Abstract
There is accumulating evidence that physical fitness influences the gut microbiome and as a result, promotes health. Indeed, exercise-induced alterations in the gut microbiome can influence health parameters crucial to athletic performance, specifically, immune function, lower susceptibility to infection, inflammatory response and tissue repair. Consequently, maintenance of a healthy gut microbiome is essential for an athlete’s health, training and performance. This review explores the effect of exercise on the microbiome while also investigating the effect of probiotics on various potential consequences associated with over-training in athletes, as well as their associated health benefits.
Keywords: athletes, probiotic, microbiome, overtraining, fitness, exercise
1. A Brief Introduction to Exercise and the Microbiome
Our gut microbiome, an elusive and recently designated organ, is under ever greater scrutiny. Uncovering the significance of the microbiome in human health and disease has become possible with the advent of various genomic tools accompanied by improvements in culture-based approaches (culture-omics). The gut contains a rich and diverse microbial ecosystem whose activities can influence the health of the host [1]. More specifically the gut is home to trillions of microbial species [2] that co-exist with human cells. The dominant microbial populations in the gut are bacteria from the Bacteroidetes and Firmicutes phyla, followed by members of the phyla Actinobacteria, Proteobacteria and Verrucomicrobia. The microbiome also consists of a fungal community, including species of Candida and Saccharomyces [3], viruses (primarily bacteriophage) [4], as well as members from the archaeal domain [2]. Despite the microbiome being stable in adults, certain factors can alter its structure, including, diet, antibiotics, probiotics and indeed exercise/fitness (for review see [5,6]). The microbiome appears to have an effect on most aspects of human health. In addition to a role for specific microbes, or groups of microbes, the importance of microbial diversity has been recognized in many studies, with a decreased diversity associated with a number of gastrointestinal (GIT) conditions such as Crohn’s disease [7], certain cancers [8] and Type 1 diabetes [9]. An altered microbiome can affect energy metabolism, immune function and oxidative stress, all of which are vital for athlete’s performance and overall health (for review see [10]). A potential, non-medical, route to maintaining athlete’s health is through probiotics. Probiotics are defined as live bacteria that, when ingested in adequate amounts, confer a benefit to the host [11]. Although such health benefits have frequently been credited to specific bacterial strains from the genera Bifidobacterium and Lactobacillus, the potential of next-generation probiotic (NGP) candidates and designer probiotics is also being recognized.
Although it is generally accepted that microbial diversity is an indicator of human health, this hypothesis has been challenged recently by Shade, who postulated that “mechanisms maintaining or changing microbial diversity are many and complex” and assumptions that an increased diversity is better may oversimplify complex mechanisms of health and disease [12]. As previously stated, certain factors including an individual’s lifestyle can alter the structure of the microbiome. It has been noted across a number of studies that athletes have increased gut microbial diversity compared with more sedentary controls [13,14,15]. Clarke et al. [14] demonstrated significant gut microbiome differences between male professional elite rugby players and a cohort of non-athlete male controls. Notably, the athletes had significantly higher gut microbial diversity accompanied by elevated levels of the Akkermansia genus. A subsequent study of the same cohort [13] concluded that athletes had a higher abundance of short-chain fatty acids (SCFA) metabolic pathways. In another instance, Petersen et al. [15], investigated the gut microbiome of cyclists and concluded that 30 out of 33 cyclists also had an increased abundance of Akkermansia. It was also observed that exercise regime is proportional to the abundance of Prevotella, which in turn has been associated with increased branched-chain amino acid (BCAA) pathways, important in muscle recovery. There was also an increase in Methanobrevibacter smithii within the professional cyclist group compared to amateur cyclists, a microbe that utilises H2 in the colon to make SCFA and Adenosine triphosphate (ATP) [15]. More recently, Cronin et al. investigated the impact of exercise and/or whey protein supplementation over an eight-week period on a cohort of sedentary adults. In this instance, the differences between the microbial communities of the three test populations were more subtle, a fact that could be attributed to the shorter period over which time these interventions took place relative to the potentially life-long practices of some athletes, i.e., microbial architecture is not easily changed and it may take more time to notice significant impacts [16]. Recently, Jang et al. compared the gut microbiome of bodybuilders, distance runners and controls (n = 45), each of the groups were ingesting a different sport specific diet. The results show that bodybuilders ingesting a high-protein and fat diet had increased relative abundance of Faecalibacterium, Sutterella, Clostridium, Haemophilus and Eisenbergiella, but decreased abundances of Bifidobacteria, Parasutterella and Eubacterium. The study also concluded that individuals practicing anaerobic sports show similar gut microbiome patterns to bodybuilders [17]. A pioneering study by Scheiman et al. discovered that the genus Veillonella was enriched in the gut microbiome of marathon runners, compared to non-runners. Furthermore, using a mouse model they demonstrated that Veillonella atypica increased endurance, reduced inflammatory cytokines, and converted lactate to acetate/propionate [18]. The present studies suggest that exercise can alter the microbiome in a beneficial manner, where the alteration in the composition of the microbiome can be associated with increased health parameters and thus possibly affect athletic performance in a beneficial way. Reported gut microbiome changes/differences associated with exercise and fitness are summarized in Table 1.
Table 1.
Studies of exercise and the microbiome.
| Subject Group | Microbiome Change | Key Findings | Study Reference |
|---|---|---|---|
| Rugby players |
↑Akkermansia, Prevotella, ↓Ruminococcaceae
Bacteroides Lactobacillus |
Akkermanisa was associated with better immunity and gut barrier function while Prevotella was correlated to biosynthesis of branched-chain amino acid (BCAA) pathways which help with muscle recovery | [14] |
| Professional male athletes | ↑Akkermansia metabolic pathways/higher short-chain fatty acids (SCFA) metabolic pathways | Rugby players had a higher abundance of health-promoting Akkermansia genus, which has been associated with an improved gut barrier function and immune function stimulation. | [13] |
| Cyclists |
↑Prevotella, Methanobrevibacter
Smithii |
Prevotella was correlated to biosynthesis of BCAA pathways which help with muscle recovery, M. smithii has been associated with degradation of H2 which is used to make ATP/SCFA resulting in a more energetically efficient body | [15] |
| Sedentary adults challenged to eight week exercise regime |
↓in Archaea species and an ↑ in microbial diversity | Microbial diversity has been linked to an overall better health | [16] |
| Marathon runners | ↑Veillonella | Veillonella has been shown to metabolize lactate to SCFA, lower inflammation and increase performance in murine models | [18] |
| Bodybuilders and Distance runners |
↑Faecalibacterium, Sutterella, Clostridium, Haemophilus, Eisenbergiella
↓Bifidobacterium, Parasutterella and Eubacterium |
Different sports and their sport specific diets can affect the gut microbiome in different ways | [17] |
Whilst exercise confers numerous physiological effects on the host including mood regulation [19], improving cardiovascular symptoms [20], alleviation of fatigue [21] and anti-inflammatory effects, a healthy balance between training load and recovery needs to be maintained to prevent the condition of overtraining occurring [22]. Studies in the past have observed the effect of overtraining on the microbiome; Allen et al. concluded that voluntary and forced exercise altered the mice microbiome in different ways. Of particular interest were the phyla Tenericutes and Proteobacteria, both elevated in forced exercised mice, compared to mice that exercised voluntarily. Tenericutes spp. have been linked to intestinal inflammation in human subjects and Proteobacteria bacteria are known for their lipopolysaccharide production [23]. Similarly, Karl et al. observed that there was a large increase in pathogenic taxa post-exercise in soldiers including, Peptostreptococcus, Staphylococcus, Peptoniphilus, Acidaminococcus, and Fusobacterium and a decrease in more beneficial taxa Bacteroides, Faecalibacterium, Collinsellaa and Roseburia. The authors postulated that the increase in more toxic species may possibly explain the observation of increased intestinal permeability within the soldier cohort [24]. More recently, Yuan et al. concluded that excessive exercise had a negative impact on microbial diversity in murine models [25].
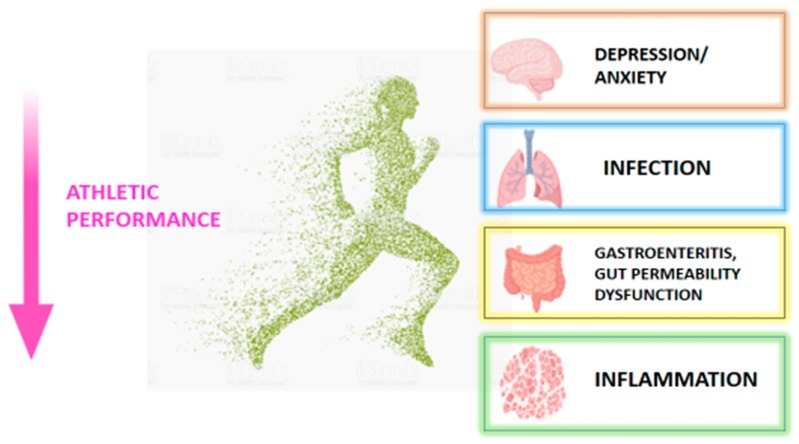
Various studies throughout the years have investigated overtraining and its effect on human health particularly host immunity [26,27] and susceptibility to infection [22,28,29]. The potential side effects of overtraining are illustrated in Figure 1. Furthermore, overtraining has been associated with an increased incidence of mental illnesses in athletes [30,31,32,33] and oxidative stress [34,35], where studies in animal models have shown the relationship between the microbiome and oxidative stress [36,37]. One of the most common side effects of overtraining is gut dysfunction and it has been frequently noted in athletes [38,39]. Gastrointestinal (GIT) symptoms are frequently observed with athletes who tend to travel for training or competition, purposes [40,41]. Antibiotics can be used to overcome the GIT infections, however, certain complications may arise when using antibiotics. Firstly, studies have shown that the microbiome takes about six weeks to resemble its original state post antibiotic therapy [42], it increases possible tendon rupture [43] and can cause antibiotic associated diarrhea [44].
