Abstract
Yingyangbao (YYB) is a nutrient-dense complementary food supplement for infants and young children in China. There has been considerable interest and research on the potential effects of YYB on hematological and anthropometric outcomes in China, but limited effort has been made to consolidate and synthesize the evidence to inform the research and policy agendas. Eight English databases and three Chinese databases were searched from January 2001 to June 2019 to identify YYB intervention studies. A total of 32 quasi-experimental, post-only, concurrent-control studies or pre-post studies were identified, and 26 were included in the meta-analyses. A pooled analysis of post-only studies with concurrent-control determined that YYB was associated with an increase of 4.43 g/L (95% confidence interval (CI) 1.55, 7.30) hemoglobin concentration, 2.46 cm (CI 0.96, 3.97) in height, and 0.79 kg (CI 0.25, 1.32) weight in infants and young children. YYB was also associated with reductions in the prevalence of anemia (risk ratio (RR) = 0.55; 95% CI: 0.45, 0.67), stunting (RR = 0.60; 95% CI: 0.44, 0.81), and underweight (RR = 0.51; 95% CI: 0.39, 0.65). Overall, YYB was found to be associated with improved hematological and anthropometric indicators among infants and young children in China; however, randomized trials are needed to causally assess the efficacy of YYB due to the inherent risk of bias in existing quasi-experimental studies; rigorous implementation and cost-effectiveness evaluations are also needed.
Keywords: Yingyangbao, food supplement, infant and children, hematological status, anthropometric status
1. Introduction
China is home to 83 million children under 5 years of age, accounting for 13% of the global population in that age category in 2017 [1,2]. Despite rapid economic growth, China still has large number of undernourished children. In 2013, around seven-million Chinese children were stunted and two-million were underweight [3,4]. Anemia also remains a severe problem in China, particularly in rural areas: 28% of rural children between 6 and 12 months old and 21% between 13 and 24 months are estimated to be anemic [5].
To improve child nutritional status in rural China, Yingyangbao (YYB) was developed as a nutrient-dense complementary food supplement for infants and young children 6–36 months old. The base of YYB is full fat soybean powder with multiple additional micronutrients, including calcium, iron, zinc, vitamin B1, vitamin B2, vitamin B12, vitamin A, and vitamin D. The composition of YYB varies slightly by the manufacturing company [6,7,8,9,10,11], and in some formulations, folic acid, and omega-3 or omega-6 fatty acids are added. YYB also provides calories (usually around 50 kcal per sachet) and protein (3 g per sachet) [6,7,8].
The first YYB study was launched in 2001 in five poor counties of Gansu Province, China, among children 4–12 months old [8]8. After the pilot, the provision of YYB has gradually expanded to other poor rural areas of China and regions affected by natural disasters. Early YYB programs provided YYB to children from 4 months old due to national-exclusive feeding guidelines that were later changed to 6 months in 2007 [12]. Consequently, YYB programs implemented post 2007 were primarily among children 6–36 months old. In 2011, the Chinese government invested ¥100 million (USD 16 million) in the YYB program to cover 300,000 children in 100 counties across 10 provinces [13]. This investment was expanded to ¥500 million (USD 81 million) for 1.4 million children across 21 provinces. In 2017, the Chinese government issued a national nutrition strategy for 2017–2030 that prioritized the nutritional status of children in their first 1000 days, and YYB was included as a key intervention for rural and poor populations [14]. In 2019, the Chinese government launched the “Upgrade of YYB Plan” which will provide YYB to infants and children in 823 poor counties [15].
Multiple quasi-experimental research studies have estimated the impact of the YYB program on anthropometric indicators and hematological parameters [6,7,8,9,16,17]; however, the results have been heterogeneous. For example, Wang J et al. (2017) found that YYB significantly reduced stunting prevalence; however, another study by Zhang Y et al. (2016) reported no significant effect [6,7]. Two prior meta-analyses have been conducted to estimate the effects of YYB program [9,17]; however, they did not include several recently published studies and incorrectly included a single study multiple times in pooled analyses [9,17,18]. In addition, the prior reviews did not consider differences in study design or selection of control participants, and they did not assess the full range of anthropometric and hematologic indicators. Therefore, this systematic review was conducted to address these limitations. The authors also summarize the state of the evidence on YYB, and propose the next steps for advancing research and policy.
2. Materials and Methods
This systematic review of YYB was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [19]. This article sought to examine the effectiveness of providing YYB to infants and children on all health and nutrition outcomes assessed in published studies, including: (1) anthropometric outcomes including height (cm), weight (kg), height-for-age z score (HAZ), weight-for-age Z score (WAZ), weight-for-height Z score (WHZ), stunting prevalence (defined as HAZ ≤ −2 standard deviations [SD]), underweight prevalence (defined as WAZ ≤ −2 SDs), and wasting prevalence (defined as WHZ ≤ −2 SDs); (2) hematological parameters, including hemoglobin (Hb) concentration (g/L) and anemia prevalence (defined as Hb concentration <110 g/L); (3) child developmental outcomes including intelligence quotient and developmental quotient; and (4) disease prevalence, including the prevalence of diarrhea and the prevalence of respiratory infections in prior two-weeks.
2.1. Study Selection
Studies were eligible for inclusion if they: (1) included YYB as an intervention, (2) compared the effect of YYB between intervention group and a control group (quasi-experimental study), or (3) assessed the prevalence of health and nutrition outcomes between before and after YYB implementation (pre-post study). Studies were excluded if they were not academic studies, implemented a non-YYB complementary feeding intervention (like a soybean powder based, or micronutrient supplementation without energy), did not target children under 5 years old, or did not contain health or nutrition outcomes. The YYB program has not been implemented outside China, and therefore, this review only included studies published after 2001, which is when the first YYB study was carried out in China.
2.2. Data Sources and Search Strategy
A total of 11 databases were searched, including eight English databases covering nutrition, social sciences, and rural development: Cochrane Library, Medline, Econlit, PubMed, Embase, Web of Science, CAB Abstracts (EBSCO, Ipswich, MA, USA), CINAHL, and three Chinese databases including Wanfang data, China National Knowledge Infrastructure, and VIP—over the period from January 2001 to June 2019. Separate search strategies consisting of a combination of free text words (tw), words in titles/abstracts (tiab) and medical subject headings (mesh) for interventions, participants, and study designs were developed, and then combined by using “AND.” The following search strategy was run across in PubMed and tailored to each database when necessary: (“nutritional package” (tiab) or “micronutrient package” (tiab) or “micronutrient supplementation” (tiab) or “multi-micronutrient supplementation” (tiab) or “multi-nutrient powder” (tiab) or “complementary supplementation” (tiab) or “micronutrient supplement” (tiab) or “multi-micronutrient supplement” (tiab) or “complementary supplement” (tiab) or “Yingyangbao” (tiab) or “YYB” (tiab) or “Ying Yang Bao” (tiab) or “nutritional intervention” (tiab) or “Nutrition Policy” (Mesh) or “Dietary Supplements” (Mesh)) and (“China” (tiab)) and (“infant” (tiab) or “infancy” (tiab) or “child” (tiab) or “children” (tiab)) and 1 January 2001–1 June 2019. No language restriction was placed. The reference lists of included studies, and previous systematic reviews were also searched to identify additional eligible studies.
