Abstract
Near-monodisperse zinc ferrite nanoparticles (ZnFe2O4 NPs) are synthesized by a co-precipitation method and deposited on the surface of silver nanowires (AgNWs), employing a stepwise solution method. The resulting hybrid nanostructures (ZnFe2O4@AgNWs) show a thin and uniform layer of ZnFe2O4 NPs at an optimum weight ratio of 1:6 between the two component nanostructures. The hybrid nanostructures retain the high crystal quality and phase purity of their constituents. It is demonstrated that the ZnFe2O4@AgNWs hybrid nanostructures are effective at inhibiting the biofilm formation of Candida albicans cells. The biofilm inhibition activity of the hybrid nanostructures is estimated to be more than 50% at a low concentration of 100 µg/mL from both crystal violet assay and XTT assay, which are more than 8-fold higher than those of pure AgNWs and ZnFe2O4 NPs. This greatly enhanced biofilm inhibition activity is attributed to the ZnFe2O4 NPs-carrying membrane penetration by AgNWs and the subsequent interaction between Candida cells and ZnFe2O4 NPs. These results indicate that the ZnFe2O4@AgNWs hybrid nanostructures have great potential as a new type of novel antibiofilm agent.
Keywords: zinc ferrite nanoparticles, hybrid nanostructures, Candida albicans, biofilm inhibition, crystal violet assay
1. Introduction
Typical candidiasis can proliferate into a severe fungal infection known as invasive candidiasis [1], which stimulates common oral thrush, vaginal and neonatal candidiasis, and bloodstream infections [2,3]. Candida albicans (hereafter abbreviated as C. albicans) is the prevailing species, responsible for almost 90% of invasive candidiasis [4], and usually resides as a normal commensal of the human oral, vaginal, and gut microflora [2]. Naturally, C. albicans grows in a symbiotic association within the human ecosystem that regulates the overgrowth and invasion of other tissues by communalistic colonization [5]. The regulation of overgrowth is an important factor to suppress parasitic interactions in order to develop into infections. Another known phenomenon that indicates the proliferation rate of C. albicans cells through intercellular signaling is quorum sensing [6]. This phenomenon is crucial for phenotypic changes in normal yeast cells. It is known that immunocompromised people, people with HIV or diabetes, and catheter-treated and long-term hospitalized patients are vulnerable to invasive candidiasis [7]. The normal C. albicans cells are easily transformed to hyphal morphogenesis (yeasttohyphae transition), promoting the formation of biofilm, a thick extracellular polymeric matrix (EPM) mainly consisting of proteins, lipids, and nucleic acid components [8]. The biofilm formation of C. albicans increases the resistance to commercial antifungal drugs due to the increased fungal activity in the film [9,10]. In the past two decades, there have been drastic morphological changes due to the yeasttohyphae transition of Candida species, and the associated risk of antifungal-drug resistance seems to be gradually increasing [11]. Epidemiological changes and ever-growing drug resistance can cause a high mortality rate of up to 60% for hospitalized patients with invasive candidiasis and related infections [12,13,14]. Therefore, it is necessary to regulate the proliferation from normal yeast infection to life-threatening invasive candidiasis, which is capable of infecting multiple organs including the central nervous system, brain, blood, heart, bones, etc.
The applications of nanoparticles (NPs) over conventional therapeutics and diagnostics are important since they can touch every area of human microbiological infection. However, there have been limited studies about Candida infection treatment with conventional NPs of silver (Ag), gold (Au), titanium (Ti), zinc (Zn), and their oxides [15,16,17]. For instance, Pierce et al. synthesized silver nanoparticles (AgNPs) to evaluate the C. albicans antifungal–biofilm activity at submicrogram concentrations, and showed good biocompatibility [18]. Another study on C. albicans cells using zinc oxide nanoparticles (ZnO NPs) turned out to be effective only for the inhibition of planktonic C. albicans growth [19]. New metal-resistant bacterial strains have, however, appeared with rapid industrialization and growing exposure to heavy metals, which could be a serious threat to fungal infection treatment in the future [20,21,22]. As an alternative, magnetic ferrite NPs such as cobalt (Co) ferrites, Ag-doped Co ferrites, and Zn ferrites have been evaluated for antifungal activity [23,24,25], although their study areas were limited [26]. For example, Jadhav et al. studied the anti-Candida adhesion activity of Zn ferrite (ZnFe2O4) NPs with an average size of ~40 nm [27]. Furthermore, the quorum sensing-mediated inhibition of biofilm using silver nanowires (AgNWs) was disclosed to be effective in both fungi and Gram-negative bacteria, but the high inhibitory concentration weakened their potential application as anti-Candida agents [28].
In this work, we synthesized Zn ferrite NPs-deposited AgNWs (ZnFe2O4@AgNWs) hybrid nanostructures to evaluate their biofilm inhibition activity for C. albicans at relatively low concentrations. To the best of our knowledge, these hybrid nanostructures have been explored for the first time for antifungal activity. AgNWs alone did not exhibit an antifungal biofilm effect, whereas the biofilm inhibition activity of AgNWs was greatly enhanced with the addition of ZnFe2O4 NPs, indicating that ZnFe2O4@AgNWs had synergistic effects on C. albicans as an effective antibiofilm agent.
2. Materials and Methods
2.1. Chemicals and Reagents
All the necessary chemicals, including zinc nitrate hexahydrate (Zn(NO3)2·6H2O), iron nitrate nonahydrate (Fe(NO3)2·9H2O), silver nitrate (AgNO3), polyvinylpyrrolidone (PVP), ethylene glycol (EG, C2H6O2), copper (II) chloride dihydrate (CuCl2·2H2O), and sodium borohydride (NaBH4), were purchased from Sigma Aldrich (Yongin, South Korea). Other reagents like acetone, sodium hydroxide (NaOH), and ethyl alcohol (C2H5OH) were purchased from Daejung Chem. (Siheung, South Korea). All the chemicals and reagents were pure and used with no further treatment.
