Abstract
Background: Plants belonging to the genus Kaempferia (family: Zingiberaceae) are distributed in Asia, especially in the southeast region, and Thailand. They have been widely used in traditional medicines to cure metabolic disorders, inflammation, urinary tract infections, fevers, coughs, hypertension, erectile dysfunction, abdominal and gastrointestinal ailments, asthma, wounds, rheumatism, epilepsy, and skin diseases. Objective: Herein, we reported a comprehensive review, including the traditional applications, biological and pharmacological advances, and phytochemical constituents of Kaempheria species from 1972 up to early 2019. Materials and methods: All the information and reported studies concerning Kaempheria plants were summarized from library and digital databases (e.g., Google Scholar, Sci-finder, PubMed, Springer, Elsevier, MDPI, Web of Science, etc.). The correlation between the Kaempheria species was evaluated via principal component analysis (PCA) and agglomerative hierarchical clustering (AHC), based on the main chemical classes of compounds. Results: Approximately 141 chemical constituents have been isolated and reported from Kaempferia species, such as isopimarane, abietane, labdane and clerodane diterpenoids, flavonoids, phenolic acids, phenyl-heptanoids, curcuminoids, tetrahydropyrano-phenolic, and steroids. A probable biosynthesis pathway for the isopimaradiene skeleton is illustrated. In addition, 15 main documented components of volatile oils of Kaempheria were summarized. Biological activities including anticancer, anti-inflammatory, antimicrobial, anticholinesterase, antioxidant, anti-obesity-induced dermatopathy, wound healing, neuroprotective, anti-allergenic, and anti-nociceptive were demonstrated. Conclusions: Up to date, significant advances in phytochemical and pharmacological studies of different Kaempheria species have been witnessed. So, the traditional uses of these plants have been clarified via modern in vitro and in vivo biological studies. In addition, these traditional uses and reported biological results could be correlated via the chemical characterization of these plants. All these data will support the biologists in the elucidation of the biological mechanisms of these plants.
Keywords: Kaempferia, traditional medicine, diterpenoids, flavonoids, phenolic, biosynthesis
1. Introduction
From the first known civilization, medicinal plants have met primary care and health needs around the world [1,2,3]. Natural products, derived from plants, have enriched the pharmaceutical industry since time immemorial. So far, people of the developing countries depend upon the traditional medicines to cure daily aliments [4]. The medicinal plants are characterized by a diversity of chemical and pharmacological constituents, owing to their complicity and the abundance of secondary metabolites. There are several factors that caused the variations of the secondary metabolites such as ecological zones, weather, climates, and other natural factors via the effects on the biosynthetic pathways [1,2,3].
Zingiberaceae (the ginger family) is distributed worldwide comprising 52 genera and more than 1300 plant species [5,6]. Kaempferia is a diverse family with members distributed widely throughout Southeast Asia and Thailand, including some 60 species [5]. Several Kaempferia species are used widely in folk medicine, including K. parviflora, K. pulchra, and K. galanga, (Figure 1). In Laos and Thai, traditional medicines derived from K. parviflora rhizomes are reported for the treatment of inflammation, hypertension, erectile dysfunction, abdominal ailments [6,7], and improvement of the vitality and blood flow [8]. Japanese use the extract of K. parviflora as a food supplement and for the treatment of metabolic disorders [9]. K. pulchra is used extensively as a carminative, diuretic, deodorant, and euglycemic, as well as for the treatment of urinary tract infections, fevers, and coughs [4]. The rhizomes of K. galanga are used as an anti-tussive, expectorant, anti-pyretic, diuretic, anabolic, and carminative, as well as for the curing of gastrointestinal ailments, asthma, wounds, rheumatism, epilepsy, and skin diseases [10].
Figure 1.
Traditional medicinal used Kaempheria species.
Extracts and purified compounds from select Kaempferia species are used for the treatment of knee osteoarthritis and the inhibition of a breast cancer resistance protein (BCRP), anti-inflammatory, anti-acne, anticholinesterase, anti-obesity-induced dermatopathy, wound healing, anti-drug resistant strains of Mycobacterium tuberculosis, neuroprotective, anti-nociceptive, human immunodeficiency virus type-1 (HIV-1) inhibitory activity, in vitro anti-allergenic, and larvicidal activity against Aedes aegypti [4,6,7,8,9,10,11]. The scientific literature such as, Google Scholar, Scifinder, PubMed, Springer, Elsevier, Wiley, Web of Science, were screened in the period between 1972–2019 in order to collect the up-to-date information of the traditional uses/applications, biological studies, and chemical characterization of Kaempheria species. All these collected data were addressed and summarized in our review article to highlight the potential ethnopharmacological importance of these plants.
2. Materials and Methods
The scientific literature such as Google Scholar, Scifinder, PubMed, Springer, Elsevier, Wiley, Web of Science, etc., including all the traditional uses/applications, biological studies, and chemical characterization of Kaempheria species were collected between 1972–2019. All these collected data were adjusted and summarized in our review article due to the potential ethnopharmacological importance of these plants.
The correlation between the Kaempheria species was evaluated based on the main chemical classes of compounds. The data matrix of seven Kaempferia species (K. angustifolia, K. elegans, K. galanga, K. marginata, K. parviflora, K. pulchra, and K. roscoeana) and six chemical classes (abietanes, labdanes and clerodanes, flavonoids, phenolic compounds, and chalcones) were subjected to principal component analysis (PCA) to identify correlation between different Kaempferia species. In addition, the similarity based on the Pearson correlation coefficient was determined via subjecting the dataset to an agglomerative hierarchical cluster (AHC). The PCA and AHC were performed using an XLSTAT statistical computer software package (version 2018, Addinsoft, NY, USA, www.xlstat.com).
3. Distribution
Zingiberaceae (the ginger family) comprises 52 genera and more than 1300 plant species. Kaempferia is distributed worldwide with diverse members occurring throughout southeast tropical Asian countries such as Indonesia, India, Malaysia, Myanmar, Cambodia, and China, as well as Thailand, including some 60 species [5]. K. pulchra is a perennial herbal plant and widely cultivated in numerous tropical countries, involving Indonesia, Malaysia, Myanmar, and Thailand [12].
4. Traditional Uses
Several Kaempferia species are used widely in folk medicine, including K. parviflora, K. pulchra, and K. galanga (Figure 1). In Laos and Thai, traditional medicines derived from K. parviflora rhizomes are reported for the treatment of inflammation, hypertension, erectile dysfunction, abdominal ailments [6,7], and improvement of the vitality and blood flow [8]. Japanese folk medicine documented a positive effect of K. parviflora extract when used as a food supplement and for the treatment of metabolic disorders [9]. K. pulchra is used extensively as a carminative, diuretic, deodorant, and euglycemic, as well as for the treatment of urinary tract infections, fevers, and coughs [4]. K. galanga is sold as an industrial crop in the market, and its rhizome has been used as a flavor spice of various cooking [13]. The rhizomes of K. galanga is used as an anti-tussive, expectorant, anti-pyretic, diuretic, anabolic, carminative, as well as for curing of gastrointestinal ailments, asthma, wounds, rheumatism, epilepsy, and skin diseases [10]. In Malaysian folk medicines, several gingers belonging to the Zingiberaceae family especially, Kaempheria genus, are used in the treatment of several diseases such as stomach ailments, vomiting, cough, bruises, epilepsy, nausea, rheumatism, sore throat, wounds, eyewash, sore eyes, childbirth, liver complaints, muscular pains, ringworm, asthma, fever, malignancies, swelling, and several other disorders [14].
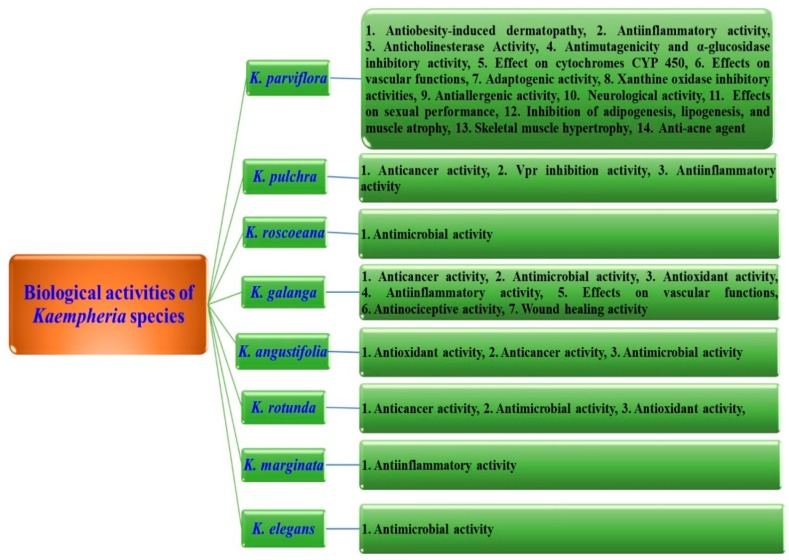
5. Biological Activity
Extracts and purified compounds of Kaempferia species are used for the treatment of knee osteoarthritis and the inhibition of a breast cancer resistance protein (BCRP), anti-inflammatory, anti-acne, anticholinesterase, anti-obesity-induced dermatopathy, wound healing, anti-drug resistant strains of Mycobacterium tuberculosis, neuroprotective, anti-nociceptive, human immunodeficiency virus type-1 (HIV-1) inhibitory activity, in vitro anti-allergenic, and larvicidal activity against Aedes aegypti [11]. Kaempheria plant extracts and isolated compounds demonstrate numerous and promising biological and pharmaceutical activities, which are summarized in Figure 2.
Figure 2.
Reported biological activities for Kaempheria species.
5.1. Anticancer Activity
Rhizome ethanolic extracts of K. galanga and the purified component ethyl trans p-methoxycinnamate (105) demonstrate moderate cytotoxic activity against human cholangiocarcinoma (CL-6) cells with IC50 of 64.2 and 49.4 μg mL−1, respectively. Significant cholangiocarcinoma (CCA) efficacy as indicated by suppressing tumor growth and lung metastasis in CL6-xenografed mice [15] is also observed. Swapana et al. [16] documented that K. galanga isopimarene diterpenoids, sandaracopimaradiene-9α-ol (2), kaempulchraol I (14), and kaempulchraol L (17) exhibit promising activity against human lung cancer with IC50 of 75 µM, 74 µM, and 76 µM, respectively, and mouth squamous cell carcinoma (HSC-2) inhibition with IC50 of 70 µM, 53 µM, and 58 µM, respectively [16]. The latter compound, isolated from K. pulchra, is reported to have weak anti-proliferative activity against human pancreatic and cervix cancers [17]. Chawengrum et al. [18] stated that K. pulchra labdene diterpenoids, (−)-kolavelool (81), and (−)-2β-hydroxykolavelool (82) exhibit cytotoxic activity against human leukemia cells (HL-60) with IC50 values of 9.0 ± 0.66 and 9.6 ± 0.88 μg mL−1, respectively [18]. Acetone, petroleum ether, chloroform, and MeOH extracts of K. galanga rhizomes show moderate cytotoxicity in a brine shrimp lethality bioassay compared with vincristine sulfate as the reference compound [19]. Moreover, a methanolic extract of K. galanga rhizomes induces Ehrlich ascites carcinoma (EAC) cell death in a dose-dependent manner [20]. 5,7-Dimethoxyflavone (86) isolated from K. galanga was found to reduce cancer resistance to tyrosine kinase inhibitors (TKI) by inhibiting breast cancer resistance protein (BCRP), one of the efflux transporters that increased efflux of TKI out of cancer cells. This was observed both in vitro with a dose-dependent increase in the intracellular concentration of sorafenib in MDCK/BCRP1 breast cancer resistance cells, with an EC50 of 8.78 μM as well as in vivo by increasing sorafenib AUC in mice tissues when co-administered with compound 88, as reported by kinetic results [21]. The isolated methyl-β-D-galactopyranoside specific lectin from the rhizome of K. rotunda exhibited in vitro antitumor activity against Ehrlich ascites carcinoma cells at a pH between 6–9 and a temperature range between 30–80 °C. Tumor inhibition was also observed in vivo in EAC-bearing mice [22].
