Abstract
Arctoscopus japonicus is a cold-water marine fish. The present study investigated the fatty acid composition of A. japonicus egg lipids and their anti-inflammatory effects on LPS-stimulated RAW246.7 macrophages. The results showed that A. japonicus egg lipids contained primarily polyunsaturated fatty acids (52.9% of the total fatty acid content; mostly eicosapentaenoic acid [EPA, 21.2 ± 0.5%] and docosahexaenoic acid [DHA, 25.9 ± 0.1%]), followed by monounsaturated fatty acids and saturated fatty acids (23.7% and 23.4%, respectively). A. japonicus egg lipids significantly decreased nitric oxide (NO) production and suppressed the expression of immune-associated genes such as iNOS, COX-2, IL-1β, IL-6, and TNF-α LPS-stimulated RAW246.7 macrophages in dose-dependent manner. A. japonicus egg lipids also reduced the phosphorylation levels of NF-κB p-65, p38, ERK1/2, and JNK, key components of the NF-κB and MAPK pathways, suggesting that the lipid-induced anti-inflammatory activity is related to these signaling pathways. These results indicate that the lipids extracted from A. japonicus eggs have potential biofunctions and might be useful for regulating inflammation in macrophages.
Keywords: Arctoscopus japonicus, egg, lipid, anti-inflammation, macrophage, NF-κB, and MAPK
1. Introduction
Lipids, more specifically fatty acids, are key components of fish eggs, as they are the source of metabolic energy used for swimming, growth, and reproduction, and the fatty acid proportions in the cell membranes of fish, especially marine fish, are very high [1,2]. The major lipids in fish eggs are composed of saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), and cholesterol [3]. Because of their various bioactivities, lipids from fish eggs have been proposed as nutrients for human health [4]. Lipid mediators derived from PUFAs have been shown to have beneficial effects on inflammatory diseases, such as Alzheimer’s disease [5,6,7], cardiovascular diseases [8,9], asthma [10], inflammatory bowel disease [11], and cancer [12]. They are also involved in fetal development [9], play crucial roles in inflammatory regulation, and contribute to overall health [13,14]. DHA has been shown to reduce interleukin-1β (IL-1β) and tumor necrosis factor (TNF)-α levels in LPS-stimulated peripheral blood mononuclear cells (PBMCs) [15], and linoleic acid (LA; 18:2n-6), α-linolenic acid (ALA, 18:3n-3), and DHA inhibited IL-1β, IL-6, and TNF-α gene expression in human THP-1 macrophages [16].
LPS stimulation of macrophages induces inflammation by activating the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways [17]. NF-κB activity is modulated by phosphorylation and regulatory proteins such as p-65 and IκBα, and upon their degradation, NF-κB translocate to the nucleus where it functions as a transcription factor to regulate inflammation [18]. The MAPK signaling pathway, which includes extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase/stress-activated protein kinase (JNK), and p38, is involved in various cellular functions, such as cell proliferation, differentiation, and survival [19]. This signaling pathway regulates the expression of various inflammatory mediators, including nitric oxide (NO), inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2, and pro-inflammatory cytokines, such as IL-1β, and IL-6, and TNF-α [20]. Therefore, both the NF-κB and MAPK signaling pathways are primary targets for regulating inflammatory cytokine expression and inflammation-related processes.
The anti-inflammatory activities of lipids derived from marine sources, such as Katsuwonus pelamis [21], Perna canaliculus [22,23], Virgularia gustaviana [24], Mytilus coruscus [25], and Gracilaria sp. [26], have been studied. Arctoscopus japonicus, a popular commercial fish that is widely distributed in the northwestern Pacific Ocean, including the east coast of Korea [27,28], has been reported to contain functional peptides (in the meat and eggs) with antioxidant [29,30] and anti-inflammatory biological activities [31,32]. Although A. japonicus has also been shown to possess high lipid contents with biofunctional fatty acids, especially EPA and DHA, few studies have explored the lipids extracted from A. japonicus eggs and their anti-inflammatory effects on immune cells [33].
Therefore, the present study analyzed the fatty acid composition of lipids extracted from A. japonicus eggs and their anti-inflammatory effects on the immune system using LPS-stimulated RAW264.7 cells.
