Abstract
25-hydroxyvitamin D (25(OH)D) is commonly measured to assess vitamin D status. Other vitamin D metabolites such as 24,25-dihydroxyvitamin D (24,25(OH)2D) provide additional insights into vitamin D status or metabolism. Earlier studies suggested that the vitamin D metabolite ratio (VMR), calculated as 24,25(OH)2D/25(OH)D, could predict the 25(OH)D increase after vitamin D supplementation. However, the evidence for this additional value is inconclusive. Therefore, our aim was to assess whether the increase in 25(OH)D after supplementation was predicted by the VMR better than baseline 25(OH)D. Plasma samples of 106 individuals (25(OH)D < 75 nmol/L) with hypertension who completed the Styrian Vitamin D Hypertension Trial (NC.T.02136771) were analyzed. Participants received vitamin D (2800 IU daily) or placebo for 8 weeks. The treatment effect (ANCOVA) for 25(OH)D3, 24,25(OH)2D3 and the VMR was 32 nmol/L, 3.3 nmol/L and 0.015 (all p < 0.001), respectively. Baseline 25(OH)D3 and 24,25(OH)2D3 predicted the change in 25(OH)D3 with comparable strength and magnitude. Correlation and regression analysis showed that the VMR did not predict the change in 25(OH)D3. Therefore, our data do not support routine measurement of 24,25(OH)2D3 in order to individually optimize the dosage of vitamin D supplementation. Our data also suggest that activity of 24-hydroxylase increases after vitamin D supplementation.
Keywords: vitamin D metabolites; vitamin D supplementation; vitamin D metabolite ratio; randomized controlled trial; 24,25-dihydroxy vitamin D
1. Introduction
Vitamin D plays an essential role in calcium and phosphate homeostasis [1]. Vitamin D status is most commonly assessed by determining the 25-hydroxyvitamin D (25(OH)D) concentration in serum or plasma. However, several other vitamin D-related metabolites can be measured to provide a better understanding of individual vitamin D status and metabolism. Among them, 24,25(OH)2D has emerged as a metabolite with potentially high utility [2].
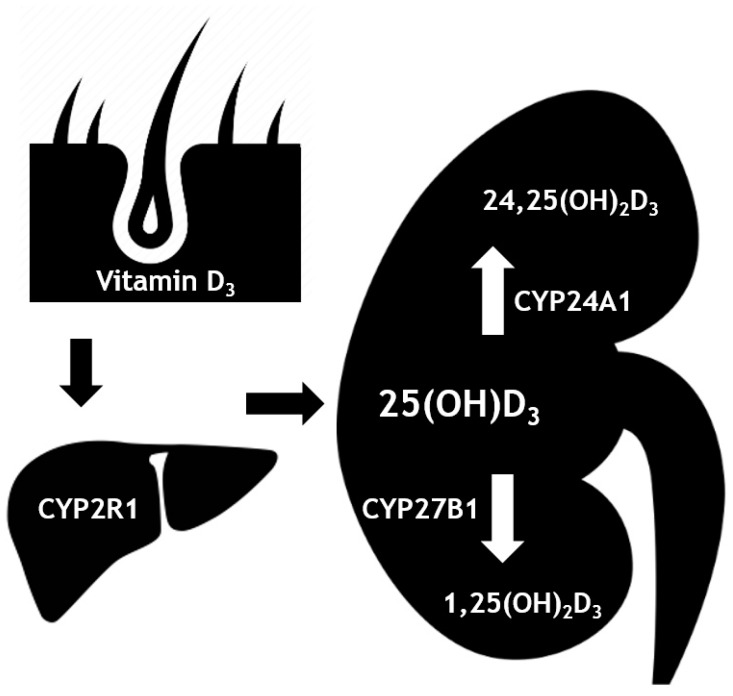
In the kidneys, 25(OH)D is converted by 1-alpha-hydroxylase (CYP27B1) into 1,25-dihydroxyvitamin D (1,25(OH)2D; also called active vitamin D or calcitriol) (Figure 1). 1,25(OH)2D can bind to the vitamin D receptor (VDR) with high affinity. The subsequent signaling results in an increase in serum calcium and phosphate concentrations, mainly mediated by an increased intestinal uptake. In addition, 1,25(OH)2D has effects on the parathyroid gland, kidneys and bones, all resulting in an increase in serum calcium and phosphate concentrations [1]. Furthermore, 1,25(OH)2D has major effects on modulating the immune system, which might be relevant for the treatment of autoimmune diseases, infections, cancer and cardiovascular diseases [3]. An excess of both 1,25(OH)2D and/or 25(OH)D lead to their catabolism by the enzyme 24-hydroxylase (CYP24A1). This results in the formation of metabolites 1,24,25(OH)2D and 24,25(OH)2D, respectively [4]. It is still unclear whether 24,25(OH)2D has a physiological role in humans [5].
Figure 1.