The cytotoxicity of MeOH, petroleum ether, and EtOAc extracts against C33A cancer cells via MTT and scratch assays compared with essential oils of K. galanga rhizomes showed activity for the EtOAc and MeOH fractions at 1000 μg mL−1 with 11% and 14% cell viability and weak efficacy with petroleum ether extracted essential oils in a MTT assay. Cell growth inhibition was observed with all extracts in the scratch assay [23]. Compound (140) isolated from K. angustifolia was described to have strong activity with an IC50 of 1.4 µg mL−1, which was comparable to 5-fluorouracil as a reference drug. Compound (138) also showed moderate inhibition against human lung cancer. 2′-Hydroxy-4,4′,6′-trimethoxychalcone (flavokawain A; 119) exhibited potent activity against HL-60 and MCF-7 cell lines. The results of Tang et al. [24] revealed that flavokawain A (119) exhibited cytotoxic activity against MCF-7 and HT-29 cell lines with GI50 values of 17.5 µM (5.5 µg mL−1) and 45.3 μM (14.2 µg mL−1), respectively. Kaempfolienol (65) and zeylenol (133) were also found to have moderate activity against HL-60 and MCF-7 cells with IC50 values <30 µg mL−1 and against HL-60 only with an IC50 value of 11.6 µg mL−1 respectively [24].
5.2. Anti-Obesity Activity
An ethanolic extract, a polymethoxyflavonoid-rich fraction (PMF) and a polymethoxyflavonoid-poor fraction from K. parviflora were screened against an obesity-induced dermatopathy system using Tsumura Suzuki obese diabetes (TSOD) mice as an obesity model (Hidaka, Horikawa, Akase, Makihara, Ogami, Tomozawa, Tsubata, Ibuki, and Matsumoto) [11]. The ethanolic extract reduced mouse body weight and the thickness of the subcutaneous fat layer more than the PMF fraction that is used as a dietary supplement in controlling skin disorders caused by obesity [11].
5.3. Anti-HIV Activity
Viral protein R (Vpr) is one of the HIV accessory proteins that can be targeted for controlling viral replication and pathogenesis. A CHCl3 fraction of K. pulchra exhibits Vpr-inhibitory activity at 25l g mL−1. In addition, isopimarene type diterpenoids isolated from the rhizomes of the plants, kaempulchraol B (43), kaempulchraol D (45), kaempulchraol G (46), kaempulchraol Q (20), kaempulchraol T (36), kaempulchraol U (50), and W (22) inhibit the expression of Vpr at concentrations from 1.56 to 6.25 µM [25].
5.4. Antimicrobial Activity
Arabietatriene (62) isolated from K. roscoeana exhibits antibacterial activity against Gram-positive bacteria Staphylococcus epidermidis and Bacillus cereus [26]. Anticopalic acid (72), anticopalol (77), and 8(17)-labden-15-ol (68) isolated from K. elegans also exhibited antibacterial activity against B. cereus [18]. Acetone, petroleum ether, chloroform, and MeOH extracts of K. galanga rhizomes exhibit moderate antibacterial activity against Gram-positive and Gram-negative bacteria in comparison with ciprofloxacin [19]. Ethyl p-methoxycinnamate (105) also isolated from K. galanga rhizomes have been shown based on a resazurin micro-titer assay to inhibit Mycobacterium tuberculosis H37Ra, H37Rv, multidrug-resistant, and drug-susceptible isolates with MIC 0.242–0.485 mM [27]. Its essential oil also displays strong antibacterial activity against Staphylococcus aureus and Salmonella typhimurium, and weak activity against Escherichia coli [28]. Moreover, essential oils extracted from three varieties of K. galanga exhibited potent larvicidal activity [29]. An ethyl acetate extract of K. rotunda inhibits S. aureus and E. coli [30]. A rhizomes extract of K. galanga inhibits Epstein–Barr virus with no cytotoxic effect in Raji cells [14]. In contrast, isolated diterpenoids from K. roscoeana exhibited no activity against Plasmodium falciparum (Chloroquine-resistant) [26]. Fauziyah et al. [31] described that an ethanolic extract of K. galanga alone exhibits 100% growth inhibition of the multi-drug resistant (MDR) Mycobacterium tuberculosis (isolates at 500 µg mL−1). However, a combination of this extract with streptomycin, ethambutol, and isoniazid showed inhibition values of 55%, 76%, and 50%, respectively. Ethanol, methanol, petroleum ether, chloroform, and aqueous extracts of K. galanga rhizome showed antimicrobial activity against human pathogenic bacteria and fungi, while the ethanolic extract exhibited the strongest inhibition of S. aureus using an inhibition zone assay [32]. However, flavokawain A (119) and other compounds reported from K. angustifolia had no antimicrobial activity against tested microbes [24].
5.5. Antioxidant Activity
The CHCl3 and MeOH extracts of the rhizomes of K. angustifolia showed strong antioxidant activity against DPPH expressed with 615.92 mg trolox equivalent (TE)/g of extract. In an azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) assay, MeOH extracts showed good antioxidant properties with a value of 38.87 mg TE/g. However, n-hexane extract exhibited significant antioxidant activity with 901.76 mg TE/g in a cupric-reducing antioxidant capacity assay, while EtOAc extract exhibited significant reduction ability against ferric reducing antioxidant power (FRAP) with a value of 342.23 mg TE/g. Also, kaempfolienol (65) showed potent free radical scavenging activity in a DPPH assay, as well as, 2′-hydroxy-4,4′,6′-trimethoxychalcone (119) in ABTS, CUPRAC, and FRAP assays [33,34]. A methanol extract of rhizomes of K. galanga exhibited a concentration-dependent antioxidant activity in DPPH, ABTS, and nitric oxide (NO) radical scavenging assays [20]. Moreover, the essential oil extracts of conventionally propagated and in vitro propagated K. galanga had significant DPPH radical scavenging activity [35]. As well, the ethanol extract of K. rotunda exhibited antioxidant activity in a DPPH assay with IC50 (67.95 μg mL−1) [30].
5.6. Anti-Inflammatory Activity
The cyclohexane, chloroform, and ethyl acetate extracts with diarylheptanoids isolated from K. galanga showed a pronounced inhibition of Lipopolysaccharides (LPS)-induced nitric oxide in macrophage RAW 264.7 cells compared with indomethacin [13]. The EtOH extract and compounds (1, 52, 53, 119, 120) isolated from K. marginata had promising anti-inflammatory activity based on the suppression of NO production and inducible nitric oxide synthase (iNOS) mRNA and cyclooxygenase-2 (COX-2) genes expression [36,37]. Diterpenoids (9–10) isolated from K. pulchra had topical anti-inflammatory activity in 12-O-tetradecanoylphorbol-13-acetate-induced ear edema in rats with ID50 330 and 50 µg/ear, respectively. Biological activity may be due to the activation of Maxi-K channels in neurons and smooth muscles [38]. The ethanol extract of K. parviflora exhibited potent inhibition of PGE2. The plant extract and 3′,4′,5,7-tetramethoxyflavone (86) were also reported to exhibit a dose-dependent inhibition of iNOS-mRNA expression. Additionally, H2O, EtOH, EtOAC, CHCl3, and n-hexane soluble sub-fractions exhibited good in vivo anti-inflammatory activity by decreasing rat paw edema [39]. An 80% EtOH extract reduced UV-induced COX-2 expression in mice skin that was attributed to the anti-oxidative activity of polyphenolics against the oxidizing properties of UV radiation [40]. A 60% EtOH and EtOAc-soluble fraction of 100% methanol extracts of K. parviflora decreased knee osteoarthritis, which was likely due to methoxylated flavones [41]. Ethyl p-methoxycinnamate (105) isolated from K. galana inhibited cytokines as IL-1 and TNFα and endothelial function in rats [42].
Tewtrakul, et al. [43] found that the isolated methoxylated flavonoids from K. parviflora, 5-hydroxy-3,7,3′,4′-tetramethoxyflavone (96), 5-hydroxy-7,4′-dimethoxyflavone (93), and 5-hydroxy-3,7,4′-trimethoxyflavone (95) exhibited anti-inflammatory activity against the PGE2 production, with IC50 values of 16.1 μM, 24.5 μM, and 30.6 μM, respectively [43]. Tewtrakul and Subhadhirasakul [44] described methoxyflavones 96, 93, and 95 from a hexane extract of K. parviflora rhizomes that exhibited activity against NO release in RAW264.7 cells with IC50 values of 16.1 μM, 24.5 μM, and 30.6 μM, respectively. In addition, 5-hydroxy-3,7,3′,4′-tetramethoxyflavone (96) inhibited PGE2 release with an IC50 value of 16.3 μM, with negative activity on Tumor Necrosis Factor alpha (TNF-α) with IC50 >100 μM [44]. Petroleum ether extract from K. galanga was active against acute inflammation at 300 mg/kg in rats and inhibited the inflammation and MPO levels at 100 mg kg−1 in the chronic model [45].
5.7. Anticholinesterase Activity
According to Sawasdee et al. [46], a MeOH extract as well as compounds (86–87) isolated from K. parviflora rhizomes inhibited acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) with greater cholinesterase inhibitory toward AChE and BChE for (86), which was an observation of significance in the treatment of Alzheimer’s disease [46].
5.8. Anti-Mutagenicity Activity
CH2Cl2 and EtOAc soluble fractions of K. parviflora showed anti-mutagenicity and α-glucosidase inhibitory activity. Isolated methoxylated compounds (86, 97, 84, and 92) from these extracts exhibited potent activity with IC50 values of 0.40, 0.40, 0.42, and 0.47 nmol/plate, respectively. Compounds (88, 87, and 91), also showed significant activity with IC50 values of 20.4 μM, 54.3 μM, and 64.3 μM, respectively [47].
5.9. Effect on Cytochromes CYP 450
The results listed by Ochiai et al. [48] stated that the continued ingestion of (88) isolated from K. parviflora decreases liver CYP3A expression, which in turn increased levels of compounds metabolized by CYP3As such as midazolam [48].
5.10. Vascular Activity
The oral administration of CH2Cl2 extract of K. parviflora in middle-aged rats was found to decrease vascular responses to phenylephrine, increase acetylcholine-induced vasorelaxation and the production of nitric oxide (NO) from blood vessels, and decrease visceral, subcutaneous fat, fasting serum glucose, triglyceride, and liver lipid accumulation [49]. The effect of intravenous administration of a CH2Cl2 extract of K. galanga to rats reduced the mean arterial blood pressure [50]. This anti-hypertensive effect was attributed to ethyl cinnamate, which is a major compound in the extract [50]. The ethanol extract of rhizomes of K. parviflora caused dose-dependent relaxation on aortic rings as well as ileum pre-contracted with phenylephrine and acethylcholine [51].
