cryptococcus.
C. neoformans is the principal causative agent of cryptococcosis, a disease distributed worldwide. After inhalation of fungal cells, immunosuppressed individuals, such as those infected with HIV, can develop the invasive form of this disease (39, 40). EVs derived from C. neoformans carry many virulence factors, including its major capsular antigen, glucuronoxylomannan (GXM), and laccase, the enzyme responsible for melanin production (12, 41). GXM exerts an immunosuppressive action over macrophages, monocytes, neutrophils, and T lymphocytes (42). This polysaccharide enhances IL-10 production by monocytes, subsequently impairing IL-12 production and intracellular killing (43). The lack of IL-12 may be due to the low levels of production of gamma interferon (IFN-γ) by peripheral blood mononuclear cells (PBMC), which in turn hampers the development of the Th1 protective response (43). GXM also exerts a direct in vivo and in vitro cytotoxic effect on macrophages due to activation of the Fas/FasL pathway (44, 45). Indeed, it has been demonstrated that macrophages stimulated in vitro with EVs derived from C. neoformans produce anti-inflammatory cytokines, such as transforming growth factor β (TGF-β) and IL-10 (46). Interestingly, the production of both TNF-α and nitric oxide (NO), as well as an increased ability to phagocytize and kill fungal cells, suggests that several molecules present in EVs derived from C. neoformans play dual roles: positive and negative stimulation of macrophages (46). These findings reinforce the suggestion that the effect of C. neoformans EVs is more protective than deleterious for the host. The presence of specific antibodies against EV proteins in the sera of patients diagnosed with cryptococcosis confirms the activation of the humoral immune response by these EVs in vivo (41). The degradation of cryptococcal EVs in human sera might be highly efficient, since the vesicles are disrupted in the presence of albumin and galectin-3 (47, 48).
EVs derived from a virulent strain of C. gattii also modulate macrophage responses, ultimately facilitating the intracellular multiplication of less virulent strains without interfering with the phagocytosis rate (49). Therefore, the presence of fungal virulence factors might be critical for the protective or deleterious effect of EVs. Heat inactivation of EVs abolished this effect, suggesting that, besides the virulence factors, a plethora of compounds derived from EVs (lipids, RNA, and proteins) could play a pivotal role in the modulation of macrophage functions (49).
Candida.
C. albicans is the most common human commensal fungus. This fungus primarily colonizes the gastrointestinal tract and the oral and vaginal mucosa of healthy individuals. In immunosuppressed individuals, C. albicans can become an opportunistic pathogen, ultimately causing a range of diseases from mucosal infections to candidemia and disseminated candidiasis (50, 51). Collected findings show that C. albicans EVs exhibit immunomodulatory effects that benefit the activation of the innate response. When stimulated with EVs, RAW 264.7 macrophages produce NO, IL-12p40, and lower concentrations of IL-10 and TGF-β, while bone marrow-derived macrophages (BMDM) produce NO, IL-12p40, tumor necrosis factor alpha (TNF-α), and IL-10. Bone marrow-derived dendritic cells (BMDC) produce IL-12p40, TNF-α, and TGF-β and undergo cell maturation that coincides with a higher level of expression of major histocompatibility complex class II and CD86. The hallmark of dendritic cell (DC) activation by C. albicans EVs suggests that vesicle constituents might be important for the immune adaptive response against the pathogen (52). The phospholipids contained in C. albicans EVs may also play a role in immune cell activation, as EVs derived from phosphatidylserine synthase (CHO1)-knockout fungi were unable to activate NF-κB in BMDM and J774.14 macrophages (53). EVs produced by C. albicans may be more stable in human sera than other fungal vesicles due to the presence of the glucose transmembrane transporter Hgt1p, which binds to complement factor H to allow for evasion of complement alternative pathway activation and, ultimately, opsonization (54). Indeed, blockage of Hgt1p in fungal cells results in an increase in neutrophil phagocytosis and intracellular killing (54). The EVs produced by C. albicans also played a protective effect in a Galleria mellonella model of fungal infection. Inoculation of EVs into the larval form of this invertebrate 2 days prior to lethal challenge with live C. albicans fungal cells resulted in a lower fungal burden and an increased life span in the EV-treated larvae (52).
Paracoccidioides.