Figure 1.
The effects of overtraining on the wellbeing of an athlete.
The review aims to provide a panoramic view of what is currently known about probiotics and their effect on gut microbiome of athletes. A recent systematic review by Moller et al. [45] has provided an overview of the effects of traditional probiotics on physically active individuals and athletes, with a particular focus on the clinical trials performed to date. Here we explore the relationship between fitness and the gut microbiome focusing particularly on overtraining. We also explore the available literature on how, possibly, probiotics can help to alleviate or prevent the different side effects of overtraining. We also discuss next generation and designer probiotics, as well as the safety and toxicity considerations associated with novel strains.
2. Traditional Probiotics
Traditionally, the majority of probiotic strains were representatives of the lactic acid bacteria group (LAB) i.e., Bifidobacterium, Lactobacillus [46], however other bacterial and yeast strains are also commonly used such as Escherichia coli and Saccharomyces respectively. Although probiotics have existed for quite some time, it is only recently that their commercial potential has been realized, with the market expected to exceed $67 billion by 2024 [47]. Probiotics can consist of one or a combination of a few strains, either as capsules, powders or as a component of a food e.g., yoghurt [48]. One of the main concerns relating to the probiotic market is that some strains do not have substantial proof of efficacy. It is also important for consumers to be aware that the benefits of one probiotic strain cannot be inferred to another [48,49]. Nonetheless, there is increasing evidence of the ability of specific probiotics to, for example, modulate the immune system, impact on tight-junction proteins and inhibit pathogen colonization; this list is not exhaustive, and the benefits of probiotics are strain-dependent. Focusing on athletes as noted above, extreme exercise can be associated with undesirable symptoms and specific probiotics can help to ease or prevent certain gastrointestinal disorders, help with the brief immunosuppression period, and reduce susceptibility to infections. Ways in which existing and novel probiotics could contribute to athlete health are provided in Table 2.
Table 2.
Traditional probiotics and next-generation probiotics and their benefits.
| Probiotic Genus * | Found in the Body | Dietary Source: | Potential Benefits Attributed to Specific Strains | References |
|---|---|---|---|---|
| Lactobacillus | Colon, gut and vagina | Yoghurt, fermented foods, bread, sauerkraut, wine etc. | Gastroenteritis, easing lactose intolerance, immune system modulation, alleviating inflammation, lowering cholesterol, cancer protection, modulating brain activity, preventing pathogen colonisation, bile resistant. | [60,61,62,68,82,83,84,85,86,87] |
| Bifidobacterium | Colon, oral cavity, breast milk and vagina | Yoghurt, kombucha, sauerkraut, kefir etc. | Bile resistant, easing lactose intolerance, antibiotic-associated diarrhoea, eczema, immune system modulation, cholesterol lowering abilities | [68,71,88,89,90] |
| Saccharomyces | Colon, decaying fruit, plants, soil, insects | Wine, yoghurt, kombucha, sauerkraut etc. | Travellers’ diarrhoea, antibiotic-associated diarrhoea, preventing recurring Clostridium difficile infections, irritable bowel syndrome | [77,78,79] |
| Escherichia coli | Colon | Capsules | Antagonistic properties against a variety of pathogens, production of defensin, tight-junction protein modification, irritable bowel disorder, constipation, pro-inflammatory properties and colon cancer | [50,51,52,53,54,91] |
| Bacteroidetes | Colon | - | Immune system modulation, intestinal homeostasis | [92,93,94] |
| Akkermansia | Colon | - | Gut barrier function, fat mass storage, glucose homeostasis, immune system stimulation, production of Vitamin B12 | [95,96,97,98,99,100,101] |
| Faecalibacterium | Colon | - | Immune system modulation, ease inflammation | [102,103] |
| Eubacterium | Colon | - | Improve insulin sensitivity, increase energy production, produce Vitamin B12, maintain intestinal homoestasis, colon detoxification, reducing the symptoms of colitis | [104,105,106,107] |
* Some of the mentioned genera are regarded as potential probiotics.
2.1. Escherichia Coli
Escherichia coli, is the most diversely studied prokaryotic model organism in science. E. coli belongs to the Enterobacteriaceae, a large family of Gram-negative bacteria consisting of commensal and pathogenic bacteria, E. coli is a facultative anaerobe, meaning it can either respire or ferment, depending on the environment, which maximises its growth in the gut. E. coli Nissle 1917 (EcN) possesses antagonistic properties against Salmonella, Yersinia, Shigella and Listeria [50]. A study by Wehkamp found that EcN induces the production of defensin in epithelial cells [51]. Various studies have also demonstrated that E. coli affects the intestinal epithelial barrier [52,53], additionally EcN can be used for irritable bowel disorder [53], constipation (for review see [54]), and has pro-inflammatory potential [55]. EcN has been shown to repair the gut barrier function in vitro [53] and in murine models [52]. Henker et al, investigated whether EcN is an effective therapeutic agent against acute diarrhea in infants and toddlers. The randomized double blind placebo controlled trial, including 113 subjects concluded that, EcN successfully reduced the duration and incidence of diarrhea compared to placebo, and improved the general health of the subject [56].
EcN has shown great promise in mitigating effects of gut barrier dysfunction, diarrhea and impairment immune system, experienced often with the condition of overtraining. In order to prove its efficacy more research is needed, especially involving athlete cohorts.
2.2. Lactobacillus
Lactobacillus species are Gram-positive bacteria belonging to the lactic acid bacteria group, capable of lactic acid fermentation metabolism. Lactobacilli are frequently resistant to bile salts; an important probiotic trait as it allows for the probiotic to survive in the hostile acidic environment of the gastrointestinal tract [57]. Lactobacilli are frequently found in various fermented foodstuffs, silage, human gut and the vagina [49,50,51,52,53,54,55,56,57,58]. Lactobacilli are amongst the most widely used and characterized probiotics to date. Various strains from the species: Lactobacillus rhamnosus, Lactobacillus plantarum and Lactobacillus acidophillus have been considered important probiotics, each strain exhibiting individual functions. A small selection of very many examples are provided here, Lactobacillus casei GG has been found to shorten diarrheal distress in subjects suffering from viral gastroenteritis [59], Lactobacillus johnsonii BFE 6128 and Lb. plantarum BFE 1685 have been shown to aid in modulating the immune system by inducing the secretion of the cytokine IL-8 in vitro [60], Lb. rhamnosus 4B15 and Lactobacillus gasseri 4M13 have been suggested to inhibit the expression of inflammatory cytokines at transcriptional level in vitro thus showing anti-inflammatory potential [61]. Among the aforementioned benefits, lactobacilli are capable of inhibiting enteric pathogens through the production of lactic acid, bacteriocins and hydrogen peroxide [62]. It is worth to mention that lactobacilli are biofilm matrix formers, which makes the bacteria more resistant to antibiotics, and could potentially allow for longevity of the bacteria in the gut [63].
Most studies to date have investigated the potential impact of lactobacilli in either animal models or non-active human populations and, as of today, little has been done in athletes. Cox et al investigated whether Lb. fermentum VRI-003 had any effect on mucosal immunity in elite male distance runners. They observed a significant decrease in the duration and severity of respiratory illness, and a two-fold increase in interferon gamma (INFƴ) [64]. Another study examined Lb. fermentum (PCC®) and its effects on gastrointestinal and respiratory health in competitive cyclists. In this study West and colleagues deduced that Lb. fermentum (PCC®) was successful in reducing the severity of gastrointestinal symptoms in male cyclists, in addition, it significantly reduced (20%–60%) cytokine imbalance caused by acute exercise. Moreover, the study also concluded that the severity and duration of lower respiratory illness decreased in male cyclists but increased in female cyclists, taking the probiotic compared to placebo [65]. It is also worth noting that a recent study by Shing et al, reported that a multi-strain probiotic formulation consisting of Lb. acidophilus, Lb. rhamnosus, Lb. casei, Lb. plantarum, Lb. fermentum, B. lactis, B. breve, B. bifidum and Streptococcus thermophilus increased run to fatigue time in male runners, and showed small to moderate improvement in gut permeability [66]. Overtraining can result in a compromised immune system and gut barrier function; in particular lactobacilli are capable of reducing the severity and duration of upper respiratory tract infections (URTI) and gastrointestinal problems, as well as modulating the immune system and increasing performance and thus a good candidate probiotic for athletes.