5.11. Adaptogenic Activity
Hexane, chloroform, methanol, and ethanol extracts of K. parviflora exhibited adaptogenic activity compared with a crude ginseng root powder used as a reference [52]. A single oral dose of K. parviflora rhizome (60% EtOH extract) increased the whole-body potential expenditure in humans [53]. K. parviflora was also found to improvement physical fitness and health by decreasing oxidative stress [54].
5.12. Xanthine Oxidase Inhibitory Activity
Among the isolated methoxylated flavonoids from K. parviflora, (87 and 86) inhibit xanthine oxidase activity with IC50 values of 0.9 and >4 mM, respectively [9].
5.13. Allergenic Activity
Isolated polymethoxyflavones from K. parviflora (86, 97), in addition to CH2Cl2, EtOAc, and H2O extracts, alleviated type I allergy symptoms through suppressing Rat Basophilic Leukemia cells (RBL-2H3) cell degranulation, with (92) and (94) showing the highest anti-allergenic activity [55].
5.14. Neurological Activity
A methanolic extract (95% MeOH) of K. parviflora exhibited neuroprotective activity by increasing rat hippocampus serotonin, norepinephrine, and dopamine levels in comparison with a vehicle-treated group [56]. An acetone extract of K. galanga rhizomes and leaves also exhibited central nervous system depressant activity [57].
5.15. Nociceptive Activity.
A K. galanga rhizome extract exhibited anti-nociceptive activity in rats that was stronger than aspirin but weaker than morphine. The efficacy was abolished by naloxone, suggesting that the analgesic effect may be centrally and peripherally mediated [58].
5.16. Wound-Healing Activity
The co-administration of a K. galanga rhizomes extract (95% EtOH) with dexamethazone was found to have wound-healing activity in mice comparable to dexamethazone only [59].
5.17. Effects on Sexual Performance
Several 7-methoxyflavones (86, 87, 89, 91, 93–95) isolated from K. parviflora rhizomes improved sexual activity in males through the inhibition of PDE5, with 86 being the most potent [60]. The activity was attributed to methoxyls present at positions C5 and C7 [60]. K. parviflora rhizome extracts, standardized to 5% DMF, also improve erectile function in healthy men [61]. A K. parviflora extract as well as 5,7-dimethoxyflavones augment testosterone production, which decreases age-related diseases and hypogonadism [62]. Improved testosterone levels, sperm count, and sexual performance was observed in streptozotocin (STZ)-induced diabetic rats when treated with a K. parviflora extract (aqueous with 1% Tween-80) [63].
5.18. Miscellaneous
The rhizome extract (95% ethanolic) of K. parviflora reduced obesity via the inhibition of adipogenesis, lipogenesis, and muscle atrophy in mice [64]. In contrast, the K. parviflora derivatives of 5-hydroxy-7-methoxyflavone induce skeletal muscle hypertrophy [65]. A K. parviflora extract (95% EtOH) served as a potential anti-acne agent with anti-inflammatory, sebostatic, and anti-propioni bacteria activity [66].
Recently, K. parviflora alcoholic extract at 3–30 µg mL−1 was evaluated regarding the molecular mechanisms associated with rheumatoid arthritis for up to 72 h compared with the dexamethasone as positive control [67]. They documented that the EtOH extract significantly decreased the gene expression levels of pro-inflammatory cytokines, inflammatory mediators, and matrix-degraded enzymes, but neither induced apoptosis nor altered the cell cycle. They also reported that the alcoholic extract inhibits cell migration, reduces the mRNA expression of cadherin-11, and selectively reduces the phosphorylation of mitogen-activated protein kinases (P38, MAPKs), signal transducers, and activators of transcription 1 (STAT1) and 3 (STAT3) signaling molecules, without interfering with the NF-κB pathway [67].
A K. galanga extract (acetone, petroleum ether, chloroform, or methanolic) exhibited dose-dependent anthelmintic activity with strong paralytic activity within one hour and death within 80 min at a 25 mg mL−1 concentration [68].
6. Chemical Metabolites of Kaempferia Species
Chemical profiles of Kaempferia exhibited the presence of different types of secondary metabolites such as terpenoids, especially isopimarane phenolic compounds, diarylheptanoids [13], flavonoids [69,70,71], and essential oils [72,73]. This review summarized the reported variety of compound types, including isopimarane, abietane, labdan, and clerodane diterpenoids, flavonoids, phenolic acids, phenyl-heptanoids, curcuminoids, tetrahydropyrano-phenolic, and steroids. Diterpenoids, especially isopimarane types, were the most reported compounds from the plants of this genus, in addition to phenolics, flavonoids, and essential oils. Each class will be described and listed in the following items, and the structures will be summarized in Table 1, Table 2 and Table 3.
Table 1.
Diterpenoids.
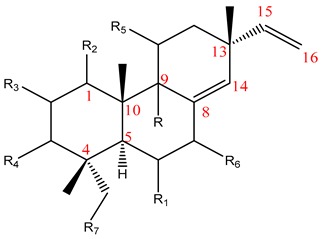
| |||||||||||
| No | Name | R | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Plant | Ref |
| 1 | Sandaracopimaradiene | H | H | H | H | H | H | H | H |
K. galanga
K. roscoeana K. marginata |
[4,16,17,25,26,36,74,75,76] |
| 2 | Sandaracopimaradiene-9α-ol | α-OH | H | H | H | H | H | H | H | ||
| 3 | 8(14),15-Sandaracopimaradiene-1α,9α-diol | α-OH | H | α-OH | H | H | H | H | H |
K. galanga K. pulchra K. sp. |
|
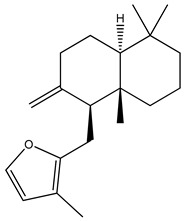
| 4 | 1,11-Dihydroxypimara-8(14),15-diene | H | H | α-OH | H | H | α-OH | H | H | ||
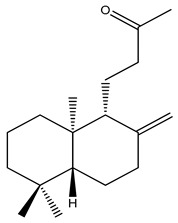
| 5 | 6β-Hydroxypimara-8(14),15-diene-1-one | H | β-OH | =O | H | H | H | H | H |
K. galanga
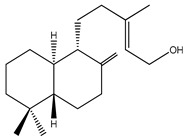
K. marginata |
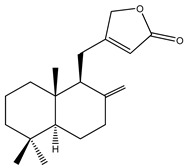
|
| 6 | Sandaracopimaradien-6β,9α-diol-l-one | α-OH | β-OH | =O | H | H | H | H | H | K. galanga | |
| 7 | Boesenberol I | α-OH | H | =O | H | H | H | α-OH | H | ||
| 8 | Boesenberol J | α-OH | β-OH | =O | H | H | H | H | H | K. galanga | |
| 9 | Sandaracopimaradien-1α,2α-diol | H | H | α-OH | α-OH | H | H | H | H |
K. roscoeana
K. pulchra K. marginata |
[26,38,75] |
| 10 | 2α-Acetoxy-sandaracopimaradien-1α-ol | H | H | α-OH | α-OAc | H | H | H | H |
K. pulchra
K. marginata |
|
| 11 | Kaempulchraol E | α-H | β-OH | α-OH | H | H | H | H | H |
K. galanga
K. pulchra |
|
| 12 | Kaempulchraol F | H | H | α-OH | H | α-OH | H | H | H | K. pulchra | [4,16,17,25,26,74] |
| 13 | Kaempulchraol H | H | β-OH | α-OH | H | α-OH | H | H | H | ||
| 14 | Kaempulchraol I | H | H | α-OH | H | H | H | H | H |
K. galanga
K. pulchra K. roscoeana |
|
| 15 | Kaempulchraol J | H | H | α-OH | H | H | H | =O | K. pulchra | ||
| 16 | Kaempulchraol K | α-OH | β-OAc | H | H | H | H | H | H | ||
| 17 | Kaempulchraol L | α-OMe | β-OH | H | H | H | H | H | H |
K. galanga
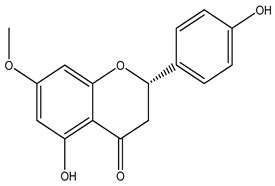
K. pulchra |
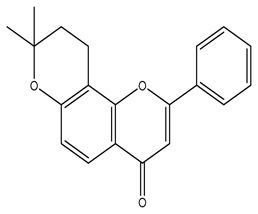
|
| 18 | Kaempulchraol M | α-OH | H | α-OH | α-OH | H | H | H | H | K. pulchra | |
| 19 | Kaempulchraol P | H | β-OH | H | H | H | H | H | H | ||
| 20 | Kaempulchraol Q | α-OAc | β-OH | H | H | H | H | H | H | ||
| 21 | Kaempulchraol R | α-OH | H | H | H | H | H | α-OAc | H | ||
| 22 | Kaempulchraol T | H | β-OH | H | H | H | H | α-OAc | H | ||