Metabolism of vitamin D. Vitamin D3 (cholecalciferol) is produced in the skin when exposed to sunlight. The hepatic enzyme CYP2R1 then converts this into 25(OH)D3 (calcifediol). In the kidneys, 25(OH)D3 can be converted into the active form, 1,25(OH)2D3 (calcitriol), by CYP27B1 (1-α-hydroxylase). In the kidneys, CYP24A1 (24-hydroxylase) can catabolize the 25(OH)D3 into 24,25(OH)2D3.
Using an LC-MS/MS method, 25(OH)D and 24,25(OH)2D can be measured simultaneously, which allows for determination of the 24,25(OH)2D/25(OH)D ratio, also known as the vitamin D metabolite ratio (VMR) [2]. The VMR is an indicator of CYP24A1 activity and thereby of vitamin D catabolism. It is currently used for diagnosing idiopathic infantile hypercalcemia, a rare genetic disorder in which a mutation in CYP24A1 results in severe hypercalcemia and suppressed parathyroid hormone (PTH) levels [5]. The VMR may also reflect vitamin D receptor (VDR) activity since CYP24A1 expression is upregulated in response to 1,25(OH)2D [2].
In recent years, there has been an increasing interest in the use of the VMR when assessing vitamin D status. For example, it has been postulated to better reflect vitamin D deficiency [6]. In addition, it has been speculated that the ratio could provide useful information regarding bone health [7]. Interestingly, several studies show that the VMR can predict the change seen in 25(OH)D after vitamin D supplementation, although results are inconclusive [6,8,9,10,11]. The CYP24A1 activity could be partially responsible for the individual differences seen in the effect of vitamin D supplementation on serum levels of 25(OH)D. Theoretically, if CYP24A1 activity is a major predictor of the effect of vitamin D supplementation, the VMR could be used to personalize the treatment dosage. At present, 25(OH)D concentrations at the start of supplementation, as well as BMI, age, ethnicity and genetic background have been most commonly studied in regard to predicting the response to vitamin D supplementation, and studies involving 1,25(OH)2D, 24,25(OH)2D, free and bioavailable 25(OH)D and the VMR are scarce [12].
Therefore, we set out to determine whether baseline VMR measurements can predict changes in vitamin D-related metabolite levels after vitamin D supplementation. To that extent, we measured 25(OH)D3, 1,25(OH)2D and 24,25(OH)2D3 in a randomized clinical trial of patients (25(OH)D < 75 nmol/L) receiving vitamin D supplementation [13]. We hypothesized that measurements of baseline VMR would be advantageous over baseline 25(OH)D measurements for the prediction of the change in 25(OH)D upon supplementation.
2. Materials and Methods
2.1. Study Cohort
The present post-hoc analysis was conducted in adults (>18 years old) with 25(OH)D levels <75 nmol/L and hypertension, who completed the randomized, placebo-controlled Styrian Vitamin D Hypertension Trial (NC.T.02136771). The participants of this trial were treated with either placebo or 2800 IU daily of vitamin D3 (Oleovit D3, Fresenius Kabi, Graz, Austria) for 8 weeks. A total of 188 study participants completed the original study and sufficient material for analysis from both study visits was available for 106 of these subjects. The details regarding the study, including inclusion and exclusion criteria, can be found in the publication of the original study by Pilz et al. [13].
Study participants provided written informed consent. The study complied with the Declaration of Helsinki and was approved by the ethics committee of the Medical University of Graz, Austria.
2.2. Measurements
For the original study by Pilz et al, the 25(OH)D levels were determined with the ChemiLuminescence assay (IDS-iSYS 25-hydroxyvitamin D assay; Immunodiagnostic Systems Ltd., Boldon, UK) on an IDS-iSYS multidiscipline automated analyser [13]. The intra- and inter-assay CVs were 6.2% and 11.6%, respectively.
In the present study, 25(OH)D3 and 24,25(OH)2D3 were measured in plasma samples by isotope dilution liquid chromatography-tandem mass spectrometry at the Endocrine Laboratory of the Amsterdam UMC, as described previously [14]. For 25(OH)D3, the lower limit of quantitation (LLOQ) was 1.2 nmol/L and the inter- and intra-assay coefficients of variation (CV) were 6% and 3%, respectively. For 24,25(OH)2D3, the LLOQ was 0.1 nmol/L and the inter- and intra-assay coefficients of variation (CV) were 9% and 5%, respectively. 25(OH)D2 was also measured, but as the concentrations were all very low (<7.9 nmol/L) and supplementation was given as vitamin D3, these data were not taken into account in this paper. In order to calculate the VMR and as it is the golden standard, the LC/MS-MS method was used for the current study. Using this method, 7 subjects had 25(OH)D levels >75 nmol/L at baseline. Measurements of other study parameters have been described previously [13].
To calculate free and biologically available 25(OH)D3 we used the equations from Powe et al. [15].