2.3. Data Extraction and Assessment of Methodological Quality
Two reviewers (X.L. and Y.L.) independently extracted data using a pilot tested data extraction form. Information from each study on study design, setting, participants, intervention, control, and outcomes were collected. Intervention details included the composition of YYB, the frequency of taking YYB (per day, or per 10 days), and intervention duration in months. No individually or cluster-randomized controlled trials were identified but multiple quasi-experimental studies were included. Two types of quasi-experimental study designs were distinguished: those that had a non-randomized control group (post-only with concurrent-control study) and those that compared outcomes before and after the YYB intervention in the same communities. Mean and standard deviation values were collected for continuous outcomes, as were the number of cases and non-cases in each arm for binary outcomes.
The methodological index for non-randomized studies (MINORS) [20] was used to assess the quality of post-only studies with concurrent-control and pre-post studies. The quality of post-only studies with concurrent-control was assessed with 12 criteria: (1) the stated aim of the study, (2) consecutive inclusion of participants, (3) prospective collection of data, (4) appropriate endpoints, (5) unbiased evaluation of endpoints, (6) appropriate follow-up period, (7) loss to follow-up ≤5%, (8) appropriate control group, (9) contemporary groups, (10) baseline equivalence of groups, (11) prospective calculation of sample size, and (12) statistical analyses adapted to the study design. The first seven criteria were considered when assessing the quality of pre-post studies, with each criterion assigned a score of 0 to 7. The potential range of total score was 0 to 84 for studies with concurrent-control, and 0 to 49 for pre-post studies. Articles were categorized into high, moderate, and low quality based on total score tertiles. Concurrent control studies with scores higher than 53 were defined as high quality; those with a score of 50–53 as moderate-quality; and those with scores of lower than 50 as low-quality. As for pre-post studies, studies with scores higher than 35, in the range of 31–35, and less than 31, were categorized as high, moderate, and low quality, respectively. Quality scores are presented in Tables S1 and S2.
2.4. Statistical Analyses
All analyses were stratified by study design due to differences in risk of bias. For post-only with concurrent-control YYB studies, the comparison of outcomes was between intervention and control groups at the end of the intervention period, since pre-intervention assessments or difference-in-difference estimates were not provided in most studies. For pre-post studies, outcomes were compared before and after the YYB intervention. Estimates were pooled separately for concurrent control studies and pre-post studies for outcomes where three or more studies provided estimates. Between-study heterogeneity was assessed by I2 statistics. Random effects meta-analysis was used if substantial heterogeneity was present, indicated by p < 0.1 in χ2 test and an I2 > 50%, otherwise the fixed effects were applied [21]. Pooled mean differences (MD) were reported for continuous outcomes and risk ratios (RR), and risk differences (RD) for binary outcomes and their corresponding 95% confidence intervals (CIs).
Two pre-specified subgroup analyses were conducted to explore sources of heterogeneity: (1) control type (active control; i.e., providing rice flour with the same energy as YYB; or a blank control where no intervention was given) and (2) intervention duration (≤12 months or >12 months).
To detect the robustness of the results, sensitivity analyses were also conducted by excluding studies with low quality. When an adequate number of studies (number of studies ≥ 10) were available [21], the potential for publication bias was assessed by the visual inspection of funnel plots for asymmetry and through Egger’s linear regression tests. Stata (version 14.0, STATA, College Station, TX, USA) and Cochrane Review Manager (RevMan) (version 5.3 Cochrane Community, London, UK) were used for all analyses.
3. Results
3.1. Literature Search and Selected Studies
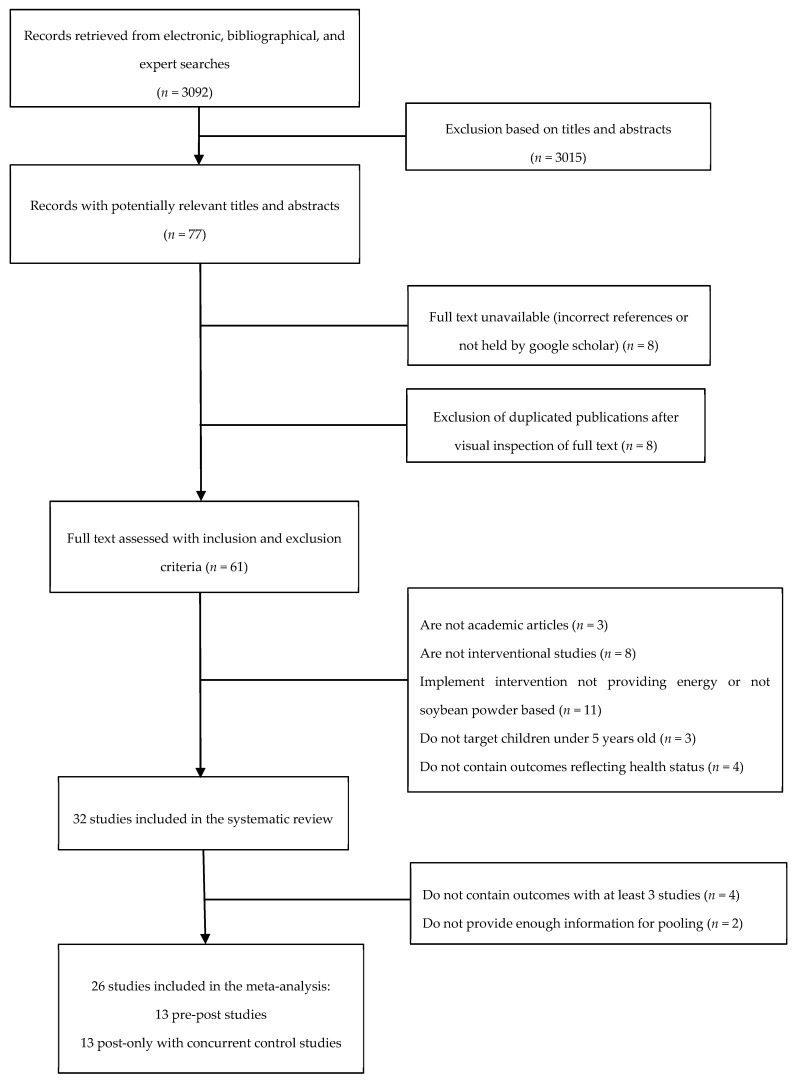
A total of 3092 reports were identified in our systematic search of the literature (Figure 1). Seventy-seven of them were identified as potentially relevant reports for review after screening the titles and abstracts. A full text review of these papers yielded 32 studies for inclusion in the systematic review, including 18 post-only studies with concurrent-control and 14 pre-post studies. Only outcomes reported in three or more studies were examined in meta-analysis, and as a result, 26 studies were in the meta-analyses (13 post-only studies with concurrent-control and 13 pre-post studies).
Figure 1.
Flow diagram of search and selection process.
Table 1 and Table 2 present the characteristics of included post-only studies with concurrent-control and pre-post studies, respectively. Among the 18 concurrent control studies, 17 provided YYB on a daily basis (one sachet per day) and one study provided one sachet of YYB every 10 days; 11 studies had a blank control and seven included isocaloric rice flour as the active control. All the 14 pre-post studies provided one sachet of YYB per day. The composition of YYB varied slightly across studies, with three major types (referred to as YYB-A, YYB-B, and YYB-C). Table 1 and Table 2, and Table S3, present composition details for the YYB supplement in each study. A majority of the included studies were conducted among children aged 6–24 months old.
Table 1.