| 23 | Kaempulchraol V | α-OH | β-OH | H | H | H | H | β-OAc | H | ||
| 24 | Kaempulchraol W | α-OH | β-OH | H | H | H | H | β-OH | H | ||
| 25 | 9 α-Hydroxyisopimara-8(14),15-dien-7-one | α-OH | H | H | H | H | H | =O | H | ||
| 26 | 7β,9 α-Dihydroxypimara-8(14),15-diene | α-OH | H | H | H | H | H | β-OH | H | ||
| 27 | Isopimara-8(14),15-dien-7-one | H | H | H | H | H | H | =O | H | K. roscoeana | [26] |
| 28 | (1S,5S,9S,10S,11R,13R)-1,11-Dihydroxypimara-8(14),15-diene | H | H | α-OH | H | H | α-OH | H | H |
K. roscoeana
K. marginata K. pulchra |
[4,17,25,26,74,75] |
| 29 | (1R,2S,5S,9S,10S,11R,13R)-1,2,11-Trihydroxypimara-8(14),15-diene | H | H | α-OH | α-OH | H | α-OH | H | H | ||
| 30 | 7α-Hydroxyisopimara-8(14),15-diene | H | H | H | H | H | H | α-OH | H |
K. roscoeana
K. pulchra |
|
| 31 | Sandaracopimaradien- 9α-ol-l-one | α-OH | H | =O | H | H | H | H | H | K. sp | [76] |
| 32 | 6β-Acetoxysandaracopimaradien-9α-ol-l-one | α-OH | β-OAc | =O | H | H | H | H | H | ||
| 33 | Sandaracopimaradien-6β,9α-diol-l-one | α-OH | β-OH | =O | H | H | H | H | H | ||
| 34 | 6β-Acetoxysandaracopimaradien-lα,9α-diol | α-OH | β-OAc | α-OH | H | H | H | H | H | ||
| 35 | Sandaracopimaradien- lα,6β,9α-triol | α-OH | β-OH | α-OH | H | H | H | H | H | ||
| 36 | Roscorane B | H | H | H | H | H | α-OH | H | OH | K. roscoeana | [26] |
| 37 | Roscorane C | H | β-OH | H | OH | H | H | OH | H | ||
| 38 | Roscorane D | H | H | H | OH | H | H | OH | OH | ||
| 39 | (1R,2S,5S,7S,9R,10S,13R)-1,2,7-Trihydroxypimara-8(14),15-diene | H | H | H | α-OH | H | H | β-OH | H | K. marginata | [75] |
| 40 | (1S,5S,7R,9R,10S,11R,13R)-1,7,11-Trihydroxypimara-8(14),15-diene | H | β-OH | H | H | H | H | α-OH | H | ||
| 41 | (1R,2S,5S,7S,9R,10S,13R)-1,2-Dihydroxypimara-8(14),15diene-7-one | H | H | H | α-OH | H | H | H | H | ||
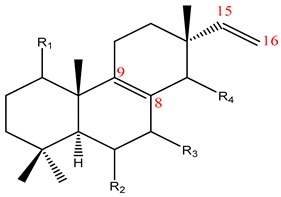
52–54 |
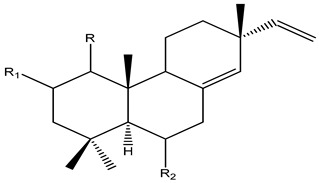
42–51 |
||||||||||
| No | Name | R1 | R2 | R3 | R4 | Plant | Ref | ||||
| 42 | Kaempulchraol A | H | β-OH | H | α-OMe | K. pulchra | [4,17,25,74] | ||||
| 43 | Kaempulchraol B | H | β-OH | H | β-OMe | ||||||
| 44 | Kaempulchraol C | H | β-OH | H | α-OH | ||||||
| 45 | Kaempulchraol D | H | β-OH | H | β-OH | ||||||
| 46 | Kaempulchraol G | H | β-OH | H | =O | ||||||
| 47 | Kaempulchraol N | α-OH | β-OH | H | α-OH | ||||||
| 48 | Kaempulchraol O | α-OH | β-OH | H | β-OMe | ||||||
| 49 | Kaempulchraol S | H | H | =O | α-OH | ||||||
| 50 | Kaempulchraol U | H | H | H | α-OH | ||||||
| 51 | Isopimara-8(9),15-dien-7-one | H | H | =O | H | K. roscoeana | [26] | ||||
| 52 | 8(14),15-Isopimaradiene-6α-ol | H | α-OH | H | --- | K. marginata | [36] | ||||
| 53 | 1α-Acetoxy-sandaracopimaradiene | α-OAc | H | H | - | ||||||
| 54 | 1α-Acetoxy-sandaraco pimaradien-2-one | α-OAc | =O | H | - | ||||||
| No | Name | Structure | Plant | Ref | |||||||
| 55 | (2R)-ent-2-Hydroxyisopimara-8(14),15-diene |
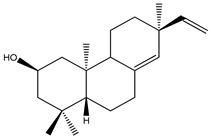
|
K. pulchra | [4,17,25,74] | |||||||
| 56 | Kaemgalangol A |
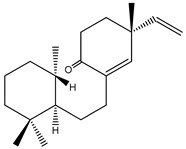
|
K. galanga | [16] | |||||||
| 57 | Roscorane A |
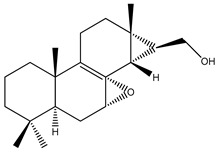
|
K. roscoeana | [26] | |||||||
| 58 | R=OMe; Roscotane A |
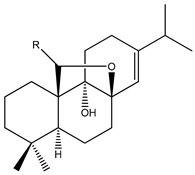
|
K. roscoeana | [26] | |||||||
| 59 | R=H; Roscotane B | ||||||||||
| 60 | Roscotane C |
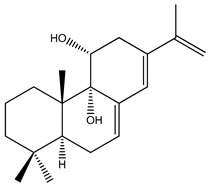
|
|||||||||
| 61 | Roscotane D |
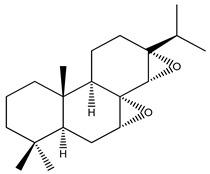
|
|||||||||
| 62 | R=H; Ar-abietatriene |
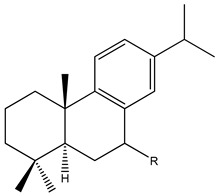
|
|||||||||
| 63 | R=[=O]; 7-Dehydroabietanone | ||||||||||
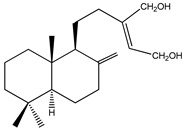
| 64 | R=α-OH; Abieta-8,11,13-trien-7α-ol | ||||||||||
| 65 | Kaempfolienol |
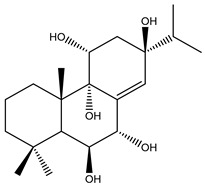
|
K. angustifolia | [33,34] | |||||||
| 66 | (12Z,14R)-Labda-8(17),12-dien-14,15,16-triol |
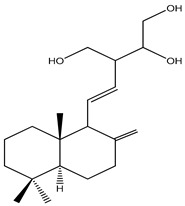
|
K. roscoeana | [26] | |||||||
| 67 | Propadane A |
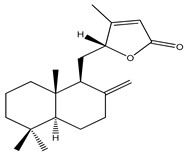
|
K. elegans | [18] | |||||||
| 68 | R=H --- 8(17)-Labden-15-ol |
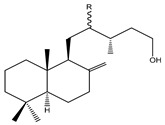
|
|||||||||
| 69 | R=OH; Propadane B | ||||||||||
| 70 | Propadane C |
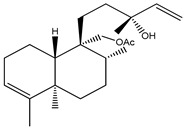
|
K. pulchra | ||||||||
| 71 | Cleroda-2,4(18),14-trien-13-ol |
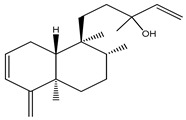
|
K. pulchra | ||||||||
| 72 | R=H; Anticopalic acid |
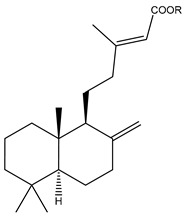
|
K. elegans | ||||||||
| 73 | R=Me; Methyl anticopalate | ||||||||||
| 74 | (+)-15,16-Eoxy-8(17),13(16),14-labdatriene |
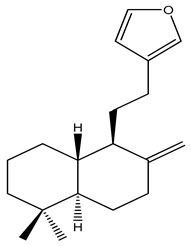
|
[18] | ||||||||
| 75 | (+)-Pumiloxide |
|
|||||||||
| 76 | 13-Oxo-14,15-bis-nor-labd-8(17)-ene |
|
|||||||||
| 77 | Anticopalol |
|
K. elegans | ||||||||
| 78 | Labda-8(17),13(14)-diene-15,16-olide |
|
|||||||||
| 79 | (+)-Labda-8(17),13(Z)-diene-15,16-diol |
|
|||||||||
| 80 | Calcaratarin A |
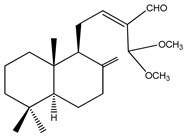
|
K. pulchra | ||||||||
| 81 | R=H; (-)-Kolavelool |
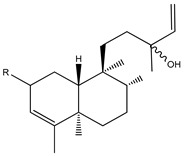
|
[18] | ||||||||
| 82 | R= β-OH; (-)-2β-Hydroxykolavelool | ||||||||||
| 83 | R=β-OMe; Dysoxydensin E | ||||||||||
| 84 | 13-Epi-roseostachenone |
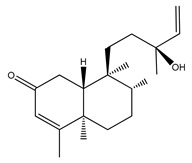
|
|||||||||
| 85 | (+)-13-Epi-2α-hydroxykolavelool (13-epi-roseostachenol) |
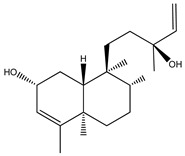
|
K. pulchra | ||||||||
Table 2.
Flavonoids and phenolics (Flavonoids).
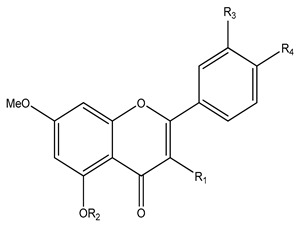
86–97 |
98–101 |
102–103 |
|||||
| No | Name | R1 | R2 | R3 | R4 | Plant | Ref |
| 86 | 5,7-Dimethoxyflavone | H | Me | H | H | K. parviflora | [9,55,71,77] |
| 87 | 4‘,5,7-Trimethoxyflavone | H | Me | H | OMe | ||
| 88 | 3‘,4‘,5,7-Tetramethoxyflavone | H | Me | OMe | OMe | ||
| 89 | 3,5,7-Trimethoxyflavone | OMe | Me | H | H | ||
| 90 | 3,5,7,4‘-Tetramethoxyflavone | OMe | Me | H | OMe | ||
| 91 | 3,5,7,3‘,4‘-Pentamethoxyflavone | OMe | Me | OMe | OMe | ||
| 92 | 5-Hydroxy-7-methoxyflavone | H | H | H | H | ||
| 93 | 5-hydroxy-7,4‘-dimethoxyflavone | H | H | H | OMe | ||
| 94 | 5-Hydroxy-3,7-dimethoxyflavone | OMe | H | H | H | ||
| 95 | 5-Hydroxy-3,7,4‘-trimethoxyflavone | OMe | H | H | OMe | ||
| 96 | 5-Hydroxy-3,7,3‘,4‘-tetramethoxy flavone | OMe | H | OMe | OMe | ||
| 97 | 5,3‘-Dihydroxy-3,7,4‘-trimethoxyflavone | OMe | H | OH | OMe | ||
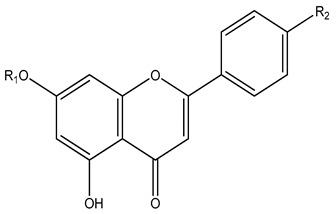
| 98 | Kaempferol | H | OH | - | - | K. galanga | [42] |
| 99 | Kaempferide | H | OMe | - | |||
| 100 | Tectochrysin | Me | H | - | - | K. parviflora | |
| 101 | Genkwanin | Me | OH | - | - | [46] | |
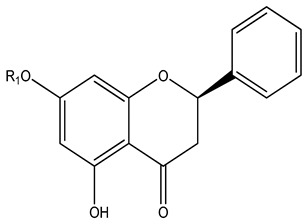
| 102 | Pinocembin | H | - | - | - |
K. parviflora
K. angustifolia |
[71] |
| 103 | Pinostrobin | Me | - | - | - | ||
| 104 | Sakuranetin |
|
|||||
| 105 | 2″,2″-Dimethylpyrano-[5″,6″:8,7]-flavone |
|
K. pulchra | [18] | |||
106–109 |
110–113 |
114–115 |
|||||
116–117 |
118–119 |
||||||
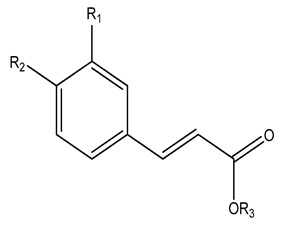
| No | Name | R1 | R2 | R3 | Plant | Ref | |
| 106 | Ethyl trans-p-methoxycinnamate | H | OMe | CH2Me | K. galanga | [13] | |
| 107 | Ferulic acid | OMe | OH | H | |||
| 108 | trans-p-Hydroxy-cinnamic acid | H | OH | H | |||
| 109 | trans-p-Methoxy cinnamic acid | H | H | CH2Me | |||
| 110 | p-Hydroxybenzoic acid | H | OH | COOH | [13,50] | ||