2.2. Synthesis of Hybrid Nanostructures
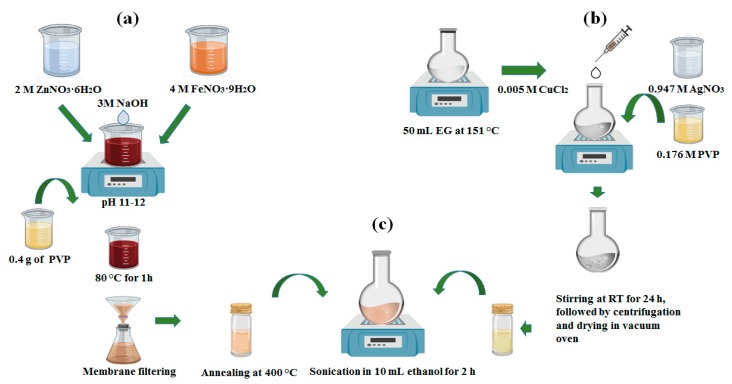
The synthesis of ZnFe2O4@AgNWs was performed in a stepwise manner, as schematically represented in Figure 1. A conventional coprecipitation method with PVP capping was followed for the synthesis of ZnFe2O4 NPs [29]. As presented in Figure 1a, FeNO3·9H2O (4 M) and ZnNO3·6H2O (2 M) were dissolved in 30 mL of deionized (DI) water, followed by the slow addition of NaOH (3 M). During the addition, the mixture solution was maintained at constant stirring until the pH reached 11–12. Then, a few drops of PVP solution (4 M) were added before heating at 80 °C for 1 h. Shortly, thick brown precipitates were cooled down to room temperature and collected by centrifugation after thorough washing with ethanol and DI water. Afterwards, the obtained product was dried in a heating oven at 105 °C and ground to fine particles with a mortar and pestle. Finally, the collected powder was annealed at 400 °C for 3 h and again collected by centrifugation. Before the final collection, the powder was filtrated using a 0.2-µm pore size membrane to achieve uniform size distribution by removing agglomerated particles. In parallel, AgNWs were synthesized using a polyol method, as depicted in Figure 1b. The detailed synthesis procedures were previously reported elsewhere [30]. Briefly, 50 mL of EG was stabilized by heating at 151.5 °C for 1 h, and AgNO3 solution (0.947 M) and PVP solution (0.176 M) were prepared using EG as a solvent. Next, 0.4 mL of CuCl2 solution (0.005 M) was added to the preheated EG. In 15 min, the AgNO3 and PVP solutions were added dropwise into the solution at a constant rate over 30 min, leading to the gradual color change from transparent to gray. Then, the solution was kept overnight with magnetic stirring and AgNWs were finally collected through repeated centrifugation and washing. The final step was the deposition of ZnFe2O4 NPs over the surface of AgNWs, as shown in Figure 1c. For that, collected powders of both ZnFe2O4 NPs and AgNWs were dispersed together in 10 mL of ethanol and sonicated for 2 h at room temperature. The ZnFe2O4 NPs and AgNWs were mixed at two different weight ratios (1:2 and 1:6). Finally, the ZnFe2O4@AgNWs hybrid nanostructures were collected by centrifugation after drying.
Figure 1.
Synthesis procedures of (a) ZnFe2O4 NPs with PVP capping, (b) AgNWs, and (c) AgNWs combined with ZnFe2O4 NPs (ZnFe2O4@AgNWs hybrid nanostructures).
2.3. Characterization of Hybrid Nanostructures
As-synthesized powders of pure ZnFe2O4 NPs, pure AgNWs, and ZnFe2O4@AgNWs hybrid nanostructures were all characterized. For morphological examination, a field emission scanning electron microscope (FE-SEM, JEOL JSM-7500F, Tokyo, Japan) was used. The crystal structure and phase purity were analyzed by X-ray diffraction (XRD, Rigaku D/MAX 2200, Tokyo, Japan) with Cu Kα radiation. The oxidation state and the nature of bonding of constituents (Zn, Fe, O and Ag) in individual samples were examined by X-ray photoelectron spectroscopy (XPS, K-alpha Thermo Electron, Thermo Fisher Scientific, Waltham, MA, USA). The optical characteristics of the samples were checked using a UV-Vis spectrophotometer (UV-Vis Cary 50 Bio, San Diego, CA, USA). In addition, the photocatalytic activity of the hybrid nanostructures was evaluated under AM1.5 conditions, using a solar simulator (XEC-301S, Osaka, Japan) as a light source and methylene blue (MB) as a probe dye. For this test, 20 mg of ZnFe2O4@AgNWs were dispersed in 50 μL of MB solution (10 μM) and stirred well.
2.4. Strains, Media, and Culture Conditions
The Candida albicans strain SC5314 was purchased from ATCC (Incheon Koram, South Korea) and stored at 80 °C until further experimental use. A loop of inoculum was streaked into yeast‒peptone‒dextrose agar (YPD, consisting of 2% yeast extract, 2% peptone, 1% dextrose, and 1.5% agar) on a Petri dish and incubated at 30 °C overnight. Then, the overnight grown culture was subcultured into a conical flask containing 30 mL of YPD broth media and incubated at 30 °C for 12–14 h in an orbital shaker at 180 rpm. After washing with phosphate-buffered saline (1× PBS), the cells were again resuspended in RPMI-1640 media (RPMI) with L-glutamine (Sigma Aldrich, Yongin, South Korea) and buffered in 0.165 mM 3-(N-morpholino) propanesulfonic acid (MOPS, Sigma Aldrich, Yongin, South Korea) as an essential supplement for Candida biofilm study [31]. The evolution of the Candida biofilms was evaluated by high-throughput screening, using a flat-bottomed 96-well microtiter plate (Corning, Inc., Wujiang, China) method [32]. Furthermore, the biofilm quantification was studied by a widely used crystal violet staining assay [33] and the colorimetric analysis using an 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) cell proliferation assay [34]. The detailed processes for the crystal violet assay and XTT cell proliferation assay are described in the Supplementary Materials, along with fluorescent dye staining assay. The starting culture of 1 × 106 (CFU/mL) suspension was prepared using a hemocytometer cell-counting technique with 0.4% trypan blue dye to maintain cell density as a McFarland standard. For the biofilm treatment, the colloids of pure ZnFe2O4 NPs, pure AgNWs (0, 50, 100, 150, 200, 250, and 300 µg/mL), and ZnFe2O4@AgNWs (0, 10, 20, 40, 60, 80, and 100 µg/mL) were prepared in RPMI medium with varying the concentrations and stored at 4 °C. The comparative quantification analyses were performed using optical densities from control wells and treated wells at their inhibitory concentrations.