2. Results
2.1. Fatty Acid Analysis of A. japonicus Lipids
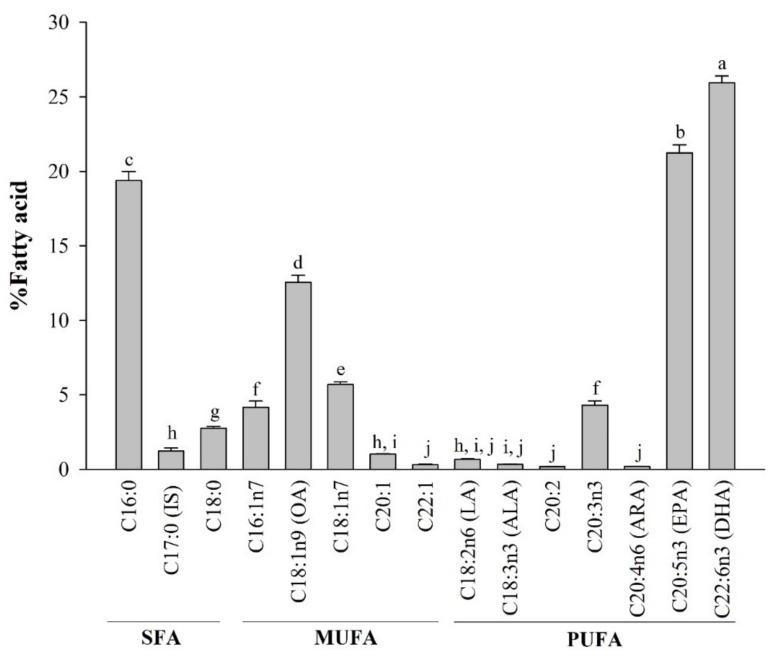
The fatty acid composition of lipids extracted from A. japonicus eggs is shown in Figure 1. The fatty acids were first analyzed according to type, i.e., SFA, monounsaturated fatty acids (MUFA), and PUFA. The lipids were mostly composed of PUFAs (52.9%), followed by MUFAs (23.7%) and SFAs (23.4%). Further analysis showed that A. japonicus egg lipids contained 19.4 ± 0.6% palmitic acid (C16:0), 2.6 ± 0.1% oleic acid (C18:0), 21.2 ± 0.5% eicosapentaenoic acid (DHA, C20:5n-3), and 25.9 ± 0.5% docosahexaenoic acid (DHA, C22:6n-3).
Figure 1.
Fatty acid composition of lipids extracted from A. japonicus eggs. Data are the mean ± SD (n = 5). Lowercase letters (a–j) indicate significant differences (p < 0.05) between the amounts of total fatty acid from A. japonicus lipids (where, a > b > c > d > e > f > g > h > i > j). SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
2.2. Cytotoxicity of A. japonicus Egg Lipids
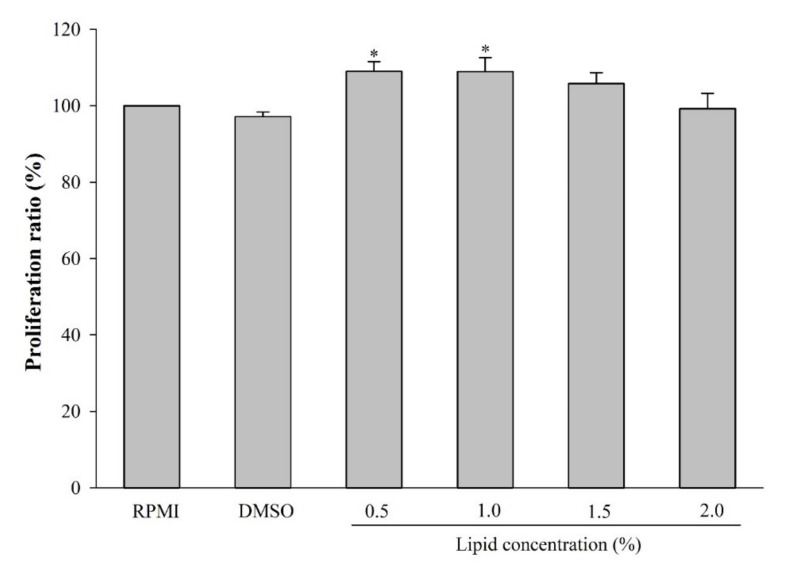
To examine the potential toxicity of A. japonicus egg lipids on RAW264.7 cells, the cells were incubated with different concentrations of A. japonicus egg lipids (0%, 0.5%, 1.0%, 1.5%, and 2.0%), and cell viability was assessed. As shown in Figure 2, A. japonicus egg lipids did not decrease cell viability, but certain concentrations moderately stimulated the proliferation of RAW264.7 cells.