| 111 | p-Methoxybenzoic acid | H | OMe | COOH | |||
| 112 | Vanillic acid | OMe | OH | COOH | |||
| 113 | Methyl 3,4-dihydroxybenzoate | OH | OH | COOMe | |||
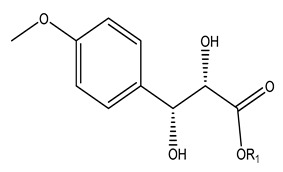
| 114 | Methyl (2R,3S)-2,3-dihydroxy-3-(4-methoxyphenyl) propanoate | Me | - | - | |||
| 115 | Ethyl-(2R,3S)-2,3-dihydroxy-3-(4-methoxyphenyl) propanoate | CH2Me | - | - | |||
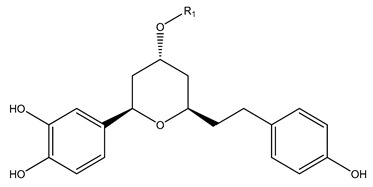
| 116 | (1R,3R,5R)-1,5-Epoxy-3-hydroxy-1-(3,4-dihydroxyphenyl)-7-(3,4-dihydroxy phenyl) heptane | H | - | - | [13] | ||
| 117 | (1R,3R,5R)-1,5-Epoxy-3-hydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl) heptane 3-O-β-D-glucopyranoside | D-glc | - | - | |||
| 118 | 2‘-hydroxy-4‘,6‘-dimethoxychalcone | H | - | - |
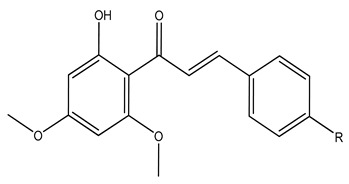
K. parviflora
K. angustifolia |
[24,71] | |
| 119 | 2‘-hydroxy-4,4‘,6‘-trimethoxychalcone | Me | - | - | |||
| No | Name | Structure | Plant | Ref | |||
| 120 | Desmethoxyyangonin |
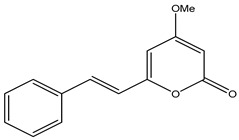
|
K. marginata | [36] | |||
| 121 | Bisdemethoxycurcumin |
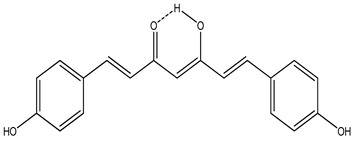
|
|||||
| 122 | 1-(4-Hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)heptane-1,2,3,5,6-pentaol |
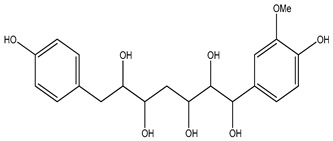
|
K. galanga | [13] | |||
| 123 | (3R,5S)-3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxy phenyl) heptane |
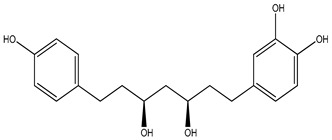
|
K. galanga | ||||
| 124 | Phaeoheptanoxide |
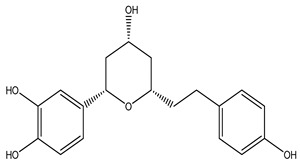
|
|||||
| 125 | Hedycoropyran B |
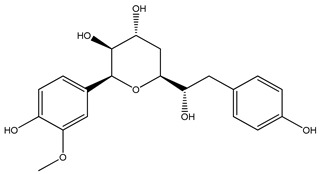
|
|||||
| 126 | 1-O-4-Carbonxylphenyl-(6-O-4-hydroxybenzoyl)-β-D-glucopyranoside |
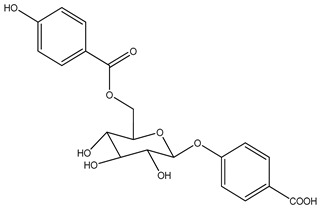
|
|||||
| 127 | Dihydro-5,6-dehydrokawain |
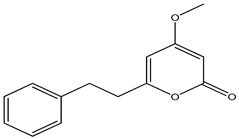
|
K. parviflora | [13,50] | |||
| 128 | R=OH; (-)-Hydroxypanduratin A |
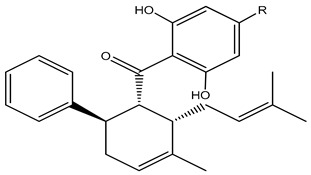
|
|||||
| 129, | R=OMe; (-)-Panduratin A | ||||||
| 130 | Cinnamaldehyde |
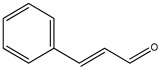
|
K. galanga | [78] | |||
| 131 | R=Me; Crotepoxide |
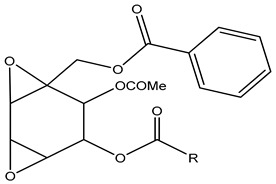
|
K. angustifolia | [24,33] | |||
| 132 | R=Benzen; Boesenboxide | ||||||
| 133 134 | R=H; Zeylenol R=Me; 6-methylzeylenol |
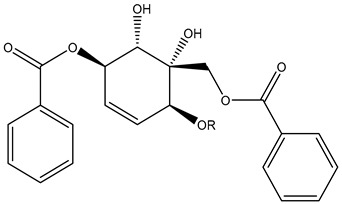
|
|||||
| 135 | rel-(5aS,10bS)-5a,10b-Dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1–d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside] |
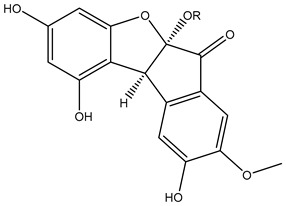 R=α-L-rha-(1→6)-β-D-glc |
K. parviflora | [79] | |||
| 136 | rel-(5aS,10bR)-5a,10b-Dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1–d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside] |
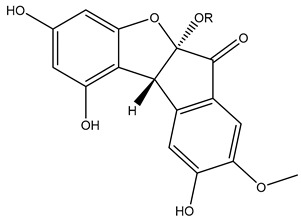 R=α-L-rha-(1→6)-β-D-glc |
|||||
| 137 | (2R,3S,4S)-3-O-[α-l-Rhamnopyranosyl-(1 → 6)-β-d-glucopyranosyl]-3′-O-methyl-ent-epicatechin-(2α→O→3,4α→ 4)-(5aS,10bS)-5a,10b-dihydro-1,3,5a,9-tetrahydroxy-8-methoxy-6H-benz[b]indeno[1,2-d]furan-6-one 5a-O-[α-l-rhamnopyranosyl-(1→ 6)-β-d-glucopyranoside] |
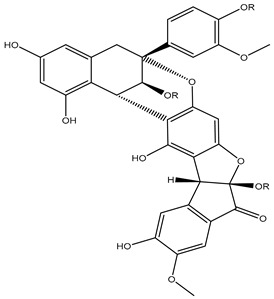 R=α-L-rha-(1→6)-β-D-glc |
|||||
Table 3.
Steroid and triterpenes.
6.1. Diterpenoids
Kaempferia plants were characterized with a predominance of diterpenoids, especially the isopimaranes in addition to abietane, labdane, and clerodane types (Table 1).
6.1.1. Isopimarane-Type Diterpenoids
The isopimaranes reported from the Kaempheria species (Table 1) are characterized with the presence of two double bonds; one is mostly ∆15(16), while the other is between ∆8(9) or ∆8(14) [4,25,74]. From the rhizomes of K. galanga, 12 usual isopimarenes (1–8, 10, 11, 14, and 17) were observed that contained a ∆8(14),15 motif in addition to the rarely reported oxygenated seco-isopimarane (56) [16]. From the rhizomes of K. marginata, five isopimarenes with a ∆8(14),15 motif were observed (1, 2, 52–54) [36]. Only one thumbing isopimarenes, roscorane A (57), was reported from K. roscoeana, which was characterized by only one double bond ∆8(9) and (7-8)-epoxy, as well as the absence of the exomethylene ∆15(16) [26].
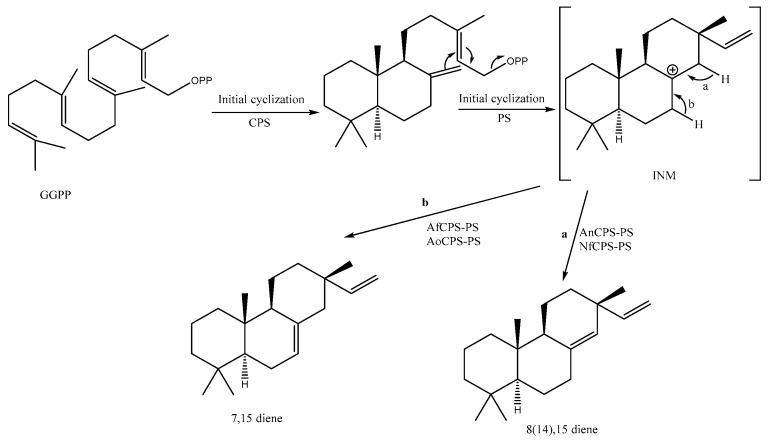
Biosynthesis of Isopimarane-Type Diterpenoids
Isopimarane diterpenoids are the most characteristic compounds for Kaempheria plants. (E,E,E)-Geranylgeranyl diphosphate (GGPP) is a well-known biosynthesized intermediate of diterpenoids as described by [80]. GGPP is firstly cyclized via copalyl diphosphate (CPP) synthases (CPS), and then by the unknown enzyme (PS), affording a charged intermediate (INM). Then, this intermediate is completely cyclized by the enzymatic reactions via the bifunctional (iso) pimaradiene synthases (AoCPS-PS, NfCPS-PS, and AfCPS-PS) (Scheme 1), as described by Xu et al. [81].
Scheme 1.
Plausible isopimaradiene biosynthesis [81] starting with (E,E,E)-geranylgeranyl diphosphate (GGPP): (E,E,E)-geranylgeranyl diphosphate; CPS: copalyl diphosphate (CPP) synthases; PS: Unknown enzyme; AoCPS-PS, NfCPS-PS, and AfCPS-PS: bifunctional (iso) pimaradiene synthases.
6.1.2. Abietane-Type Diterpenoids
Seven abietanes (58–64) (Table 1) have been isolated and characterized from the rhizomes of K. roscoeana, and one (65) was isolated and characterized from K. angustifolia [26,33,34]. These highly oxygenated metabolites contain one or more double bonds and an absence of exomethylenes, except for roscotane D (61), which contains no double bonds.
6.1.3. Labdane and Clerodane Diterpenoids
After isopimarenes, labdane and clerodane represent major diterpenoid classes from the Kaempheria species. Nineteen highly oxygenated labdanes and clerodanes (66–86) have been reported from Kaempheria rhizomes (Table 1) [18,26]. From these isolated labdanes, only (12Z,14R)-labda-8(17),12-dien-14,15,16-triol (66) has been isolated from K. roscoeana rhizomes. In contrast, several labdane and clerodane types of diterpenoids have been isolated from K. elegans and K. pulchra rhizomes collected in Thailand.
6.1.4. Flavonoids
Kaempheria species are characterized by rich biological activity due in part to the presence of a diversity of flavonoids (86–105) and phenolic compounds (106–137) (Table 2). K. parviflora rhizomes with flavonoid nuclei contain methoxy groups in specific positions (86–97) [9,55]. Pyrano-flavone, 2”,2”-dimethylpyrano-[5”,6”:8,7]-flavone (105), has been isolated from K. pulchra rhizomes collected from Thailand [18], and flavanones (97–99) have been isolated and identified from K. parviflora rhizomes [70,71]. K. galanga contains kaempferol and kaempferide (98, 99) [78].
6.1.5. Phenolic Compounds
From K. galanga rhizomes, diarylheptanoid compounds (116, 117, 122–125) are reported by Yao, Huang, Wang, and He [13]. From K. marginata rhizomes, curcuminoid (121) was characterized by Kaewkroek, Wattanapiromsakul, Kongsaeree, and Tewtrakul [36]. From K. galanga, rhizomes phenolic acids (106–113) were the major compounds isolated, including methoxylated cinnamic acid derivatives. Two (4-methoxyphenyl)-propanoates (114–115) were also isolated from the K. galanga rhizomes [13,50]. S- and R-isomers at C-4 of phenolic glycosides (135 and 136) as well as a rare phenolic glycoside (137) were observed in K. previflora rhizomes [79]. All the phenolic compounds (106–137) are summarized in Table 2.