Paracoccidioidomycosis is a fungal infection endemic in Latin America that is caused by the thermally dimorphic fungi Paracoccidioides brasiliensis and P. lutzii. The infection initiates in the lungs but can spread to other organs (55). The activation of a Th1 immune response is an indicator of a good prognosis, while the activation of the Th2 and Th9 immune responses causes an uncontrolled, deleterious inflammatory process (56). It is well-known that classically activated macrophages (M1) play a protective role against P. brasiliensis (55, 57). Interestingly, it has been demonstrated that in vitro stimulation with P. brasiliensis EVs can induce the differentiation of M1 macrophages (58). The EV-induced stimulus leads to the production of proinflammatory cytokines and to the increased relative expression of the inducible nitric oxide synthase (iNOS) gene. Additionally, macrophage phagocytosis and intracellular killing were comparable to those that were observed after IFN-γ stimulation (58). Mammalian lectin microarray assays demonstrated that the interaction between EVs and DCs was dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) dependent, with no involvement of Dectin-1 and -2 (59). Sera obtained from patients diagnosed with paracoccidioidomycosis reacted with EVs through a process that required fungal α-galactosyl epitopes (15). Despite this, it remains unclear if the α-galactosyl epitopes in EVs participate in the generation of immune responses, as observed in Trypanosoma cruzi (15). As previously reported for C. neoformans, galectin-3 disrupts P. brasiliensis EVs, ultimately limiting the functionality of those vesicles (60).
Histoplasma.
Histoplasma capsulatum is a thermally dimorphic fungus that causes histoplasmosis. H. capsulatum is distributed worldwide and is highly endemic in North and South America (61). In immunocompetent hosts, a Th1/Th17 immune response limits the progression of the infection (62). However, a severe form of histoplasmosis might occur in immunocompromised individuals (63, 64). Similar to the findings for other fungal EVs, vesicular proteins of H. capsulatum were recognized by sera from patients with histoplasmosis, with a dominant serological reactivity of heat shock protein 60 (Hsp60) and histone 2B (13). The in vivo generation of anti-Hsp60 antibodies might be of significance, as it has been demonstrated that in vitro incubation of EVs with anti-Hsp60 antibodies alters the morphology, protein cargo, and enzymatic activities (65). Although the interplay of H. capsulatum EVs with host leukocytes remains to be better investigated, those EVs reduced the phagocytic rates and intracellular fungal killing by BMDM (66), suggesting that they may be more suppressive than protective in the infection.
Malassezia.
Malassezia spp. are dimorphic fungi that colonize the human skin as commensal organisms and are typically harmless; however, these fungi can cause skin-related disorders (67). Malassezia sympodialis and M. furfur are two of the many Malassezia species associated with atopic eczema (AE) (68). M. sympodialis produces EVs that carry allergens recognized by IgE from AE patients, and those EVs might induce the production of IL-4 and TNF-α by peripheral blood mononuclear cells (PBMC) obtained from AE patients or from healthy individuals (14). M. sympodialis EVs also exert an indirect immune effect, as DCs are able to phagocytize these fungal structures and produce their own EVs containing M. sympodialis antigens. DC-derived vesicles also induce the production of IL-4 and TNF-α by PBMCs from AE patients (14). In addition to DCs, monocytes and keratinocytes could actively internalize M. sympodialis EVs (69). M. furfur EVs may also play a role in immune modulation and AE development. Collected evidence from in vitro and in vivo experiments showed that M. furfur EVs stimulated NF-κB activation and increased IL-1β gene expression and IL-6 production by keratinocytes (70).
Trichophyton.
Dermatophytes are associated with infections of keratin-rich tissues that represent the most common superficial mycoses in humans (24, 71). Dermatophyte infections are also called ringworms or tinea. Trichophyton interdigitale and T. rubrum are established as the most important species that cause skin and nail infections (71). T. interdigitale EVs induce a strong inflammatory response in BMDM and keratinocytes. Both types of cells produce NO, TNF-α, IL-6, and IL-1β in response to EVs. An increase in iNOS relative expression suggests that EV-treated macrophages differentiate into M1 macrophages, which exhibit a higher phagocytic and intracellular killing index than other macrophages when incubated with fungal conidia. Additionally, Toll-like receptor 2 activation was found to be required for EV-induced macrophage activation (18). These data suggest that T. interdigitale EVs positively modulate inflammatory and microbicidal innate responses.
Sporothrix.