2.3. Bifidobacterium
Bifidobacteria are Gram-positive anaerobic bacteria from the phylum Actinobacteria. Associated strains are typically among the first colonisers of the infant gut and contribute to the infants’ immune system maturation and the utilisation of certain dietary components [67]. They are generally bile-acid resistant [68] and represent a large proportion of the bacterial microbiome obtained from infant faecal samples, on average about 60%–91% present in breast fed infants, but proportions decrease greatly later in life [69]. Numerous strains from the species: Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium bifidum exhibit probiotic properties, several examples are listed below. The Bifidobacteria genus is represented by many different strains that have been assigned probiotic properties, shown here is a small selection of strains, although many more have been noted in literature A mixture of B. breve, B. longum and Bifidobacterium infantis has been associated with the treatment of antibiotic associated diarrhea [70], while two Bifidobacterium strains: B. breve strain Yakult and B. bifidum strain Yakult have been shown by in vitro studies to have a possible role in modulating the immune system [71]. Even though bifidobacteria are frequently used in probiotic preparations and many studies have elucidated their benefits in vivo, little has been studied in athlete populations. West et al investigated whether a single strain of a probiotic (B. animlais subsp. lactis BI-04) compared against a multi-strain probiotic (Lb. acidophilus NCFM and B. animalis subsp. lactis BI-04) was effective in reducing the risk of upper respiratory tract infection in physically active individuals. The study concluded that B. animalis subsp. lactis BI-04 reduced the risk of upper respiratory illness by 27%. The multi-strain probiotic did not show a significant decrease in risk of URTI [72]. Another worthwhile study investigated a multi-strain probiotic composed of B. bifidum, B. longum and Lb. gasseri and its effect on the duration and incidence of infection in elite rugby players. The findings were that the multi-strain probiotic formulation reduced both the incidence and duration of URTI and gastrointestinal symptoms [73]. In addition to the aforementioned studies, Jäger and colleagues concluded that a probiotic composed of B. breve BR03 and S. thermophilus FP4 has a positive effect on the reduced performance and range of motion followed by intense muscle damaging exercise [74]. More recently, Pugh et al. demonstrated that a four week supplementation of Lb. acidophilus (CUL 60/ CUL 21), B. bifidum (CUL20) and B. animalis subsp. Lactis (CUL34) successfully lowered the incidence and severity of GIT symptoms both during training and a marathon race [75]. Bifidobacteria supplementation can positively help with the burden of gastrointestinal distress found often in overtrained athletes.
2.4. Saccharomyces
Saccharomyces is another commonly used probiotic; it is a non-pathogenic yeast, and is one of the most common fungal species found in the microbiome [3]. The gut mycobiome represents about 0.1% of the total microbiome [76]. Saccharomyces boulardii is most widely used for the treatment of traveler’s diarrhea [77]; it can prevent antibiotic-associated diarrhoea and treat recurrent Clostridium difficile infections [78]. Additionally, research suggests that taking S. boulardii benefits irritable bowel syndrome sufferers [79]. It is not to be overlooked that immunocompromised patients are at risk of fungemia, if supplementing with Saccharomyces [78]. The effect of S. boulardii on diarhoea was elucidated by Kurugol and Koturoglu, where they enrolled 200 young children in a randomized placebo study. The study concluded that S. boulardii successfully reduced the duration of diarrhea and hospital stay associated with it, compared to placebo [80]. In a similar study, Billoo et al. investigated S. boulardii and its possible impact on diarrheal episodes in 100 children, the study concluded that the probiotic reduced the number of diarrhea episodes by 50%, lowered stool frequency and the duration of the illness [81].
It can be postulated that S. boulardii could possibly be an effective treatment for athletes and their reoccurring gastrointestinal problems.
3. Next-Generation Probiotics
Next-generation probiotics still merit the definition of a probiotic and although they have not been used as health modulators to date, have potential to be of importance to the probiotic market. Next-generation probiotics are outside of the commonly used probiotic spectrum (Lactobacilli, Bifidobacteria etc.), however large-scale genomic initiatives have identified putative probiotic strains with potential health benefit, mainly from the genera Bacteroides, Akkermasia, Faecalibacterium and Eubacterium.
One promising candidate is a strain of Bacteroides fragilis which has been proposed to stimulate T-cell immune responses in vitro [108], this is of interest as athletes have decreased numbers of T cells following intensive anaerobic exercise [109]. Similarly, strains of, Bacteroides acidifaciens have been suggested to induce IgA production in murine models and as a consequence elevating the production of IgA+ B cells and B cells; this is important because IgA plays a pivotal role in upholding intestinal homeostasis namely preventing the adherence of pathogens in the intestine [94], studies in the past have demonstrated the decrease in salivary IgA in endurance athletes [110,111] as well as when decreased it has been linked to a higher incidence of URTI [112]. Studies have illustrated the potential role of Akkermanisa in obesity, diabetes and inflammation (for review see [96]). Akkermansia muciniphilia has been associated with maintaining gut barrier function, which can be compromised in endurance athletes [113,114], and glucose homeostasis [97,98] and its direct correlation to athletic performance [115] and additionally it has the potential to stimulate the immune system [97,99,100,101]. The most recent study has concluded that A. muciniphilia is capable of synthesising Vitamin B12 de novo, however whether humans can benefit from the product remains unclear [95]. Considering that recent studies demonstrated an increase in Akkermansia in athletes this is a species that definitely warrants further investigation for use in athletes [13,14,15]. Another potential probiotic is the Faecalibacterium prausnitzii. F. prausnitzii A2-165 has been proposed to have immunodulatory capabilities, through induction of IL-10 and T-cell responses in human and murine dendric cells [102,103,116]. Another worthy strain belongs to the genus Eubacterium. Eubacterium hallii L2-7 has been demonstrated to improve insulin sensitivity and increase energy metabolism in obese and diabetic murine models [104], E. hallii DMS 3353 and E. hallii DSM 17630 have been indicated to produce Vitamin B12 and maintain intestinal homeostasis through the utilisation of glucose and various fermentation intermediates like acetate and lactate in vitro studies [105], while, Eubacterium limosum JCM 6421 has been suggested to produce SCFAs that have been shown to decrease the levels of the pro-inflammatory cytokine IL-6 and increase mucosal integrity [107].
Preliminary research has shown the benefit of the various new generation probiotics however more proof is required to ensure their efficacy, as well as testing for their safety in humans. The potential benefits of next-generation probiotics are also summarized in Table 2.
4. Designer Probiotics
In recent years, research has uncovered numerous functions provided by probiotics, including antagonistic activities, anti-inflammatory and tight-junction modification all of which are of particular benefit to athlete health. Designer probiotics are simply commensal strains of bacteria that have been engineered or modified in a way that they are able to resist the countless stresses that they meet both outside and inside our bodies (lyophilisation/manufacturing/acids/temperature) or simply just to improve the functions most beneficial to the host. The advent of synthetic biology has allowed for engineering of both probiotic strains and commensal strains to acquire and execute new functions (for review see [117]). The most recent concept in the probiotic field is the idea of a “biodrug”, which essentially allows for oral administration of a live recombinant probiotic strain of bacteria for the treatment/prevention of diseases. In the past 10 years, recombinant probiotics have been engineered, for the delivery of therapeutic molecules (usually proteins, fragile in nature, easily denatured by changes in the environment) such as DNA, peptides, single-chain variable fragments, various enzymes or cytokines. Probiotics are excellent vectors of transmission for such therapeutics, due to; a) their ease of colonization and direct delivery to the mucosa; b) resistance to gastric acid and bile salts; c) continued colonization and longevity of protection against pathogens; d) cost of delivery being relatively non-expensive; e) prolonged shelf-life and stability (for review see [118]). Designer probiotics could potentially be an attractive proposition to athletes due to their amenability to being tailored to the athletes specific needs.
Lb. plantarum NC8 which has been engineered to possess angiotensin-converting enzyme inhibitory peptides (ACEIPs), showed reduction of high blood pressure in rats [117]. It has also been shown that engineering Lactococcus lactis NZ29000, B. longum and Lb. gasseri ATCC 33323, managed the symptoms of diabetes and induced insulin secretion. More probiotics are currently under investigation for pathogen infection, cancer or human immunodeficiency virus (HIV), however certain issues arise with engineering probiotics [117]. Tackling the problem of biocontainment should be addressed to prevent the spread of genetically modified microorganisms (GMO) organisms into the natural environment [118] and, of course, the consumer acceptance of GMO products is still a major issue. With that being said, designer probiotics are a great alternative to todays, often impermanent medication.
5. Safety Considerations
When a novel probiotic strain is being considered, evidence needs to be presented to prove its efficacy and safety for human consumption. Even though probiotics are generally considered safe for use, certain side effects may arise. In a statement released by the World Health Organisation (WHO) and the Food and Agriculture Organisation of United Nations (FAO) in 2002, probiotics in theory can have four possible side effects: 1) they can be responsible for lateral gene transfer of antibiotic resistant genes i.e. Lactobacillus spp. 2) They can be responsible for systematic infections i.e. Fungemia. 3) They can stimulate the immune system excessively due to their immune system modulation potential, possibly causing inflammation; and 4) they are detrimental to metabolic activities, as some strains produce D-lactate which can be linked to d-acidosis (for review see [119]). However, with the advent of whole genome sequencing some of these issues can be identified or at least indicated using bioinformatics tools. Genus, species and strain level need to be provided, the nomenclature must agree with the International Committee of Systemics of Prokaryotes, and covered by the International Code of Nomenclature (ICN) of Prokaryotes for bacteria, for fungi, the nomenclature is covered by the ICN for algae, fungi and plants, the organism under consideration should be deposited in a recognized culture collection and identified by the means of current methodologies such as whole-genome sequencing (WGS). As well as using WGS to identify the organism, sequencing should be used to fully analyse the genetic content of the probiotic (both chromosomal and extra-chromosomal) to determine the presence of any transferable antimicrobial resistance loci, virulence factors or production of adverse metabolites that might deem the strain unsafe for human consumption [120]. The European Food Safety Authority (EFSA) guidelines also require full antimicrobial resistance (AMR) testing. AMR testing requires two sets of data, firstly the phenotypic testing determining the minimum inhibitory concentration (MIC) of the antimicrobial, using internationally recognized standards (ISO or similar), serial two-fold dilutions should be applied using relevant controls. The results should be compared to established and published MIC cut off values. Secondly, WGS is required to test for the presence of known AMR genes. Probiotic supplements are sold in many different doses depending on the product and strain. Most commercially available probiotics contain about 1–10 billion colony forming units (CFU) per serving. Generally, the CFU of the product should be equivalent to human studies, showing a positive effect. As of now no strict guidelines exist on the dosage of probiotics [121,122]. Consumers should also be aware that for the most part multi-strain probiotics are more effective than monostrain preparations [123]. It is to be assumed that, new probiotic strains with the intent of consumption, should be of human origin (although Saccharamyces spp., is an exception), their safety, benefits, and dose ratio, should be clearly stated and assessed (for review see [124]) Before the consumer purchases a probiotic it is recommended to research the probiotic strain best suited for their needs paying particular attention to studies performed in human subjects. Customers should ensure the commercial probiotic dosage is consistent with the dosage used in human studies. An important consideration to keep in mind is that the use of probiotics in sport is still a novel area; therefore, most studies to date are preliminary pilot studies, where the dosage and exact benefit of the strain are not fully optimized, and therefore should be reasoned with caution.