2.3. Statistical Analysis
Continuous data following a normal distribution are reported as means with standard deviations (SD). Variables with a skewed distribution are shown as medians with interquartile ranges. Categorical variables are shown as percentages of observations. Groups at baseline were compared using the unpaired Students t-test, the Mann–Whitney U test or the chi-squared test. Skewed variables were log transformed before being used in parametric analyses.
The changes from baseline for 25(OH)D3, 1,25(OH)2D and 24,25(OH)2D3 in the vitamin-D-treated group were calculated as the difference between the value at the final study visit and the value at baseline. They are depicted as Δ25(OH)D3, Δ1,25(OH)2D and Δ24,25(OH)2D3. The VMR was calculated as the ratio between 24,25(OH)2D3 and 25(OH)D3.
Analysis of covariance (ANCOVA) was used to calculate the treatment effects with adjustment for baseline values. Pearson correlation analysis was used to determine the strength of associations between vitamin-D-related parameters and Δ25(OH)D3, Δ1,25(OH)2D, as well as Δ24,25(OH)2D3. Bonferroni correction was applied to account for multiple testing. Univariate linear regression analysis was used to determine the relation between Δ25(OH)D3 and baseline 25(OH)D3, 24,25(OH)2D3 and VMR.
Using the LC/MS-MS method, 7 subjects had 25(OH)D levels >75 nmol/L at baseline. Therefore, we explored whether inclusion of these subjects had an effect on the analyses. In addition, we also investigated whether the inclusion of only subjects with 25(OH)D levels <50 nmol/L at baseline would affect the analyses.
If outliers were detected in the analyses by the software, defined as cases with standardized residuals greater than 3 standard deviations for ANCOVA analyses or as cases with values higher or lower than 1.5*IQR (interquartile range) for correlation analyses, they were removed and the analysis repeated to determine their potential effect on the analysis. In the case of Pearson correlation analyses, one extreme outlier was removed (25(OH)D > 4xSD at baseline) because of its significant effect on all of the analyses. This is marked in the results section. If the outliers had no significant effect on the analysis, the results including the outliers are reported. A p-value < 0.05 was considered statistically significant. All analyses were performed using S.P.SS version 25 (S.P.SS, Chicago, IL, USA).
3. Results
The baseline characteristics of study participants can be found in Table 1. There were no differences between the placebo and vitamin-D-treated groups in any of the parameters at baseline.
Table 1.
Baseline characteristics.
| Parameter | All (n = 106) | Placebo (n = 54) | Vitamin D (n = 52) | p-value |
|---|---|---|---|---|
| Age (years) | 62.0 (51.3–68.7) | 64.8 (50.8–70.2) | 59.6 (52.4–66.6) | 0.318 |
| Body mass index (kg/m2) | 30.0 ± 5.4 | 29.7 ± 5.9 | 30.3 ± 4.9 | 0.562 |
| Gender (% female) | 57 | 57 | 56 | 0.865 |
| 24,25(OH)2D3 (nmol/L) | 3.5 ± 1.6 | 3.4 ± 1.5 | 3.6 ± 1.5 | 0.419 |
| 25(OH)D3 (nmol/L) | 48 ± 18 | 46 ± 19 | 49 ± 18 | 0.401 |
| VMR ((nmol/L)/(nmol/L)) | 0.073 ± 0.017 | 0.072 ± 0.018 | 0.073 ± 0.017 | 0.768 |
| PTH (pmol/L) | 5.5 (4.1–6.7) | 5.5 (4.0–6.7) | 5.3 (4.1–6.7) | 0.779 |
| 1,25(OH)2D (pmol/L) | 126 ± 53 | 118 ± 52 | 133 ± 52 | 0.142 |
| Serum phosphate (mmol/L) | 0.94 ± 0.17 | 0.96 ± 0.17 | 0.92 ± 0.16 | 0.282 |
| Serum calcium (mmol/L) | 2.26 (2.21–2.33) | 2.26 (2.21–2.34) | 2.26 (2.20–2.33) | 0.773 |
| eGFR (mL/min/1.73m2) | 72 ± 17 | 69 ± 16 | 74 ± 18 | 0.152 |
| 24h urinary calcium excretion (mmol/24h) | 3.30 (1.90–5.00) | 2.95 (1.83–4.78) | 3.70 (2.10–6.30) | 0.222 |
| Calculated free 25(OH)D3 (pmol/L) | 15 (9–21) | 12 (8–21) | 17 (11–20) | 0.153 |
| Vitamin D binding protein (µg/mL) | 247.1 ± 109.5 | 254.8 ± 110.6 | 239.3 ± 109.0 | 0.772 |
| Calculated bioavailable 25(OH)D3 (nmol/L) | 5.9 (3.9–8.2) | 5.2 (3.2–8.5) | 6.6 (4.1–8.0) | 0.149 |
| 1,25(OH)2D /25(OH)D3 ((nmol/L)/(nmol/L)) | 0.0023 (0.0019–0.0036) | 0.0027 (0.0018–0.0039) | 0.0028 (0.0021–0.0035) | 0.753 |
| 1,25(OH)2D /24,25(OH)2D3 ((nmol/L)/(nmol/L)) | 0.036 (0.025–0.05) | 0.036 (0.024–0.051) | 0.035 (0.026–0.050) | 0.893 |
The calculated treatment effects after vitamin D supplementation are depicted in Table 2. We observed significant treatment effects for all included vitamin-D-related parameters. For 25(OH)D3, the treatment effect was 32 nmol/L (95% CI: 26 to 39; p < 0.001), for 1,25(OH)2D 26 pmol/L (9 to 42; p = 0.003), for 24,25(OH)2D3 3.3 nmol/L (2.7 to 3.9; p < 0.001), for the VMR 0.015 (nmol/L)/(nmol/L) (0.010–0.019; p < 0.001), for calculated free 25(OH)D3 12 pmol/L (6 to 18; p < 0.001), for calculated bioavailable 25(OH)D3 4.66 nmol/L (2.63 to 6.68; p < 0.001), for the 1,25(OH)2D/25(OH)D3 ratio −0.0010 (nmol/L)/(nmol/L) (−0.0013 to −0.0006; p < 0.001) and for the 1,25(OH)2D/24,25(OH)2D3 ratio −0.020 (nmol/L)/(nmol/L) (−0.026 to −0.015; p < 0.001). In the subgroup of subjects with 25(OH)D3 levels below 50 nmol/L, the treatment effects and p-values were comparable for all parameters.