Sporotrichosis, caused by Sporothrix spp., is a mycosis endemic in tropical and subtropical areas (72). In Brazil, Sporothrix brasiliensis is the main etiological agent of sporotrichosis, but Sporothrix spp. may be distributed worldwide (73, 74). After contact with Sporothrix species, both immunocompetent and immunosuppressed individuals can develop sporotrichosis, which varies from cutaneous manifestations to colonization of multiple organs (63, 72). EVs derived from S. brasiliensis exhibited immunoreactivity with sera obtained from mice with experimental sporotrichosis, revealing a humoral response against vesicle proteins (19). S. brasiliensis EVs played no role in cytokine production by BMDC. However, pretreatment of BMDC with EVs and subsequent exposure to S. brasiliensis yeast cells resulted in the differential production of IL-12 p40, TNF-α, and IFN-γ compared with that by nontreated BMDC and higher levels of phagocytosis but not intracellular killing of S. brasiliensis yeast cells by pretreated BMDC than by nontreated BMDC. The in vivo administration of EVs impaired the initial immune response, as initial skin lesions were larger and contained higher levels of fungal colonization (19). These results suggest that S. brasiliensis EVs may be more suppressive than protective in the infection.
FUNGAL EVs AS TARGETS FOR IMMUNE INTERVENTIONS
The urgent need to develop new strategies to prevent and combat fungal infections is efficiently illustrated by the global number of deaths (1.5 million annually) caused by invasive mycoses. There are no licensed antifungal vaccines, and only two vaccine candidates targeting vulvovaginal candidiasis are currently under development (75, 76).
Several groups have demonstrated that fungal EVs act as immunomodulators, as they carry a number of immunogenic molecules that elicit inflammatory and microbicidal responses, suggesting the possibility of their use as candidates for adjuvants, vaccines against invasive fungal infections, or immunotherapies. In this sense, liposomes resembling EVs could be promising structures for the development of vaccines. Liposomes are laboratory-generated round vesicles of various sizes that are formed by one or more lipid bilayers. They can be engineered to carry selected antigens in association with the lipid bilayer or within the vesicle core. Addition of pathogen-associated molecular patterns (PAMP) or damage-associated molecular patterns (DAMP) in liposomes promotes adjuvant-like properties (77, 78). In this context, EVs would play a role of natural liposomes, delivering fungal PAMP for leukocytes of the innate system and, possibly, driving the differentiation of CD4+ subsets. P10 is an epitope derived from P. brasiliensis glycoprotein 43 (gp43) that exerts a protective effect against experimental paracoccidioidomycosis. gp43, which is present in EVs, is the most well studied antigen from P. brasiliensis (79). The immunization of healthy or immunosuppressed mice with P10 decreased P. brasiliensis fungal loads and increased the survival of mice lethally challenged with P. brasiliensis by a mechanism dependent on the Th1 immune response (80–82). This protection is achieved through the establishment of a Th1 response (80). Additionally, concomitant treatment of mice with P10 and antifungal drugs efficiently diminished the fungal burden in infected mice (81). Two facts—that P10 is a component of EVs and that EVs induce the differentiation of M1 macrophages—suggest that the protective potential of P. brasiliensis EVs as a candidate vaccine could be assessed.
The C. albicans protein 1,3-β-glucosyltransferase (Bgl2) is involved in cell wall biosynthesis and virulence. Bgl2 proteins are present in C. albicans EVs and elicit humoral immune responses, as concluded from their reactivity with sera from patients suffering from systemic candidiasis (83). Additionally, treatment of mice with Bgl2 in a vaccine model led to the increased survival of mice challenged with C. albicans (84). C. albicans EVs used as immunogens in G. mellonella reduced the fungal burden and increased larva survival after challenge with yeast cells (52). In this sense, G. mellonella provides a useful experimental model, as its innate immune response resembles the human innate immune response. Unlike humans, however, no adaptive immune response is present in this invertebrate. G. mellonella exhibits a short period of immune boost after an immune challenge. This period is called “primary immunization” or “primitive immunity” and is characterized by higher counts of hemocytes and the increased production of antimicrobial peptides (85). Thus, the protective effect of C. albicans EVs may be due to primary immunization rather than adaptive immunization.
In C. neoformans, fungal protein extracts or the recombinant proteins were able to induce protection in mice challenged with C. neoformans or C. gattii (86, 87). Similarly, the vaccination of mice with protein extracts derived from the cell wall or cytoplasm of C. gattii increased the survival of mice and decreased the fungal burden after challenge with C. gattii (88). The recruitment of lymphocytes to the infection sites was also reported (88). The association of these proteins with EVs has not been demonstrated, but on the basis of the complexity of the protein composition of EVs, results similar to those described above for protein extracts from C. gattii would not be surprising. Indeed, EVs produced by C. neoformans containing GXM and sterylglucosides induced protection in G. mellonella (89). Similar to what has been proposed for C. albicans, this effect could be related to a primary immunization mechanism.