Characteristics of post-only studies with concurrent-control.
| First Author, Year | Target Age (Months) | Intervention Group 1 | Control Group | Intervention Duration (Months) | Age When Outcomes Measured (Months) | Outcomes Measured 2 |
|---|---|---|---|---|---|---|
| Shuhua Ni, 1995 [22] | 5–13 | 2 counties; n = 76; YYB-C |
2 counties; n = 77; Blank control |
8 months | 13 | Height, weight, Kaup index, Vervaeck index, urine hydroxyproline index |
| Yuying Wang, 2004 [10] | 4–12 | 5 counties; n = 670; YYB-B |
5 counties; n = 307; Energy matched rice flour |
12 months | 16–24 | Hb concentration and prevalence of anemia |
| Yuying Wang, 2006 [23] | 4–12 | 5 counties; n = 232; YYB-B |
5 counties; n = 116; Energy matched rice flour |
Until 24 months of age for each child | 24 | Developmental quotient |
| Dongmei Yu, 2007 [24] | 4–12 | 5 counties; n = 978; YYB-B |
5 counties; n = 500; Energy matched rice flour |
12 months | 16–24 | Prevalence of diarrhea and respiratory infection in past two weeks |
| Yuying Wang, 2007 [25] | 4–12 | 5 counties; n = 978; YYB-B |
5 counties; n = 500; Energy matched rice flour |
Until 24 months of age | 24 | WAZ, HAZ |
| Yuying Wang, 2009 [26] | 6–12 | 5 counties; n = 978; YYB-B |
5 counties; n = 500; Energy matched rice flour |
12 months | 18–24 | Hb concentration and prevalence of anemia |
| Chunming Chen, 2010 [27] | 4–12 | 5 counties; n = 232; YYB-B |
5 counties; n = 116; Energy matched rice flour |
Until 24 months of age | 24 | Development quotient, intelligent quotient |
| Zhifeng Fang, 2010 [28] | 6–24 | 3 counties; n = 146; YYB-A |
3 counties; n = 107; Blank control |
6 months | 12–30 | Prevalence of stunting, underweight, wasting and anemia |
| China Development and Research Foundation, 2011 [29] | 6–24 | 6 counties; n = 1034; YYB-A |
6 counties; n = 449; Blank control |
24 months | 24–36 | Prevalence of underweight, stunting and wasting, Hb concentration and anemia prevalence |
| Wenli Zhao, 2012 [30] | 0–60 | 2 counties; n = 676; YYB-A |
2 counties; n = 536; Blank control |
12 months | 12–72 | Height, weight, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Songli Fan, 2013 [31] | 6–24 | 27 counties; n = 113; YYB-A |
27 counties; n = 328; Blank control |
3 months | 9–27 | Hb concentration and prevalence of anemia |
| Wenhao Li, 2013 [32] | 4–30 | 3 counties; n = 146; YYB-A |
3 counties; n = 146; Blank control |
3 months | 7–33 | Height, weight, anemia prevalence, and Hb concentration |
| Lingyun Ren, 2013 [33] | 6–11 | One county; n = 76; YYB-A |
One county; n = 78; Blank control |
Until 24 months of age for each child | 24 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Shangming Li, 2014 [34] | 0–36 | One county; n = 387; YYB-A |
One county; n = 240; Blank control |
Until 36 months of age for each child | 36 | Prevalence of stunting, underweight and anemia |
| Qin Hu, 2016 [35] | 6–36 | One county; n = 589; YYB-A |
One county; n = 300; Blank control |
6 months | 12–42 | Height, weight, head circumference, Hb concentration and prevalence of anemia and rickets |
| Xiaoting Ding, 2016 [36] | 6–18 | 3 counties; n = 483; YYB-A |
3 counties; n = 248; Blank control |
6 months | 12–24 | Height, weight, WAZ, HAZ, WHZ, Hb concentration, prevalence of underweight, wasting, stunting and anemia |
| Yanfeng Zhang, 2016 [7] | 6–23 | One county; n = 2186; YYB-A |
One county; n = 760; Blank control |
Until 24 months of age for each child | 6–23 | Prevalence of stunting and anemia |
| Shuai Li, 2017 [37] | 6–24 | One county; n = 450; YYB-A |
One county; n = 450; Blank control |
12 months | 18–36 months | Height, weight, WAZ, HAZ, WHZ, Hb concentration, prevalence of underweight, wasting, stunting and anemia |
1 Composition of YYB-A, B, C presented in Table S1. 2 Abbreviations: YYB, Yingyangbao; T, intervention group; C, control group; WAZ, weight-for-age z score; HAZ, height-for-age z score; WHZ, weight-for-height z score; Hb, hemoglobin.
Table 2.
Characteristics of pre-post studies.
| First Author, Year | Target Age (Months) | Intervention 1 | Intervention Duration | Age When Outcomes Measured (Months) | Outcomes Measured 2 |
|---|---|---|---|---|---|
| Hong Shen, 2011 [38] | 0–36 | One county; Pretest: n = 143 Posttest: n = 148; YYB-A |
8 months | 8–44 | Prevalence of anemia |
| Jing Sun, 2011 [39] | 6–24 | 2 counties; Pretest: n = 226 Posttest: n = 221; YYB-A |
20–24 months of age for each child | 6–24 | Prevalence of anemia |
| Lijuan Wang, 2011 [40] | 6–29 | One county; Pretest: n = 257 Posttest: n = 253; YYB-A |
15 months | 6–29 | Height, weight, WAZ, HAZ, prevalence of stunting and underweight, Hb concentration and prevalence of anemia |
| Caixia Dong, 2012 [41] | 6–18 | One county; Pretest: n = 314 Posttest: n = 242; YYB-A |
18 months until 24 months of age for each child |
6–24 | WAZ, HAZ, WHZ, prevalence of underweight, stunting and wasting, Hb concentration and anemia prevalence |
| Linjiang Wang, 2012 [42] | 6–24 | One county; Pretest: n = 327 Posttest: n = 300; YYB-A |
18 months until 24 months of age for each child |
6–24 | WAZ, HAZ, Hb concentration |
| Lixiang Li, 2012 [43] | 6–24 | One county; Pretest: n = 327 Posttest: n = 307; YYB-A |
6 months | 6–24 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Zengkang Xu, 2012 [44] | 6–24 | One county; Pretest: n = 327 Posttest: n-300; YYB-A |
18 months | 6–24 | Heigh, weight, WAZ, HAZ, WHZ, Hb concentration, and prevalence of underweight, wasting, stunting and anemia |
| Zuyang Liu, 2013 [45] | 6–24 | 5 counties; Pretest: n = 659 Posttest: n = 506; YYB-A |
18 months until 24 months of age |
6–24 | Prevalence of stunting, underweight, wasting and anemia |
| Jianhong Qin, 2014 [46] | 6–24 | One county; Pretest: n = 159 Posttest: n = 206; YYB-A |
12 months until 24 months of age for each child |
6–24 | Prevalence of underweight and stunting, Hb concentration and anemia prevalence, prevalence of diarrhea and respiratory infection |
| Junsheng Huo, 2015 [47]47 | 6–23 | 8 counties; Pretest: n = 1290 Posttest: n = 1040; YYB-A |
18 months until 24 months of age for each child |
6–23 | Hb concentration and prevalence of anemia |
| Qiannan Zhang, 2015 [9]9 | 6–23 | 2 counties; Pretest: n = 596 Posttest: n = 589; YYB-A |
12 months until 24 months of age for each child |
6–23 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Qiujing Jiang, 2016 [48] | 6–24 | 3 counties; Pretest: n = 596 Posttest: n = 589; YYB-A |
12 months until 24 months of age for each child |
6–24 | Height, weight, prevalence of stunting and underweight, Hb concentration and prevalence of anemia |
| Jie Wang, 2017 [6] | 6–23 | 3 counties; Pretest: n = 823 Posttest: n = 693; YYB-A |
18 months until 24 months of age for each child |
6–23 | Prevalence of underweight, stunting, wasting and anemia, micronutrient status |
| Ping Wu, 2017 [49] | 6–24 | One county; Pretest: n = 156 Posttest: n = 156; YYB-A |
24 months until 24 months of age |
6–24 | Prevalence of stunting, underweight, wasting and anemia |
1 Composition of YYB presented in Table S1. 2 Abbreviations: YYB, Yingyangbao; T, intervention group; C, control group; WAZ, weight-for-age z score; HAZ, height-for-age z score; WHZ, weight-for-height z score; Hb, hemoglobin.