Table 2.
Analysis of covariance (ANCOVA) analysis for the effect of vitamin D or placebo treatment on vitamin-D-related parameters.
| Parameter | Group | Baseline | Follow-up | Treatment Effect (95% CI) | p-value |
|---|---|---|---|---|---|
| 25(OH)D3 (nmol/L) | Placebo, n=54 | 46 ± 19 | 45 ± 20 | 32 (26 to 39) | <0.001 |
| Vitamin D, n=52 | 49 ± 18 | 79 ± 19 | |||
| 1,25(OH)2D (pmol/L) | Placebo, n=52 | 118 ± 52 | 114 ± 39 | 26 (9 to 42) | 0.003 |
| Vitamin D, n=52 | 133 ± 52 | 150 ± 63 | |||
| 24,25(OH)2D3 (nmol/L) | Placebo, n=54 | 3.4 ± 1.5 | 3.3 ± 1.8 | 3.3 (2.7 to 3.9) | <0.001 |
| Vitamin D, n=52 | 3.6 ± 1.6 | 6.8 ± 1.7 | |||
| VMR | Placebo, n=54 | 0.072 ± 0.018 | 0.071 ± 0.017 | 0.015 (0.010 to 0.020) | <0.001 |
| Vitamin D, n=52 | 0.073 ± 0.017 | 0.087 ± 0.018 | |||
| Calculated free 25(OH)D3 (pmol/L)* | Placebo, n=53 | 12 (8–21) | 12 (8–18) | 12 (6 to 18) | <0.001 |
| Vitamin D, n=51 | 17 (11–20) | 21 (17–31) | |||
| Calculated bioavailable 25(OH)D3 (nmol/L) * | Placebo, n=53 | 5.22 (3.15–8.51) | 4.99 (2.95–6.83) | 4.66 (2.63 to 6.68) | <0.001 |
| Vitamin D, n=51 | 6.60 (4.10–8.02) | 8.69 (6.58–12.51) | |||
| 1,25(OH)2D/ 25(OH)D3 * | Placebo, n=52 | 0.0027 (0.0018–0.0039) | 0.0026 (0.0019–0.0036) | −0.0010 (−0.0013 to −0.0006) | <0.001 |
| Vitamin D, n=52 | 0.0028 (0.0021–0.0035) | 0.0019 (0.0014–0.0026) | |||
| 1,25(OH)2D /24,25(OH)2D3 * | Placebo, n=52 | 0.036 (0.024–0.051) | 0.037 (0.026–0.052) | −0.020 (−0.026 to −-0.015) | <0.001 |
| Vitamin D, n=52 | 0.035 (0.026–0.050) | 0.022 (0.016–0.028) |
* Log-transformed parameters.