EVs carry several virulence factors, suggesting that detrimental activity against the host cannot be ruled out. EVs transport pathogen antigens that can interact with the host immune system, ultimately resulting in immune memory. The controlled administration of fungal EVs in combination with an appropriate three-dimensional culture or animal model could be exploited to provide an adjuvant, a vaccine platform, or an immunotherapy platform. However, there is an equivoque with two commercially available vaccines derived from extracts of outer membrane vesicles from Neisseria meningitidis processed into a liposomal form (Bexsero and VA-Mengoc-BC); although these vaccines exhibit satisfactory efficacy (90–93), these materials cannot be considered EVs since these vaccines do not derive from secretions produced by bacterial cells (94). However, these previous successful approaches support the search for vaccines derived from fungal EVs.
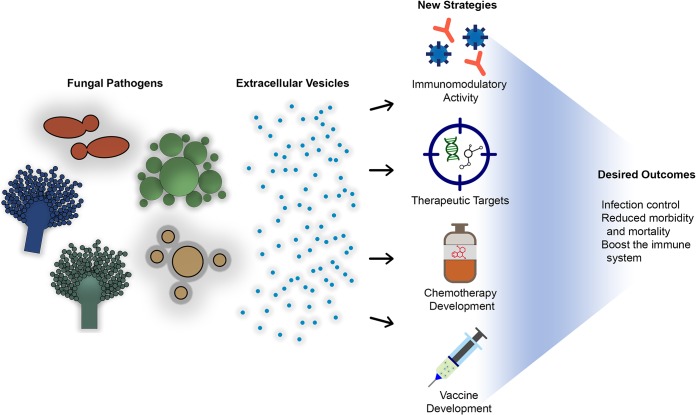
The field of fungal EVs is still in its infancy, which agrees with the existence of many gaps in knowledge on how these structures can impact disease. Dissection of the physiological and pathogenic roles of fungal EVs will enhance our understanding of their potential applicability in models of vaccination. In this sense, we propose strategies to explore fungal EVs as potential prophylactic or therapeutic targets (Fig. 1).
FIG 1.
Fungal EVs may play a pivotal role in the establishment of fungal infections and can alter the infection process. These EVs are potential targets for new antifungal agents, as well as potential candidates for chemotherapy and vaccine development.
We described above the interactions between various pathogenic fungal EVs and leukocytes of the innate system. The diversity of molecules present within these structures results in a diverse immune response. In some cases, the interactions between EVs and the immune system are beneficial to the host, and in other situations, the vesicles support disease development. It is clear that not all EV interactions are well established. Indeed, fungal EVs are important factors in immune response modulation, and they act at different levels, depending on the pathogen and the EV cargo. More studies are necessary to further elucidate this relationship and the possible use of such vesicles in host protection (23). The complexity of fungal EVs is enormous, but their potential to impact the interaction of fungi with host cells suggests that they have great potential in the development of new strategies to combat fungal infections.
ACKNOWLEDGMENTS
We acknowledge support from the Fundação de Amparo a Pesquisa do Estado de São Paulo (grant 2016/03322-7), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças de Populações Negligenciadas (INCT-IDPN).
REFERENCES
- 1.Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NHH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers E-M, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen ENM, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, et al. . 2015. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Niel G, D'Angelo G, Raposo G. 2018. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 4.Brock CK, Wallin ST, Ruiz OE, Samms KM, Mandal A, Sumner EA, Eisenhoffer GT. 2019. Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat Commun 10:1044. doi: 10.1038/s41467-019-09010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malloci M, Perdomo L, Veerasamy M, Andriantsitohaina R, Simard G, Martinez MC. 2019. Extracellular vesicles: mechanisms in human health and disease. Antioxid Redox Signal 30:813–856. doi: 10.1089/ars.2017.7265. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas SLN, Breakefield XO, Weaver AM. 2017. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson RK, Peberdy JF. 1972. Fine structure of protoplasts of Aspergillus nidulans. J Gen Microbiol 72:529–538. doi: 10.1099/00221287-72-3-529. [DOI] [PubMed] [Google Scholar]