6. Probiotics in Treating the Overtraining Syndrome
Overtraining can put the athlete at risk of developing asthma [125], infection such as upper respiratory illness [126,127], gastrointestinal complaints [128] as well as depression and anxiety [129]. Various other symptoms such as immunity suppression and chronic fatigue are also very common [26]. The symptoms can have negative effects for the athlete; impeding progress and decreasing performance. Various studies have explored the positive relationship between athletes and probiotics, and how they can possibly be used to ease or prevent symptoms associated with overtraining. The effects of probiotics on athletes and physically active individuals are summarized in Table 3.
Table 3.
Summary of studies using probiotics on physically active and athlete cohorts.
| Subject Group | Intervention | Result | Limitations of the Study | References |
|---|---|---|---|---|
| 20 male elite distance runners. Randomized double-blinded, placebo controlled trial, | Lb. fermentum VRI-003 | ↓ risk and severity of respiratory systems ↑ INFƴ |
A small sample size | [64] |
| 99 male and female competitive cyclists. Randomized, double-blinded, placebo trial | Lb. fermentum (PCC®) | ↓ severity of GIT symptoms ↓ severity/duration of lower respiratory illness ↓ cytokine imbalance |
Inclusion criteria for antibiotics was only four weeks, study relied on self-reported illness, reported a higher rate of lower respiratory illness in females. | [65] |
| 10 male runners. Randomized, double-blinded, placebo trial |
Lb. acidophilus, Lb. rhamnosus, Lb. casei, Lb. plantarum, Lb. fermentum, B. lactis, B. breve, B.bifidum and Streptococcus thermophilus | ↑ increased run time to fatigue, small to moderate improvement in gut permeability |
Study only investigated males, sample size was too small, short study duration of 4 weeks. | [66] |
| 465 physically active males and females. Randomized double blind placebo controlled trial | B. animalis subsp. lactis BI-04 (BI-04), Lb. acidophilus NCFM and B.animalis subsp. lactis BI-04 (NCFM and BI-04) | ↓ the risk of URTIs by 27% | No separation between recreational and professional athletes, study relied on self-reported illness data, inclusion criteria for antibiotic use was only four weeks. | [72] |
| 30 male elite male rugby players. Randomized, double-blinded, Placebo trial. |
B. bifidum B. longum Lb. gasseri | ↓ in the incidence of URTI/GIT | Relatively small sample size, study only looked at males, short study duration of 4 weeks, relied on self-reported illness data as opposed to measuring immune system markers | [73] |
| 15 resistance-trained men. Randomized, double-blinded, placebo trial. |
B. breve BR03 and S. thermophilus FP4 | positive effect on the reduced performance and range of motion followed by intense muscle damaging exercise | Small sample size, looked at males only, didn’t include antibiotic use in inclusion criteria, short study duration of 3 weeks | [74] |
| 24 amateur athletes | Lb. acidophilus (CUL60/CUL21), B. bifidum (CUL20), B. animalis subsp. Lactis (CUL34) | ↓ incidence and severity of GIT symptoms, both during training and a marathon race | Small sample size, ratio of males to females was skewed, athletic levels weren’t standardized, not double-blinded. | [75] |
| 24 amateur male athletes | Lb. rhamnosus IMC 501 and Lb. paracasei IMC 502 | ↓antioxidant levels followed by exercise | Small sample size, short study duration of 4 weeks, studied males only, study did not include placebo in control group | [130] |
| 23 endurance male athletes Randomized, double-blind, placebo controlled trial. |
EcologicWPerformance or OMNi-BiOTiCWPOWER, | ↓ zonulin ↓TNF-alpha |
Small sample size, only looked at men, looked at only one marker or impaired intestinal permeability | [131] |
| 27 trained amateur athletes | Lb. acidophilus LAFTI® | ↓fatigue | Small sample size, sample size of control group was significantly lower than test group, the ratio of male to females was skewed, not randomized or double blinded. | [132] |
| 44 university student athletes. Randomized, double-blind, placebo controlled trial. |
Lb. gasseri OLL2809 combined with alpha-lactalbumin | prevents the exercise induced drops in Natural Killer cells positive effect on minor fatigue ↑mood from a depressive state |
Study only looked at males, all participants were at a university level, relied on self-reported illness, short study duration of 4 weeks | [133] |
| 33 highly trained individuals. Randomized, double-blinded, placebo controlled trial. |
B. bifidum W23, B. W51, Enterococcus faecium W54, Lb. acidophilus W22, Lb. brevis W63, and Lactococcus lactis W58 |
↓drops of tryptophan levels caused by intense exercise ↓incidence of URTI’s |
Relatively small sample size, women were overrepresented; the severity of illness could not be calculated due to lack of replies from participants. | [134] |
6.1. Antioxidant Boosting
Oxidative stress is the imbalance between oxidant and antioxidant levels in the body; it results in the creation of reactive oxygen species (ROS). Our bodies counteract them with special enzymes that neutralize the reactive species and if the species are not neutralized then they can compromise the cell [135]. Diseases such as rheumatoid arthritis, heart disease, Parkinson’s disease and aging can all be linked to oxidative stress. Intense physical regime coupled to the increased oxygen consumptions results in athletes having a higher abundance of reactive oxygen species [130]. Various probiotic species have been reported to have antioxidant activities; Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus delbrueckii ssp. lactis, Lb. acidophilus, Lb. casei [136]. Studies have also demonstrated the antioxidant activity of B. animalis, which was found to absorb hydroxyl radicals and superoxidase anions in vivo, as well as, increasing the general antioxidase activity in murine species [135]. Certain probiotic strains have also been found to manufacture metabolites that have antioxidant abilities such as; Lb. fermentum E3 and E18 have been found to produce glutathionine (GSH), and Clostridium butyricum has been detected to produce the SCFA butyrate [135]. SCFAs have health-aiding properties and are thought to help with the immune system [137,138] as well as insulin resistance [139]. Martarelli et al has investigated if two probiotic strains (Lb. rhamnosus IMC 501 and Lb. paracasei IMC 502) have any effect on the oxidative stress of athletes during a four-week time frame of intense training [130]. The study came to two findings; firstly, intense exercise significantly increased the oxidative stress in athletes, and secondly, the probiotic course successfully lowered the oxidative stress caused by the intense exercise.
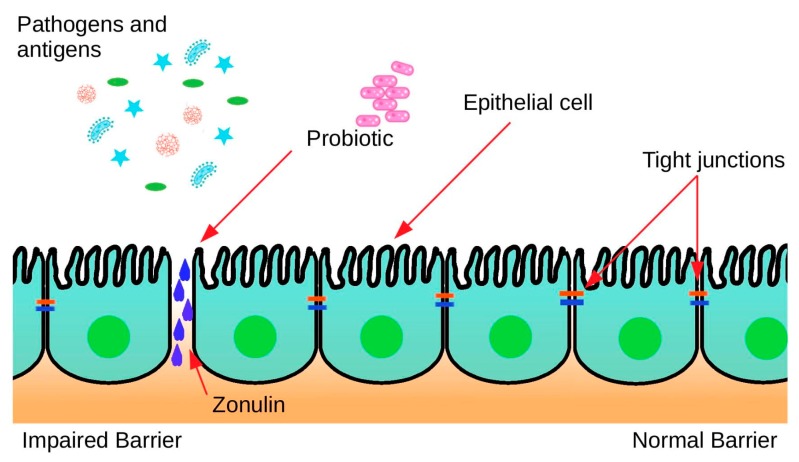
6.2. Tight-Junction Protein Modification
The mucosal layer of the intestine is made up of several constituents namely; enterocytes and epithelial cells, which are interconnected by tight junctions. This intestinal barrier is pivotal in nutrient and water absorption, while preventing harmful substances entering the bloodstream [140,141]. During intense continuous exercise, cardiovascular and thermoregulatory responses augment the blood flow, increasing the phosphorylation state of the tight-junction proteins consequently increasing their permeability (Figure 2). Increased permeability can cause; vulnerability to allergies and infections, along with various gastrointestinal complaints [142]. A randomized, double-blinded, placebo-controlled trial has investigated two probiotic brands in relation to markers of intestinal barrier integrity on 23 endurance male athletes. The probiotics of choice were Ecologic®Performance (B. bifidum W23, B. lactis W51, Enterococcus faecium W54, Lb. acidophilus W22, Lb. brevis W63, L. lactis W58) and OMNi-BiOTiC®POWER (E. faecium W54, Lb. acidophilus W22, Lb. brevis W63, L. lactis W58, B. bifidum W23, B. lactis W51). The study investigated the concentration of Zonulin in faeces, a marker of impaired gut barrier. The study concluded that the probiotic treatment effectively decreased Zonulin concentrations in faeces from above normal to below normal, during the 14 days of the study, when compared to a placebo [142].