Figure 2.
Effect of lipid extracts from A. japonicus eggs on the proliferation of RAW264.7 cells. Data are the mean ± SD (n = 3). Asterisks indicate a significant difference (p < 0.05) compared to cells incubated with RPMI (set at 100%).
2.3. Effects of A. japonicus Egg Lipids on NO Production
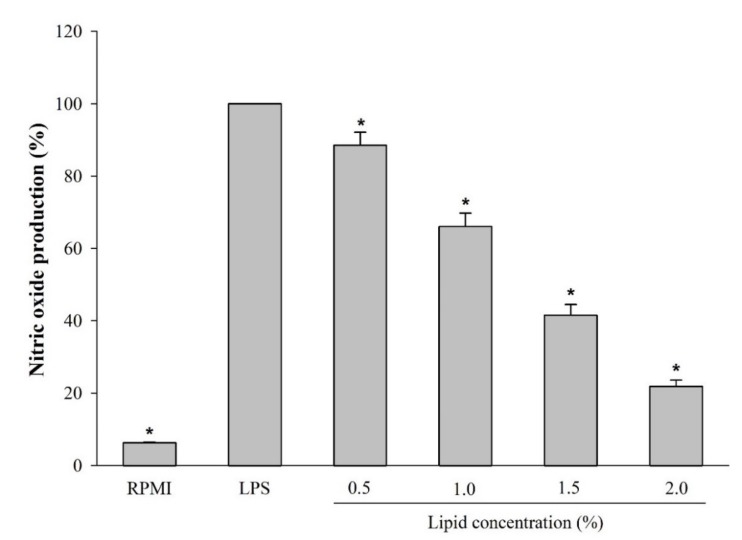
To evaluate the effect of A. japonicus lipids on immune regulation, NO production by RAW264.7 cells was assessed in the presence of extracted A. japonicus egg lipids. Figure 3 shows that NO production was significantly reduced in the presence of 0.5–2.0% lipids in a concentration-dependent manner.
Figure 3.
Effect of lipids extracted from A. japonicus eggs on NO production in LPS-stimulated RAW264.7 cells. Data are the mean ± SD (n = 3). Asterisks indicate a significant difference (p < 0.05) compared to LPS.
2.4. Anti-Inflammatory Effect of A. japonicus Egg Lipids Mediated by Modulation of Immune-Associated Gene Expression
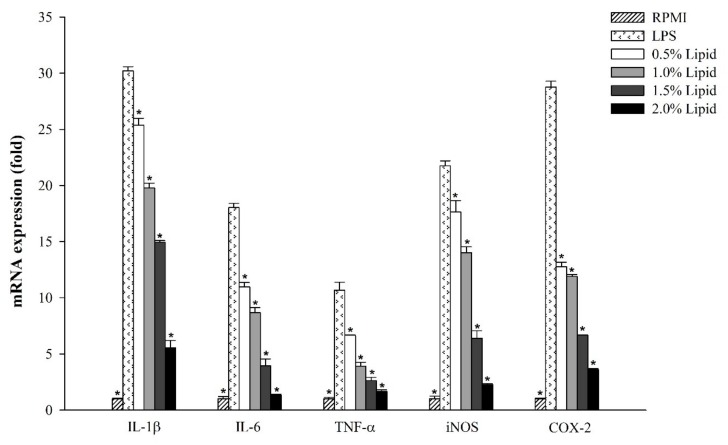
The effects of lipids extracted from A. japonicus eggs on the expression levels of immune-associated genes in LPS-stimulated RAW264.7 cells were examined by quantitative real-time PCR. The results showed that A. japonicus lipids decreased the expression levels of most tested genes and significantly reduced the expression levels of the inflammatory mediators iNOS and COX-2 as well as the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in an A. japonicus egg lipid concentration-dependent manner (Figure 4).
Figure 4.
Effects of lipids extracted from A. japonicus eggs on the expression levels of immune-associated genes in LPS-stimulated RAW264.7 cells. Data are the mean ± SD fold difference compared to unstimulated cells (n = 3). Asterisks indicate significant differences (p < 0.05) versus LPS alone.