2.5. SEM Examination of C. albicans Biofilms
In order to prepare the samples for SEM examination, the Candida cells were treated with effective inhibitory concentrations of pure ZnFe2O4 NPs, pure AgNWs, and ZnFe2O4@AgNWs. A biofilm was developed at the bottom of the microtiter plate, using the method explained above. The biofilm formed at the bottom of microtiter plate was gently washed with PBS, and further sample preparation was conducted following a method reported previously [35]. In brief, the freshly grown biofilm was rinsed and fixed with 4% formaldehyde and 1% glutaraldehyde in PBS, followed by rinsing twice with a phosphate buffer (0.1 M). After a few minutes, 1% osmium tetroxide (OsO4) solution was added to the wells and kept there for 1 h. Then, the wells were progressively dried with solutions of increasing ethanol concentration (20%, 30%, 50%, 70%, 95%, and absolute ethanol, 10 min each). The SEM images were collected from both treated and untreated wells for the evaluation of biofilm morphologies.
3. Results and Discussion
3.1. Morphologies
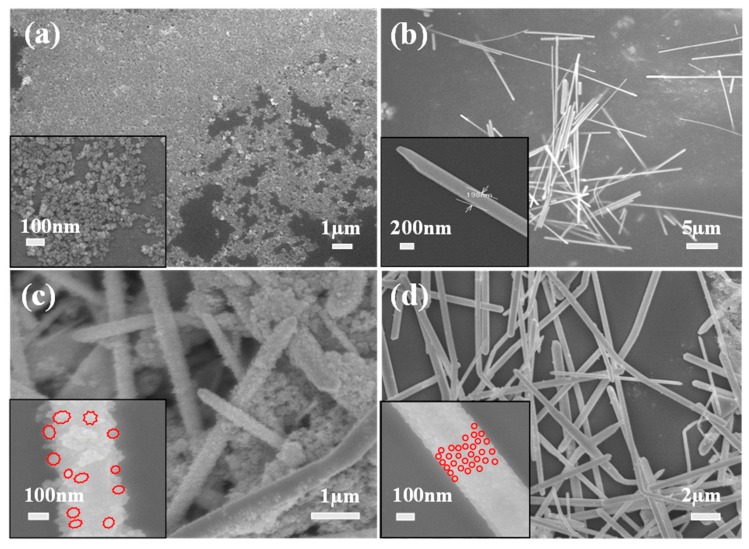
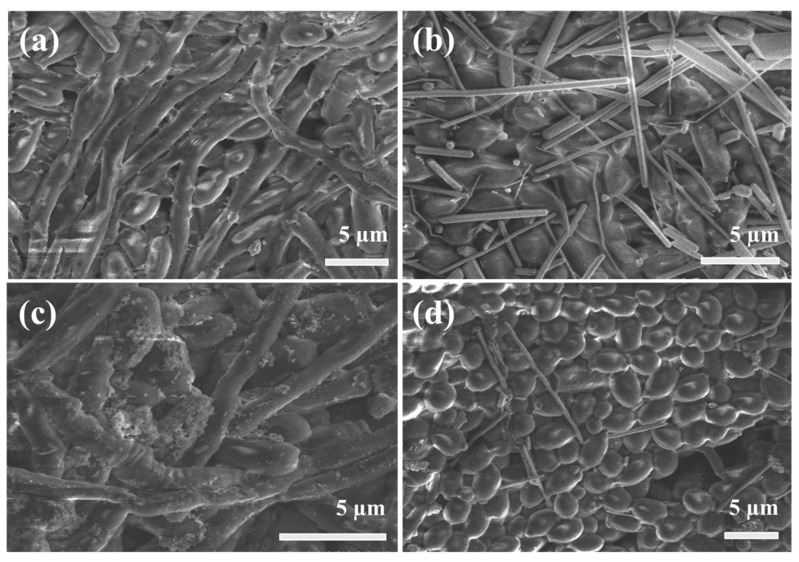
The morphologies of all the nanostructures were characterized, and the representative SEM images are shown in Figure 2. The as-synthesized ZnFe2O4 NPs coated with PVP look monodisperse, but some degree of agglomeration is also observed due to the inter-particle magnetic interaction (Figure 2a). The average size of the nanoparticles is estimated at 33 nm. A SEM-EDX profile in Figure S1 clearly shows the presence of three elements (Z, Fe, O) in the nanoparticles. From Figure 2b, it is found that as-synthesized AgNWs are also uniform and well-faceted. There are no AgNPs found, indicating the dimensional purity of the nanostructures. The average diameter and length of AgNWs are estimated to be 200 nm and 15 µm, respectively. It is revealed that the morphologies of ZnFe2O4@AgNWs hybrid nanostructures depend on the weight ratio of ZnFe2O4 NPs to AgNWs, as shown in Figure 2c,d. At a lower ratio (ZnFe2O4 NPs:AgNWs = 1:2), ZnFe2O4 NPs completely cover the surface of AgNWs, and those NPs seem to be agglomerated even on the AgNW surface, leading to the rough surface (Figure 2c). The estimated particle size (~70 nm) is larger than that of as-synthesized ZnFe2O4 NPs. Moreover, many independent agglomerates of ZnFe2O4 NPs are also found. On the other hand, a thin and relatively smooth layer of ZnFe2O4 NPs is coated on the surface of AgNWs at a higher ratio (ZnFe2O4 NPs:AgNWs = 1:6) (Figure 2d). Consequently, the general shape of the hybrid nanostructures resembles that of pure AgNWs. From the inset of Figure 2d, no significant agglomeration of ZnFe2O4 NPs is observed on the surface. Based on this difference in morphologies, ZnFe2O4@AgNWs with a weight ratio of 1:6 were used for further examinations and biofilm inhibition studies.
Figure 2.
SEM images of (a) as-synthesized ZnFe2O4 NPs coated with PVP, (b) pure AgNWs, (c,d) ZnFe2O4@AgNWs hybrid nanostructures with different weight ratios: (c) ZnFe2O4 NPs:AgNWs = 1:2, (d) ZnFe2O4 NPs:AgNWs = 1:6. The red contours in the insets of (c,d) represent the general shapes of ZnFe2O4 NPs deposited on the surface of AgNWs.