Figure 2.
Decreased intestinal permeability.
6.3. Immune System Modulation
It has been documented that overtraining can lead to immunosuppression within the individual [26]. The tissue trauma associated with excessive training results in overproduction of cytokines (IL6, TNF-alpha) which subsequently lead to chronic fatigue-like behaviour in athletes, followed by the induction of a humoral response, resulting in the suppression of cell-mediated immunity resulting in a higher chance of infections (for review see [143]).
Much research has focused on potential probiotics and their strain specific effect on the immune system. Throughout the years several different probiotics have been found to influence the host immune system. Studies have shown that Lb. plantarum 299v is capable of inducing mucin, MUC2 and MUC3, which is important in hindering pathogen colonization [144,145]. Mucin is a large glycosylated protein which protects the intestines by a minimum of three mechanisms: a) it creates a gel-like structure to trap various molecules; and b) it can bind to various molecules, pathogens or proteins through very specific binding sites and c) through disposal of trapped organisms or proteins [146]. Escherichia coli have also been assigned some immunomodulatory functions in recent years, EcN has been found to induce beta-defensins, defensins are small peptides which are the innate immune systems first line of defence, they are produced by epithelial cells of the intestinal tract, skin and lung. Defensins have a broad antagonistic spectrum against various pathogens and are active against both Gram-positive and negative bacteria as well as fungi [147]. A cheese product containing live a culture of Lb. rhamnosus HN001 and Lb. acidophillus NCFM has been proven to improve the ability of natural killer cells (NK Cells) to kill cancerous cells and phagocytosis in elderly subjects. Clancy et al. [132] investigated if a daily dose of Lb. acidophilus could reverse a defect in INF-y secretion in fatigued athletes. The study has concluded that treatment with Lb. acidophillus has significantly increased IFN-y secretion in fatigued athletes to comparable levels of the control cohort. Another worthy study has investigated the effect of probiotic supplementation on respiratory infections and gastrointestinal symptoms of 141 marathon runners. The probiotic of choice was Lb. rhamnosus GG, compared with a placebo pill. The findings of the study were as follows: there was no significant improvement in the URTI, however, Lb. rhamnosus GG decreased the duration of the gastrointestinal symptoms by 33% during the training period, compared to the placebo [148]. Various studies have elucidated immune modulation induced by Akkermansia. A study by Derrien et al. determined that A. muciniphilia is capable of up regulating genes involved in antigen presentation, as well as aiding in B and T cell maturation [100]. Ottman also investigated A. muciniphilia and its immune modulation activity, and concluded that Akkermansia could activate Toll like receptor 2 (TLR) and TLR4 and induce IL8 and IL10 [101].
6.4. Infection
Overtraining can lower the immune system and in turn result in an “open window” period, where sensitivity to infection is increased [126,149]. Athletes are vulnerable to GI, URTI, and skin infections [150].
To benefit the residential flora, probiotic strains need to show antagonistic activities against pathogens. There are two types of antimicrobials produced by certain probiotic strains: high molecular weight compounds called bacteriocins, and low molecular weight compounds including, but not restricted to: hydrogen peroxide, lactic acid, acetic acid and reuterin [151]. Lactic acid, acetic acid and hydrogen peroxide are the most common antimicrobial compounds secreted by probiotic species. LMW compound production is specific to its species, and the effectiveness of microbial inhibition is directly proportional to the amount of organic acid produced [152]. Bacteriocins are small peptides that have been ribosomally-synthesized which possess antagonistic properties against other bacteria, to which the producing strain is immune (for review see [153]). Bacteriocins can either be narrow spectrum (bactericidal against closely related bacteria) or broad spectrum (bactericidal against a wide variety of strains (for review see [151,154]). They can be classified into three groups, Class 1 bacteriocins are lantibiotics which possess lanthionine or beta-metyllanthionine residues, like nisin, Class 2 can be classified as non-lanthionine containing bacteriocins such as lactacin F, and finally bacteriolysins which are non-bacteriocin lytic proteins like lysostaphin [153]. Bacteriocins are an important trait for a probiotic to possess, they can aid in the survival of the producer within a gut [155], help inhibit the proliferation of other bacteria and function as sensing molecules (for review see [155,156]). The production of bacteriocins is dependent on a variety of factors including, the bacterial species producing the antimicrobial compound, and the environmental conditions present. Different bacteria produce various types of bacteriocins, for example, Lb. reuteri is known to produce reuterin, L. lactis produces Nisin A, E. faecalis DS16 makes Cytolisin, Lb. plantarum manufactures Plantaricin S and Lb. acidophillus makes acidophilicin. The production of antimicrobial entities is an important probiotic trait. It allows for longer survival of the producer in the intestine, as well as having the potential to outcompete pathogenic bacteria maintaining a healthy bacterial balance in the gut. Athletes tend to be prone to gastroenteritis and other infections, and the antimicrobial compounds produced by the probiotics can help in easing or preventing the symptoms.
6.5. Mental Health
The ubiquity of depressive symptoms in elite athletes ranges from 4%–68%, with the consensus that female athletes are more likely to develop depressive episodes than male athletes. Athletes, who take part in individual sports, rather than team-based sports, tend to be more susceptible to depressive symptoms. Elite athletes face risk factors that contribute to depression including, genetic factors, environmental factors, injury, competitive failure, retirement from sport, pain, concussion and of course overtraining [157].
A study by Sashihara [133] and colleges investigated if Lb. gasseri OLL2809 combined with alpha-lactalbumin decreases symptoms of depression. The study has found that Lb. gasseri OLL2809 has successfully elevated symptoms of depression in college athletes. Similarly, Strasser et al. investigated the effect of a multi-species probiotic formulation (B. bifidum W23, B. lactis W51, E. faecium W54, Lb. acidophilus W22, Lb. brevis W63, L. lactis W58) and its possible effect on tryptophan levels, often implicated in the etiology of depression [158]. The study concluded that the multispecies probiotic formulation had a positive effect on the tryptophan-kynurenine pathway by successfully reducing the exercise-induced depletion of tryptophan levels that occur after excessive exercise [134].
7. Conclusion and Future Prospects
In conclusion, the relationship between physical fitness and the microbiome is a complex one. Exercise and physical fitness can positively alter the composition of the microbiome and benefit host health, consequently maintaining athletic potential. Studies suggest that different forms of exercise may influence the abundance of different bacterial populations and, hence, alter the microbiome in a different way and, therefore, studies in the future should consider this. As addressed in this review, strenuous exercise can put an athlete in a predicament where they are more prone to infection, inflammation, depression and gut permeability dysfunction. Probiotics offer an effective strategy to prevent or improve those symptoms and allow for continued athletic success without the drawback of the aforementioned symptoms. Future research should aim to clarify the optimal dosage for each individual strain with proven efficacy, as well as establishing whether the probiotic strains colonize our intestine or are simply transient microorganisms with beneficial effects. The impact of probiotics on the athletic population is a new and exciting area, where a limited amount of research has been conducted to date, and although research has shown great promise, little is known about the benefits of probiotics in highly active individuals and ultimately if they benefit from them.