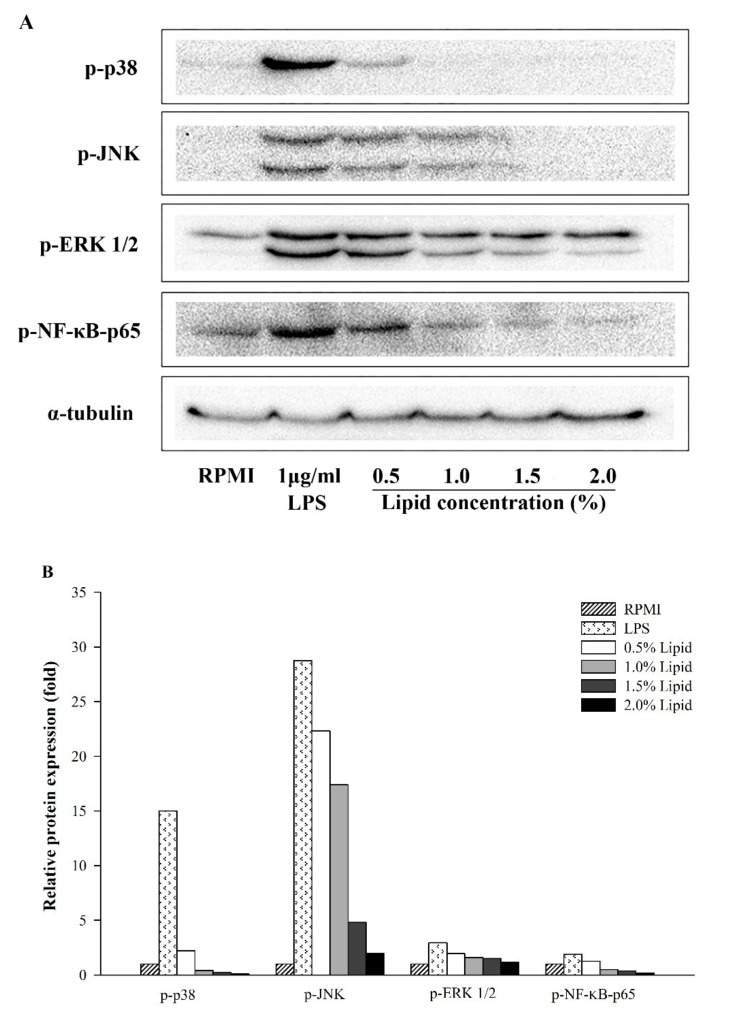
2.5. Anti-Inflammatory Effects of A. japonicus Egg Lipids Involve the NF-ĸB and MAPK Signaling Pathways
To investigate whether the lipid extracts from A. japonicus eggs influence immune-associated signaling, the pathways involving NF-κB and MAPKs were evaluated by western blotting. As shown Figure 5, A. japonicus egg lipids decreased the phosphorylation levels of the NF-κB p65 subunit in a concentration-dependent manner, when compared to that in the control. The levels of phosphorylated ERK, JNK, and p38, which are the biomarkers of the MAPK signaling pathway, were also reduced in lipid-treated RAW264.7 cells.
Figure 5.
Effect of lipids extracted from A. japonicus eggs on the protein levels in the NF-κB and MAPK pathways in LPS-stimulated RAW264.7 cells as determined by western blotting. (A) Western blot; (B) quantification of relative band intensity.
3. Discussion
A. japonicus, a cold-water fish found in the northwestern Pacific Ocean, the East Sea of Korea, and the Northern Sea of Japan, possesses lipids containing high levels of PUFAs, especially EPA and DHA, which are commonly found in fish and marine foods [34] and are useful in the pharmaceutical and food industries for their beneficial effects on human health [35]. However, no studies have examined the lipids from A. japonicus eggs and their anti-inflammatory effects on murine RAW264.7 cells.
The total fatty acid composition of the lipids extracted from A. japonicus eggs was analyzed by GC-FID (Figure 1), which showed that the predominant fatty acids were DHA, (22:6n-3), EPA (20:5n-3), and ALA (18:3n-3). In addition, the major SFAs and MUFAs were palmitic acid (16:0) and oleic acid (OA, 18:1n-9), respectively. Similar to A. japonicus egg lipids, lipids extracted from Pollock eggs were shown to include fatty acids with high levels of palmitic acid and oleic acid as well as DHA and EPA [36], whereas tuna eggs contained much higher levels of DHA (26.19%) than those of EPA (3.80–4.62%) when compared with A. japonicus egg lipids [37].