3.2. Continuous Hematologic and Anthropometric Outcomes
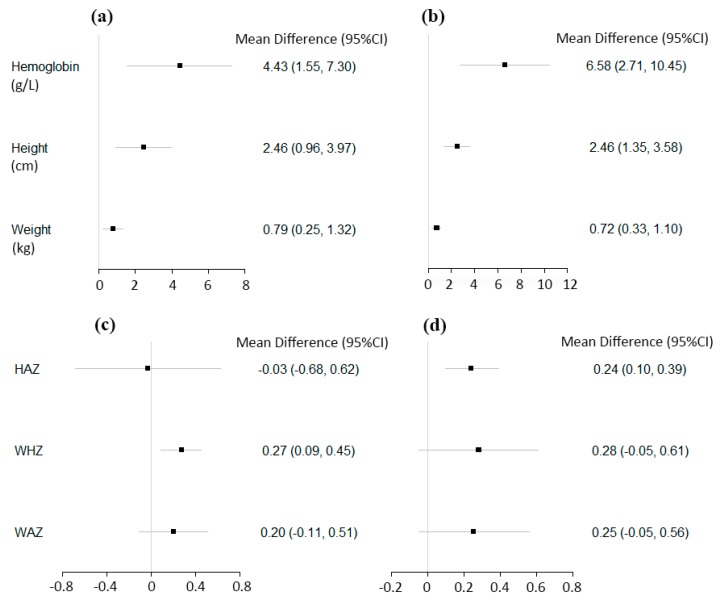
The results of meta-analyses on Hb concentration, weight, and height are summarized in Figure 2 and Table 3. A pooled analysis of seven post-only studies with concurrent-control on Hb concentration indicated that YYB increased Hb concentrations by 4.43 g/L (95% CI: 1.55, 7.30; p = 0.003) compared to control; however, it is important to note there was large heterogeneity in effect sizes across studies (I2 = 96%, p < 0.001). Consistently, the pooled results of seven pre-post studies indicated a 6.58 g/L (95% CI: 2.71, 10.45; p < 0.001) increase in Hb concentration after the YYB intervention (Table 3 and Figure S1).
Figure 2.
The effect of Yingyangbao (YYB) on continuous hematologic and anthropometric outcomes among post-only studies with concurrent-control (a,c), and pre-post studies (b,d) in China.
Table 3.
The effect of YYB on children’s hemoglobin concentration and continuous, anthropometric outcomes in China.
| Post-Only Studies with Concurrent-Control | Number of Studies | Total Intervention Participants | Total Control Participants | Mean Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Hemoglobin concentration (g/L) | 7 | 2810 | 1714 | 4.43 (1.55, 7.30) | 0.003 | 96 |
| Height (cm) | 6 | 2061 | 1689 | 2.46 (0.96, 3.97) | 0.001 | 94 |
| Weight (kg) | 6 | 2061 | 1689 | 0.79 (0.25, 1.32) | 0.004 | 97 |
| Height-for-age Z score (SD) | 3 | 1009 | 776 | −0.03 (−0.68, 0.62) | 0.94 | 95 |
| Weight-for-height Z score (SD) | 3 | 1009 | 776 | 0.27 (0.09, 0.45) | 0.003 | 66 |
| Weight-for-age Z score (SD) | 3 | 1009 | 776 | 0.20 (−0.11, 0.51) | 0.21 | 88 |
| Pre-Post Studies | Number of Studies | Total Posttest Participants | Total Pretest Participants | Mean Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Hemoglobin concentration (g/L) | 7 | 3370 | 3817 | 6.58 (2.71, 10.45) | <0.001 | 99 |
| Height (cm) | 5 | 2088 | 2213 | 2.46 (1.35, 3.58) | <0.001 | 90 |
| Weight (kg) | 5 | 1800 | 2212 | 0.72 (0.33, 1.10) | <0.001 | 94 |
| Height-for-age Z score (SD) | 5 | 1691 | 1821 | 0.24 (0.10, 0.39) | <0.001 | 70 |
| Weight-for-height Z score (SD) | 4 | 1438 | 1564 | 0.28 (−0.05, 0.61) | 0.10 | 95 |
| Weight-for-age Z score (SD) | 5 | 1691 | 1821 | 0.25 (−0.05, 0.56) | 0.11 | 95 |
The effect of YYB on children’s height and weight was assessed in six post-only studies with concurrent-control. Children’s height was 2.46 cm greater (95% CI: 0.96, 3.97; p = 0.001) and weight was on average 0.79 kg heavier (95% CI: 0.25, 1.32; p = 0.004) in the YYB groups compared to control groups. Heterogeneity was also high in height and weight meta-analyses (I2 = 94%, p < 0.002, and I2 = 97%, p < 0.001, respectively). The pre-post studies showed comparable results; children after the YYB intervention were on average 2.46 cm (95% CI: 1.35, 3.58; p < 0.001) taller and 0.72 kg (95% CI: 0.33, 1.10; p < 0.001) heavier than before intervention (Table 3, and Figures S2 and S3).
Table 3 and Figures S4–S6 present the effect of YYB on HAZ, WHZ, and WAZ. Only three post-only studies with concurrent-control reported effect estimates on HAZ, and in pooled analyses there was no effect of YYB on continuous HAZ (mean difference: −0.03; 95% CI: −0.68, 0.62; p = 0.94). Yet, the pooled result from five pre-post studies indicated that HAZ significantly increased by a large 0.24 SD (95% CI: 0.10, 0.39; p < 0.001) after YYB intervention with moderately high heterogeneity (I2 = 70%, p = 0.01). Results from three concurrent control studies showed that children in the YYB group had 0.27 of a SD (95% CI: 0.09, 0.45; p = 0.003) higher WHZ than children in the control group. There was a similar point estimate of 0.28 SD in pre-post studies, but the results were not statistically significant. In terms of WAZ, there was no effect of YYB in the three concurrent control studies or the five pre-post studies (Table 3 and Figure S6).