The overall correlation between 25(OH)D3 and 24,25(OH)2D3 at baseline was r = 0.815, p < 0.001. Results of the regression analyses of the Δ25(OH)D3 in the vitamin-D-supplemented group are shown in Figure 2. The slope of the linear regression, p-values and R2 values are highly similar for baseline 25(OH)D3 and 24,25(OH)2D3. The VMR, however, could not predict the increase in 25(OH)D3 concentration. The results of the correlation analyses in the vitamin-D-treated group are summarized in Table 3. None of the vitamin-D-related parameters correlated significantly with Δ25(OH)D3 or Δ1,25(OH)2D after Bonferroni correction. Also, in the subgroup of subjects with 25(OH)D levels below 50 nmol/L, none of the parameters correlated significantly with Δ25(OH)D3 or Δ1,25(OH)2D after Bonferroni correction. For Δ25(OH)D3, a trend was seen for baseline 25(OH)D3 and baseline 24,25(OH)2D3 (r = −0.388, p = 0.056 and r = −0.374, p = 0.056). This trend with Δ25(OH)D3 was also observed for calculated free 25(OH)D3 and calculated bioavailable 25(OH)D3 (r = −0.373, p = 0.056 and r = −0.375, p = 0.056). Δ24,25(OH)2D3 was significantly associated with baseline 25(OH)D3, 24,25(OH)2D3, calculated free 25(OH)D3 and calculated bioavailable 25(OH)D3 (r = −0.562, p < 0.001; r = −0.476, p = 0.003; r = −0.382, p = 0.048 and r = −0.393, p = 0.032, respectively), but not with other parameters. In the subgroup of subjects with 25(OH)D3 levels below 50 nmol/L, none of the parameters correlated significantly with Δ24,25(OH)2D3 after Bonferroni correction.
Figure 2.
Univariate linear regression analysis for the change in 25(OH)D3 concentration in the vitamin D intervention group and (a) baseline 25(OH)D3, (b) baseline 24,25(OH)2D3 and (c) baseline VMR (Vitamin D Metabolite Ratio).
Table 3.
Pearson correlations with unadjusted p-values and Bonferroni adjusted p-values of baseline vitamin-D-related parameters with the changes from baseline of 25(OH)D3, 1,25(OH)2D and 24,25(OH)2D3 after vitamin D supplementation.
| Baseline Parameters | Δ25(OH)D3 | Δ1,25(OH)2D | Δ24,25(OH)2D3 | |
|---|---|---|---|---|
| 25(OH)D3 | r | −0.388 | −0.142 | −0.562 |
| p-value | 0.005 | 0.322 | <0.001 | |
| Adjusted p-value | 0.056 | 1.000 | <0.001 | |
| 1,25(OH)2D | r | −0.287 | −0.260 | −0.272 |
| p-value | 0.041 | 0.065 | 0.053 | |
| Adjusted p-value | 0.328 | 0.520 | 0.424 | |
| 24,25(OH)2D3 | r | −0.374 | −0.122 | −0.476 |
| p-value | 0.007 | 0.392 | <0.001 | |
| Adjusted p-value | 0.056 | 1.000 | 0.003 | |
| VMR | r | −0.109 | −0.027 | −0.015 |
| p-value | 0.448 | 0.850 | 0.916 | |
| Adjusted p-value | 1.000 | 1.000 | 1.000 | |
| Calculated free 25(OH)D3 * | r | −0.373 | −0.281 | −0.382 |
| p-value | 0.007 | 0.046 | 0.006 | |
| Adjusted p-value | 0.056 | 0.368 | 0.048 | |
| Calculated bioavailable 25(OH)D3 * | r | −0.375 | −0.280 | −0.393 |
| p-value | 0.007 | 0.047 | 0.004 | |
| Adjusted p-value | 0.056 | 0.376 | 0.032 | |
| 1,25(OH)2D/25(OH)D3 * | r | −0.004 | −0.058 | 0.176 |
| p-value | 0.980 | 0.687 | 0.216 | |
| Adjusted p-value | 1.000 | 1.000 | 1.000 | |
| 1,25(OH)2D /24,25(OH)2D3 * | r | 0.053 | −0.028 | 0.181 |
| p-value | 0.711 | 0.843 | 0.204 | |
| Adjusted p-value | 1.000 | 1.000 | 1.000 | |
* Log-transformed parameters.
Correlation analyses after adjustment for gender, age, BMI, PTH, eGFR, serum phosphate and serum calcium showed that none of the vitamin-D-related parameters were significantly associated with Δ25(OH)D3 or Δ1,25(OH)2D after Bonferroni correction (Table A1). However, when corrected for the above-mentioned parameters, only baseline 25(OH)D3 was still significantly associated with Δ24,25(OH)2D3 (r = −0.657, p = 0.008). In the subgroup of subjects with 25(OH)D3 levels below 50 nmol/L, none of the parameters correlated significantly with Δ25(OH)D3, Δ1,25(OH)2D or Δ24,25(OH)2D3 after Bonferroni correction.
4. Discussion
The goal of our study was to assess whether vitamin D metabolites can predict the increase of 25(OH)D after vitamin D supplementation. As elaborated above, CYP24A1 activity (24-hydroxylase) is reflected by the ratio of 24,25(OH)2D over 25(OH)D, i.e. the VMR. In addition, the ratio between 1,25(OH)2D and 24,25(OH)2D3 was recently proposed as part of a three-dimensional model for assessing vitamin D metabolic pathways [16]. It was previously suggested that vitamin D metabolites and their ratios could provide additional information for predicting vitamin D treatment response [8,9]. The findings in this vitamin D RC.T. in patients with 25(OH)D levels <75 nmol/L and hypertension do not support this hypothesis.