- 9.Takeo K, Uesaka I, Uehira K, Nishiura M. 1973. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. J Bacteriol 113:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson J, Mihalik R, Soll DR. 1990. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol 172:224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osumi M. 1998. The ultrastructure of yeast: cell wall structure and formation. Micron 29:207–233. doi: 10.1016/S0968-4328(97)00072-3. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, Almeida IC, Nosanchuk JD. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol 10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, Gabrielsson S, Scheynius A. 2011. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—novel mechanisms for host-microbe interactions in atopic eczema. PLoS One 6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, Freymuller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. 2011. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-galactosyl epitopes. Eukaryot Cell 10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva BMA, Prados-Rosales R, Espadas-Moreno J, Wolf JM, Luque-Garcia JL, Gonçalves T, Casadevall A. 2014. Characterization of Alternaria infectoria extracellular vesicles. Med Mycol 52:202–210. doi: 10.1093/mmy/myt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leone F, Bellani L, Muccifora S, Giorgetti L, Bongioanni P, Simili M, Maserti B, Del Carratore R. 2018. Analysis of extracellular vesicles produced in the biofilm by the dimorphic yeast Pichia fermentans. J Cell Physiol 233:2759–2767. doi: 10.1002/jcp.25885. [DOI] [PubMed] [Google Scholar]
- 18.Bitencourt TA, Rezende CP, Quaresemin NR, Moreno P, Hatanaka O, Rossi A, Martinez-Rossi NM, Almeida F. 2018. Extracellular vesicles from the dermatophyte Trichophyton interdigitale modulate macrophage and keratinocyte functions. Front Immunol 9:2343. doi: 10.3389/fimmu.2018.02343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda MAK, de Almeida JRF, Jannuzzi GP, Cronemberger-Andrade A, Torrecilhas ACT, Moretti NS, da Cunha JPC, de Almeida SR, Ferreira KS. 2018. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front Microbiol 9:2286. doi: 10.3389/fmicb.2018.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Toledo Martins S, Szwarc P, Goldenberg S, Alves LR. 2019. Extracellular vesicles in fungi: composition and functions. Curr Top Microbiol Immunol 422:45–59. doi: 10.1007/82_2018_141. [DOI] [PubMed] [Google Scholar]
- 21.Bleackley MR, Dawson CS, Anderson MA. 2019. Fungal extracellular vesicles with a focus on proteomic analysis. Proteomics 19:e1800232. doi: 10.1002/pmic.201800232. [DOI] [PubMed] [Google Scholar]
- 22.Rella A, Farnoud AM, Del Poeta M. 2016. Plasma membrane lipids and their role in fungal virulence. Prog Lipid Res 61:63–72. doi: 10.1016/j.plipres.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamith-Miranda D, Nimrichter L, Rodrigues ML, Nosanchuk JD. 2018. Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect 20:501–504. doi: 10.1016/j.micinf.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 25.Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 3:E57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmakiotis D, Kontoyiannis DP. 2017. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents 50:318–324. doi: 10.1016/j.ijantimicag.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Robbins N, Caplan T, Cowen LE. 2017. Molecular evolution of antifungal drug resistance. Annu Rev Microbiol 71:753–775. doi: 10.1146/annurev-micro-030117-020345. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Wang H, Li Z, Wong AH, Wang YZ, Guo Y, Lin X, Zeng G, Liu H, Wang Y, Wang J. 2018. Candida albicans gains azole resistance by altering sphingolipid composition. Nat Commun 9:4495. doi: 10.1038/s41467-018-06944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rybak JM, Ge W, Wiederhold NP, Parker JE, Kelly SL, Rogers PD, Fortwendel JR. 2019. Mutations in hmg1, challenging the paradigm of clinical triazole resistance in Aspergillus fumigatus. mBio 10:e00437-19. doi: 10.1128/mBio.00437-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida F, Rodrigues ML, Coelho C. 2019. The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214. doi: 10.3389/fmicb.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuipers ME, Hokke CH, Smits HH, Nolte-'t Hoen ENM. 2018. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front Microbiol 9:2182. doi: 10.3389/fmicb.2018.02182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deo P, Chow SH, Hay ID, Kleifeld O, Costin A, Elgass KD, Jiang JH, Ramm G, Gabriel K, Dougan G, Lithgow T, Heinz E, Naderer T. 2018. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog 14:e1006945. doi: 10.1371/journal.ppat.1006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunsmann L, Ruter C, Bauwens A, Greune L, Gluder M, Kemper B, Fruth A, Wai SN, He X, Lloubes R, Schmidt MA, Dobrindt U, Mellmann A, Karch H, Bielaszewska M. 2015. Virulence from vesicles: novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci Rep 5:13252. doi: 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canas MA, Fabrega MJ, Gimenez R, Badia J, Baldoma L. 2018. Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front Microbiol 9:498. doi: 10.3389/fmicb.2018.