Palmitic acid is an essential source of metabolic energy in fish during growth and egg formation in female fish [38]. Oleic acid, a major MUFA, plays a key role in energy metabolism during fish spawning [38]. Likewise, A. japonicus eggs may require these SFAs for energy metabolism during embryonic development. Our results showed that the dominant fatty acids were PUFAs, which likely fulfill the nutritional requirements of the immune system during embryonic development [39]. These results suggested that the lipids extracted from A. japonicus eggs, especially the high EHA and DHA contents, may be involved in inflammation regulation in RAW264.7 cells.
Macrophages play a key role in the regulation of acute and chronic inflammation by removing antigens and increasing of NO production [40]. Prostaglandin E2 (PGE2), a key inducer of inflammatory symptoms, such as fever, swelling, and pain, was evaluated in activated macrophage cells [41]. Under inflammatory conditions, i.e., LPS stimulation, macrophages were activated to induce the production of inflammatory mediators such as NO and PGE2, which was mediated by inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, respectively, as well as pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α [42]. Our results demonstrated that A. japonicus egg lipids significantly decreased NO production and the expression of immune-associated genes such as iNOS, IL-1β, IL-6, TNF-α, and COX-2 in LPS-stimulated RAW264.7 cells. These results indicated that A. japonicus egg lipids have potential for use as anti-inflammatory regulators.
NF-κB, a well-known transcription factor, modulates the expression of genes involved in the innate and adaptive immune-associated genes such as iNOS, COX-2, and pro-inflammatory cytokine [18]. Moreover, MAPKs, such as ERK1/2, JNK, and p38, are involved in the expression regulation of these immune-associated genes under inflammatory conditions [20,43]. In addition to its effects on NO production and immune-associated gene expression, A. japonicus egg lipids also reduced the phosphorylation of NF-κB p-65 and various MAPKs (p-38, JNK, and ERK), suggesting their involvement in the observed anti-inflammatory activity. JNK signaling may be a major pathway because JNK protein phosphorylation was remarkably reduced by A. japonicus egg lipids in LPS-stimulated RAW264.7 cells.
4. Materials and Methods
4.1. Preparation of A. japonicus Lipids
A. japonicus was obtained from the East Sea near Gangwon province, South Korea, and its eggs were isolated, freeze-dried, and ground for lipid extraction using a modification of the method of Bligh and Dyer [44]. A. japonicus egg lipids were prepared by extraction using chloroform and methanol and inert gas evaporation. After dissolving the evaporated sample in dimethyl sulfoxide (DMSO), it was weighed to determine the lipid mass and stored at −20 °C.
4.2. Fatty Acid Analysis
The fatty acids were extracted from A. japonicus lipids according to the method of Garces and Mancha [45]. Fatty acid methyl esters (FAMEs) were prepared by the modified one-step lipid extraction method [46] to analyze the fatty acid composition. The FAMEs were analyzed by gas chromatography (GC)-flame ionization detection (FID) (Perkin Elmer, Waltham, MA, USA).
4.3. Cell Culture
The RAW264.7 cells line was obtained from the Korean Cell Line Bank (Korean Cell Line Research Foundation, Seoul, Korea), and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
4.4. Cell Viability
After a 24-h incubation in 96-well plates, RAW264.7 cells were cultured with five different concentrations of A. japonicus lipids (0%, 0.5%, 1.0%, 1.5%, and 2.0%) for another 24 h. After discarding the supernatant, cell proliferation was evaluated by using the EZ-Cytox Cell Viability Assay Kit (Daeil Lab Service, Korea) as described by Kim et al. [47]. The ratio of proliferating cells was calculated according to the following equation:
4.5. Nitric Oxide (NO) Production
RAW264.7 cells were cultured with the different concentrations of A. japonicas lipids for 1 h. After incubation, the cells were treated with 1 μg/ml LPS for 24 h. Then, NO production was determined based on nitrite accumulation in the culture medium using Griess reagent (Promega, USA) [48].
4.6. RNA Isolation and Real-Time PCR
Total RNA was isolated from RAW264.7 cells using TRI reagent® (Molecular Research Center, Inc., USA), and then reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The mRNA levels of inflammatory genes were quantified by quantitative real-time PCR using SYBR® Premix Ex Taq™ II (Takara Bio, Inc., Kusatsu, Japan) and a QuantStudio™ 3 FlexReal-Time PCR System (Thermo Scientific, Waltham, MA, USA). The primers used in this analysis are shown in Table 1. The results were quantified using the 2−ΔΔCT method [49]. β-Actin was included as a control gene.