3.2. Crystallographic, Chemical, and Optical Features of Hybrid Nanostructures
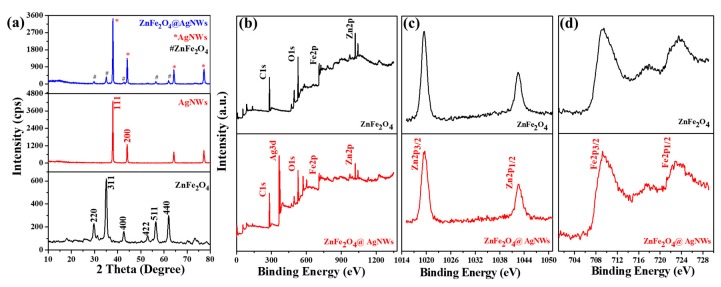
Figure 3a shows the XRD patterns of pure ZnFe2O4 NPs, pure AgNWs, and ZnFe2O4@AgNWs hybrid nanostructures. The pure ZnFe2O4 NPs exhibit characteristic diffraction peaks at 29.75°, 35.05°, 42.65°, 53.05°, 56.5°, and 62.05°, which are indexed to the (220), (311), (400), (422), (511), and (440) planes of ZnFe2O4 crystal with cubic spinel structure (JCPDS card No. 82-1049) [36]. The pure AgNWs show sharp diffraction peaks, particularly at 37.95° and 44.15°, which correspond to the (111) and (200) planes of FCC Ag crystal (JCPDS card No. 04-0783). Interestingly, the ZnFe2O4@AgNWs hybrid nanostructures reveal the major peaks of both AgNWs and ZnFe2O4 NPs at the same positions as those for the two components. For instance, the primary peaks corresponding to the Ag (111) and ZnFe2O4 (311) planes appear at 37.95° and 35.05° for both hybrid nanostructures and constituent nanostructures. Furthermore, the peak sharpness for ZnFe2O4@AgNWs hybrid nanostructures is not deteriorated at all, as compared to AgNWs and ZnFe2O4 NPs. These XRD results indicate that the hybrid nanostructures can be well synthesized by the stepwise solution method developed in this work, without any crystal damage or the formation of a mixed phase.
Figure 3.
(a) XRD patterns of pure ZnFe2O4 NPs, AgNWs, and ZnFe2O4@AgNWs hybrid nanostructures. (b–d) XPS spectra of ZnFe2O4 NPs and ZnFe2O4@AgNWs hybrid nanostructures: (b) overall scans, (c) magnified spectra focused on Zn 2p levels, (d) magnified spectra focused on Fe 2p levels.
XPS measurements were performed to further evaluate the chemical bonding states of the hybrid nanostructures. Figure 3b–d present the XPS spectra of pure ZnFe2O4 NPs and ZnFe2O4@AgNWs nanostructures. From the overall spectra shown in Figure 3b, it is found that both nanostructures include the constituents (Zn, Fe, O) of ZnFe2O4, but strong Ag peaks are observed only in the hybrid nanostructures (see Figure S2 for precise peak positions). Magnified Zn 2p and Fe 2p profiles for both samples are displayed in Figure 3c,d. Zn 2p3/2 and Zn 2p1/2 peaks are found at the same positions for pure ZnFe2O4 NPs and ZnFe2O4@AgNWs hybrid nanostructures (at 1019.6 and 1042.5 eV, respectively). Likewise, the peak positions of Fe 2p3/2 and Fe 2p1/2 for the two nanostructures are almost superposed at 709.4 and 723.4 eV, respectively. These results indicate that the bonding characteristics of ZnFe2O4 NPs remain unchanged during hybridization with AgNWs. Furthermore, the fact that the characteristic peaks of ZnO, which are supposed to appear at 1021.7 and 1044.9 eV, are not found, and the Zn 2p peaks are very sharp, indicates that Zn2+ ions at octahedral sites are surrounded by tetrahedrally positioned Fe ions [37,38,39]. In addition, of the other potential compounds, the Fe2O3 and Fe3O4 phases are not formed, the Fe 2p3/2 positions of which should be at 710.6 and 711.6 eV, respectively [40,41]. Thus, it might be stated that the ZnFe2O4 NPs are in pure spinel ferrite phase with negligible secondary compounds such as ZnO, Fe2O3, and Fe3O4.
Both ZnFe2O4 NPs and ZnFe2O4@AgNWs hybrid nanostructures appeared to optically respond over the broad spectrum range from visible light to UV, as seen from the UV-Vis absorption spectra in Figure S3. This is a desirable attribute for the photoactivated bio-applications. To evaluate the photoactivity of the hybrid nanostructures, the time-dependent degradation of methylene blue (MB) was tested. As presented in Figure S4, the MB solution is greatly discolored after 80 min of light illumination. The photoactivated decomposition of MB is estimated at 98.5% for the 80-min-long illumination, which was calculated from the relative absorbance intensities at a characteristic peak (665 nm) before and after illumination. These results support the strong photoactivity of the hybrid nanostructures, although the decomposition time is longer than the previous reports [42], presumably due to the limited fraction (1/7 of the total weight) of ZnFe2O4 NPs in the nanostructures.