3.3. Anemia, Stunting, Underweight, and Wasting
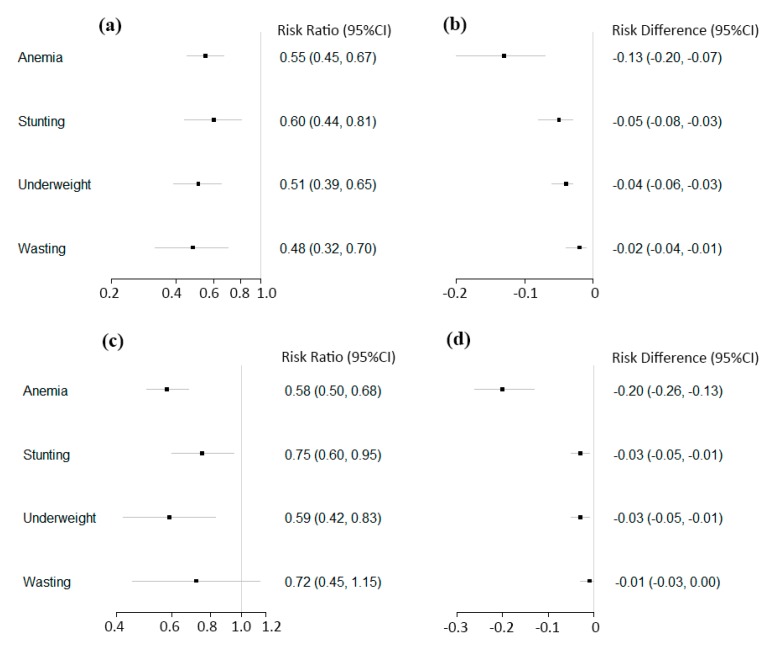
Figure 3 and Table 4 present the overall effect of YYB program on anemia prevalence. Eleven post-only studies with concurrent-control were included in the pooled analyses, and a 45% reduction in the risk of anemia (RR = 0.55; 95% CI: 0.45, 0.67; p < 0.001) was observed with high heterogeneity in effect estimates (I2 = 84%, p < 0.001). Pooled analyses of 13 pre-post studies similarly showed that anemia prevalence was reduced by 42% after the YYB intervention (RR = 0.58; 95% CI: 0.50, 0.68; p < 0.001) (Figure S7).
Figure 3.
The effect of YYB on the prevalence of anemia and child anthropometric outcomes in China among post-only studies with concurrent-control using risk ratios (a) and risk differences (b), and among pre-post studies, using risk ratios (c) and risk differences (d).
Table 4.
The effect of YYB on prevalence of child anemia and the categorical outcomes of nutritional status in China.
| Post-Only Studies with Concurrent-Control | Number of Studies | Total Intervention Participants | Total Control Participants | Risk Ratio (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) | Risk Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Anemia | 11 | 5869 | 3518 | 0.55 (0.45, 0.67) | <0.001 | 84 | −0.13 (−0.20, −0.07) | <0.001 | 95 |
| Stunting | 7 | 4368 | 2405 | 0.60 (0.44, 0.81) | <0.001 | 71 | −0.05 (−0.08, −0.03) | <0.001 | 52 |
| Underweight | 6 | 2218 | 1659 | 0.51 (0.39, 0.65) | <0.001 | 22 | −0.04 (−0.06, −0.03) | <0.001 | 0 |
| Wasting | 5 | 1831 | 1419 | 0.48 (0.32, 0.70) | <0.001 | 21 | −0.02 (−0.04, −0.01) | <0.001 | 50 |
| Pre-Post Studies | Number of Studies | Total Posttest Participants | Total Pretest Participants | Risk Ratio (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) | Risk Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Anemia | 13 | 5300 | 6019 | 0.58 (0.50, 0.68) | <0.001 | 89 | −0.20 (−0.26, −0.13) | <0.001 | 93 |
| Stunting | 10 | 3873 | 4345 | 0.75 (0.60, 0.95) | 0.02 | 67 | −0.03 (−0.05, −0.01) | 0.01 | 68 |
| Underweight | 10 | 3873 | 4345 | 0.59 (0.42, 0.83) | 0.002 | 63 | −0.03 (−0.05, −0.01) | 0.003 | 72 |
| Wasting | 7 | 2793 | 3202 | 0.72 (0.45, 1.15) | 0.17 | 60 | −0.01 (−0.03, 0.00) | 0.14 | 64 |
A pooled analyses of seven post-only studies with concurrent-control found that children’s risk of stunting in the YYB groups was reduced by 40% (RR = 0.60; 95% CI: 0.44, 0.81; p < 0.001) compared to the control groups; there was high heterogeneity in this analysis (I2 = 71%, p = 0.002). Consistently, pooled analysis of 10 pre-post studies found that YYB was associated with a 25% (RR = 0.75; 95% CI: 0.60, 0.95; p = 0.02) lower risk of being stunted after the intervention (Table 4 and Figure S8). Pooled analyses showed that YYB was associated with lower risk of underweight (Table 4 and Figure S9). In terms of wasting, YYB was associated with 52% (RR = 0.48; 95% CI: 0.32, 0.70; p < 0.001) lower risk of being wasting in the five post-only with concurrent-controll studies, yet the seven pre-post studies did not yield any significant effect (Table 4 and Figure S10).
The summary risk differences and 95% CIs for categorical outcomes were also calculated (Figures S11–S14). Overall, the findings for anemia, stunting, underweight, and wasting were consistent with the relative risk analyses.
3.4. Subgroup and Sensitivity Analysis
Subgroup analyses were conducted among post-only with concurrent studies by the type of control (active or blank). Pooled analyses of blank control studies determined that YYB was associated with a 5.54 g/L (95% CI: 1.46, 9.61; p = 0.001) increase in Hb concentration (I2 = 0%). The subgroup of active control studies also found YYB was associated with higher Hb concentration, but the effect size was 1.80 g/L (95% CI: 0.77, 2.83; p = 0.008) (I2 = 97%). The increase of Hb concentration in blank control group studies was significantly greater than that found in active control group studies (p < 0.001).
In terms of anemia prevalence, nine studies were with blank controls and two studies with active controls. Pooled analyses showed that risk of anemia was significantly lower in both blank control and active control studies (blank control: RR = 0.64; 95% CI: 0.48, 0.87; p = 0.004; I2 = 87%; active control: RR = 0.52; 95% CI: 0.41, 0.66; p < 0.001; I2 = 0%), and the risk reduction was not significantly different between two control types (p = 0.93).
No evidence of modification by intervention duration (≤12 months or >12 months) on YYB effect was observed (Tables S4 and S5). In addition, results remained consistent when excluding five concurrent-controlled and four pre-post studies that were determined to be of low quality (Tables S6 and S7).
Funnel plots (Figures S15 and S16) and Egger’s tests for analyses of anemia prevalence did not suggest the presence of publication bias for concurrent control studies (p = 0.493 for Egger’s test) or pre-post studies (p = 0.388).
4. Discussion
In this study, YYB complementary food supplement was found to be associated with significant increases in children’s Hb concentration, height, and weight, and significant reductions in children’s risk of anemia and stunting, in quasi-experimental studies conducted in China. Subgroup analyses by control group and with sensitivity analyses limited to high-quality studies were consistent with the main results.
Consistent with other studies of multi-micronutrient supplementation (e.g., Sprinkles, Foodlet, and lipid-based nutrient supplements (LNS)) [9,50,51,52,53,54,55], YYB resulted in a significant improvement in children’s hematological outcomes in the current study. As a previous study suggested [56]56, the iron composition of the supplement might be the major contributor to reducing anemia’s prevalence and increasing Hb concentration. Despite the importance of iron supplementation, the unpleasant taste of iron has been widely discussed. A study on the YYB program showed that 45.3% of the children did not like the taste of YYB, which may have affected adherence to YYB [6]. Sprinkles have reduced the metallic taste by encapsulating iron within a thin lipid layer to prevent the iron from interacting with food [53]. As a result, the improved taste may improve adherence to and the impact of YYB.