Table 1.
Oligonucleotide primers used in this study.
| Gene | Accession No. | Primer Sequence (5’ to 3’) |
|---|---|---|
| IL-1β | NM_008361.4 | Forward: GGGCCTCAAAGGAAAGAATC Reverse: TACCAGTTGGGGAACTCTGC |
| iNOS | BC062378.1 | Forward: TTCCAGAATCCCTGGACAAG Reverse: TGGTCAAACTCTTGGGGTTC |
| IL-6 | NM_031168.2 | Forward: AGTTGCCTTCTTGGGACTGA Reverse: CAGAATTGCCATTGCACAAC |
| COX-2 | NM_011198.4 | Forward: AGAAGGAAATGGCTGCAGAA Reverse: GCTCGGCTTCCAGTATTGAG |
| TNF-α | D84199.2 | Forward: ATGAGCACAGAAAGCATGATC Reverse: TACAGGCTTGTCACTCGAATT |
| β-Actin | NM_007393.5 | Forward: CCACAGCTGAGAGGGAAATC Reverse: AAGGAAGGCTGGAAAAGAGC |
4.7. Western Blotting
Cultured RAW264.7 cells were lysed using RIPA buffer (Tech & Innovation, Hebei, China) to extract total proteins from the cells, and the protein was quantified with the Pierce™ BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Total proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene fluoride (PVDF) membrane, and an immunoblot assay was carried out as described by Narayanan et al. [50]. To investigate the NF-ĸB and MAPK signaling pathways, related proteins were detected with antibodies against phosphorylated NF-ĸB p65 (Cell Signaling Technology, #3033), p38 (Cell Signaling Technology, #9211), JNK (Cell Signaling Technology, #9251), and ERK1/2 (Cell Signaling Technology, #9101) along with α-tubulin (Abcam, #ab15246) as a control, and goat anti-rabbit IgG (H+L)-HRP (GenDEPOT, SA006-500). The signals were measured using Pierce® ECL Plus Western Blotting Substrate (Thermo Scientific, Waltham, MA, USA). The blot was quantitatively analyzed using a ChemiDoc XRS+ imaging system (Bio-Rad, Hercules, CA, USA) and ImageLab software (version 4.1, Bio-Rad).
4.8. Statistical Analysis
SPSS 24.0 software (SPSS, Chicago, IL, USA) was used to analyze the study results. Data are reported as mean ± standard deviation (SD) and were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test, with statistical significance set at p values less than 0.05.
5. Conclusions
Our study demonstrated that lipids extracted from A. japonicus eggs reduced the LPS-induced expression and levels of inflammatory mediators and pro-inflammatory cytokines by suppressing the NF-κB and MAPK signaling pathways in RAW264.7 macrophages. These results suggest that A. japonicus egg lipids have anti-inflammatory properties and are a potential biofunctional, anti-inflammatory, marine lipid material. Further studies are needed to confirm whether total lipids from A. japonicus eggs exhibit anti-inflammatory effects in inflammatory disease models.
Author Contributions
W.R.-i. designed and performed the experiments, analyzed data, and wrote the manuscript. C.M. performed the experiments. S.-m.L., S.-K.J., and S.Y edited the manuscript. W.J.P. designed the experiments, analyzed data, and wrote the manuscript.