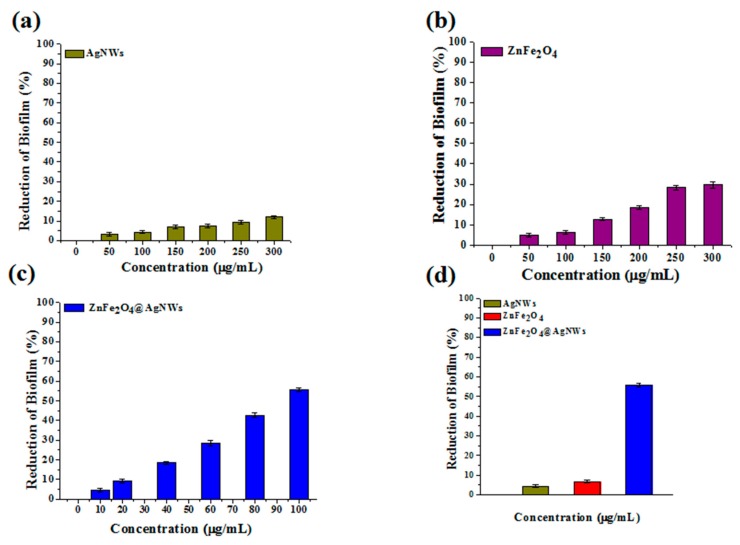
3.3. Quantification of Biofilms by Crystal Violet Assay
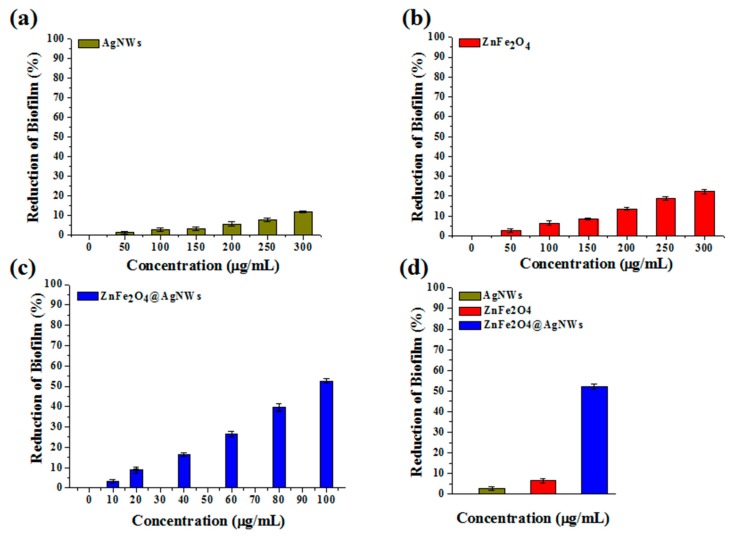
Quantification of biofilms was first made using crystal violet staining assay. Figure 4 shows the reduction of biofilm of control and treated wells depending on the concentration of individual nanostructures. The reduction charts were converted from the optical density charts, which are presented in Figure S5. For all the nanostructures, it is disclosed that the reduction of biofilm consistently increases as the nanostructure concentration increases. However, the degree of biofilm reduction, which represents the biofilm inhibition activity, differs with the type of nanostructures employed. Here, the biofilm inhibition activity (I) is calculated as Equation (1):
| I(%) = (OD0 − ODn)/OD0 × 100 | (1) |
where OD0 and ODn are optical densities from control wells and nanostructure-treated wells. The crystal violet staining assay treated with pure AgNWs exhibits only 11% of inhibition activity at a 300 µg/mL concentration (Figure 4a). Even this inhibition seems better than that of a previous report [28]. The inhibition activity of pure ZnFe2O4 NPs is estimated at 22.3% of the same concentration (Figure 4b). The assay treated with ZnFe2O4@AgNWs hybrid nanostructures reveals 52.2% of biofilm inhibition at a concentration of 100 µg/mL (Figure 4c), which is just one-third of the maximum concentration for the other two nanostructures. To better represent the relative inhibition activities of the three nanostructures, the optical densities of nanostructure-treated wells are compared at the same concentration of 100 µg/mL in Figure 4d. From the comparative data, the antibiofilm activities toward C. albicans are calculated to be 2.8%, 6.4%, and 52.2% for pure AgNWs, pure ZnFe2O4 NPs, and ZnFe2O4@AgNWs hybrid nanostructures, respectively. The enhanced biofilm inhibition activity of ZnFe2O4@AgNWs hybrid nanostructures is attributed to the synergistic effect of AgNWs and ZnFe2O4 NPs. Even though ZnFe2O4 NPs may play a greater role than AgNWs do, increasing the weight fraction of ZnFe2O4 NPs did not lead to improved biofilm inhibition activity, as confirmed in Figure S6.
Figure 4.
Reduction of biofilm versus nanostructure concentration bar charts obtained from crystal violet staining assays: (a) AgNWs, (b) ZnFe2O4 NPs, (c) ZnFe2O4@AgNWs hybrid nanostructures, (d) comparative reduction of biofilm at a specific concentration of 100 µg/mL. The reduction of biofilm was calculated from the optical density at the wavelength of 595 nm.
3.4. Quantification of Biofilms by XTT Assay
The XTT cell proliferation assay was used to quantify the metabolic activity of C. albicans cells [43]. Like in the crystal violet staining assay method, varying concentrations of nanostructures were tested separately, and the results are presented in Figure 5 (also see Figure S7 for the corresponding optical density charts). Similar to the previous method, the reduction of biofilm gradually increases with an increase in the nanostructure concentration, indicating that all the nanostructures have the more or less biofilm inhibition activity. From Figure 5a,b, the biofilm inhibition activities of pure AgNWs and pure ZnFe2O4 NPs are estimated at 12% and 29.7%, respectively, at a concentration of 300 µg/mL. In contrast, the ZnFe2O4@AgNWs hybrid nanostructures show a great enhancement in the inhibition activity even at a reduced concentration: 55.7% at 100 µg/mL (Figure 5c). This inhibition activity is in very close agreement with that estimated from the crystal violet staining assay. Figure 5d clearly demonstrates the relative effects of individual nanostructures on the biofilm inhibition at a specific concentration of 100 µg/mL. The biofilm inhibition activities of pure AgNWs, pure ZnFe2O4 NPs, and ZnFe2O4@AgNWs hybrid nanostructures are calculated to be 4.3%, 6.2%, and 55.7%, respectively.
Figure 5.
Reduction of biofilm versus nanostructure concentration bar charts obtained from XTT cell proliferation assays: (a) AgNWs, (b) ZnFe2O4 NPs, (c) ZnFe2O4@AgNWs hybrid nanostructures, (d) comparative reduction of biofilm at a specific concentration of 100 µg/mL. The reduction of biofilm was converted from the optical density at a wavelength of 490 nm.