6.1.6. Steroids and Triterpenes
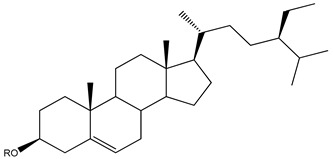
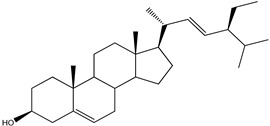
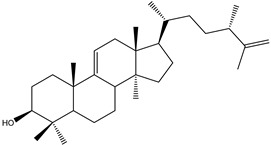
Steroids represent a minor class of compounds reported from Kaempheria species. Only three steroids, β-sitosterol (138), β-sitosterol-β-D-glucoside (139), and stigmasterol (140) (Table 3) have been reported from K. marginata rhizomes [36]. Moreover, only one lanostane type triterpene, (24S)-24-methyl-lanosta-9(11), 25-dien-3β-ol (141), was isolated from K. angustifolia [24].
6.1.7. Volatile Oils
Kaempheria species were documented as very rich plants with volatile oils such as K. galanga [29,73,82,83], K. angustiflora [29], and K. marginata [29]. The volatile oil of K. galanga has been reported as a potential market product in India and over all the world with market values around 600–700 US$/kg on the international market [83]. Phenylpropanoids and/or cinamates were represented as major constituents of volatile oils derived from Kaempheria species followed by monoterpenes [29,73,82]. The phenylpropanoid compound, trans-ethyl cinnamate, was documented as a principal component of volatile oils of all the studied Kaempheria species up to date with concentrations varied from 16–35% of the total identified [29,73,82,83]. The volatile oils of Kaempheria species were reported to have numerous biological activities such as anti-microbial [83], antioxidant [35], nutraceutical [83], nematicidal toxicity [82], and larvicide activities [29]. Table 4 summarized the main components (142–157) of the reported volatile oils of Kaempheria species.
Table 4.
Main components of volatile oils of Kaempferia species.
| No | Name | Plant | Ref |
|---|---|---|---|
| 142 | δ-3-Carene | K. galanga | [29,35,82,83] |
| 143 | E-Ethyl cinnamate | ||
| 144 | Ethyl-p-methoxycinnamate | ||
| 145 | γ-Cadinene | ||
| 146 | 1,8-Cineole | ||
| 147 | Trans-cinnamaldehyde | ||
| 148 | Borneol | ||
| 149 | Pentadecane | ||
| 150 | γ-car-3-ene | ||
| 151 | Linoleoyl chloride | ||
| 152 | Caryophyllene oxide | ||
| 153 | Cubenol | ||
| 154 | Caryophyllene | ||
| 155 | Limonene | ||
| 156 | Camphene | ||
| 157 | α-Pinene |
7. Principal Components Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC) for Kaempferia Species
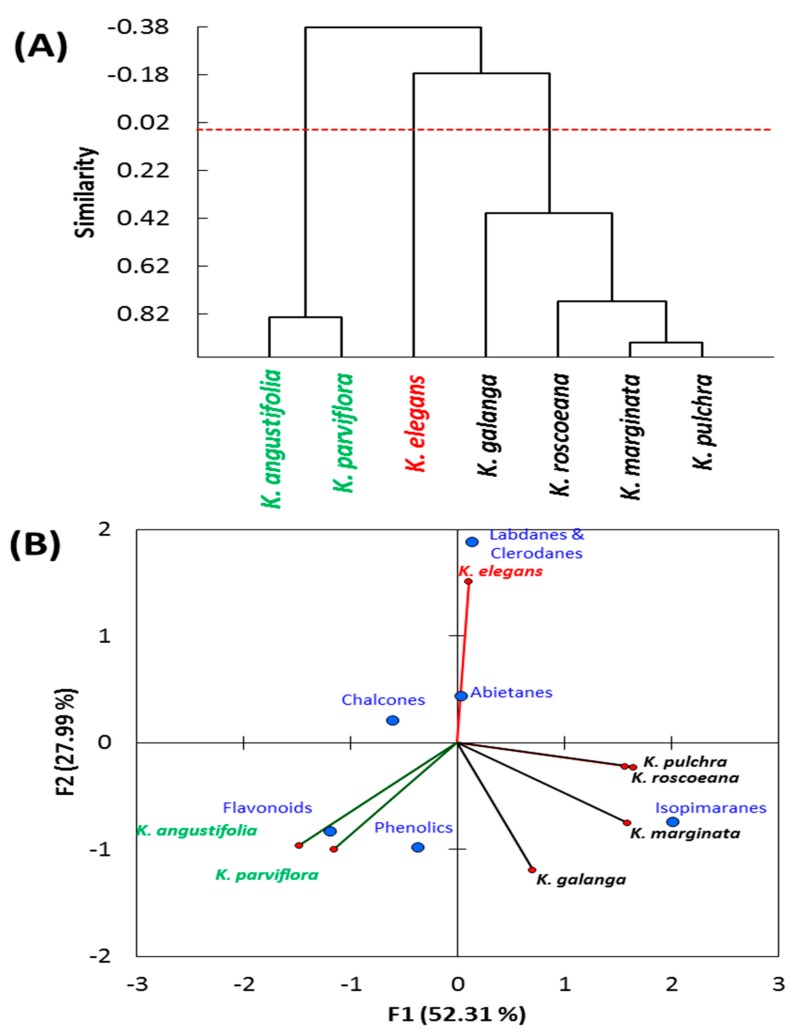
To assess the correlation between the various Kaempferia species, chemical classes of different compounds were subjected to PCA and AHC (Figure 3). According to the similarity, the analysis showed that we can group the Kaempferia species under three groups: the first group comprised K. galanga, K. marginata, K. pulchra, and K. roscoeana, and these species are correlated to isopimaranes compounds. The Pearson correlation coefficient (r) between K. marginata and K. pulchra was the highest with r = 0.938, while between K. marginata and K. roscoeana, it was 0.771, between K. roscoeana and K. pulchra, it was 0.766, and between K. marginata and K. galanga, it was 0.615 (Table 5).
Figure 3.
(A) Agglomerative hierarchical clustering (AHC) and (B) Principal component analysis (PCA) based on the chemical composition of different chemical classes of seven Kaempferia species (K. angustifolia, K. elegans, K. galanga, K. marginata, K. parviflora, K. pulchra, and K. roscoeana).
Table 5.
Proximity matrix (Pearson correlation coefficient) of the seven Kaempferia species based on the chemical classes reported.
| K. angustifolia | K. elegans | K. galanga | K. marginata | K. parviflora | K. pulchra | |
|---|---|---|---|---|---|---|
| K. elegans | −0.539 | |||||
| K. galanga | −0.042 | −0.339 | ||||
| K. marginata | −0.500 | −0.241 | 0.615 | |||
| K. parviflora | 0.833 | −0.312 | 0.075 | −0.280 | ||
| K. pulchra | −0.675 | 0.053 | 0.378 | 0.938 | −0.372 | |
| K. roscoeana | −0.643 | −0.225 | 0.206 | 0.771 | −0.513 | 0.766 |
The second group contained K. angustifolia and K. parviflora (r = 0.833), and this group showed a close correlation to flavonoids and phenolics. However, the K. elegans was separated alone, and showed a close relation to labdane and clerodane compounds. The similarities within each group might be ascribed to the genetic relations, as well as the environmental and microclimatic conditions [1,2,3].
In a study of a genetic variation of Kaempferia species based on chloroplast DNA [5], K. marginata and K. galanga were grouped together, which is agreeable with our results (r = 0.615) according to the PCA data of the present study based on the chemical composition. However, in contrast to the data from the PCA, K. angustifolia and K. parviflora were separated in different groups, but K. elegans and K. parviflora were grouped together. In another recent study, based on the DNA and morphological characteristics [84], K. angustifolia and K. parviflora were grouped together in agreement with the chemical variation of the present study.
8. Conclusions
Kaempheria species are widely used plants in traditional medicine worldwide. All the biological activity data for these plants and their isolated constituents have resulted in numerous leads for medicinal drugs. Mainly, seven rhizomes of Kaempheria plants afforded a vast array of diterpenoids, especially the isopimarane type, along with significant bioactive methoxylated flavonoids. From all these documented chemical and biological results, these plants have been and continue to be a promising source for medicinal natural products and food industrial products.
Acknowledgments
The authors are thankful to the Deanship of the Scientific Research and Research Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Dr. Elshamy gratefully acknowledges the Takeda Science Foundation, Japan for financial support. Prof. Mohamed Hegazy gratefully acknowledges the financial support from Alexander von Humboldt Foundation “Georg Foster Research Fellowship for Experienced Researcher”.