Funding
This study was supported by the Marine Bio-Regional Specialization Leading Technology Development Program (D11413914H480000100) funded by the Ministry of Oceans and Fisheries in Korea and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2019R1A2B5B01070542). This research project was also supported by the University Emphasis Research Institute Support Program (No.2018R1A61A03023584) through the National Research Foundation of Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Sargent J.R., Tocher D.R., Bell J.G. 4—The Lipids. In: Halver J.E., Hardy R.W., editors. Fish Nutrition. 3rd ed. Academic Press; San Diego, CA, USA: 2003. pp. 181–257. [Google Scholar]
- 2.Tocher D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003;11:107–184. doi: 10.1080/713610925. [DOI] [Google Scholar]
- 3.Rao P.P., Balaswamy K., Jyothirmayi T., Karuna M., Prasad R. Processing and Impact on Active Components in Food. Elsevier; Amsterdam, The Netherlands: 2015. Fish roe lipids: Composition and changes during processing and storage; pp. 463–468. [Google Scholar]
- 4.Shirai N., Higuchi T., Suzuki H. Analysis of lipid classes and the fatty acid composition of the salted fish roe food products, Ikura, Tarako, Tobiko and Kazunoko. Food Chem. 2006;94:61–67. doi: 10.1016/j.foodchem.2004.10.050. [DOI] [Google Scholar]
- 5.Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C., Galvin J.E., Emond J., Jack C.R., Jr., Weiner M., et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canhada S., Castro K., Perry I.S., Luft V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018;21:529–538. doi: 10.1080/1028415X.2017.1321813. [DOI] [PubMed] [Google Scholar]
- 7.Yassine H.N., Braskie M.N., Mack W.J., Castor K.J., Fonteh A.N., Schneider L.S., Harrington M.G., Chui H.C. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: A review. JAMA Neurol. 2017;74:339–347. doi: 10.1001/jamaneurol.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holub D.J., Holub B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell. Biochem. 2004;263:217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 9.Swanson D., Block R., Mousa S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mickleborough T.D., Lindley M.R., Ionescu A.A., Fly A.D. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Schwanke R.C., Marcon R., Bento A.F., Calixto J.B. EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. Eur. J. Pharmacol. 2016;785:156–164. doi: 10.1016/j.ejphar.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Gu Z., Shan K., Chen H., Chen Y.Q. n-3 polyunsaturated fatty acids and their role in cancer chemoprevention. Curr. Pharmacol. Rep. 2015;1:283–294. doi: 10.1007/s40495-015-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariel A., Serhan C.N. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.K., Mendis E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006;39:383–393. doi: 10.1016/j.foodres.2005.10.010. [DOI] [Google Scholar]
- 15.Kelley D.S., Branch L.B., Love J.E., Taylor P.C., Rivera Y.M., Iacono J.M. Dietary α-linolenic acid and immunocompetence in humans. Am. J. Clin. Nutr. 1991;53:40–46. doi: 10.1093/ajcn/53.1.40. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G., Etherton T.D., Martin K.R., Vanden Heuvel J.P., Gillies P.J., West S.G., Kris-Etherton P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005;336:909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 17.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 18.Tak P.P., Firestein G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Luo C., Wang P., He Q., Zhou J., Peng H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW264.7 cells. Exp. Ther. Med. 2013;5:1345–1350. doi: 10.3892/etm.2013.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong D.H., Kim K.B.W.R., Kim M.J., Kang B.K., Ahn D.H. Skipjack tuna (Katsuwonus pelamis) eyeball oil exerts an anti-inflammatory effect by inhibiting NF-κB and MAPK activation in LPS-induced RAW 264.7 cells and croton oil-treated mice. Int. Immunopharmacol. 2016;40:50–56. doi: 10.1016/j.intimp.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Halpern G.M. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol) Allerg. Immunol. 2000;32:272–278. [PubMed] [Google Scholar]
- 23.Whitehouse M.W., Macrides T.A., Kalafatis N., Betts W.H., Haynes D.R., Broadbent J. Anti-inflammatory activity of a lipid fraction (lyprinol) from the NZ green-lipped mussel. Inflammopharmacology. 1997;5:237–246. doi: 10.1007/s10787-997-0002-0. [DOI] [PubMed] [Google Scholar]
- 24.Sharifi S., Safaeian S. Anti-inflammatory effect of lipid extract of sea pen (Virgularia gustaviana) in mice. Asian J. Pharm. Clin. Res. 2015;8:332–334. [Google Scholar]