3.5. Nanostructure-Dependent Biofilm Inhibition
The development of biofilms in a control sample and nanostructure-treated samples was examined by SEM. Figure 6 displays SEM images of Candida biofilms after 24 h of biofilm development. As can be seen in Figure 6a, the fully matured Candida biofilm with a long, thick, and complex hyphae structure is observed in the control sample without any treatment. There is no clear appearance of budding cells due to the biofilm covering almost all the surface. When treated with a 300 µg/mL of AgNWs, the nanowires are randomly distributed over the surface and partially penetrate inside the biofilm matrix (Figure 6b). Unfortunately, the pure AgNWs are not so effective for biofilm inhibition, even though some penetrating AgNWs can participate in the early cell death or adhesion competition to the surface of the microtiter plate. Likewise, an equal concentration of pure ZnFe2O4 NPs was not capable of inhibiting the biofilm growth. From Figure 6c, it is apparent that the ZnFe2O4 NPs are not able to penetrate the biofilm matrix as they stick to the upper surface due to the severe agglomeration. On the contrary, the growth of budding cells with clear round cell boundaries is clearly found in the sample treated with ZnFe2O4@AgNWs hybrid nanostructures, while the hyphal growth is effectively suppressed, demonstrating the synergistic effect of the hybrid nanostructures (Figure 6d). Additional SEM measurement and EDX analysis were performed for another sample treated in the same way, but after making a scratch. It is noted from Figure S8 that an AgNW is located below the surface, and all elements of ZnFe2O4@AgNW are detected near the AgNW, although the dominating element is C, which is the main component of polymeric matrix.
Figure 6.
SEM images of (a) control Candida biofilm and biofilms treated with (b) 300 µg/mL of AgNWs, (c) 300 µg/mL of ZnFe2O4 NPs, and (d) 100 µg/mL of ZnFe2O4@AgNWs hybrid nanostructures. The biofilms were formed for 24 h.
3.6. Biofilm Inhibition Mechanism
Figure 7 shows a plausible biofilm inhibition mechanism and a high magnification SEM image of the sample treated with a 100 µg/mL of ZnFe2O4@AgNWs hybrid nanostructures. As depicted in Figure 7a, the hybrid nanostructures may penetrate through the periphery of the Candida biofilm, reach the native Candida cells, and cause damage to the cells. Through this process, the hybrid nanostructures cause physical deformation and rupture in both the biofilm matrix and the cell membrane, and may interrupt the cell membrane’s function [18,44,45]. At the same time, the ZnFe2O4 NPs deposited on the surface of AgNWs play a significant role in the cell-damaging process by generating reactive oxygen species (ROS), which attack the cells. The ZnFe2O4 NPs are known to have unique properties such as high surface energy, good anti-adhesion, and easy binding with cell membrane components [46]. The ZnFe2O4 NPs, which may be bound to or released from the AgNW surface, exert oxidative stress on cells’ organelles, leading to the generation of ROS. Subsequently, the generated ROS damage the biological metabolism of the cells by enzyme deactivation and DNA damage, ultimately causing cell death [47]. As a consequence, the Candida cells become rougher and swollen, as confirmed in Figure 7b. The ZnFe2O4@AgNWs hybrid nanostructures can transport the ZnFe2O4 NPs inside the Candida biofilm without any agglomeration. Consequently, they show overwhelming biofilm inhibition activity at a relatively lower concentration, proving their effectiveness as a new antibiofilm agent.
Figure 7.
(a) Schematic illustration of the biofilm inhibition mechanism. (b) High-resolution SEM image of Candida biofilm treated with ZnFe2O4@AgNWs hybrid nanostructures (100 µg/mL) after 24 h of biofilm formation.
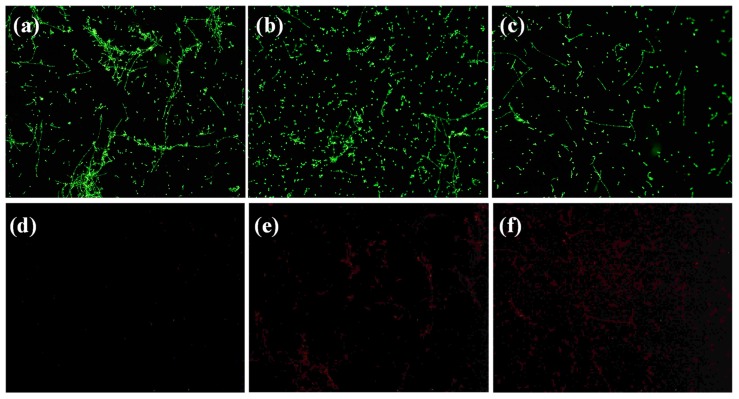
3.7. Visualization of Candida Biofilms by Fluorescent Microscopy
We stained a control biofilm and other biofilms treated with ZnFe2O4@AgNWs hybrid nanostructures (100 µg/mL) for different times, following the protocol described in the yeast visibility kit (Molecular Probes, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The biofilm images obtained by a fluorescent microscope are shown in Figure 8. For this imaging, two types of dyes (SYTO9 and propidium iodide) were used, which behave differently. SYT09 can penetrate cell membranes in both healthy and dead cells/hyphae, while propidium iodide is unable to pass through the cell membrane and binds to dead or damaged cells/hyphae. As a consequence, healthy cells appear green, whereas dead or damaged cells show red fluorescence. As can be seen in Figure 8a, the control biofilm shows strong, streaked green fluorescence, indicating active hyphal growth. Less hyphal growth and more C. albicans cells without hyphal development are observed for the 12-h-treated biofilm (Figure 8b). Further inhibition of hyphal growth and a lower density of C. albicans cells resulted in the biofilm treated for 24 h (Figure 8c). The distributions of dead or damaged cells (red fluorescence) for the same samples have the opposite trend to these healthy cell distributions (see Figure 8d–f). These results agree well with the observations from crystal violet assay, XTT assay, and SEM studies.
Figure 8.
Fluorescent microscope images of Candida albicans biofilms, showing (a–c) live cell distributions and (d–f) dead cell distributions. (a,d) Control biofilms, (b,e) biofilms treated with ZnFe2O4@AgNWs hybrid nanostructures for 12 h, (c,f) biofilms treated with ZnFe2O4@AgNWs hybrid nanostructures for 24 h. Measurements were made using a 10× objective lens.