Abbreviations
| K. | Kaempheria |
| Sp. | Species |
| PCA | Principal component analysis |
| AHC | Agglomerative hierarchical clustering |
| CCA | Significant Cholangiocarcinoma |
| HSC-2 | Mouth squamous cell carcinoma |
| EAC | Ehrlich ascites carcinoma cancer cells |
| HL-60 | Human leukemia cancer cells |
| CL-6 | Human cholangiocarcinoma cells |
| TKI | Tyrosine kinase inhibitors |
| BCRP | Breast cancer resistance protein |
| MTT | (3-[4,5,[4,5-Dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) assay |
| CH2Cl2 | Dichloromethane |
| BChE | Butyrylcholinesterase |
| NO | Nitric oxide |
| CUPRAC | Modified cupric reducing antioxidant capacity |
| PDE5 | Phosphodiesterase type 5 inhibitor |
| AP | Aerial parts |
| AChE | Acetylcholinesterase |
| RBL-2H3 | Rat Basophilic Leukemia cells |
| P38 | Type of mitogen-activated protein kinases |
| STAT1 and 3 | Signal transducers and activators of transcription 1 and 3 |
| MeOH | Methanol |
| EtOAc | Ethyl acetate |
| MCF-7 | Breast cancer cells |
| HT-29 | Colorectal adenocarcinoma cell |
| PMF | Polymethoxyflavonoid-rich fraction |
| TSOD | Tsumura Suzuki obese diabetes |
| GGPP | (E,E,E)-Geranylgeranyl diphosphate |
| CPP | Copalyl diphosphate |
| CPS | Copalyl diphosphate synthases |
| EtOH | Ethanol |
| AChE | Acetylcholinesterase |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) assay |
| Vpr | Viral protein R |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl assay |
| FRAP | Ferric reducing antioxidant power |
| CYP3A | Cytochrome P450, family 3, subfamily A |
| NF-κB | Nuclear factor pathway |
| Rh | Rhizomes |
| BChE | Butyrylcholinesterase |
| STZ | Streptozotocin |
| MPO | myeloperoxidase |
| DMF | Dimethylformamide |
| MAPKs | Type of mitogen-activated protein kinases |
| PGE2 | Prostaglandin E2 |
Author Contributions
A.I.E., T.A.M. and M.-E.F.H. suggested and designed the study. A.I.E., T.A.M., A.F.E., and A.M.A.-E.G. did the data preparation and structure drawing. A.I.E., T.A.M., A.M.A.-E.G., and M.-E.F.H. collected the data. A.S.A., A.A.S., T.Y., A.R.H.F., and M.N. wrote the original manuscript. A.I.E., T.A.M., M.-E.F.H., P.W.P., A.U., A.S.A., A.A.S., and H.R.E.-S. edited the final manuscript. All the authors reviewed and approved the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vinceti B., Loo J., Gaisberger H., van Zonneveld M.J., Schueler S., Konrad H., Kadu C.A., Geburek T. Conservation priorities for Prunus africana defined with the aid of spatial analysis of genetic data and climatic variables. PLoS ONE. 2013;8:e59987. doi: 10.1371/journal.pone.0059987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Sánchez R., Gálvez C., Ubera J.L. Bioclimatic influence on essential oil composition in South Iberian Peninsular populations of Thymus zygis. J. Essent. Oil Res. 2012;24:71–81. doi: 10.1080/10412905.2012.646025. [DOI] [Google Scholar]
- 3.Elshamy A., Mohamed T.A., Al-Rowaily S.L., Abd El-Gawad A.M., Dar B.A., Shahat A.A., Hegazy M.F. Euphosantianane E–G: Three new premyrsinane type 2 diterpenoids from Euphorbia sanctae-catharinae with 3 contribution to chemotaxonomy. Molecules. 2019;24:2412. doi: 10.3390/molecules24132412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Techaprasan J., Klinbunga S., Ngamriabsakul C., Jenjittikul T. Genetic variation of Kaempferia (Zingiberaceae) in Thailand based on chloroplast DNA (psbA-trnH and petA-psbJ) sequences. Genet. Mol. Res. 2010;9:1957–1973. doi: 10.4238/vol9-4gmr873. [DOI] [PubMed] [Google Scholar]
- 6.Wutythamawech W. Encyclopedia of Thai Herbs. OS Printing; Bangkok, Thailand: 1997. p. 365. [Google Scholar]
- 7.Yenjai C., Prasanphen K., Daodee S., Wongpanich V., Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75:89–92. doi: 10.1016/j.fitote.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Akase T., Shimada T., Terabayashi S., Ikeya Y., Sanada H., Aburada M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011;65:73–80. doi: 10.1007/s11418-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 9.Nakao K., Murata K., Deguchi T., Itoh K., Fujita T., Higashino M., Yoshioka Y., Matsumura S.-I., Tanaka R., Shinada T. Xanthine oxidase inhibitory activities and crystal structures of methoxyflavones from Kaempferia parviflora rhizome. Biol. Pharm. Bull. 2011;34:1143–1146. doi: 10.1248/bpb.34.1143. [DOI] [PubMed] [Google Scholar]
- 10.Sivarajan V., Balachandran I. Ayurvedic Drugs and their Plant Sources Oxford and IBH Publishing Co. Oxford & IBH Pub. Co; New Delhi, India: 1994. [Google Scholar]
- 11.Hidaka M., Horikawa K., Akase T., Makihara H., Ogami T., Tomozawa H., Tsubata M., Ibuki A., Matsumoto Y. Efficacy of Kaempferia parviflora in a mouse model of obesity-induced dermatopathy. J. Nat. Med. 2017;71:59–67. doi: 10.1007/s11418-016-1027-8. [DOI] [PubMed] [Google Scholar]
- 12.Win N.N., Ito T., Matsui T., Aimaiti S., Kodama T., Ngwe H., Okamoto Y., Tanaka M., Asakawa Y., Abe I. Isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorganic Med. Chem. Lett. 2016;26:1789–1793. doi: 10.1016/j.bmcl.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Yao F., Huang Y., Wang Y., He X. Anti-inflammatory diarylheptanoids and phenolics from the rhizomes of kencur (Kaempferia galanga L.) Ind. Crop. Prod. 2018;125:454–461. doi: 10.1016/j.indcrop.2018.09.026. [DOI] [Google Scholar]
- 14.Vimala S., Norhanom A., Yadav M. Anti-tumour promoter activity in Malaysian ginger rhizobia used in traditional medicine. Br. J. Cancer. 1999;80:110. doi: 10.1038/sj.bjc.6690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amuamuta A., Plengsuriyakarn T., Na-Bangchang K.J. Anticholangiocarcinoma activity and toxicity of the Kaempferia galanga Linn. rhizome ethanolic extract. BMC Complementary Altern. Med. 2017;17:213. doi: 10.1186/s12906-017-1713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swapana N., Tominaga T., Elshamy A.I., Ibrahim M.A., Hegazy M.-E.F., Singh C.B., Suenaga M., Imagawa H., Noji M., Umeyama A. Kaemgalangol A: Unusual seco-isopimarane diterpenoid from aromatic ginger Kaempferia galanga. Fitoterapia. 2018;129:47–53. doi: 10.1016/j.fitote.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Win N.N., Ito T., Aimaiti S., Kodama T., Imagawa H., Ngwe H., Asakawa Y., Abe I., Morita H. Kaempulchraols I–O: New isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their antiproliferative activity. Tetrahedron. 2015;71:4707–4713. doi: 10.1016/j.tet.2015.05.075. [DOI] [Google Scholar]
- 18.Chawengrum P., Boonsombat J., Kittakoop P., Mahidol C., Ruchirawat S., Thongnest S. Cytotoxic and antimicrobial labdane and clerodane diterpenoids from Kaempferia elegans and Kaempferia pulchra. Phytochem. Lett. 2018;24:140–144. doi: 10.1016/j.phytol.2018.02.009. [DOI] [Google Scholar]
- 19.Dash P.R., Nasrin M., Ali M.S. In vivo cytotoxic and In vitro antibacterial activities of Kaempferia galanga. J. Pharmacogn. Phytochem. 2014;3:172–177. [Google Scholar]
- 20.Ali H., Yesmin R., Satter M.A., Habib R., Yeasmin T. Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells. J. King Saud Univ. Sci. 2018;30:386–392. doi: 10.1016/j.jksus.2017.05.009. [DOI] [Google Scholar]
- 21.Bae S., D’cunha R., Shao J., An G. Effect of 5, 7-dimethoxyflavone on Bcrp1-mediated transport of sorafenib in vitro and in vivo in mice. Eur. J. Pharm. Sci. 2018;117:27–34. doi: 10.1016/j.ejps.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed F.R.S., Amin R., Hasan I., Asaduzzaman A., Kabir S.R. Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol. 2017;102:952–959. doi: 10.1016/j.ijbiomac.2017.04.109. [DOI] [PubMed] [Google Scholar]
- 23.Omar M.N., Rahman S.A., Ichwan S., Hasali N., Rasid F.A., Halim F.A. Cytotoxicity effects of extracts and essential oil of Kaempferia galanga on cervical cancer C33A cell line. Orient. J. Chem. 2017;33:1659–1664. doi: 10.13005/ojc/330409. [DOI] [Google Scholar]
- 24.Tang S.W., Sukari M.A., Neoh B.K., Yeap Y.S.Y., Abdul A.B., Kifli N., Cheng Lian Ee G. Phytochemicals from Kaempferia angustifolia Rosc. and their cytotoxic and antimicrobial activities. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/417674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Win N.N., Ngwe H., Abe I., Morita H. Naturally occurring Vpr inhibitors from medicinal plants of Myanmar. J. Nat. Med. 2017;71:579–589. doi: 10.1007/s11418-017-1104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonsombat J., Mahidol C., Chawengrum P., Reuk-Ngam N., Chimnoi N., Techasakul S., Ruchirawat S., Thongnest S. Roscotanes and roscoranes: Oxygenated abietane and pimarane diterpenoids from Kaempferia roscoeana. Phytochemistry. 2017;143:36–44. doi: 10.1016/j.phytochem.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmanan D., Werngren J., Jose L., Suja K., Nair M.S., Varma R.L., Mundayoor S., Hoffner S., Kumar R.A. Ethyl p-methoxycinnamate isolated from a traditional anti-tuberculosis medicinal herb inhibits drug resistant strains of Mycobacterium tuberculosis in vitro. Fitoterapia. 2011;82:757–761. doi: 10.1016/j.fitote.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Tian S., Wang F., Li Z., Liu L., Yang X., Bao Y., Wu Y., Huang Y., Sun L. Chemical composition and antibacterial activity of Kaempferia galanga essential oil. Int. J. Agric. Biol. 2018;20:457–462. doi: 10.17957/IJAB/15.0560. [DOI] [Google Scholar]
- 29.Panyakaew J., Sookkhee S., Rotarayanont S., Kittiwachana S., Wangkarn S., Mungkornasawakul P. Chemical variation and potential of Kaempferia oils as larvicide against Aedes aegypti. J. Essent. Oil Bear. Plants. 2017;20:1044–1056. doi: 10.1080/0972060X.2017.1377114. [DOI] [Google Scholar]
- 30.Malahayati N., Widowati T.W., Febrianti A. Total phenolic, antioxidant and antibacterial activities of curcumin extract of kunci pepet (Kaempferia rotunda L) Res. J. Pharm. Biol. Chem. Sci. 2018;9:129–135. [Google Scholar]
- 31.Fauziyah P.N., Sukandar E.Y., Ayuningtyas D.K. Combination effect of antituberculosis drugs and ethanolic extract of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Sci. Pharm. 2017;85:14. doi: 10.3390/scipharm85010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochuthressia K., Britto S.J., Jaseentha M., Raphael R. In vitro antimicrobial evaluation of Kaempferia galanga L. rhizome extract. Am. J. Biotechnol. Mol. Sci. 2012;2:1–5. doi: 10.5251/ajbms.2012.2.1.1.5. [DOI] [Google Scholar]
- 33.Yeap Y.S.Y., Kassim N.K., Ng R.C., Ee G.C.L., Saiful Yazan L., Musa K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017;20:1158–1172. doi: 10.1080/10942912.2017.1286508. [DOI] [Google Scholar]
- 34.Tang S.W., Sukari M.A., Rahmani M., Lajis N.H., Ali A.M. A new abietene diterpene and other constituents from Kaempferia angustifolia Rosc. Molecules. 2011;16:3018–3028. doi: 10.3390/molecules16043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahoo S., Parida R., Singh S., Padhy R.N., Nayak S. Evaluation of yield, quality and antioxidant activity of essential oil of in vitro propagated Kaempferia galanga Linn. J. Acute Dis. 2014;3:124–130. doi: 10.1016/S2221-6189(14)60028-7. [DOI] [Google Scholar]
- 36.Kaewkroek K., Wattanapiromsakul C., Kongsaeree P., Tewtrakul S. Nitric oxide and tumor necrosis factor-alpha inhibitory substances from the rhizomes of Kaempferia marginata. Nat. Prod. Commun. 2013;8:1205–1208. doi: 10.1177/1934578X1300800904. [DOI] [PubMed] [Google Scholar]