- 25.Li G., Fu Y., Zheng J., Li D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs. 2014;12:568–588. doi: 10.3390/md12020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Costa E., Melo T., Moreira A.S., Bernardo C., Helguero L., Ferreira I., Cruz M.T., Rego A.M., Domingues P., Calado R. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs. 2017;15:62. doi: 10.3390/md15030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.I., Yang J.H., Yoon S.C., Chun Y.Y., Kim J.B., Cha H.K., Choi Y.M. Biomass estimation of sailfin sandfish, Arctoscopus japonicus, in Korean waters. Korean J. Fish. Aquat. Sci. 2009;42:487–493. [Google Scholar]
- 28.Shirai S.M., Kuranaga R., Sugiyama H., Higuchi M. Population structure of the sailfin sandfish, Arctoscopus japonicus (Trichodontidae), in the Sea of Japan. Ichthyol. Res. 2006;53:357–368. doi: 10.1007/s10228-006-0356-0. [DOI] [Google Scholar]
- 29.Jang H.L., Liceaga A.M., Yoon K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods. 2016;20:433–442. doi: 10.1016/j.jff.2015.11.020. [DOI] [Google Scholar]
- 30.Jang H.L., Shin S.R., Yoon K.Y. Hydrolysis conditions for antioxidant peptides derived from enzymatic hydrolysates of sandfish (Arctoscopus japonicus) Food Sci. Biotechnol. 2017;26:1191–1197. doi: 10.1007/s10068-017-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang H.L., Liceaga A.M., Yoon K.Y. Isolation and characteristics of anti-inflammatory peptides from enzymatic hydrolysates of sandfish (Arctoscopus japonicus) protein. J. Aquat. Food Prod. Technol. 2017;26:234–244. doi: 10.1080/10498850.2016.1221015. [DOI] [Google Scholar]
- 32.Jang H.L., Young Yoon K. Optimal conditions of enzymatic hydrolysis for producing anti-inflammatory peptides from sandfish (Arctoscopus japonicus) hydrolysate. Korean J. Food Sci. Technol. 2018;50:203–208. [Google Scholar]
- 33.Ishihara Y., Watanabe F. Lipid content and fatty acid composition of Japanese sandfish Arctoscopus japonicus caught offshore of Tottori Prefecture, Japan. Nippon Suisan Gakkaishi. 2013;79:229–231. doi: 10.2331/suisan.79.229. [DOI] [Google Scholar]
- 34.Harris W.S. Fish oil supplementation: Evidence for health benefits. Clevel. Clin. J. Med. 2004;71:208–221. doi: 10.3949/ccjm.71.3.208. [DOI] [PubMed] [Google Scholar]
- 35.Hamed I., Özogul F., Özogul Y., Regenstein J.M. Marine bioactive compounds and their health benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015;14:446–465. doi: 10.1111/1541-4337.12136. [DOI] [Google Scholar]
- 36.Bechtel P.J., Chantarachoti J., Oliveira A.C.M., Sathivel S. Characterization of protein fractions from immature Alaska walleye pollock (Theragra chalcogramma) roe. J. Food Sci. 2007;72:S338–S343. doi: 10.1111/j.1750-3841.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 37.Intarasirisawat R., Benjakul S., Visessanguan W. Chemical compositions of the roes from skipjack, tongol and bonito. Food Chem. 2011;124:1328–1334. doi: 10.1016/j.foodchem.2010.07.076. [DOI] [Google Scholar]
- 38.Huynh M.D., Kitts D.D., Hu C., Trites A.W. Comparison of fatty acid profiles of spawning and non-spawning Pacific herring, Clupea harengus pallasi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:504–511. doi: 10.1016/j.cbpb.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Cejas J.R., Almansa E., Villamandos J.E., Badía P., Bolaños A., Lorenzo A. Lipid and fatty acid composition of ovaries from wild fish and ovaries and eggs from captive fish of white sea bream (Diplodus sargus) Aquaculture. 2003;216:299–313. doi: 10.1016/S0044-8486(02)00525-2. [DOI] [Google Scholar]
- 40.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 41.Hu S.S.J., Bradshaw H., Chen J.C., Tan B., Walker J.M. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NF-κB activity. Br. J. Pharmacol. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y.A., Song C.W., Koh W.S., Yon G.H., Kim Y.S., Ryu S.Y., Kwon H.J., Lee K.H. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw264.7 Cells. Phytother. Res. 2013;27:1200–1205. doi: 10.1002/ptr.4847. [DOI] [PubMed] [Google Scholar]
- 43.McGrath M.A., Harnett M.M., Thalhamer T. MAPKs and their relevance to arthritis and inflammation. Rheumatology. 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 44.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 45.Garces R., Mancha M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993;211:139–143. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- 46.Park W.J., Kothapalli K.S.D., Lawrence P., Tyburczy C., Brenna J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009;50:1195–1202. doi: 10.1194/jlr.M800630-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J.K., Cho M.L., Karnjanapratum S., Shin I.S., You S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011;49:1051–1058. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 48.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 49.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Narayanan B.A., Narayanan N.K., Simi B., Reddy B.S. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res. 2003;63:972–979. [PubMed] [Google Scholar]