Funding
Research in the group is funded by Science Foundation Ireland in the form of a centre grant (APC Microbiome Ireland grant number SFI/12/RC/2273).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Janssens Y., Nielandt J., Bronselaer A., Debunne N., Verbeke F., Wynendaele E., Van Immerseel F., Vandewynckel Y.-P., De Tré G., De Spiegeleer B. Disbiome database: Linking the microbiome to disease. BMC Microbiol. 2018;18:50. doi: 10.1186/s12866-018-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash A.K., Auchtung T.A., Wong M.C., Smith D.P., Gesell J.R., Ross M.C., Stewart C.J., Metcalf G.A., Muzny D.M., Gibbs R.A., et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017;5:153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donovan C.M., O’Sullivan O., Cotter P.D. Gut Microbiol—A Relatively Unexplored Domain. Elsevier; Amsterdam, The Netherlands: 2018. [Google Scholar]
- 6.Jandhyala S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur J.C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J.M., Fan T.-J., Campbell B.J., Abujamel T., Dogan B., Rogers A.B. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Goffau M.C., Luopajarvi K., Knip M., Ilonen J., Ruohtula T., Harkonen T., Orivuori L., Hakala S., Welling G.W., Harmsen H.J., et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach N., Fuster-Botella D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017;6:179–197. doi: 10.1016/j.jshs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 12.Shade A. Diversity is the question, not the answer. ISME J. 2017;11:1. doi: 10.1038/ismej.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E., Shanahan F., Cotter P.D., O’Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2017;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 14.Clarke S.F., Murphy E.F., O’Sullivan O., Lucey A.J., Humphreys M., Hogan A., Hayes P., O’Reilly M., Jeffery I.B., Wood-Martin R., et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 15.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H., Sodergren E., Weinstock G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronin O., Barton W., Skuse P., Penney N.C., Garcia-Perez I., Murphy E.F., Woods T., Nugent H., Fanning A., Melgar S., et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. MSystems. 2018;3:e00044. doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang L.-G., Choi G., Kim S.-W., Kim B.-Y., Lee S., Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019;16:21. doi: 10.1186/s12970-019-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheiman J., Luber J.M., Chavkin T.A., MacDonald T., Tung A., Pham L.-D., Wibowo M.C., Wurth R.C., Punthambaker S., Tierney B.T. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer J.D., Koltyn K.F., Stegner A.J., Kim J.-S., Cook D.B. Influence of exercise intensity for improving depressed mood in depression: A dose-response study. Behav. Ther. 2016;47:527–537. doi: 10.1016/j.beth.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Duscha B.D., Slentz C.A., Johnson J.L., Houmard J.A., Bensimhon D.R., Knetzger K.J., Kraus W.E. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest. 2005;128:2788–2793. doi: 10.1378/chest.128.4.2788. [DOI] [PubMed] [Google Scholar]
- 21.Puetz T.W., Flowers S.S., O’Connor P.J. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother. Psychosom. 2008;77:167–174. doi: 10.1159/000116610. [DOI] [PubMed] [Google Scholar]
- 22.Meeusen R., Duclos M., Foster C., Fry A., Gleeson M., Nieman D., Raglin J., Rietjens G., Steinacker J., Urhausen A. Prevention, diagnosis and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science (ECSS) and the American College of Sports Medicine (ACSM) Eur. J. Sport Sci. 2013;13:1–24. doi: 10.1080/17461391.2012.730061. [DOI] [PubMed] [Google Scholar]
- 23.Allen J.M., Berg Miller M.E., Pence B.D., Whitlock K., Nehra V., Gaskins H.R., White B.A., Fryer J.D., Woods J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015;118:1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- 24.Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y., Hoke A.V., Levangie M.W., Kumar R., Chakraborty N. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X., Xu S., Huang H., Liang J., Wu Y., Li C., Yuan H., Zhao X., Lai X., Hou S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports. 2018;28:1541–1551. doi: 10.1111/sms.13060. [DOI] [PubMed] [Google Scholar]
- 26.Walsh N.P., Gleeson M., Shephard R.J., Gleeson M., Woods J.A., Bishop N., Fleshner M., Green C., Pedersen B.K., Hoffman-Goete L. Position Statement Part One: Immune Function and Exercise. Loughborough University; Loughborough, UK: 2011. [PubMed] [Google Scholar]
- 27.Sina C., Gavrilova O., Förster M., Till A., Derer S., Hildebrand F., Raabe B., Chalaris A., Scheller J., Rehmann A.G. Protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 28.Shouval D.S., Biswas A., Goettel J.A., McCann K., Conaway E., Redhu N.S., Mascanfroni I.D., Al Adham Z., Lavoie S., Ibourk M. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutterwala F.S., Noel G.J., Clynes R., Mosser D.M. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E.F., Wang J., Tito R.Y., Schiweck C., Kurilshikov A., Joossens M., Wijmenga C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 32.Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 33.Maes M., Meltzer H.Y., Bosmans E., Bergmans R., Vandoolaeghe E., Ranjan R., Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J. Affect. Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-L. [DOI] [PubMed] [Google Scholar]
- 34.Margonis K., Fatouros I.G., Jamurtas A.Z., Nikolaidis M.G., Douroudos I., Chatzinikolaou A., Mitrakou A., Mastorakos G., Papassotiriou I., Taxildaris K. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007;43:901–910. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Tanskanen M., Atalay M., Uusitalo A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010;28:309–317. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- 36.Hsu Y.J., Chiu C.C., Li Y.P., Huang W.C., Te Huang Y., Huang C.C., Chuang H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015;29:552–558. doi: 10.1519/JSC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 37.Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutr. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Jeukendrup A., Vet-Joop K., Sturk A., Stegen J., Senden J., Saris W., Wagenmakers A. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000;98:47–55. doi: 10.1042/cs0980047. [DOI] [PubMed] [Google Scholar]
- 39.Holland A.M., Hyatt H.W., Smuder A.J., Sollanek K.J., Morton A.B., Roberts M.D., Kavazis A.N. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res. Notes. 2015;8:514. doi: 10.1186/s13104-015-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwellnus M.P., Derman W.E., Jordaan E., Page T., Lambert M.I., Readhead C., Roberts C., Kohler R., Collins R., Kara S. Elite athletes travelling to international destinations > 5 time zone differences from their home country have a 2–3-fold increased risk of illness. Br. J. Sports Med. 2012;46:816–821. doi: 10.1136/bjsports-2012-091395. [DOI] [PubMed] [Google Scholar]
- 41.Svendsen I.S., Taylor I.M., Tønnessen E., Bahr R., Gleeson M. Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br. J. Sports Med. 2016;50:809–815. doi: 10.1136/bjsports-2015-095398. [DOI] [PubMed] [Google Scholar]
- 42.Palleja A., Mikkelsen K.H., Forslund S.K., Kashani A., Allin K.H., Nielsen T., Hansen T.H., Liang S., Feng Q., Zhang C. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018;3:1255. doi: 10.1038/s41564-018-0257-9. [DOI] [PubMed] [Google Scholar]
- 43.Sode J., Obel N., Hallas J., Lassen A. Use of fluroquinolone and risk of Achilles tendon rupture: A population-based cohort study. Eur. J. Clin. Pharmacol. 2007;63:499–503. doi: 10.1007/s00228-007-0265-9. [DOI] [PubMed] [Google Scholar]
- 44.Wiström J., Norrby S.R., Myhre E.B., Eriksson S., Granström G., Lagergren L., Englund G., Nord C.E., Svenungsson B. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J. Antimicrob. Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Möller G.B., da Cunha Goulart M.J.V., Nicoletto B.B., Alves F.D., Schneider C.D. Supplementation of Probiotics and Its Effects on Physically Active Individuals and Athletes: Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2019;29:481–492. doi: 10.1123/ijsnem.2018-0227. [DOI] [PubMed] [Google Scholar]
- 46.Parvez S., Malik K.A., Ah Kang S., Kim H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 47.PRNewswire Probiotics Market Will Register 7.5% CAGR to Exceed $67 Billion by 2024: Global Market Insights, Inc. [(accessed on 20 November 2018)]; Available online: https://www.prnewswire.com/news-releases/probiotics-market-will-register-75-cagr-to-exceed-67-billion-by-2024-global-market-insights-inc-681334981.html.
- 48.Santosa S., Farnworth E., Jones P.J. Probiotics and their potential health claims. Nutr. Rev. 2006;64:265–274. doi: 10.1111/j.1753-4887.2006.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 49.Ramos C.L., Thorsen L., Schwan R.F., Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013;36:22–29. doi: 10.1016/j.fm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Altenhoefer A., Oswald S., Sonnenborn U., Enders C., Schulze J., Hacker J., Oelschlaeger T.A. The probioticEscherichia colistrain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med Microbiol. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 51.Wehkamp J., Harder J., Wehkamp K., Wehkamp-von Meissner B., Schlee M., Enders C., Sonnenborn U., Nuding S., Bengmark S., Fellermann K. NF-κB-and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ukena S.N., Singh A., Dringenberg U., Engelhardt R., Seidler U., Hansen W., Bleich A., Bruder D., Franzke A., Rogler G., et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zyrek A.A., Cichon C., Helms S., Enders C., Sonnenborn U., Schmidt M.A. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 54.Chmielewska A., Szajewska H. Systematic review of randomised controlled trials: Probiotics for functional constipation. World J. Gastroenterol. 2010;16:69. doi: 10.3748/wjg.v16.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ukena S.N., Westendorf A.M., Hansen W., Rohde M., Geffers R., Coldewey S., Suerbaum S., Buer J., Gunzer F. The host response to the probiotic Escherichia coli strain Nissle 1917: Specific up-regulation of the proinflammatory chemokine MCP-1. BMC Med Genet. 2005;6:43. doi: 10.1186/1471-2350-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henker J., Laass M., Blokhin B.M., Bolbot Y.K., Maydannik V.G., Elze M., Wolff C., Schulze J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 2007;166:311–318. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Smet I., Van Hoorde L., Vande Woestyne M., Christiaens H., Verstraete W. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 1995;79:292–301. doi: 10.1111/j.1365-2672.1995.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 58.Pavlova S., Kilic A., Kilic S., So J.S., Nader-Macias M., Simoes J., Tao L. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 2002;92:451–459. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- 59.Isolauri E., Kaila M., Mykkänen H., Ling W.H., Salminen S. Oral bacteriotherapy for viral gastroenteritis. Dig. Dis. Sci. 1994;39:2595–2600. doi: 10.1007/BF02087695. [DOI] [PubMed] [Google Scholar]