- 37.Kaewkroek K., Wattanapiromsakul C., Matsuda H., Nakamura S., Tewtrakul S. Anti-inflammatory activity of compounds from Kaempferia marginata rhizomes. Songklanakarin J. Sci. Technol. 2017;39:91–99. [Google Scholar]
- 38.Sematong T., Reutrakul V., Tuchinda P., Claeson P., Pongprayoon U., Nahar N. Topical antiinflammatory activity of two pimarane diterpenes from Kaempferia pulchra. Phytother. Res. 1996;10:534–535. doi: 10.1002/(SICI)1099-1573(199609)10:6<534::AID-PTR890>3.0.CO;2-C. [DOI] [Google Scholar]
- 39.Sae-wong C., Tansakul P., Tewtrakul S. Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J. Ethnopharmacol. 2009;124:576–580. doi: 10.1016/j.jep.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 40.Lee M.-h., Han A.-R., Jang M., Choi H.-K., Lee S.-Y., Kim K.-T., Lim T.-G. Antiskin inflammatory activity of black ginger (Kaempferia parviflora) through antioxidative activity. Oxidative Med. Cell. Longev. 2018;2018:5967150. doi: 10.1155/2018/5967150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi H., Suzuki R., Sato K., Ogami T., Tomozawa H., Tsubata M., Ichinose K., Aburada M., Ochiai W., Sugiyama K. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018;72:136–144. doi: 10.1007/s11418-017-1121-6. [DOI] [PubMed] [Google Scholar]
- 42.Umar M.I., Asmawi M.Z., Sadikun A., Majid A.M.S.A., Al-Suede F.S.R., Hassan L.E.A., Altaf R., Ahamed M.B.K. Ethyl-p-methoxycinnamate isolated from Kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α, and angiogenesis by blocking endothelial functions. Clinics. 2014;69:134–144. doi: 10.6061/clinics/2014(02)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tewtrakul S., Subhadhirasakul S., Karalai C., Ponglimanont C., Cheenpracha S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009;115:534–538. doi: 10.1016/j.foodchem.2008.12.057. [DOI] [Google Scholar]
- 44.Tewtrakul S., Subhadhirasakul S. Effects of compounds from Kaempferia parviflora on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha productions in RAW264. 7 macrophage cells. J. Ethnopharmacol. 2008;120:81–84. doi: 10.1016/j.jep.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 45.Jagadish P.C., Latha K.P., Mudgal J., Nampurath G.K. Extraction, characterization and evaluation of Kaempferia galanga L.(Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016;194:434–439. doi: 10.1016/j.jep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Sawasdee P., Sabphon C., Sitthiwongwanit D., Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009;23:1792–1794. doi: 10.1002/ptr.2858. [DOI] [PubMed] [Google Scholar]
- 47.Azuma T., Kayano S.-i., Matsumura Y., Konishi Y., Tanaka Y., Kikuzaki H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011;125:471–475. doi: 10.1016/j.foodchem.2010.09.033. [DOI] [Google Scholar]
- 48.Ochiai W., Kobayashi H., Kitaoka S., Kashiwada M., Koyama Y., Nakaishi S., Nagai T., Aburada M., Sugiyama K. Effect of the active ingredient of Kaempferia parviflora, 5, 7-dimethoxyflavone, on the pharmacokinetics of midazolam. J. Nat. Med. 2018;72:607–614. doi: 10.1007/s11418-018-1184-z. [DOI] [PubMed] [Google Scholar]
- 49.Yorsin S., Kanokwiroon K., Radenahmad N., Jansakul C. Effects of Kaempferia parviflora rhizomes dichloromethane extract on vascular functions in middle-aged male rat. J. Ethnopharmacol. 2014;156:162–174. doi: 10.1016/j.jep.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Othman R., Ibrahim H., Mohd M.A., Mustafa M.R., Awang K. Bioassay-guided isolation of a vasorelaxant active compound from Kaempferia galanga L. Phytomedicine. 2006;13:61–66. doi: 10.1016/j.phymed.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Wattanapitayakul S.K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Chansuvanich N. Vasorelaxation and antispasmodic effects of Kaempferia parviflora ethanolic extract in isolated rat organ studies. Fitoterapia. 2008;79:214–216. doi: 10.1016/j.fitote.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 52.Pripdeevech P., Pitija K., Rujjanawate C., Pojanagaroon S., Kittakoop P., Wongpornchai S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012;132:1150–1155. doi: 10.1016/j.foodchem.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Matsushita M., Yoneshiro T., Aita S., Kamiya T., Kusaba N., Yamaguchi K., Takagaki K., Kameya T., Sugie H., Saito M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. 2015;61:79–83. doi: 10.3177/jnsv.61.79. [DOI] [PubMed] [Google Scholar]
- 54.Wattanathorn J., Muchimapura S., Tong-Un T., Saenghong N., Thukhum-Mee W., Sripanidkulchai B. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid. Based Complementary Altern. Med. 2012;2012:732816. doi: 10.1155/2012/732816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi S., Kato T., Azuma T., Kikuzaki H., Abe K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods. 2015;13:100–107. doi: 10.1016/j.jff.2014.12.029. [DOI] [Google Scholar]
- 56.Plaingam W., Sangsuthum S., Angkhasirisap W., Tencomnao T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complementary Med. 2017;7:538–552. doi: 10.1016/j.jtcme.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali M.S., Dash P.R., Nasrin M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Complementary Altern. Med. 2015;15:158. doi: 10.1186/s12906-015-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridtitid W., Sae-Wong C., Reanmongkol W., Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J. Ethnopharmacol. 2008;118:225–230. doi: 10.1016/j.jep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Shanbhag T.V., Sharma C., Adiga S., Bairy K., Shenoy S., Shenoy G. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J. Physiol. Pharmacol. 2006;50:384–390. [PubMed] [Google Scholar]
- 60.Temkitthawon P., Hinds T.R., Beavo J.A., Viyoch J., Suwanborirux K., Pongamornkul W., Sawasdee P., Ingkaninan K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J. Ethnopharmacol. 2011;137:1437–1441. doi: 10.1016/j.jep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein R.A., Schmid K., Bolivar J., Swick A.G., Joyal S.V., Hirsh S.P. Kaempferia parviflora ethanol extract improves self-assessed sexual health in men: A pilot study. J. Integr. Med. 2018;16:249–254. doi: 10.1016/j.joim.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Horigome S., Maeda M., Ho H.-J., Shirakawa H., Komai M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Foods. 2016;26:529–538. doi: 10.1016/j.jff.2016.08.008. [DOI] [Google Scholar]
- 63.Lert-Amornpat T., Maketon C., Fungfuang W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia. 2017;49:e12770. doi: 10.1111/and.12770. [DOI] [PubMed] [Google Scholar]
- 64.Lee S., Kim C., Kwon D., Kim M.-B., Hwang J.-K. Standardized Kaempferia parviflora Wall. ex Baker (Zingiberaceae) extract inhibits fat accumulation and muscle atrophy in ob/ob mice. Evid. Based Complementary Altern. Med. 2018;2018:8161042. doi: 10.1155/2018/8161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ono S., Yoshida N., Maekawa D., Kitakaze T., Kobayashi Y., Kitano T., Fujita T., Okuwa-Hayashi H., Harada N., Nakano Y. 5-Hydroxy-7-methoxyflavone derivatives from Kaempferia parviflora induce skeletal muscle hypertrophy. Food Sci. Nutr. 2019;7:312–321. doi: 10.1002/fsn3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin S., Lee M.-Y. Kaempferia parviflora extract as a potential anti-acne agent with anti-inflammatory, sebostatic and anti-propionibacterium acnes activity. Int. J. Mol. Sci. 2018;19:3457. doi: 10.3390/ijms19113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kongdang P., Jaitham R., Thonghoi S., Kuensaen C., Pradit W., Ongchai S. Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine. 2019;59:152755. doi: 10.1016/j.phymed.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 68.Dash P.R., Mou K.M., Erina I.N., Ripa F.A., Al Masud K.N., Ali M.S. Study of anthelmintic and insecticidal activities of different extracts of Kaempferia galanga. Int. J. Pharm. Sci. Res. 2017;8:729–733. [Google Scholar]
- 69.Jaipetch T., Reutrakul V., Tuntiwachwuttikul P., Santisuk T. Flavonoids in the black rhizomes of Boesenbergia panduta. Phytochemistry. 1983;22:625–626. doi: 10.1016/0031-9422(83)83075-1. [DOI] [Google Scholar]
- 70.Herunsalee A., Pancharoen O., Tuntiwachwuttikul P. Further studies of flavonoids of the black rhizome Boesenbergia pandurata. J. Sci. Soc. Thail. 1987;13:119–122. [Google Scholar]
- 71.Trakoontivakorn G., Nakahara K., Shinmoto H., Takenaka M., Onishi-Kameyama M., Ono H., Yoshida M., Nagata T., Tsushida T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J. Agric. Food Chem. 2001;49:3046–3050. doi: 10.1021/jf010016o. [DOI] [PubMed] [Google Scholar]
- 72.Raina A.P., Abraham Z. Chemical profiling of essential oil of Kaempferia galanga L. germplasm from India. J. Essent. Oil Res. 2016;28:29–34. doi: 10.1080/10412905.2015.1077165. [DOI] [Google Scholar]
- 73.Wong K., Ong K., Lim C. Compositon of the essential oil of rhizomes of kaempferia galanga L. Flavour Fragr. J. 1992;7:263–266. doi: 10.1002/ffj.2730070506. [DOI] [Google Scholar]
- 74.Win N.N., Ito T., Aimaiti S., Imagawa H., Ngwe H., Abe I., Morita H. Kaempulchraols A–H, diterpenoids from the rhizomes of Kaempferia pulchra collected in Myanmar. J. Nat. Prod. 2015;78:1113–1118. doi: 10.1021/acs.jnatprod.5b00108. [DOI] [PubMed] [Google Scholar]
- 75.Thongnest S., Mahidol C., Sutthivaiyakit S., Ruchirawat S. Oxygenated pimarane diterpenes from Kaempferia marginata. J. Nat. Prod. 2005;68:1632–1636. doi: 10.1021/np050186l. [DOI] [PubMed] [Google Scholar]
- 76.Prawat U., Tuntiwachwuttikul P., Taylor W.C., Engelhardt L.M., Skelton B., White A. Diterpenes from a Kaempferia species. Phytochemistry. 1993;32:991–997. doi: 10.1016/0031-9422(93)85242-J. [DOI] [Google Scholar]
- 77.Sutthanut K., Sripanidkulchai B., Yenjai C., Jay M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A. 2007;1143:227–233. doi: 10.1016/j.chroma.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 78.Umar M.I., Asmawi M.Z.B., Sadikun A., Altaf R., Iqbal M.A. Phytochemistry and medicinal properties of Kaempferia galanga L. (Zingiberaceae) extracts. Afr. J. Pharm. Pharmacol. 2011;5:1638–1647. doi: 10.5897/AJPP11.388. [DOI] [Google Scholar]
- 79.Azuma T., Tanaka Y., Kikuzaki H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry. 2008;69:2743–2748. doi: 10.1016/j.phytochem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Davis E.M., Croteau R. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top. Curr. Chem. 2000;209:53–95. [Google Scholar]
- 81.Xu M., Hillwig M.L., Tiernan M.S., Peters R.J. Probing labdane-related diterpenoid biosynthesis in the fungal genus Aspergillus. J. Nat. Prod. 2017;80:328–333. doi: 10.1021/acs.jnatprod.6b00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y.C., Ji H., Li X.H., Zhang H.X., Li H.T. Isolation of nematicidal constituents from essential oil of Kaempferia galanga L rhizome and their activity against Heterodera avenae Wollenweber. Trop. J. Pharm. Res. 2017;16:59–65. doi: 10.4314/tjpr.v16i1.8. [DOI] [Google Scholar]
- 83.Munda S., Saikia P., Lal M. Chemical composition and biological activity of essential oil of Kaempferia galanga: A review. J. Essent. Oil Res. 2018;30:303–308. doi: 10.1080/10412905.2018.1486240. [DOI] [Google Scholar]
- 84.Labrooy C.D., Abdullah T.L., Stanslas J. Identification of ethnomedicinally important Kaempferia L. (Zingiberaceae) species based on morphological traits and suitable DNA region. Curr. Plant Biol. 2018;14:50–55. doi: 10.1016/j.cpb.2018.09.004. [DOI] [Google Scholar]