In our study, the VMR did not predict Δ25(OH)D3 in the treatment arm of the RC.T.. In a regression model, baseline 24,25(OH)2D3 and baseline 25(OH)D3 did, with comparable strength and magnitude, predict the increase in 25(OH)D3 upon treatment. When adjusting for multiple testing in correlation analyses, no correlations of any of the included parameters with Δ25(OH)D3 retained significance. Yet, we did observe trends for Δ25(OH)D3 with baseline 25(OH)D3, 24,25(OH)2D3, free 25(OH)D3 and bioavailable 25(OH)D3. Notwithstanding their borderline significance, the strength of the correlations is highly similar between these parameters and they do not seem to be superior to baseline 25(OH)D. According to these data, we can infer that CYP24A1 activity, measured by the VMR, does not predict the individual differences in the increase in 25(OH)D after vitamin D supplementation.
Concerning the VMR, the results of this study are in accordance with several other published reports. Saleh et al. performed an RC.T. of 4 weeks with 107 participants receiving a single 100,000 IU dose of vitamin D or placebo [11]. The VMR could not predict the increase of 25(OH)D after 4 weeks, whereas 25(OH)D did predict this increase with a similar R2-value to our data. However, their data indicated that 24,25(OH)2D3 could not predict the Δ25(OH)D3, whereas in our study it did. Aloia et al. reported on the predictive properties of the VMR in four different small samples (between 14 and 16 participants per group) of placebo or 800, 2000 or 4000 IU vitamin D daily for 10 weeks [6]. They did not show an advantage of the VMR as a predictor, compared to baseline 25(OH)D, 24,25(OH)2D3 or free 25(OH)D. Binkley et al. investigated the effect of 1800IU of vitamin D in 62 postmenopausal women after 4 months and measured vitamin D metabolites [10]. They observed that neither VMR, 25(OH)D, 24,25(OH)2D3 nor free 25(OH)D was related to the observed increase in 25(OH)D.
On the contrary, other published studies did suggest a predictive role for the VMR. The study by Wagner et al. included young adults with a mean age of around 27 years that received 28,000 IU (equivalent to 4000 IU per day) of vitamin D once per week for 8 weeks in the form of a supplement or fortified cheese. Wagner et al. showed that the VMR predicted the increase in vitamin-D-receiving subjects (R2 = −0.38, p = 0.004, n = 60) [8]. Also, Cashman et al. reported a significant correlation between the VMR and the change after vitamin D supplementation (R2 = 0.15, p < 0.01) in a study including subjects above 50 years of age that were treated for 15 weeks by 20 µg vitamin D (800 IU) per day [9]. Of note, both studies did not report the R2-value of baseline 25(OH)D with its increase after supplementation. Therefore, it is not possible to conclude whether the VMR was superior to 25(OH)D in this aspect.
Changes in other vitamin-D-related parameters after vitamin D treatment were also studied. To that end, we assessed if Δ1,25(OH)2D and Δ24,25(OH)2D3 could be predicted by baseline parameters included in the study. We found no correlation between any tested baseline parameter and Δ1,25(OH)2D. 1,25(OH)2D levels are mainly regulated by calcium levels, which could explain this observation [12]. On the other hand, baseline 25(OH)D3, 24,25(OH)2D3, calculated free 25(OH)D3 and calculated bioavailable 25(OH)D3 all showed a significant correlation with Δ24,25(OH)2D3. The clinical relevance of this observation is, in our opinion, unclear and should be further studied.
In our study, we observed an increase in the VMR upon vitamin D treatment. This suggests an increase in CYP24A1 activity and catabolism of 25(OH)D upon supplementation. A concurrent decrease in the 1,25(OH)2D/25(OH)D3 ratio implies a reduced conversion of 25(OH)D to 1,25(OH)2D. Indeed, this suggests the physiological shift from anabolic to catabolic pathways when an excess of vitamin D exists. This is also supported by the significant decrease in the 1,25(OH)2D/24,25(OH)2D3 ratio. In the present study, and all aforementioned studies, the correlation coefficients between baseline 25(OH)D and Δ25(OH)D3 after supplementation were negative, which implies that the change in 25(OH)D3 after vitamin D treatment is smaller in individuals with higher baseline 25(OH)D3 levels [8,9,10].
We acknowledge that this study has several limitations. First, the results are derived from post-hoc analyses. Second, the study population consisted of hypertensive subjects with 25(OH)D levels <75 nmol/L; therefore, the findings might not be readily extrapolated to the general population. Furthermore, for the vitamin D level inclusion criterion, the 25(OH)D concentrations were measured at study baseline using a chemiluminescence assay, while mass-spectrometry-based methods are currently the gold standard [2]. However, for the current study, 25(OH)D and 24,25(OH)2D were re-measured using a dedicated LC-MS/MS method. In addition, the intervention period of 8 weeks was relatively short and only a small number of subjects were severely vitamin D deficient. Vitamin D deficiency was defined as a 25(OH)D of <75 nmol/L in the original study by Pilz et al. [13]. There is still an ongoing debate as to whether the cut-off levels should be set at <50 nmol/L or <75 nmol/L [17,18]. In addition, vitamin D sufficiency was defined by measurements of baseline 25(OH)D3, which is currently the critical measurement for defining vitamin D status [19]. Some studies suggest that free 25(OH)D3 could be a better marker for assessing vitamin D status [20]. In our study, calculated free 25(OH)D3 did not predict Δ25(OH)D3 after supplementation better than baseline 25(OH)D3. The RC.T. design and the successful vitamin D intervention are strengths of this study. Also, a high number of parameters were measured with gold-standard methods. In contrast to the majority of exploratory studies on the VMR, p-values of the correlations were adjusted for multiple testing.