Previous studies on protein-free multi-micronutrient supplements, such as Sprinkles, Foodlets, and drops, found small or no effects on children’s weight and risk of underweight [52,57,58]. However, YYB appeared to have large effects on weight-related indicators; post-only studies with concurrent-control found a 0.8 kg increase in weight, a 0.27 SD increase in WAZ, and a 49% decrease in the risk of underweight prevalence. Nevertheless, our finding is generally consistent with studies on lipid-based nutrient supplements (LNS), which additionally provide calories, proteins, and fatty acids. LNS refers to a range of products wherein the majority of energy is provided by lipids, including products like ready-to-use therapeutic foods (RUTF) and small-quantity LNSs that provide <110 kcal/day [55]. Yet the effect size of YYB on weight-based outcomes in our meta-analysis appear to be much larger than LNS. A recent meta-analysis of RCTs found LNS reduced the risk of underweight by 15%, compared to a more than a 40% reduction found in our YYB meta-analysis of quasi-experimental studies [55]. Similarly, this study found YYB to have a larger effect on stunting reduction than LNS (20% compared to 7%) [55]. The difference in the effects of YYB and LNS is notable, particularly considering LNS provides greater calories (usually about 100–1000 kcal per day or 50%–100% of daily energy requirements)) compared to YYB (~50 kcal per day, 5% to 25% of daily energy requirements) [55,59,60]. The larger effect of YYB over LNS might be attributable to the differences in the study designs and corresponding risk of bias and the study populations. LNS trials were primarily for children with moderate to acute malnutrition [61], while YYB studies were largely among all children in a community. Therefore, YYB may not only improve growth for undernourished children, but also prevent child malnutrition in the general population, which could have contributed to the larger effect at population-level.
There are also some concerns with regards to use of LNS. Large quantity LNS has raised substantial concern about the adverse effects on excessive weight gain that may affect later-life, non-communicable disease risk in the future, particularly when the product was used for prevention purposes [62]. Small-quantity LNS provides a lower calorie quantity (∼110 kcal per day) and the evidence based on body composition and long term outcomes is evolving [60]. In addition, sugar is added to LNS primarily to increase palatability and there has been some concern in regard to fostering infant preferences for sweet foods over breastmilk [60]. An additional consideration is that the cost per serving of YYB is believed to be lower than small-quantities of LNS [63].
In addition to the effects on hematologic and nutritional outcomes, several papers have examined the association of YYB with additional child health and development outcomes. Two studies that examined developmental outcomes found that YYB program significantly improved intelligence quotient and gross motor development [23,27]. YYB contains macronutrients and micronutrients (e.g., protein, iron, zinc, etc.) that may be critical for brain development [64,65]. An additional study investigated the effect of YYB on childhood morbidities and found that the prevalence of respiratory infection and diarrhea was reduced after a 12-month YYB intervention [24]24.
This review has several limitations: First, both concurrent control and pre-post quasi-experimental studies are observational in nature and are at risk of confounding and other biases. As a result, randomized trials are needed to determine causal effects. Second, the small number of studies for several indicators, reduces the power of analysis to examine the effect on anthropometric Z-scores, and limits our ability to do meta-analyses on developmental outcomes or disease prevalences. Third, secular improvement in health and nutrition is of particular risk in pre-post studies. However, the estimates of effects in pre-post studies were generally in line with those from post-only studies with concurrent-control. Fourth, significant heterogeneity in estimated effects was found for most hematologic and anthropometric outcomes, which suggests that the magnitude of the effect of YYB may differ by setting.
Our findings suggest that YYB may improve hematological outcomes and growth status during infancy and early childhood. YYB is gaining attention both within China and from the global nutrition community [66], particularly, since complementary feeding interventions are included in the WHO Global Nutrition Targets 2025 that call for all countries to reduce the number of children under five who are stunted by 40% from 2015 levels [67]. Despite evidence indicating the potential benefits of YYB, there is clearly a need for more robust evidence, including randomized control trials and rigorously implemented evaluations. In addition, exploring the use of YYB outside of China, including assessments of acceptability, the composition of the product, and impacts, are warranted. In settings in China where YYB is already deployed, future research should be conducted to identify gaps in program implementation, and explore strategies to deliver the YYB with optimal effective coverage.
Acknowledgments
We thank Xiaoxiao Jiang for her input in collecting and organization data for this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/10/2404/s1, Table S1: Risk of bias of post-only studies with concurrent-control assessed with the methodological index for non-randomized studies (MINORS). Table S2: The risk of bias of pre-post studies assessed with methodological index for non-randomized studies (MINORS). Table S3: The composition of three types of YYB. Table S4: Effects of YYB by intervention duration on hemoglobin concentration (g/L), height (cm), weight (kg), and Z-scores. Table S5: Effects of YYB by intervention duration on the prevalence of anemia, stunting, underweight, and wasting. Table S6: Effects of YYB on hemoglobin concentration (g/L), height (cm), weight (kg), and Z-scores, among studies of high and medium quality. Table S7: Effects of YYB on prevalence of anemia, stunting, underweight, and wasting among studies of high and medium quality. Figure S1: The effects of YYB on hemoglobin level (g/L) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S2: The effects of YYB on height (cm) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S3: The effects of YYB on weight (kg) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S4: The effects of YYB on height-for-age z score (HAZ) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S5: The effects of YYB on weight-for-height z score (WHZ) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S6: The effects of YYB on weight-for-age z score (WAZ) among post-only studies with concurrent-control (a) and pre-post studies (b) using mean difference as the effect measure. Figure S7: The effects of YYB on anemia prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk ratios as the effect measure. Figure S8: The effects of YYB on stunting prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk ratios as the effect measure. Figure S9: The effects of YYB on underweight prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk ratios as the effect measurements. Figure S10: The effects of YYB on wasting prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk ratios as the effect measurements. Figure S11: The effects of YYB on anemia prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk difference as the effect measure. Figure S12: The effects of YYB on stunting prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk difference as the effect measure. Figure S13: The effects of YYB on underweight prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk difference as the effect measure. Figure S14: The effects of YYB on wasting prevalence among post-only studies with concurrent-control (a) and pre-post studies (b) using risk difference as the effect measure. Figure S15: Funnel plot of effects of YYB on anemia prevalence among post-only studies with concurrent-control. Figure S16: Funnel plot of effects of YYB on anemia prevalence among pre-post studies
Author Contributions
Z.L., X.L., Y.L., K.T., and Y.H. collected data for meta-analysis. Z.L. and W.F. conceptualized the analysis and developed the analysis plan. Z.L., X.L., and Y.L. conducted data analysis under W.F.’s supervision. Z.L. wrote the first draft. All authors interpreted findings, critically reviewed the report for important intellectual content, contributed to manuscript writing, and approved the final version.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.National Bureau of Statistics of China . China Statistical Yearbook. China Statistical Press; Beijing, China: 2017. [Google Scholar]
- 2.The United Nations Development Programme (UNDP) World Population Prospects—Population Division—United Nations. [(accessed on 2 August 2017)]; Available online: https://esa.un.org/unpd/wpp/Download/Standard/Population/
- 3.The World Bank Prevalence of Stunting, Height For Age (% of Children Under 5) Data. [(accessed on 11 February 2019)]; Available online: https://data.worldbank.org/indicator/sh.sta.stnt.zs.
- 4.The World Bank Prevalence of Underweight, Weight for Age (% of Children under 5) | Data. [(accessed on 11 February 2019)]; Available online: https://data.worldbank.org/indicator/SH.STA.MALN.ZS.
- 5.World Food Programme 10 Facts about Nutrition in China. [(accessed on 25 May 2018)]; Available online: https://www.wfp.org/stories/10-facts-about-nutrition-china.