4. Conclusions
A stepwise solution method was utilized to synthesize new hybrid nanostructures composed of ZnFe2O4 NPs and AgNWs. The ZnFe2O4@AgNWs hybrid nanostructures retained the crystal structures and phase purities of their component nanostructures. The hybrid nanostructures were tested for their biofilm inhibition activity on C. albicans cells, in comparison with pure ZnFe2O4 NPs and pure AgNWs. Using biofilm quantification assays such as crystal violet staining assay and XTT cell proliferation assay, it was demonstrated that the ZnFe2O4@AgNWs hybrid nanostructures were indeed effective at suppressing the biofilm development of C. albicans cells. The biofilm inhibition activity was estimated to be more than 50%, even at a low concentration of 100 µg/mL. From the biofilm visualizations using SEM and fluorescent microscopy, the biofilm inhibition activity of the hybrid nanostructures was further confirmed. These were made possible by the synergistic effect of ZnFe2O4 NPs and AgNWs. AgNWs could penetrate the biofilm matrix and transport ZnFe2O4 NPs inside the biofilm, where the ZnFe2O4 NPs caused damage to native Candida cells by the generation of ROS. There was no agglomeration of ZnFe2O4 NPs found during cell treatment or penetration through the biofilm. From these results, the ZnFe2O4@AgNWs hybrid nanostructures could be suggested as a new and novel antibiofilm agent.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/9/10/1431/s1, Figure S1: SEM-EDX spectrum of pure ZnFe2O4 NPs, Figure S2: Magnified XPS spectrum of ZnFe2O4@AgNWs hybrid nanostructures, Figure S3: UV-Vis absorption spectra of (a) pure ZnFe2O4 NPs and (b) ZnFe2O4@AgNWs hybrid nanostructures, Figure S4: Photocatalytic degradation of methylene blue (MB) using ZnFe2O4@AgNWs hybrid structures, Figure S5: Optical density versus nanostructure concentration bar charts obtained from crystal violet staining assays, Figure S6: Optical density versus concentration bar chart for ZnFe2O4@AgNWs hybrid nanostructures with the weight ratio of 1:2, Figure S7: Optical density versus nanostructure concentration bar charts obtained from XTT cell proliferation assays, Figure S8: SEM-EDX spectrum of a biofilm sample treated with ZnFe2O4@AgNWs hybrid nanostructures.
Author Contributions
D.T. conducted the experiments, collected the data, and wrote the draft of the manuscript. S.G. and K.Y. advised on the biological applications of nanostructures and helped D.T. to collect various assay data. J.-S.N. guided the whole experiment and edited the manuscript. All authors discussed the results and contributed to the analysis of collected data.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1A2C1008746). This work was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1D1A1B03932515).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.McCarty T.P., Pappas P.G. Invasive Candidiasis. Volume 30. W.B. Saunders; Philadelphia, PA, USA: 2016. pp. 103–124. Infectious Disease Clinics of North America. [DOI] [PubMed] [Google Scholar]
- 2.Wilson D. Candida albicans. Trends Microbiol. 2019;27:188–189. doi: 10.1016/j.tim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Lim S.-Y., Rosli R., Seow H.F., Chong P.P. Candida and invasive candidiasis: Back to basics. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:21–31. doi: 10.1007/s10096-011-1273-3. [DOI] [PubMed] [Google Scholar]
- 4.Mohandas V., Ballal M. Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in southern India. J. Glob. Infect. Dis. 2011;3:4–8. doi: 10.4103/0974-777X.77288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 6.Ramage G., Saville S.P., Wickes B.L., López-Ribot J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins N., Ferreira I.C.F.R., Barros L., Silva S., Henriques M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia. 2014;177:223–240. doi: 10.1007/s11046-014-9749-1. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X., Wang Y., Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emira N., Mejdi S., Dorra K., Amina B., Eulogio V. Comparison of the adhesion ability of Candida albicans strains to biotic and abiotic surfaces. Afr. J. Biotechnol. 2011;10:977–985. [Google Scholar]
- 10.Prasad R., De Wergifosse P., Goffeau A., Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen I.D., Hube B. Candida albicans morphology: Still in focus. Expert Rev. Anti. Infect. Ther. 2017;15:327–330. doi: 10.1080/14787210.2017.1290524. [DOI] [PubMed] [Google Scholar]
- 12.Hajjeh R.A., Sofair A.N., Harrison L.H., Lyon G.M., Arthington-Skaggs B.A., Mirza S.A., Phelan M., Morgan J., Lee-Yang W., Ciblak M.A., et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 2004;42:1519–1527. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao Y.K., Midha T., Garg A., Garg J., Dwivedi G.N., Singh N., Padhye A., Taneja A., Motara F., Ballot D.E., et al. Candidiasis in the newborn. Int. J. Pharma Biosci. 2015;3:128–129. [Google Scholar]
- 14.Sardi J.C.O., Scorzoni L., Bernardi T., Fusco-Almeida A.M., Mendes Giannini M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro D.R., Gorup L.F., Silva S., Negri M., de Camargo E.R., Oliveira R., Barbosa D.B., Henriques M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling. 2011;27:711–719. doi: 10.1080/08927014.2011.599101. [DOI] [PubMed] [Google Scholar]
- 16.Lipovsky A., Nitzan Y., Gedanken A., Lubart R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology. 2011;22:912–925. doi: 10.1088/0957-4484/22/10/105101. [DOI] [PubMed] [Google Scholar]
- 17.Haghighi F., Mohammadi S.R., Mohammadi P., Hosseinkhani S., Shidpour R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infect. Epidemiol. Med. 2013;1:33–38. [Google Scholar]
- 18.Lara H.H., Romero-Urbina D.G., Pierce C., Lopez-Ribot J.L., Arellano-Jiménez M.J., Jose-Yacaman M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015;13:1–12. doi: 10.1186/s12951-015-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalal M., Ansari M.A., Ali S.G., Khan H.M., Rehman S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018;46:912–925. doi: 10.1080/21691401.2018.1439837. [DOI] [PubMed] [Google Scholar]