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho C, Brown L, Maryam M, Vij R, Smith DFQ, Burnet MC, Kyle JE, Heyman HM, Ramirez J, Prados-Rosales R, Lauvau G, Nakayasu ES, Brady NR, Hamacher-Brady A, Coppens I, Casadevall A. 2019. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J Biol Chem 294:1202–1217. doi: 10.1074/jbc.RA118.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Codemo M, Muschiol S, Iovino F, Nannapaneni P, Plant L, Wai SN, Henriques-Normark B. 2018. Immunomodulatory effects of pneumococcal extracellular vesicles on cellular and humoral host defenses. mBio 9:e00559-18. doi: 10.1128/mBio.00559-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M. 2016. Potential roles of fungal extracellular vesicles during infection. mSphere 1:e00099-16. doi: 10.1128/mSphere.00099-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patin EC, Thompson A, Orr SJ. 2019. Pattern recognition receptors in fungal immunity. Semin Cell Dev Biol 89:24–33. doi: 10.1016/j.semcdb.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. 2016. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 14:106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulware DR. 2011. Cryptococcus: from human pathogen to model yeast. Lancet Infect Dis 11:434. doi: 10.1016/S1473-3099(11)70140-2. [DOI] [Google Scholar]
- 41.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monari C, Bistoni F, Vecchiarelli A. 2006. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res 6:537–542. doi: 10.1111/j.1567-1364.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 43.Retini C, Kozel TR, Pietrella D, Monari C, Bistoni F, Vecchiarelli A. 2001. Interdependency of interleukin-10 and interleukin-12 in regulation of T-cell differentiation and effector function of monocytes in response to stimulation with Cryptococcus neoformans. Infect Immun 69:6064–6073. doi: 10.1128/IAI.69.10.6064-6073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villena SN, Pinheiro RO, Pinheiro CS, Nunes MP, Takiya CM, DosReis GA, Previato JO, Mendonca-Previato L, Freire-de-Lima CG. 2008. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol 10:1274–1285. doi: 10.1111/j.1462-5822.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 45.Monari C, Pericolini E, Bistoni G, Casadevall A, Kozel TR, Vecchiarelli A. 2005. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of Fas ligand in macrophages. J Immunol 174:3461–3468. doi: 10.4049/jimmunol.174.6.3461. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. 2010. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun 78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf JM, Rivera J, Casadevall A. 2012. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol 14:762–773. doi: 10.1111/j.1462-5822.2012.01757.x. [DOI] [PubMed] [Google Scholar]
- 48.Almeida F, Wolf JM, da Silva TA, DeLeon-Rodriguez CM, Rezende CP, Pessoni AM, Fernandes FF, Silva-Rocha R, Martinez R, Rodrigues ML, Roque-Barreira MC, Casadevall A. 2017. Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun 8:1968. doi: 10.1038/s41467-017-02126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bielska E, Sisquella MA, Aldeieg M, Birch C, O'Donoghue EJ, May RC. 2018. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat Commun 9:1556. doi: 10.1038/s41467-018-03991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nobile CJ, Johnson AD. 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Sudbery P. 2011. Candida albicans, a major human fungal pathogen. J Microbiol 49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 52.Vargas G, Rocha JD, Oliveira DL, Albuquerque PC, Frases S, Santos SS, Nosanchuk JD, Gomes AM, Medeiros LC, Miranda K, Sobreira TJ, Nakayasu ES, Arigi EA, Casadevall A, Guimaraes AJ, Rodrigues ML, Freire-de-Lima CG, Almeida IC, Nimrichter L. 2015. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol 17:389–407. doi: 10.1111/cmi.12374. [DOI] [PubMed] [Google Scholar]
- 53.Wolf JM, Espadas J, Luque-Garcia J, Reynolds T, Casadevall A. 2015. Lipid biosynthetic genes affect Candida albicans extracellular vesicle morphology, cargo, and immunostimulatory properties. Eukaryot Cell 14:745–754. doi: 10.1128/EC.00054-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenno S, Speth C, Rambach G, Binder U, Chatterjee S, Caramalho R, Haas H, Lass-Flörl C, Shaughnessy J, Ram S, Gow NAR, Orth-Höller D, Würzner R. 2018. Candida albicans factor H binding molecule Hgt1p—a low glucose-induced transmembrane protein is trafficked to the cell wall and impairs phagocytosis and killing by human neutrophils. Front Microbiol 9:3319. doi: 10.3389/fmicb.2018.03319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, Paniago AMM, Pereira AC, da Silva JF, Fabro AT, Bosco SMG, Bagagli E, Hahn RC, Levorato AD. 2017. Paracoccidioidomycosis: current perspectives from Brazil. Open Microbiol J 11:224–282. doi: 10.2174/1874285801711010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez Molina LM, Tobón Orozco AM. 2018. Paracoccidioidomycosis: global vision of a forgotten endemic mycosis. Curr Trop Med Rep 5:138–143. doi: 10.1007/s40475-018-0148-4. [DOI] [Google Scholar]