- 6.Wang J., Chang S., Zhao L., Yu W., Zhang J., Man Q., He L., Duan Y., Wang H., Scherpbier R., et al. Effectiveness of community-based complementary food supplement (Yingyangbao) distribution in children aged 6–23 months in poor areas in China. PLoS ONE. 2017;12:e0174302. doi: 10.1371/journal.pone.0174302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Wu Q., Wang W., van Velthoven M.H., Chang S., Han H., Xing M., Chen L., Scherpbier R.W. Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6–23 months in poor areas of Qinghai Province, China: A controlled interventional study. BMJ Open. 2016;6:e011234. doi: 10.1136/bmjopen-2016-011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo J., Ying Yang Bao: Improving Complementary Feeding for Chinese Infants in Poor Regions [(accessed on 20 April 2018)];Nestle Nutrition Institute Workshop Series. 2017 :131–138. doi: 10.1159/000448962. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28315893. [DOI] [PubMed]
- 9.Zhang Q., Sun J., Jia X., Huo J. Meta analysis of the nutrition intervention effect of Yingyangbao on infants and young children in China. J. Hyg. Res. 2015;44:970–977. [PubMed] [Google Scholar]
- 10.Wang Y., Chen C., Jia M., Fang J., Wang F. Effect of food supplements on anemia in infants and young children. J. Hyg. Res. 2004;33:334–336. [PubMed] [Google Scholar]
- 11.China Development Research Foundation One Yuan Yingyangbao Program. [(accessed on 26 September 2019)];2018 Available online: https://www.cdrf.org.cn/gzbg/4447.jhtml.
- 12.China’s Ministry of Health Infant and Child Feeding Strategies. [(accessed on 26 September 2019)];2007 Available online: http://www.gov.cn/fwxx/jk/2007-08/01/content_703104.htm.
- 13.Ministry of Education of the People’s Republic of China Millions of Impoverished Children were Benefiting from Yingyangbao. [(accessed on 4 September 2018)]; Available online: http://www.moe.edu.cn/jyb_xwfb/s5147/201601/t20160112_227681.html.
- 14.The State Council of the People’s Republic of China Nutrition Strategy Plan for Chinese Citizens (2017–2030) [(accessed on 21 May 2018)]; Available online: http://www.mohrss.gov.cn/SYrlzyhshbzb/dongtaixinwen/shizhengyaowen/201707/t20170714_274000.html.
- 15.The United Nations Children’s Fund (UNICEF) China’s Experience, Global Perspective. [(accessed on 26 August 2019)];2019 Available online: https://www.unicef.cn/media/8311/file/unicef-in-china-and-beyond-cn.pdf.
- 16.Wang Y., Chen C., Wang F., Wang K. Effects of nutrient fortified complements food supplements on growth of Chinese infants and young children in poor rural area in Gansu Province. J. Hyg. Res. 2007;36:78–81. [PubMed] [Google Scholar]
- 17.Xu J., Li Y., Huo J., Sun J., Huang J. Supplementing fortified soybean powder reduced anemia in infants and young children aged 6–24 months. Nutr. Res. 2019;63:21–33. doi: 10.1016/j.nutres.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Tramèr M.R., Reynolds D.J.M., Moore R.A., McQuay H.J. Impact of covert duplicate publication on meta-analysis: A case study. BMJ. 1997;315:635–640. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Intervention. The Cochrane Collaboration; London, UK: 2011. [(accessed on 22 February 2019)]. Available online: www.handbook.cochrane.org. [Google Scholar]
- 22.Ni S., Qin X., Chen X., Yue C., Liu Y., Zhang F., Liu F. Study on nutrition intervention experiment of infants and young children in rural areas. Chinese J. Public Heal. 1995;11:337–339. [Google Scholar]
- 23.Wang Y., Wang F.-Z., Wang K., Chen C.-M., Jin M. Effect of nutritional fortified supplementary food supplement on intelligence development of infants and young children in poor rural areas of Gansu. J. Hyg. Res. 2006;35:772–774. [PubMed] [Google Scholar]
- 24.Yu D., Wang Y., Wang F. Effect of supplementary food supplement on respiratory tract infection and diarrhea in infants and young children in poor rural areas. J. Hyg. Res. 2007;36:355–357. [PubMed] [Google Scholar]
- 25.Wang Y., Chen C., Wang F., Wang K., Yu D. Effects of nutritional fortified supplementary food supplements on physical growth of infants and young children in poor rural areas of Gansu. J. Hyg. Res. 2007;36:78–81. [PubMed] [Google Scholar]
- 26.Wang Y., Chen C., Wang F., Jia M., Wang K. Effects of nutrient fortified complementary food supplements on anemia of infants and young children in poor rural of Gansu. Biomed. Env. Sci. 2009;22:194–200. doi: 10.1016/S0895-3988(09)60045-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen C.-M., Wang Y.-Y., Chang S.-Y. Effect of In-home Fortification of Complementary Feeding on Intellectual Development of Chinese Children. Biomed. Environ. Sci. 2010;23:83–91. doi: 10.1016/S0895-3988(10)60036-0. [DOI] [PubMed] [Google Scholar]
- 28.Fang Z., Yang H., Zhao L., Tang Z., Ma L., Zhao L., Yu W., Jia F., Wang Q. Interventional effect analysis of nutritional health status of infants aged 6–24 months in poor areas in 3 counties of Guangxi. Chinese J. Child Heal. Care. 2010;18:638–640. [Google Scholar]
- 29.Foundation CDR China Development Research Foundation Early Childhood Development Project Qinghai Pilot Mid-term Evaluation. [(accessed on 3 July 2018)];2011 Available online: https://cdrf.org.cn/jjh/pdf/qinghai.pdf.