- 60.Vizoso Pinto M.G., Schuster T., Briviba K., Watzl B., Holzapfel W.H., Franz C.M.A.P. Adhesive and Chemokine Stimulatory Properties of Potentially Probiotic Lactobacillus Strains. J. Food Prot. 2007;70:125–134. doi: 10.4315/0362-028X-70.1.125. [DOI] [PubMed] [Google Scholar]
- 61.Oh N.S., Joung J.Y., Lee J.Y., Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE. 2018;13:e0192021. doi: 10.1371/journal.pone.0192021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reid G. The scientific basis for probiotic strains ofLactobacillus. Appl. Environ. Microbiol. 1999;65:3763–3766. doi: 10.1128/aem.65.9.3763-3766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giwercman B., Jensen E., Høiby N., Kharazmi A., Costerton J. Induction of beta-lactamase production in Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 1991;35:1008–1010. doi: 10.1128/AAC.35.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox A.J., Pyne D.B., Saunders P.U., Fricker P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010;44:222–226. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 65.West N.P., Pyne D.B., Cripps A.W., Hopkins W.G., Eskesen D.C., Jairath A., Christophersen C.T., Conlon M.A., Fricker P.A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shing C.M., Peake J.M., Lim C.L., Briskey D., Walsh N.P., Fortes M.B., Ahuja K.D.K., Vitetta L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2013;114:93–103. doi: 10.1007/s00421-013-2748-y. [DOI] [PubMed] [Google Scholar]
- 67.Hidalgo-Cantabrana C., Delgado S., Ruiz L., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.BAD-0010-2016. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz L., Margolles A., Sanchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013;4:396. doi: 10.3389/fmicb.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Junick J., Blaut M. Quantification of Human Fecal Bifidobacterium Species by Use of Quantitative Real-Time PCR Analysis Targeting thegroELGene. Appl. Environ. Microbiol. 2012;78:2613–2622. doi: 10.1128/AEM.07749-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selinger C.P., Bell A., Cairns A., Lockett M., Sebastian S., Haslam N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J. Hosp. Infect. 2013;84:159–165. doi: 10.1016/j.jhin.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Imaoka A., Shima T., Kato K., Mizuno S., Uehara T., Matsumoto S., Setoyama H., Hara T., Umesaki Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008;14:2511–2516. doi: 10.3748/wjg.14.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West N.P., Horn P.L., Pyne D.B., Gebski V.J., Lahtinen S.J., Fricker P.A., Cripps A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014;33:581–587. doi: 10.1016/j.clnu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Haywood B.A., Black K.E., Baker D., McGarvey J., Healey P., Brown R.C. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport. 2014;17:356–360. doi: 10.1016/j.jsams.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Jäger R., Purpura M., Stone J., Turner S., Anzalone A., Eimerbrink M., Pane M., Amoruso A., Rowlands D., Oliver J. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients. 2016;8:642. doi: 10.3390/nu8100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pugh J.N., Sparks A.S., Doran D.A., Fleming S.C., Langan-Evans C., Kirk B., Fearn R., Morton J.P., Close G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Physiol. 2019;119:1491–1501. doi: 10.1007/s00421-019-04136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Naturer. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McFarland L.V. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med. Infect. Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Thygesen J.B., Glerup H., Tarp B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012 doi: 10.1136/bcr.06.2011.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi C.H., Jo S.Y., Park H.J., Chang S.K., Byeon J.-S., Myung S.-J. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: Effect on quality of life. J. Clin. Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 80.Kurugöl Z., Koturoğlu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediatr. 2005;94:44–47. doi: 10.1080/08035250410022521. [DOI] [PubMed] [Google Scholar]
- 81.Billoo A., Memon M., Khaskheli S., Murtaza G., Iqbal K., Shekhani M.S., Siddiqi A.Q. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J. Gastroenterol. 2006;12:4557. doi: 10.3748/wjg.v12.i28.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walter J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dugas B., Mercenier A., Lenoir-Wijnkoop I., Arnaud C., Dugas N., Postaire E. Immunity and probiotics. Immunol. Today. 1999;20:387–390. doi: 10.1016/S0167-5699(99)01448-6. [DOI] [PubMed] [Google Scholar]
- 84.Bezirtzoglou E., Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe. 2011;17:369–374. doi: 10.1016/j.anaerobe.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Tulumoglu S., Yuksekdag Z.N., Beyatli Y., Simsek O., Cinar B., Yaşar E. Probiotic properties of lactobacilli species isolated from children’s feces. Anaerobe. 2013;24:36–42. doi: 10.1016/j.anaerobe.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 86.Montes R., Bayless T.M., Saavedra J., Perman J. Effect of milks inoculated with Lactobacillus acidophilus or a yogurt starter culture in lactose-maldigesting children. J. Dairy Sci. 1995;78:1657–1664. doi: 10.3168/jds.S0022-0302(95)76790-X. [DOI] [PubMed] [Google Scholar]
- 87.Kim Y., Whang J.Y., Whang K.Y., Oh S., Kim S.H. Characterization of the Cholesterol-Reducing Activity in a Cell-Free Supernatant ofLactobacillus acidophilusATCC 43121. Biosci. Biotechnol. Biochem. 2014;72:1483–1490. doi: 10.1271/bbb.70802. [DOI] [PubMed] [Google Scholar]
- 88.Almeida C.C., Lorena S.L., Pavan C.R., Akasaka H.M., Mesquita M.A. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012;27:247–251. doi: 10.1177/0884533612440289. [DOI] [PubMed] [Google Scholar]
- 89.Kim J.Y., Kwon J.H., Ahn S.H., Lee S.I., Han Y.S., Choi Y.O., Lee S.Y., Ahn K.M., Ji G.E. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo-controlled trial. Pediatric Allergy Immunol. 2010;21:e386–e393. doi: 10.1111/j.1399-3038.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 90.Zanotti I., Turroni F., Piemontese A., Mancabelli L., Milani C., Viappiani A., Prevedini G., Sanchez B., Margolles A., Elviri L., et al. Evidence for cholesterol-lowering activity by Bifidobacterium bifidum PRL2010 through gut microbiota modulation. Appl. Microbiol. Biotechnol. 2015;99:6813–6829. doi: 10.1007/s00253-015-6564-7. [DOI] [PubMed] [Google Scholar]
- 91.Behnsen J., Deriu E., Sassone-Corsi M., Raffatellu M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb. Perspect. Med. 2013;3:a010074. doi: 10.1101/cshperspect.a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Troy E.B., Kasper D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. A J. Virtual Library. 2010;15:25. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curtis M.M., Hu Z., Klimko C., Narayanan S., Deberardinis R., Sperandio V. The Gut Commensal Bacteroides thetaiotaomicron Exacerbates Enteric Infection through Modification of the Metabolic Landscape. Cell Host Microbe. 2014;16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanagibashi T., Hosono A., Oyama A., Tsuda M., Suzuki A., Hachimura S., Takahashi Y., Momose Y., Itoh K., Hirayama K., et al. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA + B cells. Immunobiology. 2013;218:645–651. doi: 10.1016/j.imbio.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 95.Kirmiz N., Galindo K., Cross K.L., Luna E., Rhoades N., Podar M., Flores G.E. Comparative genomics guides elucidation of vitamin B12 biosynthesis in novel human associated Akkermansia. bioRxiv. 2019 doi: 10.1101/587527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cani P.D., de Vos W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shin N.-R., Lee J.-C., Lee H.-Y., Kim M.-S., Whon T.W., Lee M.-S., Bae J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 99.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016;23:107. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 100.De Vos W.M., Müller M., Norin E., Hooiveld G., Van Baarlen P., Derrien M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanz Y., Ottman N., Reunanen J., Meijerink M., Pietilä T.E., Kainulainen V., Klievink J., Huuskonen L., Aalvink S., Skurnik M., et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossi O., van Berkel L.A., Chain F., Tanweer Khan M., Taverne N., Sokol H., Duncan S.H., Flint H.J., Harmsen H.J.M., Langella P., et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016;6:18507. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martín R., Miquel S., Chain F., Natividad J.M., Jury J., Lu J., Sokol H., Theodorou V., Bercik P., Verdu E.F., et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Udayappan S., Manneras-Holm L., Chaplin-Scott A., Belzer C., Herrema H., Dallinga-Thie G.M., Duncan S.H., Stroes E.S., Groen A.K., Flint H.J. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes. 2016;2:16009. doi: 10.1038/npjbiofilms.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Engels C., Ruscheweyh H.-J., Beerenwinkel N., Lacroix C., Schwab C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016;7:713. doi: 10.3389/fmicb.2016.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fekry M.I., Engels C., Zhang J., Schwab C., Lacroix C., Sturla S.J., Chassard C. The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Environ. Microbiol. Rep. 2016;8:201–209. doi: 10.1111/1758-2229.12369. [DOI] [PubMed] [Google Scholar]