- 57.Leopold Wager CM, Wormley FL Jr. 2014. Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol 7:1023–1035. doi: 10.1038/mi.2014.65. [DOI] [PubMed] [Google Scholar]
- 58.da Silva TA, Roque-Barreira MC, Casadevall A, Almeida F. 2016. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci Rep 6:35867. doi: 10.1038/srep35867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peres da Silva R, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, Joshi L, Kilcoyne M, Puccia R. 2015. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep 5:14213. doi: 10.1038/srep14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatanaka O, Rezende CP, Moreno P, Freitas Fernandes F, Oliveira Brito PKM, Martinez R, Coelho C, Roque-Barreira MC, Casadevall A, Almeida F. 2019. Galectin-3 inhibits Paracoccidioides brasiliensis growth and impacts paracoccidioidomycosis through multiple mechanisms. mSphere 4:e00209-19. doi: 10.1128/mSphere.00209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods JP. 2016. Revisiting old friends: developments in understanding Histoplasma capsulatum pathogenesis. J Microbiol 54:265–276. doi: 10.1007/s12275-016-6044-5. [DOI] [PubMed] [Google Scholar]
- 62.Kroetz DN, Deepe GS. 2012. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 58:112–117. doi: 10.1016/j.cyto.2011.07.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Queiroz-Telles F, Fahal AH, Falci DR, Caceres DH, Chiller T, Pasqualotto AC. 2017. Neglected endemic mycoses. Lancet Infect Dis 17:e367–e377. doi: 10.1016/S1473-3099(17)30306-7. [DOI] [PubMed] [Google Scholar]
- 64.Limper AH, Adenis A, Le T, Harrison TS. 2017. Fungal infections in HIV/AIDS. Lancet Infect Dis 17:e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 65.Matos Baltazar L, Nakayasu ES, Sobreira TJ, Choi H, Casadevall A, Nimrichter L, Nosanchuk JD. 2016. Antibody binding alters the characteristics and contents of extracellular vesicles released by Histoplasma capsulatum. mSphere 1:e00085-15. doi: 10.1128/mSphere.00085-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baltazar LM, Zamith-Miranda D, Burnet MC, Choi H, Nimrichter L, Nakayasu ES, Nosanchuk JD. 2018. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci Rep 8:8065. doi: 10.1038/s41598-018-25665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harada K, Saito M, Sugita T, Tsuboi R. 2015. Malassezia species and their associated skin diseases. J Dermatol 42:250–257. doi: 10.1111/1346-8138.12700. [DOI] [PubMed] [Google Scholar]
- 68.Nowicka D, Nawrot U. 2019. Contribution of Malassezia spp. to the development of atopic dermatitis. Mycoses 62:588–596. doi: 10.1111/myc.12913. [DOI] [PubMed] [Google Scholar]
- 69.Johansson HJ, Vallhov H, Holm T, Gehrmann U, Andersson A, Johansson C, Blom H, Carroni M, Lehtio J, Scheynius A. 2018. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci Rep 8:9182. doi: 10.1038/s41598-018-27451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang YJ, Han Y, Sun YZ, Jiang HH, Liu M, Qi RQ, Gao XH. 2019. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J Dermatol Sci 93:168–175. doi: 10.1016/j.jdermsci.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Zhan P, Liu W. 2017. The changing face of dermatophytic infections worldwide. Mycopathologia 182:77–86. doi: 10.1007/s11046-016-0082-8. [DOI] [PubMed] [Google Scholar]
- 72.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. 2015. Global epidemiology of sporotrichosis. Med Mycol 53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 73.de Beer ZW, Duong TA, Wingfield MJ. 2016. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 83:165–191. doi: 10.1016/j.simyco.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gremiao ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA. 2017. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog 13:e1006077. doi: 10.1371/journal.ppat.1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicola AM, Albuquerque P, Paes HC, Fernandes L, Costa FF, Kioshima ES, Abadio AKR, Bocca AL, Felipe MS. 2019. Antifungal drugs: new insights in research & development. Pharmacol Ther 195:21–38. doi: 10.1016/j.pharmthera.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Nami S, Mohammadi R, Vakili M, Khezripour K, Mirzaei H, Morovati H. 2019. Fungal vaccines, mechanism of actions and immunology: a comprehensive review. Biomed Pharmacother 109:333–344. doi: 10.1016/j.biopha.2018.10.075. [DOI] [PubMed] [Google Scholar]