- 20.Sair A.T., Khan Z.A. Prevalence of antibiotic and heavy metal resistance in Gram negative bacteria isolated from rivers in northern Pakistan. Water Environ. J. 2018;32:51–57. doi: 10.1111/wej.12290. [DOI] [Google Scholar]
- 21.Matyar F., Kaya A., Dincer S. Antibacterial agents and heavy metal resistance in gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Sci. Total Environ. 2008;407:279–285. doi: 10.1016/j.scitotenv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Neethu C.S., Mujeeb Rahiman K.M., Saramma A.V., Mohamed Hatha A.A. Heavy-metal resistance in gram-negative bacteria isolated from Kongsfjord, Arctic. Can. J. Microbiol. 2015;61:429–435. doi: 10.1139/cjm-2014-0803. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P., Sharma A., Sharma M., Bhalla N., Estrela P., Jain A., Thakur P., Thakur A. Nanomaterial fungicides: In vitro and in vivo antimycotic activity of cobalt and nickel nanoferrites on phytopathogenic fungi. Glob. Chall. 2017;9:1–7. doi: 10.1002/gch2.201700041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A.R., Kumar R., Chakra C.S., Rao K. V silver doped manganese -zinc –ferrite nano flowers for biomedical applications. Int. J. Emerg. Technol. Adv. Eng. 2014;5:2250–2459. [Google Scholar]
- 25.Ramanavičius S., Žalnėravičius R., Niaura G., Drabavičius A., Jagminas A. Shell-dependent antimicrobial efficiency of cobalt ferrite nanoparticles. Nano Struct. Nano Obj. 2018;15:40–47. doi: 10.1016/j.nanoso.2018.03.007. [DOI] [Google Scholar]
- 26.Mehta R.V. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater. Sci. Eng. C. 2017;79:901–916. doi: 10.1016/j.msec.2017.05.135. [DOI] [PubMed] [Google Scholar]
- 27.Sharma R.P., Raut S.D., Jadhav V.V., Kadam A.S., Mane R.S. Anti-Candida and anti-adhesion efficiencies of zinc ferrite nanoparticles. Mater. Lett. 2019;237:165–167. doi: 10.1016/j.matlet.2018.11.073. [DOI] [Google Scholar]
- 28.Wagh M.S., Patil R.H., Thombre D.K., Kulkarni M.V., Gade W.N., Kale B.B. Evaluation of anti-quorum sensing activity of silver nanowires. Appl. Microbiol. Biotechnol. 2013;97:3593–3601. doi: 10.1007/s00253-012-4603-1. [DOI] [PubMed] [Google Scholar]
- 29.Alsayed Z., Badawi M.S., Awad R. Characterization of zinc ferrite nanoparticles capped with different PVP concentrations. J. Electron. Mater. 2019;48:4925–4933. doi: 10.1007/s11664-019-07288-2. [DOI] [Google Scholar]
- 30.Ta Q.T.H., Cho E., Sreedhar A., Noh J.-S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019;371:1–9. doi: 10.1016/j.jcat.2019.01.023. [DOI] [Google Scholar]
- 31.Kucharíková S., Tournu H., Lagrou K., van Dijck P., Bujdáková H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J. Med. Microbiol. 2011;60:1261–1269. doi: 10.1099/jmm.0.032037-0. [DOI] [PubMed] [Google Scholar]
- 32.Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Mowat E., Ramage G., Lopez-Ribot J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008;3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla S.K., Rao T.S. An improved crystal violet assay for biofilm quantification in 96-well microtitre plate. Biorxiv. 2017 doi: 10.1101/100214. [DOI] [Google Scholar]
- 34.The Colorimetric Reduction of XTT by Cellular Enzymes XTT Cell Proliferation Assay Kit Instruction Manual. [(accessed on 10 September 2019)]; Available online: https://www.atcc.org/~/media/56374CEEC36C47159D2040410828B969.ashx.
- 35.Orta-García S.T., Plascencia-Villa G., Ochoa-Martínez A.C., Ruiz-Vera T., Pérez-Vázquez F.J., Velázquez-Salazar J.J., Yacamán M.J., Navarro-Contreras H.R., Pérez-Maldonado I.N. Analysis of cytotoxic effects of silver nanoclusters on human peripheral blood mononuclear cells ‘in vitro’. J. Appl. Toxicol. 2015;35:1189–1199. doi: 10.1002/jat.3190. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhari P.R., Gaikwad V.M., Acharya S.A. Role of mode of heating on the synthesis of nanocrystalline zinc ferrite. Appl. Nanosci. 2015;5:711–717. doi: 10.1007/s13204-014-0367-5. [DOI] [Google Scholar]
- 37.Li Y., Cheng X., Zhang R., Wang Y., Zhang H. Formation of zinc ferrite by solid-state reaction and its characterization by XRD and XPS. Int. J. Appl. Ceram. Technol. 2015;12:443–450. doi: 10.1111/ijac.12174. [DOI] [Google Scholar]
- 38.Druska P., Steinike U., Šepelák V. Surface structure of mechanically activated and of mechanosynthesized zinc ferrite. J. Solid State Chem. 1999;146:13–21. doi: 10.1006/jssc.1998.8284. [DOI] [Google Scholar]
- 39.Liu X., Liu H., Zhang W., Li X., Fang N., Wang X., Wu J. Facile synthesis and photocatalytic activity of bi-phase dispersible Cu-ZnO hybrid nanoparticles. Nanoscale Res. Lett. 2015;10:195. doi: 10.1186/s11671-015-0895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosvenor A.P., Kobe B.A., Biesinger M.C., Mcintyre N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004;36:1564–1574. doi: 10.1002/sia.1984. [DOI] [Google Scholar]
- 41.Yamashita T., Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008;254:2441–2449. doi: 10.1016/j.apsusc.2007.09.063. [DOI] [Google Scholar]
- 42.Liu C., Ni Y., Zhang L., Guo F., Wu T. Simple solution-combusting synthesis of octahedral ZnFe2O4 nanocrystals and additive-promoted photocatalytic performance. RSC Adv. 2014;4:47402–47408. doi: 10.1039/C4RA06993E. [DOI] [Google Scholar]
- 43.Khan S., Alam F., Azam A., Khan A.U. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012;7:3245–3257. doi: 10.2147/IJN.S31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K.-J., Sung W.S., Suh B.K., Moon S.-K., Choi J.-S., Kim J.G., Lee D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 45.Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9:2673–2702. doi: 10.1039/C8RA08982E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanpo N., Berndt C.C., Wen C., Wang J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013;9:5830–5837. doi: 10.1016/j.actbio.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 47.Manke A., Wang L., Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013;11:23909–23918. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.