- 107.Kanauchi O., Fukuda M., Matsumoto Y., Ishii S., Ozawa T., Shimizu M., Mitsuyama K., Andoh A. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J. Gastroenterol. 2006;12:1071. doi: 10.3748/wjg.v12.i7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brubaker J.O., Li Q., Tzianabos A.O., Kasper D.L., Finberg R.W. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: Differential stimulatory effect on mouse and rat lymphocytes in vitro. J. Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 109.Hack V., Weiss C., Friedmann B., Suttner S., Schykowski M., Erbe N., Benner A., Bartsch P., Droge W. Decreased plasma glutamine level and CD4+ T cell number in response to 8 wk of anaerobic training. Am. J. Physiol. Endocrinol. Metab. 1997;272:E788–E795. doi: 10.1152/ajpendo.1997.272.5.E788. [DOI] [PubMed] [Google Scholar]
- 110.Laing S., Gwynne D., Blackwell J., Williams M., Walters R., Walsh N. Salivary IgA response to prolonged exercise in a hot environment in trained cyclists. Eur. J. Appl. Physiol. 2005;93:665–671. doi: 10.1007/s00421-004-1270-7. [DOI] [PubMed] [Google Scholar]
- 111.Mackinnon L.T., Ginn E., Seymour G.J. Decreased salivary immunoglobulin A secretion rate after intense interval exercise in elite kayakers. Eur. J. Appl. Physiol. Occup. Physiol. 1993;67:180–184. doi: 10.1007/BF00376664. [DOI] [PubMed] [Google Scholar]
- 112.Neville V., Gleeson M., Folland J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 113.Pals K.L., Chang R.-T., Ryan A.J., Gisolfi C.V. Effect of running intensity on intestinal permeability. J. Appl. Physiol. 1997;82:571–576. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]
- 114.Øktedalen O., Lunde O., Opstad P., Aabakken L., Kvernebo K. Changes in the gastrointestinal mucosa after long-distance running. Scand. J. Gastroenterol. 1992;27:270–274. doi: 10.3109/00365529209000073. [DOI] [PubMed] [Google Scholar]
- 115.Soman V.R., Koivisto V.A., Deibert D., Felig P., DeFronzo R.A. Increased insulin sensitivity and insulin binding to monocytes after physical training. N. Engl. J. Med. 1979;301:1200–1204. doi: 10.1056/NEJM197911293012203. [DOI] [PubMed] [Google Scholar]
- 116.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.-J., Blugeon S., Bridonneau C., Furet J.-P., Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chua K.J., Kwok W.C., Aggarwal N., Sun T., Chang M.W. Designer probiotics for the prevention and treatment of human diseases. Curr. Opin. Chem. Biol. 2017;40:8–16. doi: 10.1016/j.cbpa.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 118.Amalaradjou M.A., Bhunia A.K. Bioengineered probiotics, a strategic approach to control enteric infections. Bioengineered. 2013;4:379–387. doi: 10.4161/bioe.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doron S., Snydman D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015;60(suppl. 2):S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Panel E.F. Guidance on the Characterisation Microorganisms 1 Used as Feed Additives or as Production Organisms. EFSA; Parma, Italy: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Organisation W.G. Probiotics and Prebiotic. WGO; Forest City, IA, USA: 2017. [Google Scholar]
- 122.Health N.I.O. Probiotics. [(accessed on 25 June 2019)]; Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/
- 123.Timmerman H., Koning C., Mulder L., Rombouts F., Beynen A. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004;96:219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 124.Anadón A., Martínez-Larrañaga M.R., Arés I., Martínez M.A. Nutraceuticals in Veterinary Medicine. Springer; Berlin/Heidelberg, Germany: 2016. Prebiotics and Probiotics in Feed and Animal Health; pp. 3–23. [Google Scholar]
- 125.Helenius I.J., Tikkanen H.O., Sarna S., Haahtela T. Asthma and increased bronchial responsiveness in elite athletes: Atopy and sport event as risk factors. J. Allergy Clin. Immunol. 1998;101:646–652. doi: 10.1016/S0091-6749(98)70173-3. [DOI] [PubMed] [Google Scholar]
- 126.Nieman D.C., Johanssen L.M., Lee J.W., Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med. Phys. Fit. 1990;30:316–328. [PubMed] [Google Scholar]
- 127.Spence L., Brown W.J., Pyne D.B., Nissen M.D., Sloots T.P., McCormack J.G., Locke A.S., Fricker P.A. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 2007;39:577–586. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- 128.Ter Steege R.W., Van Der Palen J., Kolkman J.J. Prevalence of gastrointestinal complaints in runners competing in a long-distance run: An internet-based observational study in 1281 subjects. Scand. J. Gastroenterol. 2008;43:1477–1482. doi: 10.1080/00365520802321170. [DOI] [PubMed] [Google Scholar]
- 129.Gouttebarge V., Frings-Dresen M., Sluiter J. Mental and psychosocial health among current and former professional footballers. Occup. Med. 2015;65:190–196. doi: 10.1093/occmed/kqu202. [DOI] [PubMed] [Google Scholar]
- 130.Martarelli D., Verdenelli M.C., Scuri S., Cocchioni M., Silvi S., Cecchini C., Pompei P. Effect of a Probiotic Intake on Oxidant and Antioxidant Parameters in Plasma of Athletes During Intense Exercise Training. Curr. Microbiol. 2011;62:1689–1696. doi: 10.1007/s00284-011-9915-3. [DOI] [PubMed] [Google Scholar]
- 131.Lamprecht M., Frauwallner A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med. Sport Sci. 2012;59:47–56. doi: 10.1159/000342169. [DOI] [PubMed] [Google Scholar]
- 132.Clancy R., Gleeson M., Cox A., Callister R., Dorrington M., D’este C., Pang G., Pyne D., Fricker P., Henriksson A. Reversal in fatigued athletes of a defect in interferon γ secretion after administration of Lactobacillus acidophilus. Br. J. Sports Med. 2006;40:351–354. doi: 10.1136/bjsm.2005.024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sashihara T., Nagata M., Mori T., Ikegami S., Gotoh M., Okubo K., Uchida M., Itoh H. Effects of Lactobacillus gasseri OLL2809 and α-lactalbumin on university-student athletes: A randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 2013;38:1228–1235. doi: 10.1139/apnm-2012-0490. [DOI] [PubMed] [Google Scholar]
- 134.Strasser B., Geiger D., Schauer M., Gostner J.M., Gatterer H., Burtscher M., Fuchs D. Probiotic Supplements Beneficially Affect Tryptophan-Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2016;8:752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., Wang Y., Li W. Antioxidant Properties of Probiotic Bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saide J., Gilliland S. Antioxidative activity of lactobacilli measured by oxygen radical absorbance capacity. J. Dairy Sci. 2005;88:1352–1357. doi: 10.3168/jds.S0022-0302(05)72801-0. [DOI] [PubMed] [Google Scholar]
- 137.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 138.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., Deroos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zuhl M., Schneider S., Lanphere K., Conn C., Dokladny K., Moseley P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014;48:980–986. doi: 10.1136/bjsports-2012-091585. [DOI] [PubMed] [Google Scholar]
- 141.Wells J.M., Konstaninov S., Konings I., Karczewski J. Effects of probiotic and commensals on epithelial barrier function. Int. J. Probiotics Prebiotics. 2008;3:127–132. [Google Scholar]
- 142.Lamprecht M., Bogner S., Schippinger G., Steinbauer K., Fankhauser F., Hallstroem S., Schuetz B., Greilberger J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012;9:45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hackney A.C., Koltun K.J. The immune system and overtraining in athletes: Clinical implications. Acta Clin. Croat. 2012;51:633–641. [PubMed] [Google Scholar]
- 144.West N., Pyne D., Peake J., Cripps A. Probiotics, immunity and exercise: A review. Exerc. Immunol. Rev. 2009;15:e26. [PubMed] [Google Scholar]
- 145.Mack D.R., Michail S., Wei S., McDougall L., Hollingsworth M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 146.Snyder J.D., Walker A. Structure and Function of Intestinal Mucin: Developmental Aspects. Int. Arch. Allergy Immunol. 1987;82:351–356. doi: 10.1159/000234225. [DOI] [PubMed] [Google Scholar]
- 147.Möndel M., Schroeder B., Zimmermann K., Huber H., Nuding S., Beisner J., Fellermann K., Stange E., Wehkamp J. Probiotic E. coli treatment mediates antimicrobial human β-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kekkonen R.A., Vasankari T.J., Vuorimaa T., Haahtela T., Julkunen I., Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2007;17:352–363. doi: 10.1123/ijsnem.17.4.352. [DOI] [PubMed] [Google Scholar]
- 149.Peters E.M., Bateman E. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S. Afr. Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- 150.Romano R., Lu D., Holtom P. Outbreak of community-acquired methicillin-resistant Staphylococcus aureus skin infections among a collegiate football team. J. Athl. Train. 2006;41:141. [PMC free article] [PubMed] [Google Scholar]
- 151.Suskovic J. Antimicrobial Activity—The Most Important Property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010;48:296–307. [Google Scholar]
- 152.Shokryazdan P., Sieo C.C., Kalavathy R., Liang J.B., Alitheen N.B., Faseleh Jahromi M., Ho Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res. Int. 2014;2014:927268. doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cotter P.D., Hill C., Ross R.P. Food microbiology: Bacteriocins: Developing innate immunity for food. Nature Rev. Microbiol. 2005;3:777. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 154.Hegarty J.W., Guinane C.M., Ross R.P., Hill C., Cotter P.D. Bacteriocin production: A relatively unharnessed probiotic trait? F1000Research. 2016;5:2587. doi: 10.12688/f1000research.9615.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dobson A., Cotter P.D., Ross R.P., Hill C. Bacteriocin Production: A Probiotic Trait? Appl. Environ. Microbiol. 2012;78:1–6. doi: 10.1128/AEM.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hegarty J.W., Guinane C.M., Ross R.P., Hill C., Cotter P.D. Lack of Heterogeneity in Bacteriocin Production Across a Selection of Commercial Probiotic Products. Probiotics Antimicrob. Proteins. 2017;9:459–465. doi: 10.1007/s12602-017-9326-2. [DOI] [PubMed] [Google Scholar]
- 157.Reardon C.L., Hainline B., Aron C.M., Baron D., Baum A.L., Bindra A., Budgett R., Campriani N., Castaldelli-Maia J.M., Currie A. Mental health in elite athletes: International Olympic Committee consensus statement. Br. J. Sports Med. 2019;53:667–699. doi: 10.1136/bjsports-2019-100715. [DOI] [PubMed] [Google Scholar]
- 158.Dougherty D.M., Marsh-Richard D.M., Mathias C.W., Hood A.J., Addicott M.A., Moeller F.G., Morgan C.J., Badawy A.A.-B. Comparison of 50-and 100-g L-tryptophan depletion and loading formulations for altering 5-HT synthesis: Pharmacokinetics, side effects, and mood states. Psychopharmacology. 2008;198:431. doi: 10.1007/s00213-008-1163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]