- 77.De Serrano LO, Burkhart DJ. 2017. Liposomal vaccine formulations as prophylactic agents: design considerations for modern vaccines. J Nanobiotechnology 15:83. doi: 10.1186/s12951-017-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang N, Chen M, Wang T. 2019. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release 303:130–150. doi: 10.1016/j.jconrel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, Almeida IC, Puccia R. 2012. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res 11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taborda CP, Juliano MA, Puccia R, Franco M, Travassos LR. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun 66:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marques AF, da Silva MB, Juliano MA, Munhoz JE, Travassos LR, Taborda CP. 2008. Additive effect of P10 immunization and chemotherapy in anergic mice challenged intratracheally with virulent yeasts of Paracoccidioides brasiliensis. Microbes Infect 10:1251–1258. doi: 10.1016/j.micinf.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 82.Munoz JE, Luft VD, Amorim J, Magalhaes A, Thomaz L, Nosanchuk JD, Travassos LR, Taborda CP. 2014. Immunization with P10 peptide increases specific immunity and protects immunosuppressed BALB/c mice infected with virulent yeasts of Paracoccidioides brasiliensis. Mycopathologia 178:177–188. doi: 10.1007/s11046-014-9801-1. [DOI] [PubMed] [Google Scholar]
- 83.Pitarch A, Jimenez A, Nombela C, Gil C. 2006. Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol Cell Proteomics 5:79–96. doi: 10.1074/mcp.M500243-MCP200. [DOI] [PubMed] [Google Scholar]
- 84.Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, Gil C. 2015. Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. J Proteome Res 14:142–153. doi: 10.1021/pr5007944. [DOI] [PubMed] [Google Scholar]
- 85.Pereira TC, de Barros PP, Fugisaki LRO, Rossoni RD, Ribeiro FC, de Menezes RT, Junqueira JC, Scorzoni L. 2018. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J Fungi (Basel) 4:E128. doi: 10.3390/jof4040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Specht CA, Lee CK, Huang H, Tipper DJ, Shen ZT, Lodge JK, Leszyk J, Ostroff GR, Levitz SM. 2015. Protection against experimental cryptococcosis following vaccination with glucan particles containing Cryptococcus alkaline extracts. mBio 6:e01905-15. doi: 10.1128/mBio.01905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Specht CA, Lee CK, Huang H, Hester MM, Liu J, Luckie BA, Torres Santana MA, Mirza Z, Khoshkenar P, Abraham A, Shen ZT, Lodge JK, Akalin A, Homan J, Ostroff GR, Levitz SM. 2017. Vaccination with recombinant Cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio 8:e01872-17. doi: 10.1128/mBio.01872-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaturvedi AK, Hameed RS, Wozniak KL, Hole CR, Leopold Wager CM, Weintraub ST, Lopez-Ribot JL, Wormley FL Jr. 2014. Vaccine-mediated immune responses to experimental pulmonary Cryptococcus gattii infection in mice. PLoS One 9:e104316. doi: 10.1371/journal.pone.0104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombo AC, Rella A, Normile T, Joffe LS, Tavares PM, Araujo GRDS, Frases S, Orner EP, Farnoud AM, Fries BC, Sheridan B, Nimrichter L, Rodrigues ML, Del Poeta M. 2019. Cryptococcus neoformans glucuronoxylomannan and sterylglucoside are required for host protection in an animal vaccination model. mBio 10:e02909-28. doi: 10.1128/mBio.02909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campos JH, Soares RP, Ribeiro K, Andrade AC, Batista WL, Torrecilhas AC. 2015. Extracellular vesicles: role in inflammatory responses and potential uses in vaccination in cancer and infectious diseases. J Immunol Res 2015:832057. doi: 10.1155/2015/832057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ochoa-Azze RF. 2018. Cross-protection induced by VA-MENGOC-BC(R) vaccine. Hum Vaccin Immunother 14:1064–1068. doi: 10.1080/21645515.2018.1438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O'Hallahan J, Rosenqvist E, Black S. 2013. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother 9:1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petousis-Harris H. 2018. Impact of meningococcal group B OMV vaccines, beyond their brief. Hum Vaccin Immunother 14:1058–1063. doi: 10.1080/21645515.2017.1381810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coelho C, Casadevall A. 2019. Answers to naysayers regarding microbial extracellular vesicles. Biochem Soc Trans 47:1005–1012. doi: 10.1042/BST20180252. [DOI] [PMC free article] [PubMed] [Google Scholar]