In summary, we show that 25(OH)D3, 24,25(OH)2D3 and the VMR increase after vitamin D treatment. However, 24,25(OH)2D3 and the VMR could not predict 25(OH)D3 levels after vitamin D treatment in this cohort better than baseline 25(OH)D3. As this has been corroborated by other studies, it implicates the routine measurement of 24,25(OH)2D3 will probably be of no added value when personalizing the treatment dosage of vitamin D.
Acknowledgments
The authors thank all study participants and also Fresenius Kabi for providing the study medication.
Appendix A
Table A1.
Pearson correlations of baseline vitamin-D-related parameters adjusted for gender, age, BMI, PTH, eGFR, serum phosphate and serum calcium, with the changes from baseline of 25(OH)D, 1,25(OH)2D and 24,25(OH)2D after vitamin D supplementation. p-values without and with Bonferroni adjustment are shown.
| Baseline Parameters | Δ25(OH)D3 | Δ1,25(OH)2D | Δ24,25(OH)2D3 | |
|---|---|---|---|---|
| 25(OH)D3 | r | −0.508 | −0.277 | −0.657 |
| p-value | 0.013 | 0.201 | 0.001 | |
| Adjusted p-value | 0.104 | 1.000 | 0.008 | |
| 1,25(OH)2D | r | −0.350 | −0.171 | −0.430 |
| p-value | 0.102 | 0.435 | 0.040 | |
| Adjusted p-value | 0.816 | 1.000 | 0.320 | |
| 24,25(OH)2D3 | r | −0.490 | −0.129 | −0.597 |
| p-value | 0.018 | 0.559 | 0.003 | |
| Adjusted p-value | 0.440 | 1.000 | 0.096 | |
| VMR | r | −0.064 | 0.137 | −0.516 |
| p-value | 0.773 | 0.534 | 0.012 | |
| Adjusted p-value | 1.000 | 1.000 | 0.096 | |
| Calculated free 25(OH)D3 * | r | −0.451 | −0.363 | −0.399 |
| p-value | 0.031 | 0.089 | 0.059 | |
| Adjusted p-value | 0.248 | 0.712 | 0.472 | |
| Calculated bioavailable 25(OH)D3 * | r | −0.451 | −0.363 | −0.404 |
| p-value | 0.031 | 0.089 | 0.056 | |
| Adjusted p-value | 0.248 | 0.712 | 0.448 | |
| 1,25(OH)2D/25(OH)D3 * | r | 0.122 | 0.272 | 0.218 |
| p-value | 0.578 | 0.209 | 0.318 | |
| Adjusted p-value | 1.000 | 1.000 | 1.000 | |
| 1,25(OH)2D /24,25(OH)2D3 * | r | 0.126 | 0.136 | 0.211 |
| p-value | 0.565 | 0.536 | 0.333 | |
| Adjusted p-value | 1.000 | 1.000 | 1.000 | |
* Log-transformed parameters.
Author Contributions
Conceptualization, V.F., S.R.U., B.O.P., A.C.H.; methodology, N.F.D.; software, V.F., S.R.U., S.P., A.C.H., B.O.P., W.M., A.T.; validation, A.C.H., B.O.P.; formal analysis, V.F. and S.R.U..; investigation, M.H.K., V.T., C.T., M.P., V.B., M.R.G., N.D.V.; resources, S.P., A.C.H., B.O.P.; data curation, S.P., A.C.H., B.O.P.; writing—original draft preparation, V.F., S.R.U..; writing—review and editing, V.F., S.R.U., S.P., B.O.P., A.C.H., N.F.D.; visualization, V.F., S.R.U.; supervision, S.P., B.O.P., A.C.H.; project administration, S.P., A.C.H., B.O.P.; funding acquisition, n.a.