- 30.Zhao W., Li H., Yang H. Evaluation on the effect of nutrition intervention for children in poor rural areas of Gansu. Chinese J. Sch. Heal. 2012;33:257–258+262. [Google Scholar]
- 31.Fan S., Li J., Zhang Y., Xu C., Zhao L., Ma M., M H., Ma Q., Z L., L Y. Analysis of anemia and intervention effect of infants and young children in Hebei Province. Matern Child Heal Care China. 2013;28:2032–2033. [Google Scholar]
- 32.Li W., Zhu B., Shao J., Zhu Z. Effects of complementary food supplements on incidence of anemia among infants and young children in Zhejiang Province. Mod. Prev. Med. 2013;40:23. [Google Scholar]
- 33.Ren L. Effect evaluation of nutrition intervention for infants aged 6~24 months in Changle County. Mod Prev Med. 2014;41:1984–1986+1990. [Google Scholar]
- 34.Li S. Investigation report on implementation of early childhood nutrition intervention project in poor areas of Ledu District. Qinghai Med. J. 2014;44:51–52. [Google Scholar]
- 35.Hu Q., Du M., Liang C., Zhang R. Evaluation of the effect of infant nutrition for children aged 6 to 36 months in Shenzhen. Chinese J. Women Child Heal. 2016;7:26–29. [Google Scholar]
- 36.Ding X., Zhang F., He Q., Mao Z., Li R. Nutrition effectiveness of 6–18 months old infants in low-income rural areas in Jiangxi province. Mod. Prev. Med. 2016;43:3703–3705+3756. [Google Scholar]
- 37.Li S., Zhang K., Qiu J., Cha L., Xiang Y. Effect evaluation of nutritional intervention of infantile complementary food package in 6–24-month infants. Matern. Child Heal. Care China. 2017;32:58–61. [Google Scholar]
- 38.Shen H., Li G. Study on comprehensive nutrition intervention of infants and young children in rural areas of Shaanxi. Chinese J Clin Res. 2011;24:857–858. [Google Scholar]
- 39.Sun J., Dai Y., Zhang S., Huang J., Yang Z., Huo J., Chen C. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China. Matern. Child Nutr. 2011;7:96–111. doi: 10.1111/j.1740-8709.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Huo J., Sun J., Li W., Huang J., Huang C., Lai S., Hu J., Chen C.-M., Wang Y. Effect of nutrition package on infants and young children aged 6 to 23 months after Wenchuan earthquake in Li County, Sichuan. J. Hyg. Res. 2011;40:61–64. [PubMed] [Google Scholar]
- 41.Dong C., Ge P., Ren X., Wang J., Fan H., Yan X., Yin S.A. Prospective Study on the Effectiveness of Complementary Food Supplements on Improving Status of Elder Infants and Young Children in the Areas Affected by Wenchuan Earthquake. PLoS One. 2013;9:e72711. doi: 10.1371/journal.pone.0072711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., Xu Z., Chang F., Fu P., Zhang J., Zhang T., Zhang H., Li J., Song P. Periods study of nutrition interventions about infants aged 6–24 months in Ningqiang country affected by Wenchuan earthquake. Chinese J. Child Heal. Care. 2012;20:413–415. [Google Scholar]
- 43.Li L., Chang F., Xu Z., Wang L., Full G., Fu P., Zhang J., Song P. Half-year effect evaluation of infant nutrition package intervention for infants aged 6–24 months in Ningqiang, Shanxi. Chinese J. Child Heal. Care. 2012;20:395–397. [Google Scholar]
- 44.Xu Z., Wang L., Chang F., Fu P., Zhang J., Zhang H., Li J. Study on the effect of nutrition intervention on infants and young children aged 6 to 24 months in Ningqiang County of the earthquake-stricken area. Chinese J. Child Heal. Care. 2012;20:728–730. [Google Scholar]
- 45.Liu Z., Yan L., Wu T., Lan Z., Xu Y., Lin L. Study on Nutrition Improvement of Infants and Children in Earthquake-Stricken Areas. J. Prev. Med. Inf. 2013;29:107–110. [Google Scholar]
- 46.Qin J., Ma G. The effects of nutrition interventions about infants and children in Wulan County. Qinghai Med. J. 2014;44:63–64. [Google Scholar]
- 47.Huo J., Sun J., Fang Z., Chang S., Zhao L., Fu P., Wang J., Huang J., Wang L., Begin F., et al. Effect of Home-Based Complementary Food Fortification on Prevalence of Anemia Among Infants and Young Children Aged 6 to 23 Months in Poor Rural Regions of China. Food Nutr. Bull. 2015;36:405–414. doi: 10.1177/0379572115616001. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q., Zhang H., Su X., Zhou X. Study on Nutritional Intervention Effect of Infants and Children in 6~24 Months in Chongqing Project Areas. Matern. Child Heal. Care China. 2016;31:2641–2643. [Google Scholar]
- 49.Wu P., Shi Y. Effect of children’s food supplement nutrition package on nutrition intervention of infants and young children in poor areas in China. Heal. Care Guid. 2017;1:114. [Google Scholar]
- 50.Dewey K.G., Yang Z., Boy E. Systematic review and meta-analysis of home fortification of complementary foods. Matern. Child Nutr. 2009;5:283–321. doi: 10.1111/j.1740-8709.2009.00190.x. [DOI] [Google Scholar]
- 51.Jack S.J., Ou K., Chea M., Chhin L., Devenish R., Dunbar M., Eang C., Hou K., Ly S., Khin M., et al. Effect of Micronutrient Sprinkles on Reducing Anemia. Arch. Pediatr. Adolesc. Med. 2012;166:842. doi: 10.1001/archpediatrics.2012.1003. [DOI] [PubMed] [Google Scholar]
- 52.Smuts C.M., Lombard C.J., Benadé A.J.S., Dhansay M.A., Berger J., Hop L.T., López d.R.G., Untoro J., Karyadi E., Erhardt J., et al. Efficacy of a Foodlet-Based Multiple Micronutrient Supplement for Preventing Growth Faltering, Anemia, and Micronutrient Deficiency of Infants: The Four Country IRIS Trial Pooled Data Analysis. J. Nutr. 2005;135:631S–638S. doi: 10.1093/jn/135.3.653S. [DOI] [PubMed] [Google Scholar]
- 53.Zlotkin S.H., Schauer C., Christofides A., Sharieff W., Tondeur M.C., Hyder S.M.Z. Micronutrient Sprinkles to Control Childhood Anaemia. PLoS Med. 2005;2:e1. doi: 10.1371/journal.pmed.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchdev P.S., Ruth L.J., Woodruff B.A., Mbakaya C., Mandava U., Flores-Ayala R., Jefferds M.E.D., Quick R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: A cluster-randomized controlled trial. Am. J. Clin. Nutr. 2012;95:1223–1230. doi: 10.3945/ajcn.111.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das J.K., Salam R.A., Hadi Y.B., Sheikh S.S., Bhutta A.Z., Prinzo Z.W., Bhutta Z.A. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst. Rev. 2019;5 doi: 10.1002/14651858.CD012611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De-Regil L.M., Suchdev P.S., Vist G.E., Walleser S., Peña-Rosas J.P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review) Evid. Based Child Health Cochrane Rev. J. 2013;8:112–201. doi: 10.1002/ebch.1895. [DOI] [PubMed] [Google Scholar]
- 57.Samadpour K., Long K.Z., Hayatbakhsh R., Marks G.C. Randomised comparison of the effects of Sprinkles and Foodlets with the currently recommended supplement (Drops) on micronutrient status and growth in Iranian children. Eur. J. Clin. Nutr. 2011;65:1287–1294. doi: 10.1038/ejcn.2011.124. [DOI] [PubMed] [Google Scholar]
- 58.Jyoti V., Sharma S. Impact of micronutrients sprinkle on weight and height of children aged 6–36 months in Tonk district of Rajasthan state. Indian J. Community Health. 2014;26(Suppl. 2):294–299. [Google Scholar]
- 59.The American Heart Association Dietary Recommendations for Healthy Children American Heart Association. [(accessed on 12 April 2019)]; Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/dietary-recommendations-for-healthy-children.
- 60.Arimond M., Zeilani M., Jungjohann S., Brown K.H., Ashorn P., Allen L.H., Dewey K.G. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: Experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern. Child Nutr. 2015;11:31–61. doi: 10.1111/mcn.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gera T., Pena-Rosas J.P., Boy-Mena E., Sachdev H.S. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): A systematic review. PLoS ONE. 2017;12:e0182096. doi: 10.1371/journal.pone.0182096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaparro C.M., Dewey K.G. Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings. Matern. Child Nutr. 2010;6:1–69. doi: 10.1111/j.1740-8709.2009.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Home Fortification [(accessed on 26 September 2019)];Complementary Food Supplements (CFS) Available online: http://www.hftag.org/page.asp?content_id=33984.
- 64.Martins V.J., Toledo Florêncio T.M., Grillo L.P., Do Carmo P.F., Martins P.A., Clemente A.P.G., Santos C.D., Vieira M.D.F.A., Sawaya A.L. Long-lasting effects of undernutrition. Int. J. Environ. Res. Public Health. 2011;8:1817–1846. doi: 10.3390/ijerph8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cusick S.E., Georgieff M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S. Ying Yang Bao Story. [(accessed on 12 April 2019)];2015 Available online: http://www.chinanutri.cn/xwzx_238/xyxw/201510/t20151019_121165.html.
- 67.The World Health Organization (WHO) Interventions by Global Target. [(accessed on 11 June 2019)]; Available online: https://www.who.int/elena/global-targets/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.