Funding
The Styrian Vitamin D Hypertension Trial was supported by funding from the Austrian National Bank (Jubilaeumsfond: project no.: 13878 and 13905).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bergwitz C., Jüppner H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuckey R.C., Cheng C.Y.S., Slominski A.T. The Serum Vitamin D Metabolome: What We Know and What is Still to Discover. J. Steroid Biochem. Mol. Biol. 2019;186:4–21. doi: 10.1016/j.jsbmb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prietl B., Treiber G., Pieber T.R., Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones G., Strugnell S.A., DeLuca H.F. Current Understanding of the Molecular Actions of Vitamin D. Physiol. Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 5.Dirks N., Ackermans M., Lips P., de Jongh R., Vervloet M., de Jonge R., Heijboer A. The When, What & How of Measuring Vitamin D Metabolism in Clinical Medicine. Nutrients. 2018;10:482. doi: 10.3390/nu10040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aloia J., Fazzari M., Shieh A., Dhaliwal R., Mikhail M., Hoofnagle A.N., Ragolia L. The vitamin D metabolite ratio (VMR) as a predictor of functional biomarkers of bone health. Clin. Endocrinol. (Oxf.) 2017;86:674–679. doi: 10.1111/cen.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginsberg C., Katz R., de Boer I.H., Kestenbaum B.R., Chonchol M., Shlipak M.G., Sarnak M.J., Hoofnagle A.N., Rifkin D.E., Garimella P.S., et al. The 24,25 to 25-hydroxyvitamin D Ratio and Fracture Risk in Older Adults: The Cardiovascular Health Study. Bone. 2018;107:124–130. doi: 10.1016/j.bone.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D., Hanwell H.E., Schnabl K., Yazdanpanah M., Kimball S., Fu L., Sidhom G., Rousseau D., Cole D.E.C., Vieth R. The Ratio of Serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is Predictive of 25-hydroxyvitamin D3 Response to Vitamin D3 Supplementation. J. Steroid Biochem. Mol. Biol. 2011;126:72–77. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Cashman K.D., Hayes A., Galvin K., Merkel J., Jones G., Kaufmann M., Hoofnagle A.N., Carter G.D., Durazo-Arvizu R.A., Sempos C.T. Significance of Serum 24,25-Dihydroxyvitamin D in the Assessment of Vitamin D Status: A Double-edged Sword? Clin. Chem. 2015;61:636–645. doi: 10.1373/clinchem.2014.234955. [DOI] [PubMed] [Google Scholar]
- 10.Binkley N., Borchardt G., Siglinsky E., Krueger D. Does Vitamin D Metabolite Measurement Help Predict 25(OH)D Change Following Vitamin D Supplementation? Endocr. Pract. 2017;23:432–441. doi: 10.4158/EP161517.OR. [DOI] [PubMed] [Google Scholar]
- 11.Saleh L., Tang J., Gawinecka J., Boesch L., Fraser W.D., von Eckardstein A., Nowak A. Impact of a Single Oral Dose of 100,000 IU Vitamin D3 on Profiles of Serum 25(OH)D3 and its Metabolites 24,25(OH)2D3, 3-epi-25(OH)D3, and 1,25(OH)2D3 in Adults with Vitamin D Insufficiency. Clin. Chem. Lab. Med. 2017;55:1912–1921. doi: 10.1515/cclm-2016-1129. [DOI] [PubMed] [Google Scholar]
- 12.Mazahery H., von Hurst P. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients. 2015;7:5111–5142. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilz S., Gaksch M., Kienreich K., Grübler M., Verheyen N., Fahrleitner-Pammer A., Treiber G., Drechsler C., Hartaigh B.Ó., Obermayer-Pietsch B., et al. Effects of Vitamin D on Blood Pressure and Cardiovascular Risk Factors. Hypertension. 2015;65:1195–1201. doi: 10.1161/HYPERTENSIONAHA.115.05319. [DOI] [PubMed] [Google Scholar]
- 14.Dirks N.F., Ackermans M.T., de Jonge R., Heijboer A.C. Reference Values for 24,25-dihydroxyvitamin D and the 25-hydroxyvitamin D/24,25-dihydroxyvitamin D Ratio. Clin. Chem. Lab. Med. 2019;25:24–26. doi: 10.1515/cclm-2018-1096. [DOI] [PubMed] [Google Scholar]
- 15.Powe C.E., Ricciardi C., Berg A.H., Erdenesanaa D., Collerone G., Ankers E., Wenger J., Karumanchi S.A., Thadhani R., Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J.C.Y., Jackson S., Walsh N.P., Greeves J., Fraser W.D. Bioanalytical Facility team The Dynamic Relationships Between the Active and Catabolic Vitamin D Metabolites, their ratios, and Associations with PTH. Sci. Rep. 2019;9:6974. doi: 10.1038/s41598-019-43462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilz S., Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Keppel M.H., Grübler M.R., März W., Pandis M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019;8:R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Sempos C.T., Heijboer A.C., Bikle D.D., Bollerslev J., Bouillon R., Brannon P.M., DeLuca H.F., Jones G., Munns C.F., Bilezikian J.P., et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuprykov O., Chen X., Hocher C.-F., Skoblo R., Yin L., Hocher B. Why should we measure free 25(OH) vitamin D? J. Steroid Biochem. Mol. Biol. 2018;180:87–104. doi: 10.1016/j.jsbmb.2017.11.014. [DOI] [PubMed] [Google Scholar]